Recent Advancements in Fish-on-Chip: A Comprehensive Review
Abstract
1. Introduction
- Section 2: Automated capture and culturing of zebrafish embryos.
- Section 3: High-resolution and advanced imaging techniques.
- Section 4: Novel techniques in drug and disease studies.
- Section 5: Novel stimulation and transportation methods.
- Section 6: Microinjection techniques and sperm retention studies.
- Section 7: Development of a smart microfluidics-based fish farm for zebrafish screening.
2. Automated Capture and Culturing of Zebrafish Embryos
3. High-Resolution and Advanced Imaging Techniques
4. Novel Techniques in Drug and Disease Studies
5. Novel Stimulation and Transportation Methods
6. Microinjection Techniques and Sperm Retention Studies
7. Development of a Smart Microfluidics-Based Fish Farm for Zebrafish Screening
- Design 1: This included incubation, observation, and culturing chambers, enabling a dynamic microscale flow-through perfusion and culturing system (Figure 25a).
- Design 2: This featured a straight long channel with an inlet opening for larvae loading. Effective transportation of larvae was achieved by combining tuned water flow with in-house developed animation patterns (Figure 25b).
- Room temperature (24–26 °C).
- Controlled temperature (28.5 °C) in a Petri dish.
- Controlled temperature (28.5 °C) in the fish farm.
8. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Basu, S.; Sachidanandan, C. Zebrafish: A multifaceted tool for chemical biologists. Chem. Rev. 2013, 113, 7952–7980. [Google Scholar] [CrossRef] [PubMed]
- White, R.; Rose, K.; Zon, L. Zebrafish cancer: The state of the art and the path forward. Nat. Rev. Cancer 2013, 13, 624–636. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Stewart, A.M.; Gerlai, R. Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol. Sci. 2014, 35, 63–75. [Google Scholar] [CrossRef]
- McGrath, P.; Li, C.-Q. Zebrafish: A predictive model for assessing drug-induced toxicity. Drug Discov. Today 2008, 13, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Cassar, S.; Adatto, I.; Freeman, J.L.; Gamse, J.T.; Iturria, I.; Lawrence, C.; Muriana, A.; Peterson, R.T.; Van Cruchten, S.; Zon, L.I. Use of zebrafish in drug discovery toxicology. Chem. Res. Toxicol. 2020, 33, 95–118. [Google Scholar] [CrossRef]
- Hwang, H.; Lu, H. Microfluidic tools for developmental studies of small model organisms –nematodes, fruit flies, and zebrafish. Biotechnol. J. 2012, 8, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Bayliss, P.E.; Bellavance, K.L.; Whitehead, G.G.; Abrams, J.M.; Aegerter, S.; Robbins, H.S.; Cowan, D.B.; Keating, M.T.; O’Reilly, T.; Wood, J.M.; et al. Chemical modulation of receptor signaling inhibits regenerative angiogenesis in adult zebrafish. Nat. Chem. Biol. 2006, 2, 265–273. [Google Scholar] [CrossRef]
- D’Amora, M.; Giordani, S. The Utility of Zebrafish as a Model for Screening Developmental Neurotoxicity. Front. Neurosci. 2018, 12, 976. [Google Scholar] [CrossRef]
- Dooley, K. Zebrafish: A model system for the study of human disease. Curr. Opin. Genet. Dev. 2000, 10, 252–256. [Google Scholar] [CrossRef]
- Shi, W.; Wen, H.; Lu, Y.; Shi, Y.; Lin, B.; Qin, J. Droplet microfluidics for characterizing the neurotoxin-induced responses in individual Caenorhabditis elegans. Lab Chip 2010, 10, 2855–2863. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Z.; Ching, P.; Shi, Q.; Li, X. An integrated microfluidic platform for evaluating in vivo antimicrobial activity of natural compounds using a whole-animal infection model. Lab Chip 2013, 13, 3373–3382. [Google Scholar] [CrossRef]
- Zhang, B.; Li, Y.; He, Q.; Qin, J.; Yu, Y.; Li, X.; Zhang, L.; Yao, M.; Liu, J.; Chen, Z. Microfluidic platform integrated with worm-counting setup for assessing manganese toxicity. Biomicrofluidics 2014, 8, 054110. [Google Scholar] [CrossRef]
- Chung, K.; Kim, Y.; Kanodia, J.S.; Gong, E.; Shvartsman, S.Y.; Lu, H. A microfluidic array for large-scale ordering and orientation of embryos. Nat. Methods 2010, 8, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Hulme, S.E.; Whitesides, G.M. Chemistry and the Worm: Caenorhabditis elegans as a Platform for Integrating Chemical and Biological Research. Angew. Chem. Int. Ed. Engl. 2011, 50, 4774–4807. [Google Scholar] [CrossRef]
- Wang, X.; Hu, R.; Ge, A.; Hu, L.; Wang, S.; Feng, X.; Du, W.; Liu, B.-F. Highly efficient microfluidic sorting device for synchronizing developmental stages of C. elegans based on deflecting electrotaxis. Lab Chip 2015, 15, 2513–2521. [Google Scholar] [PubMed]
- Bakhtina, N.A.; Korvink, J.G. Microfluidic laboratories for C. elegans enhance fundamental studies in biology. RSC Adv. 2013, 4, 4691–4709. [Google Scholar] [CrossRef]
- Yanik, M.F.; Rohde, C.B.; Pardo-Martin, C. Technologies for Micromanipulating, Imaging, and Phenotyping Small Invertebrates and Vertebrates. Annu. Rev. Biomed. Eng. 2011, 13, 185–217. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Lieschke, G.J.; Currie, P.D. Animal models of human disease: Zebrafish swim into view. Nat. Rev. Genet. 2007, 8, 353–367. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Wen, Z.-H.; Lin, C.-S.; Chakraborty, C. The Zebrafish Model: Use in Studying Cellular Mechanisms for a Spectrum of Clinical Disease Entities. Curr. Neurovasc. Res. 2007, 4, 111–120. [Google Scholar] [CrossRef]
- Chan, P.K.; Lin, C.C.; Cheng, S.H. Noninvasive technique for measurement of heartbeat regularity in zebrafish (Danio rerio) embryos. BMC Biotechnol. 2009, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Volkery, S.; Siekmann, A.F. Intubation-based anesthesia for long-term time-lapse imaging of adult zebrafish. Nat. Protoc. 2015, 10, 2064–2073. [Google Scholar] [CrossRef]
- Collymore, C.; Tolwani, A.; Lieggi, C.; Rasmussen, S. Efficacy and safety of 5 anesthetics in adult zebrafish (Danio rerio). J. Am. Assoc. Lab Anim. Sci. 2014, 53, 198–203. [Google Scholar]
- Nordgreen, J.; Tahamtani, F.M.; Janczak, A.M.; Horsberg, T.E. Behavioural effects of the commonly used fish anaesthetic tricaine methanesulfonate (MS-222) on zebrafish (Danio rerio) and its relevance for the acetic acid pain test. PLoS ONE 2014, 9, e92116. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, A.; Mickoleit, M.; Weber, M.; Huisken, J. Multilayer mounting enables long-term imaging of zebrafish development in a light sheet microscope. Development 2012, 139, 3242–3247. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.-C.; Li, D.; Al-Shoaibi, A.; Bersano-Begey, T.; Chen, H.; Ali, S.; Flak, B.; Perrin, C.; Winslow, M.; Shah, H.; et al. A Student Team in a University of Michigan Biomedical Engineering Design Course Constructs a Microfluidic Bioreactor for Studies of Zebrafish Development. Zebrafish 2009, 6, 201–213. [Google Scholar] [CrossRef]
- Akagi, J.; Khoshmanesh, K.; Evans, B.; Hall, C.J.; Crosier, K.E.; Cooper, J.M.; Crosier, P.S.; Wlodkowic, D. Miniaturized Embryo Array for Automated Trapping, Immobilization and Microperfusion of Zebrafish Embryos. PLoS ONE 2012, 7, e36630. [Google Scholar] [CrossRef]
- Akagi, J.; Khoshmanesh, K.; Hall, C.J.; Cooper, J.M.; Crosier, K.E.; Crosier, P.S.; Wlodkowic, D. Fish on chips: Microfluidic living embryo array for accelerated in vivo angiogenesis assays. Sens. Actuators B Chem. 2013, 189, 11–20. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Gelinas, D.; Ciruna, B.; Sun, Y. A Fully Automated Robotic System for Microinjection of Zebrafish Embryos. PLoS ONE 2007, 2, e862. [Google Scholar] [CrossRef]
- Bischel, L.L.; Mader, B.R.; Green, J.M.; Huttenlocher, A.; Beebe, D.J. Zebrafish Entrapment by Restriction Array (ZEBRA) device: A low-cost, agarose-free zebrafish mounting technique for automated imaging. Lab Chip 2013, 13, 1732–1736. [Google Scholar] [CrossRef]
- Yang, F.; Chen, Z.; Pan, J.; Li, X.; Feng, J.; Yang, H. An integrated microfluidic array system for evaluating toxicity and teratogenicity of drugs on embryonic zebrafish developmental dynamics. Biomicrofluidics 2011, 5, 024115. [Google Scholar] [CrossRef]
- Lin, X.; Wang, S.; Yu, X.; Liu, Z.; Wang, F.; Li, W.T.; Cheng, S.H.; Dai, Q.; Shi, P. High-throughput mapping of brain-wide activity in awake and drug-responsive vertebrates. Lab Chip 2015, 15, 680–689. [Google Scholar] [PubMed]
- Choudhury, D.; van Noort, D.; Iliescu, C.; Zheng, B.; Poon, K.-L.; Korzh, S.; Korzh, V.; Yu, H. Fish and Chips: A microfluidic perfusion platform for monitoring zebrafish development. Lab Chip 2011, 12, 892–900. [Google Scholar] [CrossRef]
- Wielhouwer, E.M.; Ali, S.; Al-Afandi, A.; Blom, M.T.; Riekerink, M.B.O.; Poelma, C.; Westerweel, J.; Oonk, J.; Vrouwe, E.X.; Buesink, W.; et al. Zebrafish embryo development in a microfluidic flow-through system. Lab Chip 2011, 11, 1815–1824. [Google Scholar] [CrossRef]
- Li, Y.; Yang, F.; Chen, Z.; Shi, L.; Zhang, B.; Pan, J.; Li, X.; Sun, D.; Yang, H. Zebrafish on a Chip: A Novel Platform for Real-Time Monitoring of Drug-Induced Developmental Toxicity. PLoS ONE 2014, 9, e94792. [Google Scholar] [CrossRef]
- Noori, A.; Selvaganapathy, P.R.; Wilson, J. Microinjection in a microfluidic format using flexible and compliant channels and electroosmotic dosage control. Lab Chip 2009, 9, 3202–3211. [Google Scholar] [CrossRef]
- Bansal, T.; Lenhart, J.; Kim, T.; Duan, C.; Maharbiz, M.M. Patterned delivery and expression of gene constructs into zebrafish embryos using microfabricated interfaces. Biomed. Microdevices 2009, 11, 633–641. [Google Scholar] [CrossRef]
- Funfak, A.; Brösing, A.; Brand, M.; Köhler, J.M. Micro fluid segment technique for screening and development studies on Danio rerio embryos. Lab Chip 2007, 7, 1132–1138. [Google Scholar] [CrossRef]
- Erickstad, M.; Hale, L.A.; Chalasani, S.H.; Groisman, A. A microfluidic system for studying the behavior of zebrafish larvae under acute hypoxia. Lab Chip 2014, 15, 857–866. [Google Scholar] [CrossRef]
- Macdonald, N.P.; Zhu, F.; Hall, C.J.; Reboud, J.; Crosier, P.S.; Patton, E.E.; Wlodkowic, D.; Cooper, J.M. Assessment of biocompatibility of 3D printed photopolymers using zebrafish embryo toxicity assays. Lab Chip 2015, 16, 291–297. [Google Scholar] [CrossRef]
- Candelier, R.; Murmu, M.S.; Romano, S.A.; Jouary, A.; Debrégeas, G.; Sumbre, G. A microfluidic device to study neuronal and motor responses to acute chemical stimuli in zebrafish. Sci. Rep. 2015, 5, 12196. [Google Scholar] [CrossRef]
- Zhu, F.; Wigh, A.; Friedrich, T.; Devaux, A.; Bony, S.; Nugegoda, D.; Kaslin, J.; Wlodkowic, D. Automated Lab-on-a-Chip Technology for Fish Embryo Toxicity Tests Performed under Continuous Microperfusion (μFET). Environ. Sci. Technol. 2015, 49, 14570–14578. [Google Scholar] [CrossRef] [PubMed]
- Samuel, R.; Stephenson, R.; Roy, P.; Pryor, R.; Zhou, L.; Bonkowsky, J.L.; Gale, B.K. Microfluidic-aided genotyping of zebrafish in the first 48 h with 100% viability. Biomed. Microdevices 2015, 17, 43. [Google Scholar] [CrossRef][Green Version]
- Zheng, C.; Zhou, H.; Liu, X.; Pang, Y.; Zhang, B.; Huang, Y. Fish in chips: An automated microfluidic device to study drug dynamics in vivo using zebrafish embryos. Chem. Commun. 2013, 50, 981–984. [Google Scholar] [CrossRef]
- Li, Y.; Yang, X.; Chen, Z.; Zhang, B.; Pan, J.; Li, X.; Yang, F.; Sun, D. Comparative toxicity of lead (Pb2+), copper (Cu2+), and mixtures of lead and copper to zebrafish embryos on a microfluidic chip. Biomicrofluidics 2015, 9, 024105. [Google Scholar] [CrossRef] [PubMed]
- Sy, S.K.H.; Chan, D.C.W.; Chan, R.C.H.; Lyu, J.; Li, Z.; Wong, K.K.Y.; Choi, C.H.J.; Mok, V.C.T.; Lai, H.-M.; Randlett, O.; et al. An optofluidic platform for interrogating chemosensory behavior and brainwide neural representation in larval zebrafish. Nat. Commun. 2023, 14, 227. [Google Scholar] [CrossRef] [PubMed]
- Sy, S.K.H.; Ko, H. Fish-on-Chips: Unveiling neural processing of chemicals in small animals through precise fluidic control. Neural Regen. Res. 2024, 19, 2351–2353. [Google Scholar] [CrossRef]
- Peimani, A.R.; Zoidl, G.; Rezai, P. A microfluidic device for quantitative investigation of zebrafish larvae’s rheotaxis. Biomed. Microdevices 2017, 19, 99. [Google Scholar] [CrossRef]
- Peimani, A.R.; Zoidl, G.; Rezai, P. A microfluidic device to study electrotaxis and dopaminergic system of zebrafish larvae. Biomicrofluidics 2018, 12, 014113. [Google Scholar] [CrossRef]
- Khalili, A.; Peimani, A.R.; Safarian, N.; Youssef, K.; Zoidl, G.; Rezai, P. Phenotypic chemical and mutant screening of zebrafish larvae using an on-demand response to electric stimulation. Integr. Biol. 2019, 11, 373–383. [Google Scholar] [CrossRef]
- Khalili, A.; van Wijngaarden, E.; Zoidl, G.R.; Rezai, P. Zebrafish larva’s response and habituation to electric signal: Effects of voltage, current and pulsation studied in a microfluidic device. Sens. Actuators A Phys. 2021, 332, 113070. [Google Scholar] [CrossRef]
- Khalili, A.; Wijngaarden, E.; Youssef, K.; Zoidl, G.R.; Rezai, P. Designing microfluidic devices for behavioral screening of multiple zebrafish larvae. Biotechnol. J. 2021, 17, 2100076. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Lee, S.M.-Y.; Li, C.-W. Studying the time course of cardiac responses of the same zebrafish using scalable fish-dock microarchitecture. Sens. Actuators B Chem. 2018, 268, 245–254. [Google Scholar] [CrossRef]
- He, L.; Du, H.; Yang, Y.; Guan, Z.; Li, J.; Li, H.; Lin, X.; Zhu, L. A lateral-immobilization zebrafish microfluidic chip-based system for in vivo real-time evaluation of antithrombotic agents. Chin. Chem. Lett. 2023, 35, 109013. [Google Scholar] [CrossRef]
- Panuška, P.; Nejedlá, Z.; Smejkal, J.; Aubrecht, P.; Liegertová, M.; Štofik, M.; Havlica, J.; Malý, J. A Millifluidic Chip for Cultivationof Fish Embryos and Toxicity Testing Fabricated by 3D PrintingTechnology. RSC Adv. 2021, 11, 20507–20518. [Google Scholar]
- Cho, S.-J.; Kang, Y.J.; Kim, S. High-throughput zebrafish intramuscular recording assay. Sens. Actuators B Chem. 2019, 304, 127332. [Google Scholar] [CrossRef]
- Lee, Y.; Seo, H.W.; Lee, K.J.; Jang, J.-W.; Kim, S. A Microfluidic System for Stable and Continuous EEG Monitoring from Multiple Larval Zebrafish. Sensors 2020, 20, 5903. [Google Scholar] [CrossRef]
- Zhu, Z.; Geng, Y.; Yuan, Z.; Ren, S.; Liu, M.; Meng, Z.; Pan, D. A Bubble-Free Microfluidic Device for Easy-to-Operate Immobilization, Culturing and Monitoring of Zebrafish Embryos. Micromachines 2019, 10, 168. [Google Scholar] [CrossRef]
- Zhu, Z.; Geng, Y.; Yuan, Z.; Ren, S.; Liu, M.; Meng, Z.; Pan, D. A Bubble-Free and Low-Shear-Stress Microfluidic Device for High-Quality Monitoring of Zebrafish Embryonic Development. In Proceedings of the 2019 20th International Conference on Solid-State Sensors, Actuators and Microsystems & Eurosensors XXXIII (TRANSDUCERS & EUROSENSORS XXXIII), Berlin, Germany, 23–27 June 2019; pp. 921–924. [Google Scholar]
- Chen, Z.; Liu, X.; Tang, X.; Li, Y.; Liu, D.; Li, Y.; Huang, Q.; Arai, T. On-Chip Automatic Trapping and Rotating for Zebrafish Embryo Injection. IEEE Robot. Autom. Lett. 2022, 7, 10850–10856. [Google Scholar] [CrossRef]
- Popova, A.A.; Marcato, D.; Peravali, R.; Wehl, I.; Schepers, U.; Levkin, P.A. Fish-Microarray: A Miniaturized Platform for Single-Embryo High-Throughput Screenings. Adv. Funct. Mater. 2017, 28, 1703486. [Google Scholar] [CrossRef]
- Zhang, G.; Tong, M.; Zhuang, S.; Yu, X.; Sun, W.; Lin, W.; Gao, H. Zebrafish Larva Orientation and Smooth Aspiration Control for Microinjection. IEEE Trans. Biomed. Eng. 2020, 68, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.; Lin, W.; Zhang, A.; Qian, C.; Li, L.; Qiu, J.; Gao, H. Visual Detection and Two-Dimensional Rotation Control in Zebrafish Larva Heart Microinjection. IEEE ASME Trans. Mechatron. 2017, 22, 2003–2012. [Google Scholar] [CrossRef]
- Ye, S.; Chin, W.-C.; Ni, C.-W. A multi-depth spiral milli fluidic device for whole mount zebrafish antibody staining. Biomed. Microdevices 2023, 25, 30. [Google Scholar] [CrossRef]
- Roy, S. Fatigue Simulation Technologies of Cold Expansion Processed Aerospace Aluminum and Rail Steel Holes Using the Extended Finite Element Method; Washington State University: Pullman, WA, USA, 2021; Available online: https://hdl.handle.net/2376/119050 (accessed on 25 December 2024).
- Roy, S.; Kim, D. Effect of Material Property and Hole Cold Expansion Rate on Fatigue Crack Initiation and Propagation on 2024-T351 Aluminum Alloy Holes. 2020. Available online: https://www.researchgate.net/publication/385010113_Effect_of_Material_Property_and_Hole_Cold_Expansion_Rate_on_Fatigue_Crack_Initiation_and_Propagation_on_2024-T351_Aluminum_Alloy_Holes?channel=doi&linkId=6711d9bad796f96b8ebd7ebb&showFulltext=true (accessed on 25 December 2024).
- Chen, J.; Saili, K.S.; Liu, Y.; Li, L.; Zhao, Y.; Jia, Y.; Bai, C.; Tanguay, R.L.; Dong, Q.; Huang, C. Developmental bisphenol A exposure impairs sperm function and reproduction in zebrafish. Chemosphere 2017, 169, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Riesco, M.F.; Robles, V. Cryopreservation Causes Genetic and Epigenetic Changes in Zebrafish Genital Ridges. PLoS ONE 2013, 8, e67614. [Google Scholar] [CrossRef]
- DeBaun, M.R.; Niemitz, E.L.; Feinberg, A.P. Association of in vitro fertilization with Beckwith-Wiedemann syndrome and epigenetic alterations of LIT1 and H19. Am. J. Hum. Genet. 2003, 72, 156–160. [Google Scholar] [CrossRef]
- Scherr, T.; Knapp, G.L.; Guitreau, A.; Park, D.S.-W.; Tiersch, T.; Nandakumar, K.; Monroe, W.T. Microfluidics and numerical simulation as methods for standardization of zebrafish sperm cell activation. Biomed. Microdevices 2015, 17, 65. [Google Scholar] [CrossRef]
- Park, D.S.; Egnatchik, R.A.; Bordelon, H.; Tiersch, T.R.; Monroe, W.T. Microfluidic mixing for sperm activation and motility analysis of pearl Danio zebrafish. Theriogenology 2012, 78, 334–344. [Google Scholar] [CrossRef]
- Mattern, K.; von Trotha, J.W.; Erfle, P.; Köster, R.W.; Dietzel, A. NeuroExaminer: An all-glass microfluidic device for whole-brain in vivo imaging in zebrafish. Commun. Biol. 2020, 3, 311. [Google Scholar] [CrossRef]
- Schrödter, D.; Mozafari, M.; Fichtner, J.; von Trotha, J.W.; Köster, R.W.; Dietzel, A. A 3D tailored monolithic glass chip for stimulating and recording zebrafish neuronal activity with a commercial light sheet microscope. Front. Lab Chip Technol. 2024, 3, 1346439. [Google Scholar] [CrossRef]
- Chen, C.; Gu, Y.; Philippe, J.; Zhang, P.; Bachman, H.; Zhang, J.; Mai, J.; Rufo, J.; Rawls, J.F.; Davis, E.E.; et al. Acoustofluidic rotational tweezing enables high-speed contactless morphological phenotyping of zebrafish larvae. Nat. Commun. 2021, 12, 1118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Yu, X.; Huang, G.; Lei, D.; Tong, M. An improved automated zebrafish larva high-throughput imaging system. Comput. Biol. Med. 2021, 136, 104702. [Google Scholar] [CrossRef] [PubMed]
- Mani, K.; Chien, T.-C.C.; Panigrahi, B.; Chen, C.-Y. Manipulation of zebrafish’s orientation using artificial cilia in a microchannel with actively adaptive wall design. Sci. Rep. 2016, 6, 36385. [Google Scholar] [CrossRef]
- Subendran, S.; Kang, C.-W.; Chen, C.-Y. Comprehensive Hydrodynamic Investigation of Zebrafish Tail Beats in a Microfluidic Device with a Shape Memory Alloy. Micromachines 2021, 12, 68. [Google Scholar] [CrossRef]
- Khalili, A.; van Wijngaarden, E.; Zoidl, G.R.; Rezai, P. Multi-phenotypic and bi-directional behavioral screening of zebrafish larvae. Integr. Biol. 2020, 12, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Khalili, A.; van Wijngaarden, E.; Zoidl, G.R.; Rezai, P. Simultaneous screening of zebrafish larvae cardiac and respiratory functions: A microfluidic multi-phenotypic approach. Integr. Biol. 2022, 14, 162–170. [Google Scholar] [CrossRef]
- Lin, X.; Li, V.W.; Chen, S.; Chan, C.Y.; Cheng, S.H.; Shi, P. Autonomous system for cross-organ investigation of ethanol-induced acute response in behaving larval zebrafish. Biomicrofluidics 2016, 10, 024123. [Google Scholar] [CrossRef]
- Panigrahi, B.; Chen, C.-Y. Microfluidic Transportation Control of Larval Zebrafish through Optomotor Regulations under a Pressure-Driven Flow. Micromachines 2019, 10, 880. [Google Scholar] [CrossRef]
- Mani, K.; Hsieh, Y.-C.; Panigrahi, B.; Chen, C.-Y. A noninvasive light driven technique integrated microfluidics for zebrafish larvae transportation. Biomicrofluidics 2018, 12, 021101. [Google Scholar] [CrossRef]
- Mani, K.; Chen, C.-Y. A non-invasive acoustic-trapping of zebrafish microfluidics. Biomicrofluidics 2021, 15, 014109. [Google Scholar] [CrossRef]
- Loganathan, D.; Wu, S.-H.; Chen, C.-Y. Behavioural responses of zebrafish with sound stimuli in microfluidics. Lab Chip 2022, 23, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, B.; Chen, C.-Y. Microfluidic retention of progressively motile zebrafish sperms. Lab Chip 2019, 19, 4033–4042. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, B.; Lu, C.-H.; Huang, P.-F.; Chen, C.-Y. An artificial cilia-based micromixer towards the activation of zebrafish sperms. Sens. Actuators B Chem. 2017, 244, 541–548. [Google Scholar] [CrossRef]
- Yang, F.; Gao, C.; Wang, P.; Zhang, G.-J.; Chen, Z. Fish-on-a-chip: Microfluidics for zebrafish research. Lab Chip 2016, 16, 1106–1125. [Google Scholar] [CrossRef]
- Xie, Y.; Wahab, L.; Gill, J.J. Development and Validation of a Microtiter Plate-Based Assay for Determination of Bacteriophage Host Range and Virulence. Viruses 2018, 10, 189. [Google Scholar] [CrossRef] [PubMed]
- Buie, J. Evolution of microplate technology. Lab Manag. 2010, 5, 46. [Google Scholar]
- Fernández-Suárez, M.; Ting, A.Y. Fluorescent probes for super-resolution imaging in living cells. Nat. Rev. Mol. Cell Biol. 2008, 9, 929–943. [Google Scholar] [CrossRef]
- Hansen, A.S.; Hao, N.; O'Shea, E.K. High-throughput microfluidics to control and measure signaling dynamics in single yeast cells. Nat. Protoc. 2015, 10, 1181–1197. [Google Scholar] [CrossRef]
- Yaniv, K.; Isogai, S.; Castranova, D.; Dye, L.; Hitomi, J.; Weinstein, B.M. Live imaging of lymphatic development in the zebrafish. Nat. Med. 2006, 12, 711–716. [Google Scholar] [CrossRef]
- Huisken, J.; Swoger, J.; Del Bene, F.; Wittbrodt, J.; Stelzer, E.H.K. Optical sectioning deep inside live embryos by selective plane illumination microscopy. Science 2004, 305, 1007–1009. [Google Scholar] [CrossRef]
- Suli, A.; Watson, G.M.; Rubel, E.W.; Raible, D.W. Rheotaxis in Larval Zebrafish Is Mediated by Lateral Line Mechanosensory Hair Cells. PLoS ONE 2012, 7, e29727. [Google Scholar] [CrossRef]
- Katoch, S.; Patial, V. Zebrafish: An emerging model system to study liver diseases and related drug discovery. J. Appl. Toxicol. 2020, 41, 33–51. [Google Scholar] [CrossRef] [PubMed]
- Patton, E.E.; Zon, L.I.; Langenau, D.M. Zebrafish disease models in drug discovery: From preclinical modelling to clinical trials. Nat. Rev. Drug Discov. 2021, 20, 611–628. [Google Scholar] [CrossRef]
- Li, D.; March, M.E.; Gutierrez-Uzquiza, A.; Kao, C.; Seiler, C.; Pinto, E.; Matsuoka, L.S.; Battig, M.R.; Bhoj, E.J.; Wenger, T.L.; et al. ARAF recurrent mutation causes central conducting lymphatic anomaly treatable with a MEK inhibitor. Nat. Med. 2019, 25, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- North, T.E.; Babu, I.R.; Vedder, L.M.; Lord, A.M.; Wishnok, J.S.; Tannenbaum, S.R.; Zon, L.I.; Goessling, W. PGE2-regulated wnt signaling and N-acetylcysteine are synergistically hepatoprotective in zebrafish acetaminophen injury. Proc. Natl. Acad. Sci. USA 2010, 107, 17315–17320. [Google Scholar] [CrossRef]
- Griffin, A.; Hamling, K.R.; Knupp, K.; Hong, S.; Lee, L.P.; Baraban, S.C. Clemizole and modulators of serotonin signalling suppress seizures in Dravet syndrome. Brain 2017, 140, 669–683. [Google Scholar] [CrossRef]
- MacRae, C.A.; Peterson, R.T. Zebrafish as tools for drug discovery. Nat. Rev. Drug Discov. 2015, 14, 721–731. [Google Scholar] [CrossRef]
- Zhu, F.; Baker, D.; Skommer, J.; Sewell, M.; Wlodkowic, D. Real-time 2D visualization of metabolic activities in zebrafish embryos using a microfluidic technology. Cytom. Part A 2015, 87, 446–450. [Google Scholar] [CrossRef]
- Mackman, N. Triggers, targets and treatments for thrombosis. Nature 2008, 451, 914–918. [Google Scholar] [CrossRef]
- Zhu, X.-Y.; Liu, H.-C.; Guo, S.-Y.; Xia, B.; Song, R.-S.; Lao, Q.-C.; Xuan, Y.-X.; Li, C.-Q. A Zebrafish Thrombosis Model for Assessing Antithrombotic Drugs. Zebrafish 2016, 13, 335–344. [Google Scholar] [CrossRef]
- Gregory, M.; Hanumanthaiah, R.; Jagadeeswaran, P. Genetic Analysis of Hemostasis and Thrombosis Using Vascular Occlusion. Blood Cells Mol. Dis. 2002, 29, 286–295. [Google Scholar] [CrossRef]
- Cianciolo Cosentino, C.; Roman, B.L.; Drummond, I.A.; Hukriede, N.A. Intravenous Microinjections of Zebrafish Larvae to Study Acute Kidney Injury. J. Vis. Exp. 2010, 42, e2079. [Google Scholar] [CrossRef]
- Fetcho, J.R.; Liu, K.S. Zebrafish as a Model System for Studying Neuronal Circuits and Behavior. Ann. N. Y. Acad. Sci. 1998, 860, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Luna, J.; Al-Jubouri, Q.; Al-Nuaimy, W.; Sneddon, L.U. Reduction in activity by noxious chemical stimulation is ameliorated by immersion in analgesic drugs in zebrafish. J. Exp. Biol. 2017, 220, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- Migault, G.; van der Plas, T.L.; Trentesaux, H.; Panier, T.; Candelier, R.; Proville, R.; Englitz, B.; Debregeas, G.; Bormuth, V. Whole-brain calcium imaging during physiological vestibular stimulation in larval zebrafish. Curr. Biol. 2018, 28, 3723–3735.e6. [Google Scholar] [CrossRef]
- Messina, A.; Potrich, D.; Schiona, I.; Sovrano, V.A.; Fraser, S.E.; Brennan, C.H.; Vallortigara, G. Response to change in the number of visual stimuli in zebrafish:A behavioural and molecular study. Sci. Rep. 2020, 10, 5769. [Google Scholar] [CrossRef]
- Koide, T.; Yabuki, Y.; Yoshihara, Y. Terminal nerve gnrh3 neurons mediate slow avoidance of carbon dioxide in larval zebrafish. Cell Rep. 2018, 22, 1115–1123. [Google Scholar] [CrossRef]
- Dlugos, C.A.; Rabin, R.A. Structural and Functional Effects of Developmental Exposure to Ethanol on the Zebrafish Heart. Alcohol. Clin. Exp. Res. 2010, 34, 1013–1021. [Google Scholar] [CrossRef]
- Puttonen, H.A.J.; Sundvik, M.; Rozov, S.; Chen, Y.-C.; Panula, P. Acute ethanol treatment upregulates th1, th2, and hdc in larval zebrafish in stable networks. Front. Neural Circuits 2013, 7, 102. [Google Scholar] [CrossRef]
- Olszewski, J.; Haehnel, M.; Taguchi, M.; Liao, J.C. Zebrafish Larvae Exhibit Rheotaxis and Can Escape a Continuous Suction Source Using Their Lateral Line. PLoS ONE 2012, 7, e36661. [Google Scholar] [CrossRef]
- Chow, Y.T.; Chen, S.; Liu, C.; Liu, C.; Li, L.; Kong, C.W.M.; Cheng, S.H.; Li, R.A.; Sun, D. A High-Throughput Automated Microinjection System for Human Cells with Small Size. IEEE ASME Trans. Mechatron. 2015, 21, 838–850. [Google Scholar] [CrossRef]
- Xie, M.; Shakoor, A.; Shen, Y.; Mills, J.K.; Sun, D. Out-of-Plane Rotation Control of Biological Cells with a Robot-Tweezers Manipulation System for Orientation-Based Cell Surgery. IEEE Trans. Biomed. Eng. 2018, 66, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Latt, W.T.; Tan, S.Y.M.; Ang, W.T. Visual Servoed Three-Dimensional Cell Rotation System. IEEE Trans. Biomed. Eng. 2015, 62, 2498–2507. [Google Scholar] [CrossRef]
- Hagedorn, M.; Carter, V.L. Zebrafish Reproduction: Revisiting In Vitro Fertilization to Increase Sperm Cryopreservation Success. PLoS ONE 2011, 6, e21059. [Google Scholar] [CrossRef] [PubMed]
- Mani, K.; Chen, C.-Y. A smart microfluidic-based fish farm for zebrafish screening. Microfluid. Nanofluidics 2021, 25, 22. [Google Scholar] [CrossRef]





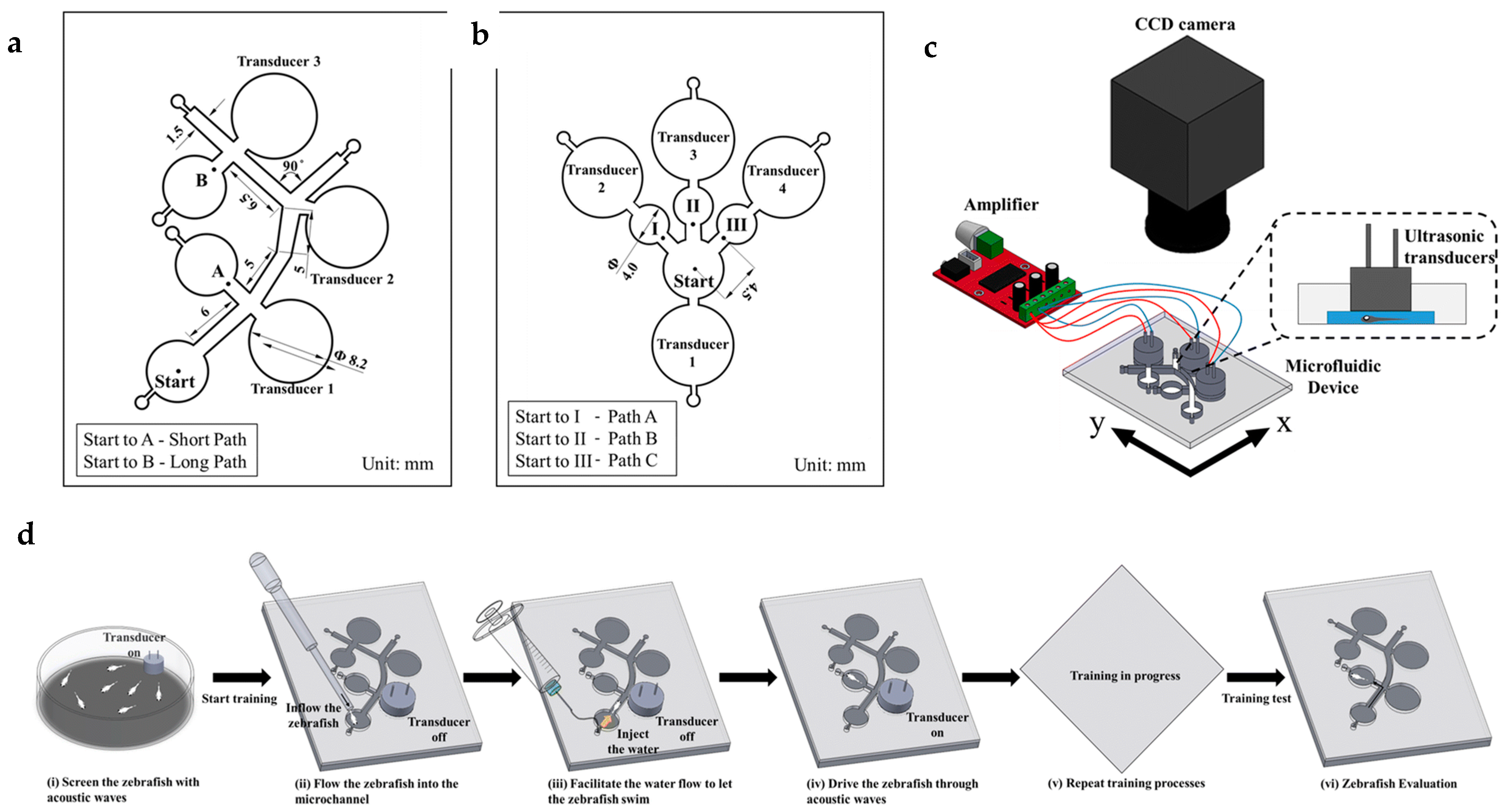


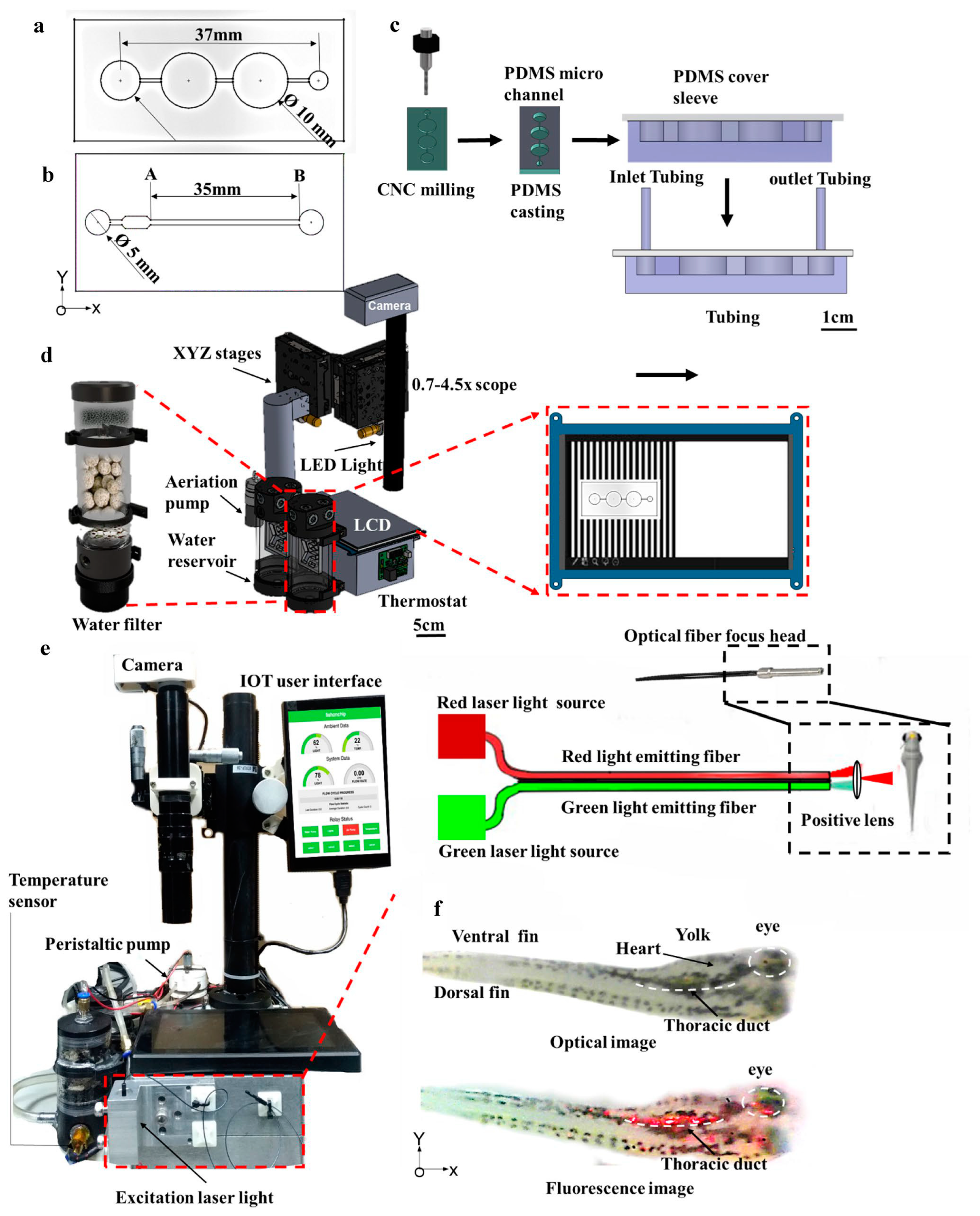

| Automated Capture and Culturing | ||
| Trapping Principle | Feature | Reference |
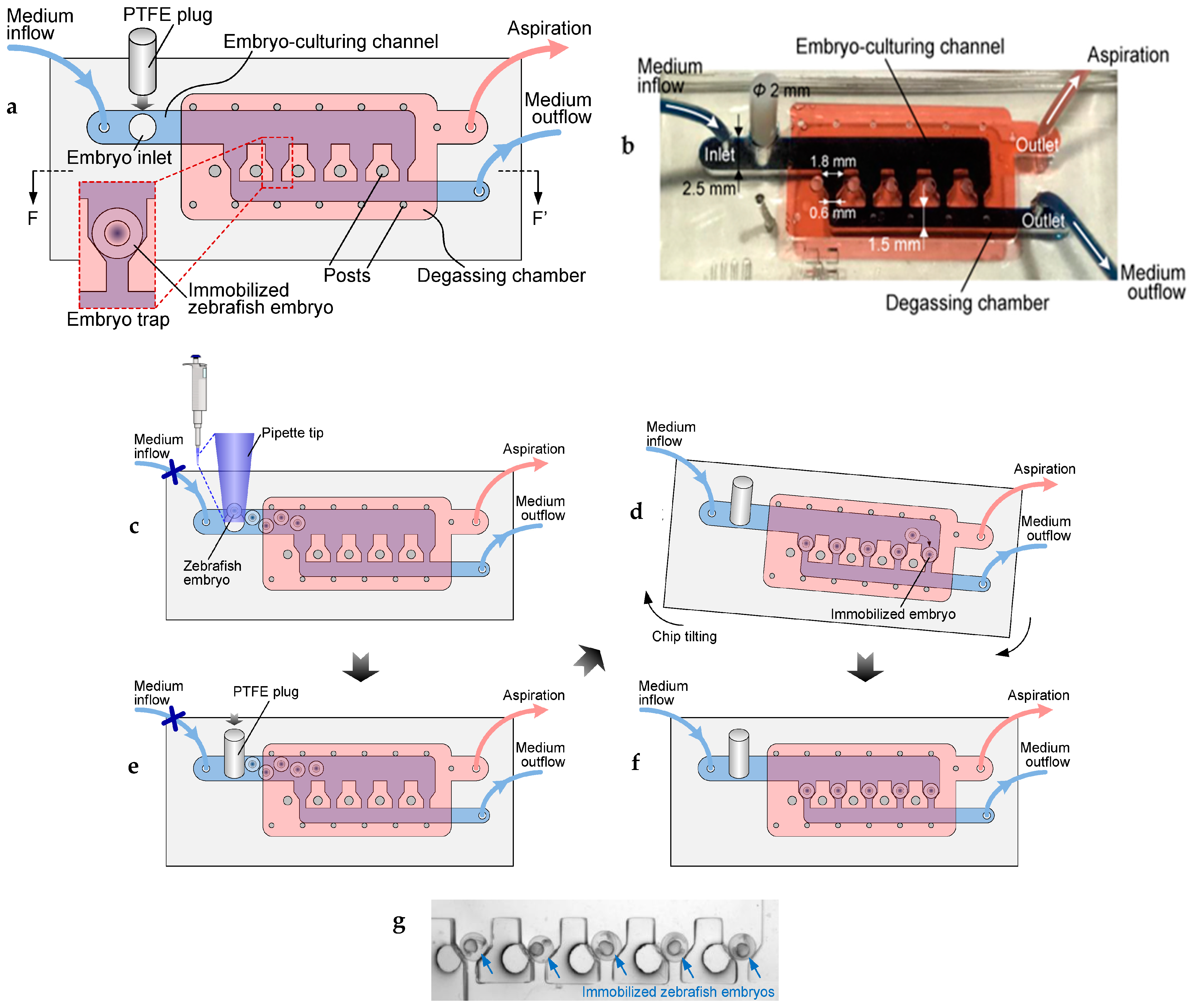
| Gravity and hydrodynamic force | Degassing chamber to remove air bubbles. | [58,59] |
| Microbubbles exposed to acoustic wave with a specific frequency | Automatic trapping and rotating of the embryo. Suitable for microinjection process. | [60] |
| Hydrodynamic suction | Whole-mount zebrafish antibody staining (ABS) by automatic tapping and immobilization of chorion-less embryos. | [64] |
| Discontinuous dewetting based on patterns of hydrophilic spots separated by superhydrophobic borders | High-throughput screening array. | [61] |
| High-Resolution and Advanced Imaging | ||
| Imaging system | Feature | Reference |
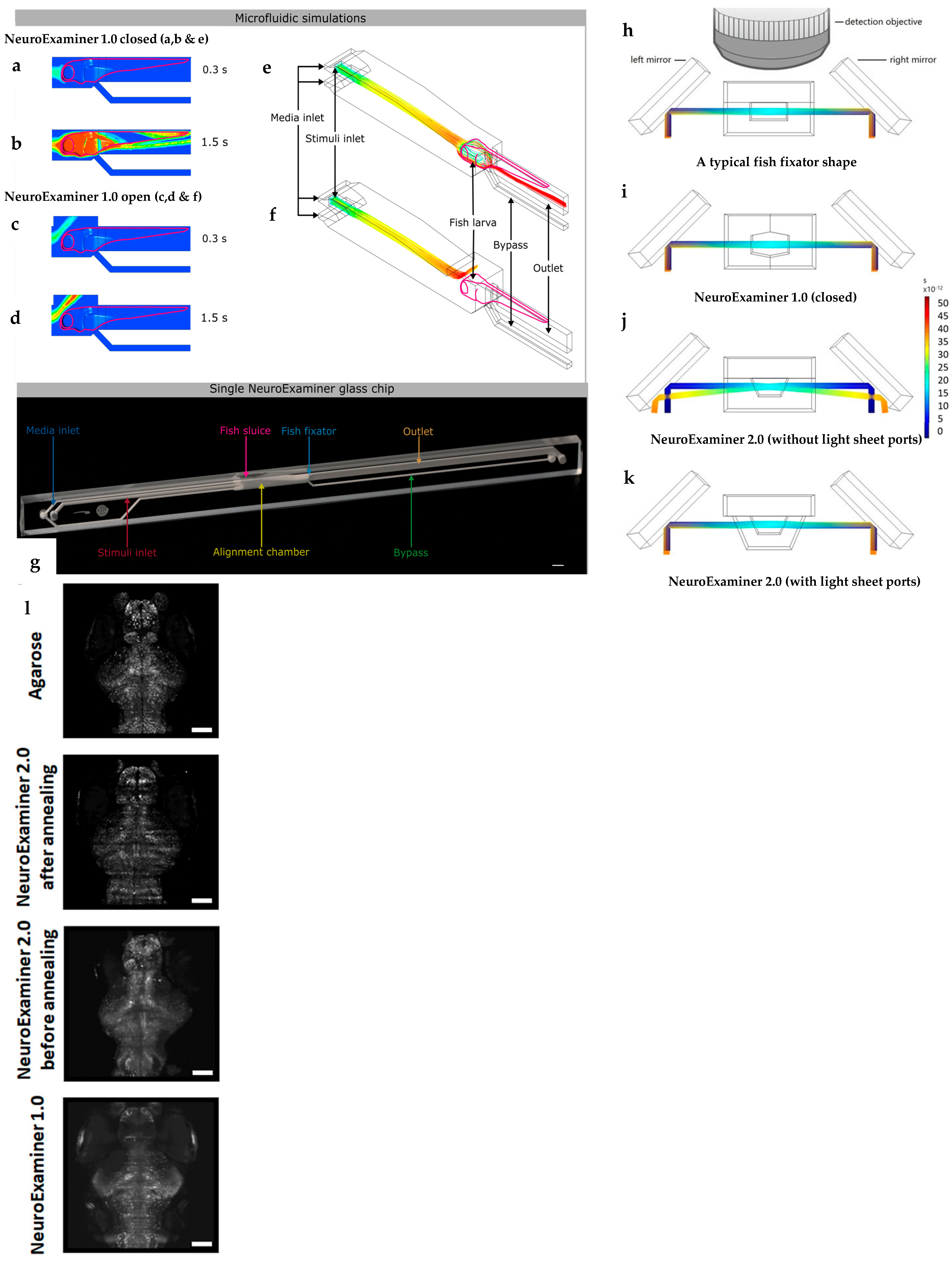
| Light sheet microscopy (LSM) | All glass microfluidic device in combination with LSM for whole brain in vivo imaging. | [72,73] |
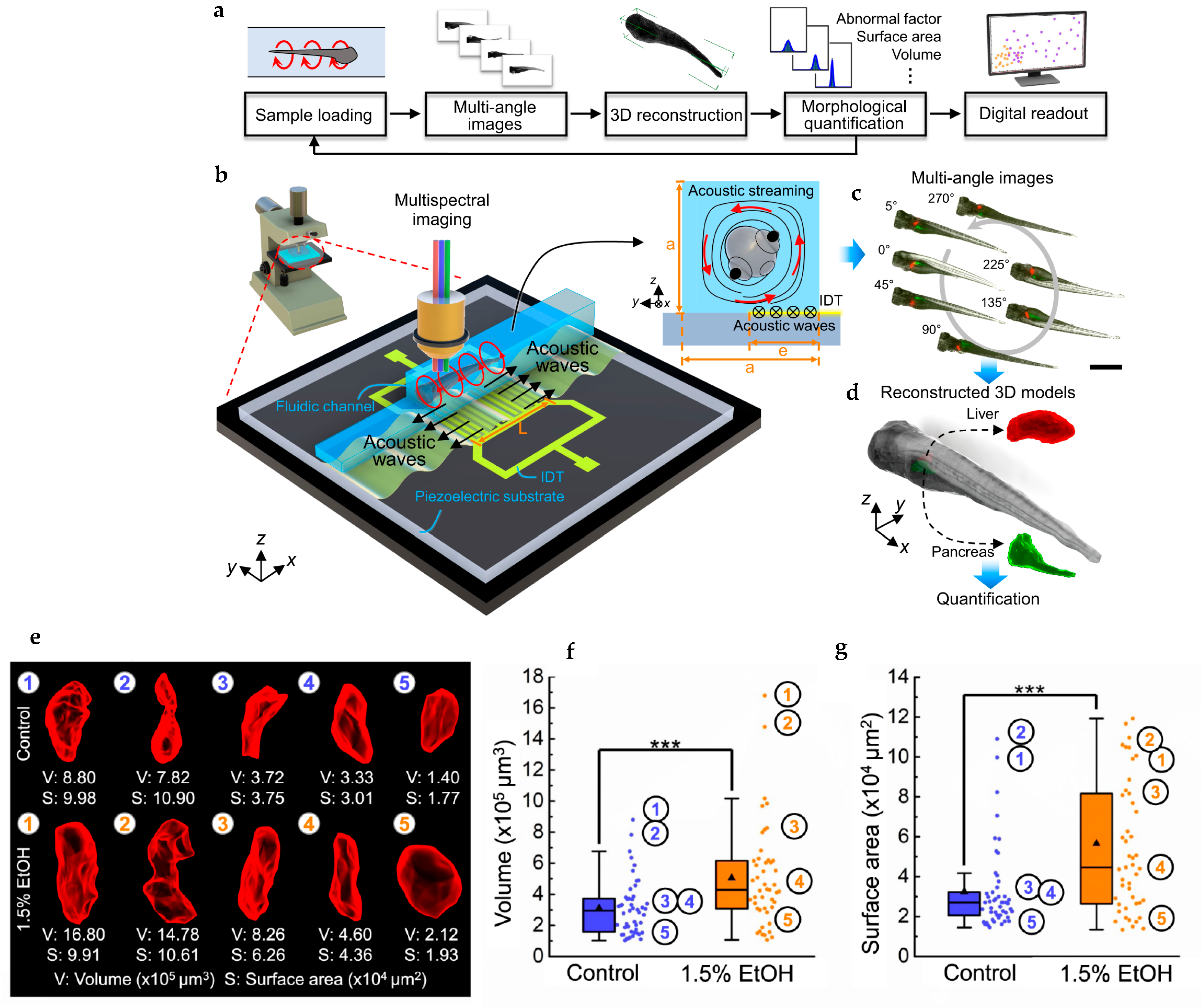
| Microscopic imaging utilizing acoustofluidic rotational tweezing (ART) | Contactless, high-speed, 3D multispectral imaging and digital reconstruction of zebrafish larvae for quantitative phenotypic analysis using acoustic-induced polarized vortex streaming. | [74] |
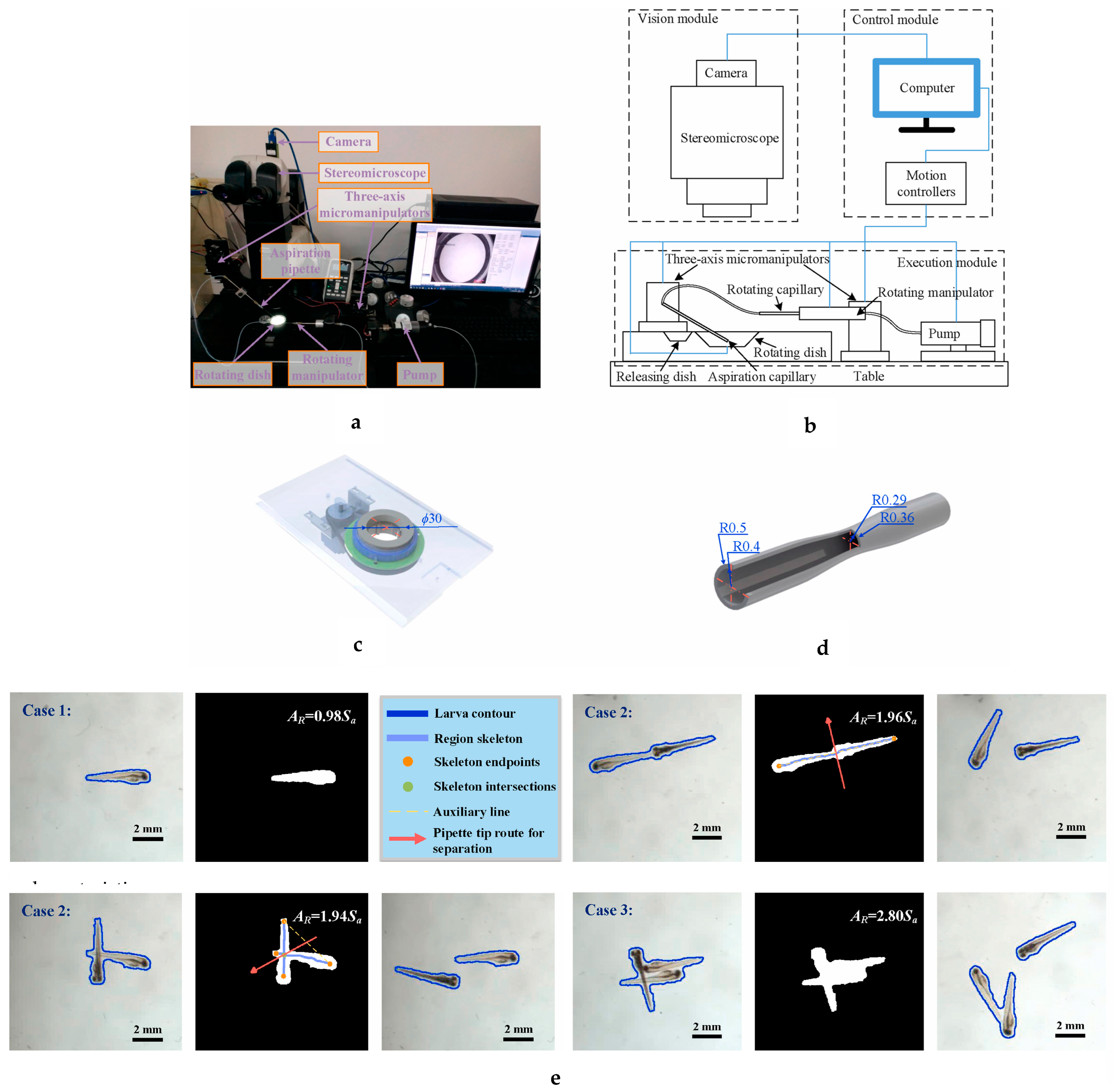
| Stereomicroscope and camera with a rotating dish and a rotating capillary | Separation of aggregated larvae with aspiration pipette or water stream and deceleration of the fast-moving larvae using narrow restricted portion of the rotating capillary to avoid damage followed by rotation of the larvae to the desired position using rotating capillary. | [75] |
| Microscope with high-speed camera in combination with artificial cilia for orientation control | Artificial cilia based microchannel for orientation control of the larvae with moving wall structure operated by shape memory alloy (SMA) wire for accommodating the rapid morphological changes of zebrafish under developing. | [76] |
| Microparticle image velocimetry facilitated by microscope and high-speed camera | Immobilization of the larvae using SMA actuator in the observation region to quantify tail beating behavior under the influence of cochineal red additive. | [77] |
| Simultaneous lateral and dorsal imaging with microscope | Integration of an optical prism into the microfluidic device to enable lateral imaging in parallel to the dorsal imaging by adding a compensating PDMS layer on top of the prism to accommodate the difference between the focal lengths of the lateral and dorsal optical paths. | [78,79] |
| Novel Techniques in Drug and Disease Studies | ||
| Area of study | Feature | Reference |
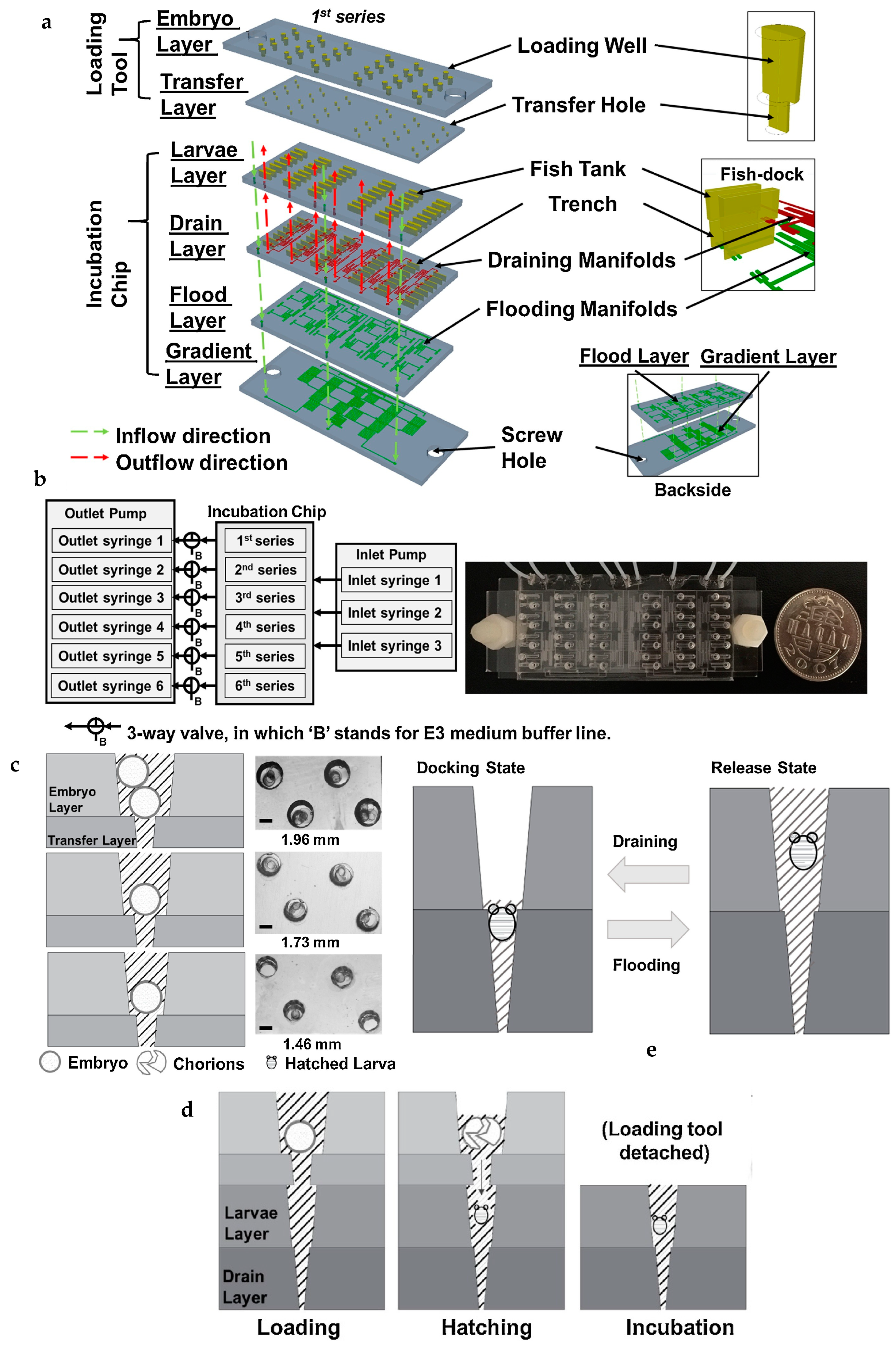
| Time course of cardiac responses of the same zebrafish | PMMA-based microfluidic device with detachable loading tool to separate the chorions post hatching of the embryo, optimized culturing chamber geometry for long-term incubation and “Fish-dock” architecture to maintain a particular orientation of the same larvae for time-based study | [53] |
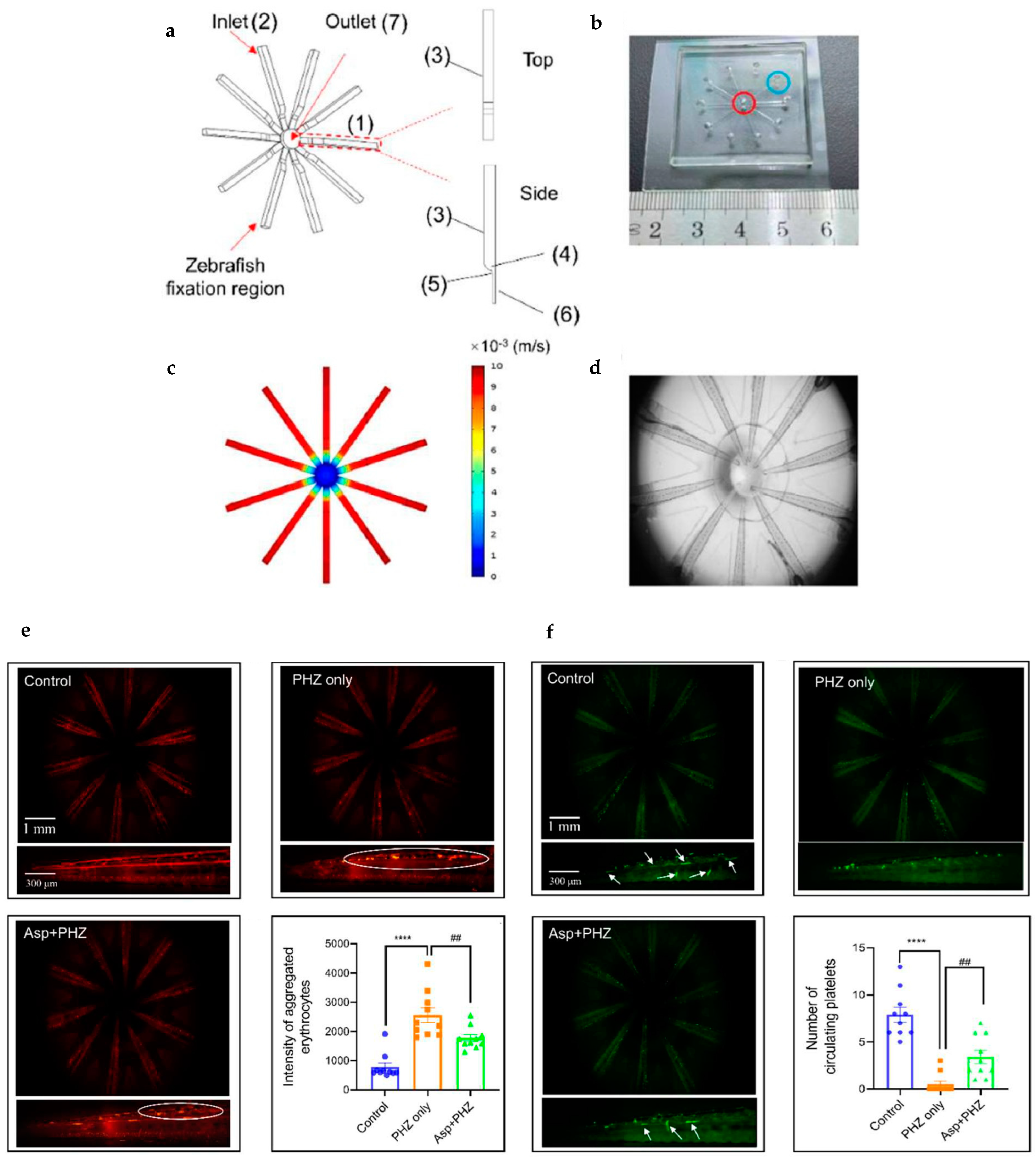
| In vivo real-time evaluation of antithrombotic agents in multiple larvae simultaneously | Lateral-immobilization zebrafish microfluidic chip (LIZMC) for the study of peripheral blood circulation in the tail of 10 larvae simultaneously. | [54] |
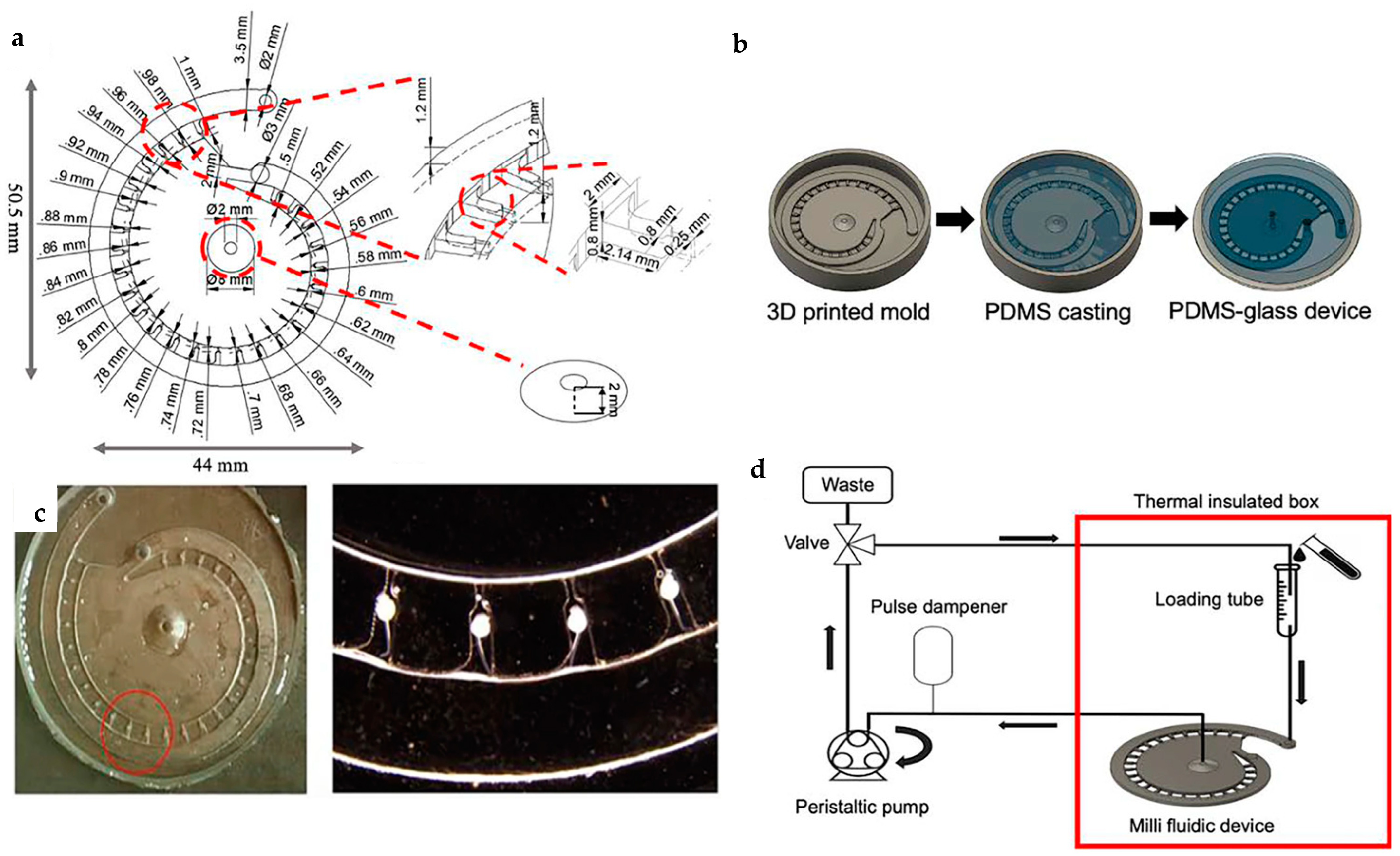
| Toxicity testing under the influence of diluted ethanol as a teratogen | A 3D printed embryo cultivation chip fabricated using digital light processing (DLP) technology provides a separate embryo removal functionality, which makes it ideal for the observation of the embryos individually. | [55] |
| High-throughput muscular activity measurement | Microfluidic chip for trapping zebrafish with a single inlet, eight outlets, and eight trapping channels facilitating electromyography (EMG) monitoring under the influence of different chemicals using electrodes attached to the fish head. | [56] |
| High-throughput EEG monitoring | Microfluidic chip for monitoring EEG of 4 larvae at a time under the influence of different chemicals without the necessity of anesthesia or agarose gel. | [57] |
| Novel Stimulation and Transportation Methods | ||
| Stimulation Technique/Medium | Feature | Reference |
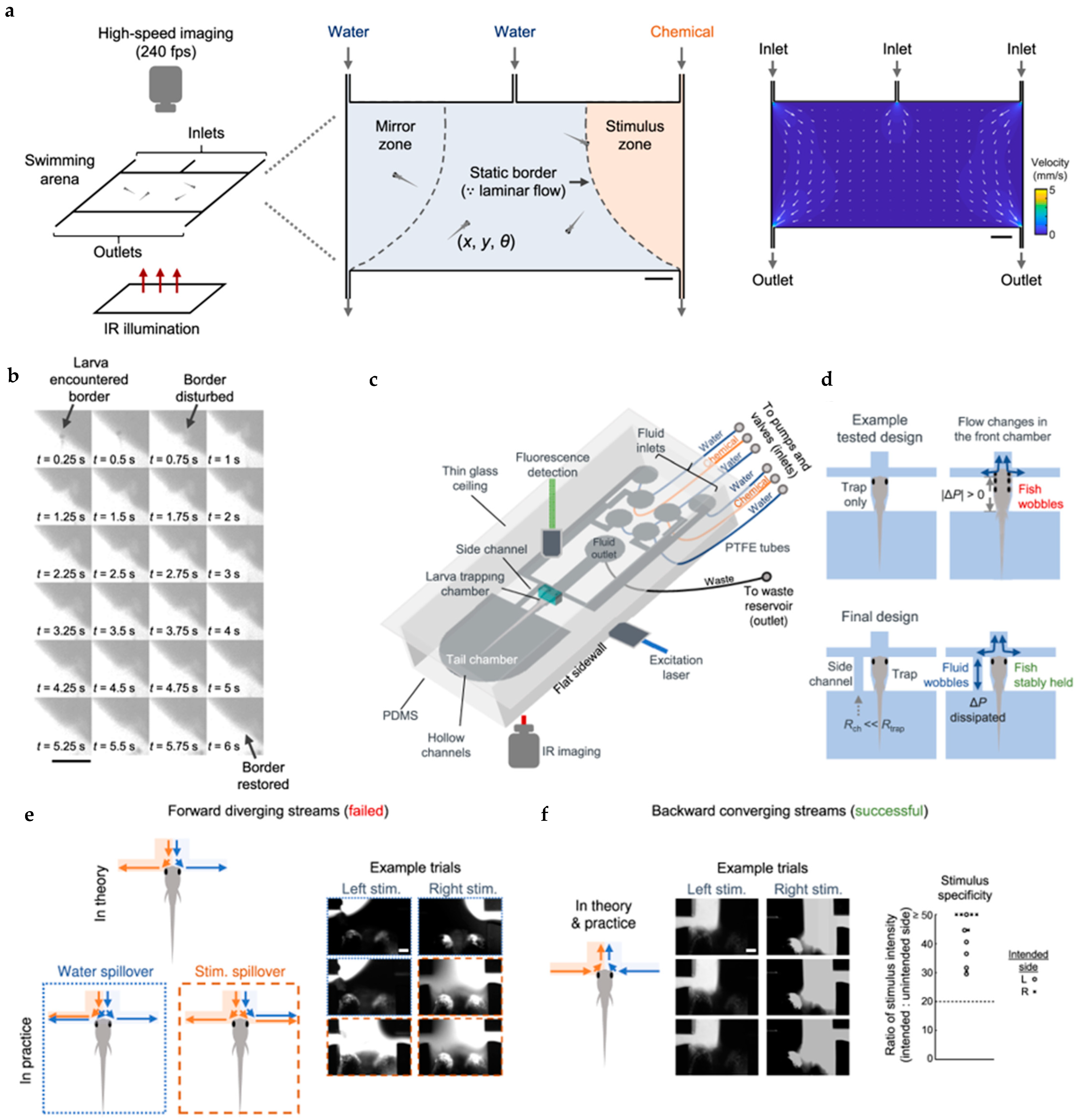
| Chemical: cadaverine (a death associated odor) | A fluidics-based swimming arena and an integrated microfluidics-light sheet fluorescence microscopy (μfluidics-LSFM) system, both of which utilize laminar fluid flows to achieve spatiotemporally precise chemical cue presentation. | [46,47] |
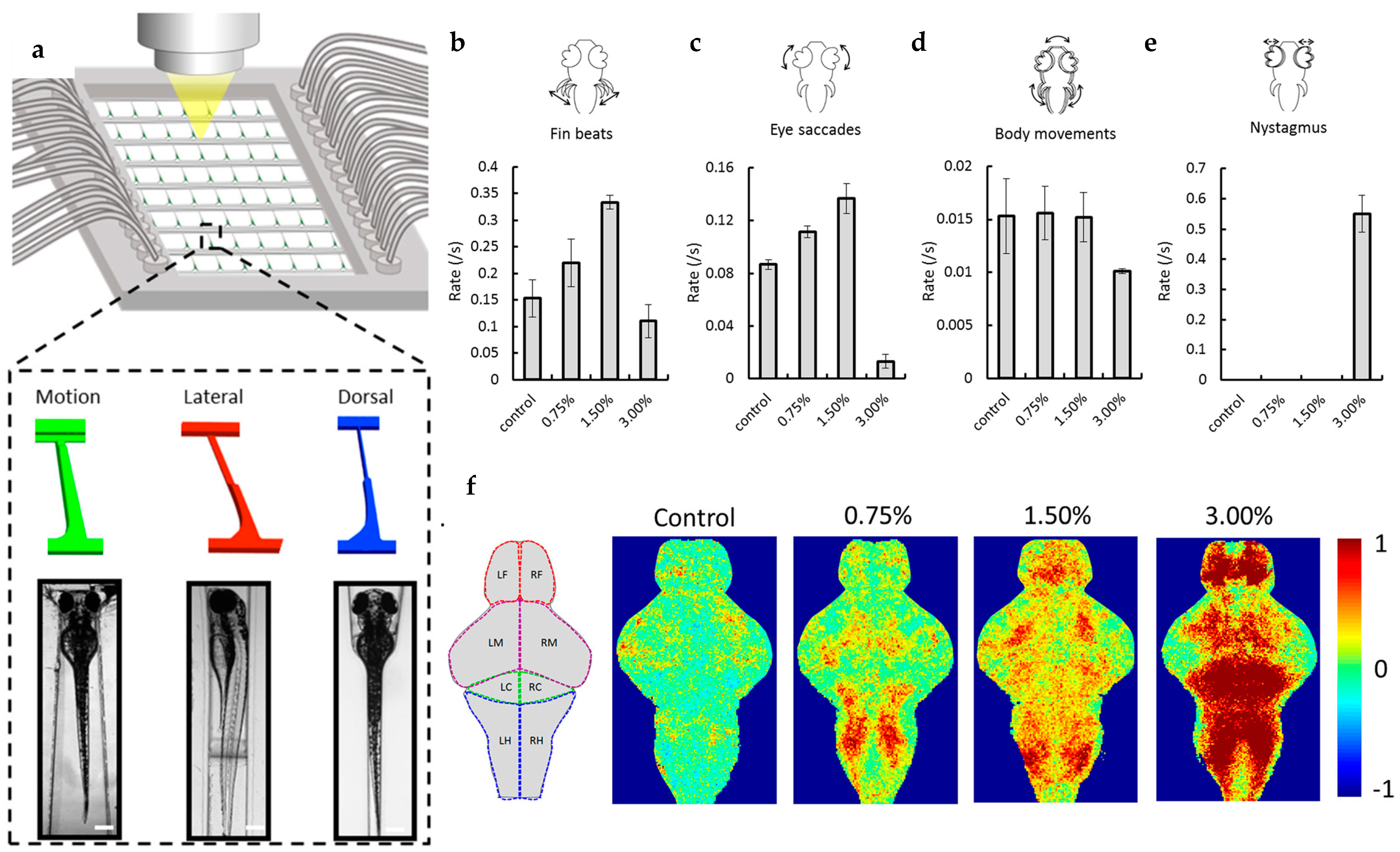
| Chemical: ethanol | Microfluidic chip with 3 distinct designs (“motion”, “lateral” and “dorsal”) to study ethanol induced behavioral responses, and associated physiological changes (cardiac and brain functionality) at cellular resolution within specific organs. | [80] |
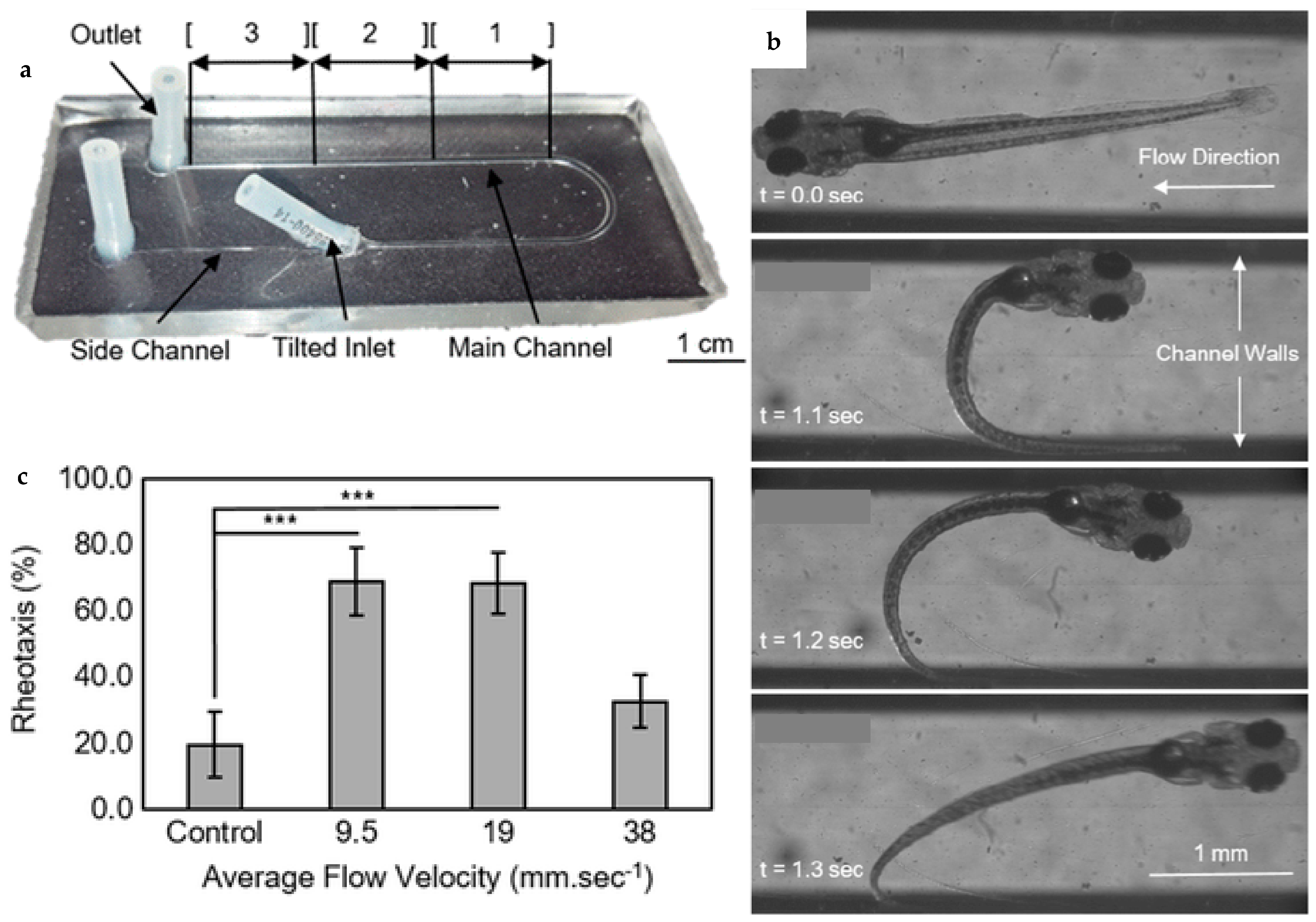
| Flow velocity | Microfluidic device to apply the flow stimulus precisely and repeatedly along the longitudinal axis of individual zebrafish larvae to study their coaxial rheotaxis (the ability of zebrafish to orient and swim against the water stream). | [48] |
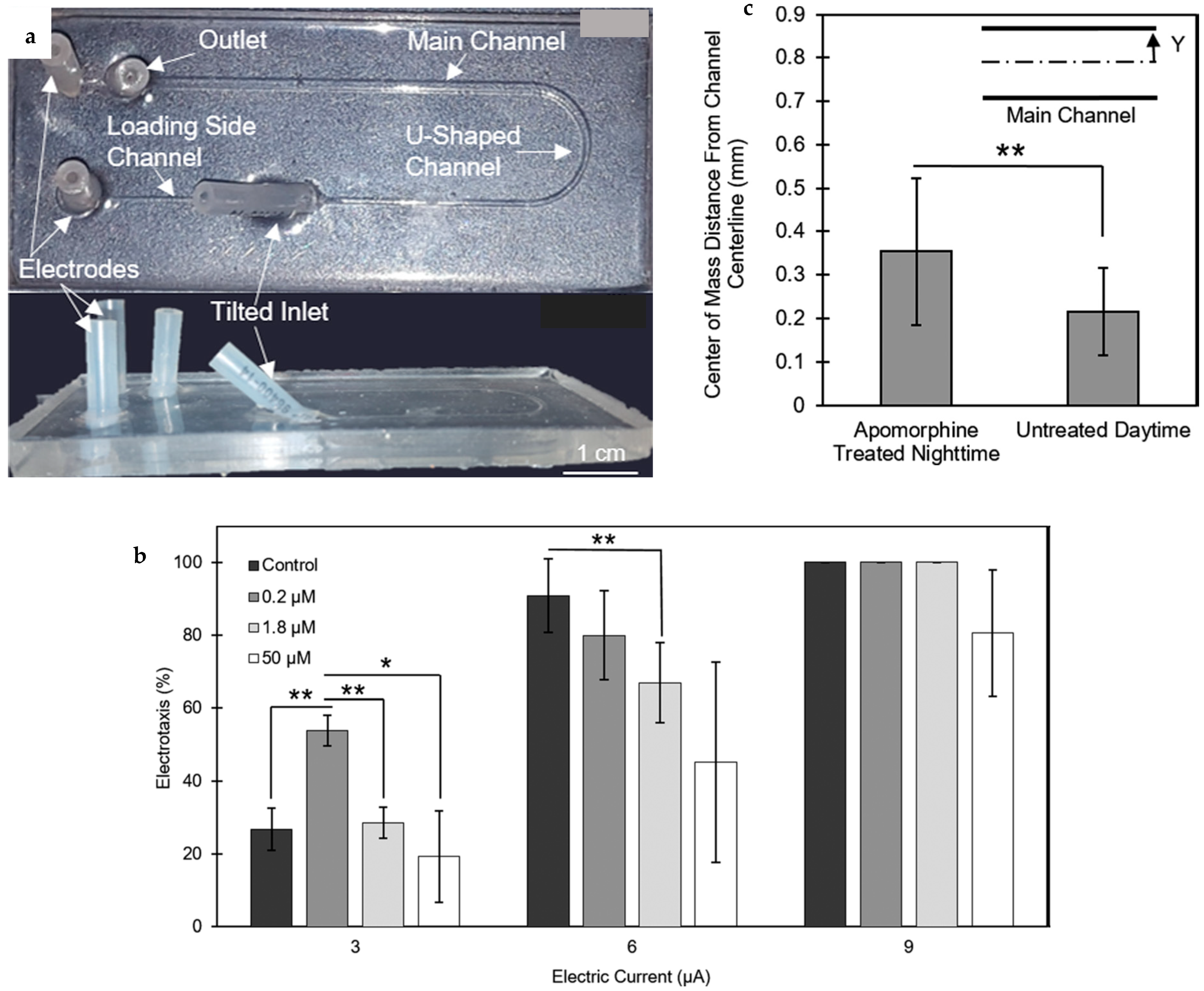
| Electric field | Microfluidic device to study the sensing and movement response of zebrafish under electrical signal, the day time dependency of these responses and the influence of dopamine agonists on these responses. | [49] |
| Electric field with trapping region | Modification of the previously mentioned device in reference with the facility for partial immobilization of the larva head to quantify the tail movement under electric signal and chemicals. | [50] |
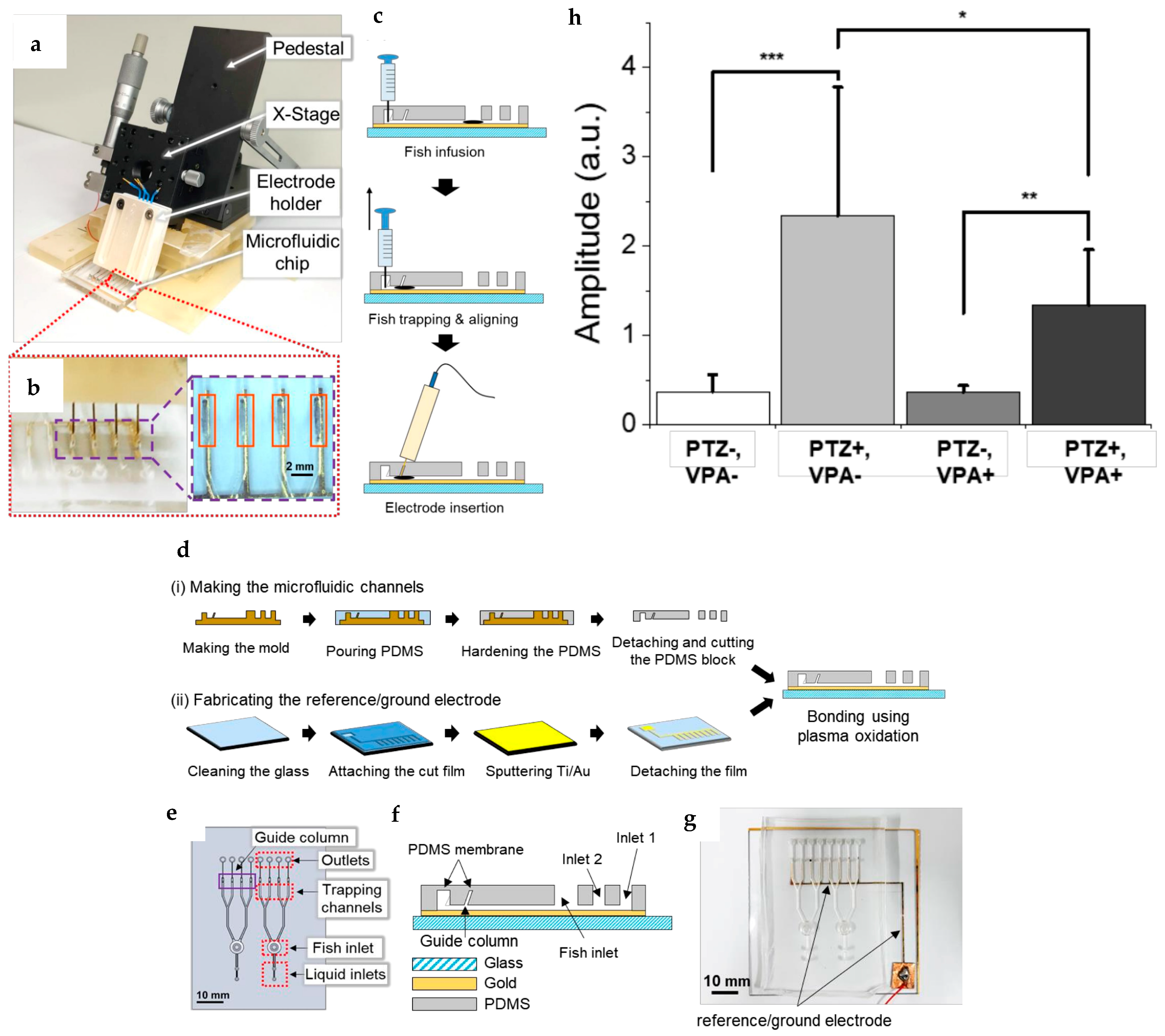
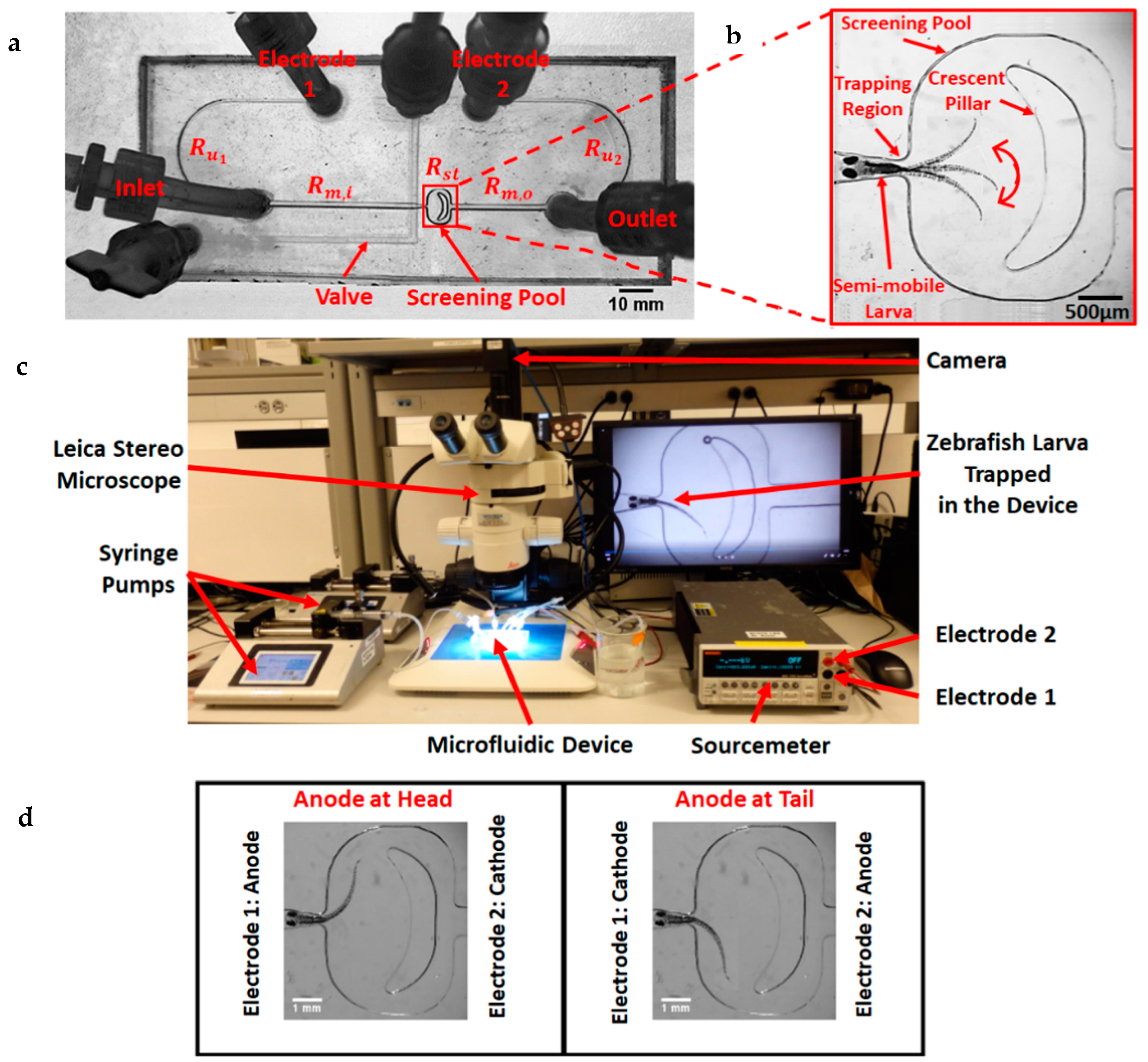
| Electric field with trapping region and varying voltage | Modification of the device mentioned in reference with the system for changing the voltage across the device and fish body by altering the solution used inside the channel to study the effects of change in the direction of the electric current and voltage magnitude on the larvae. Also study different habituation and dishabituation situations to repeatedly applied electric signals. | [51,52] |
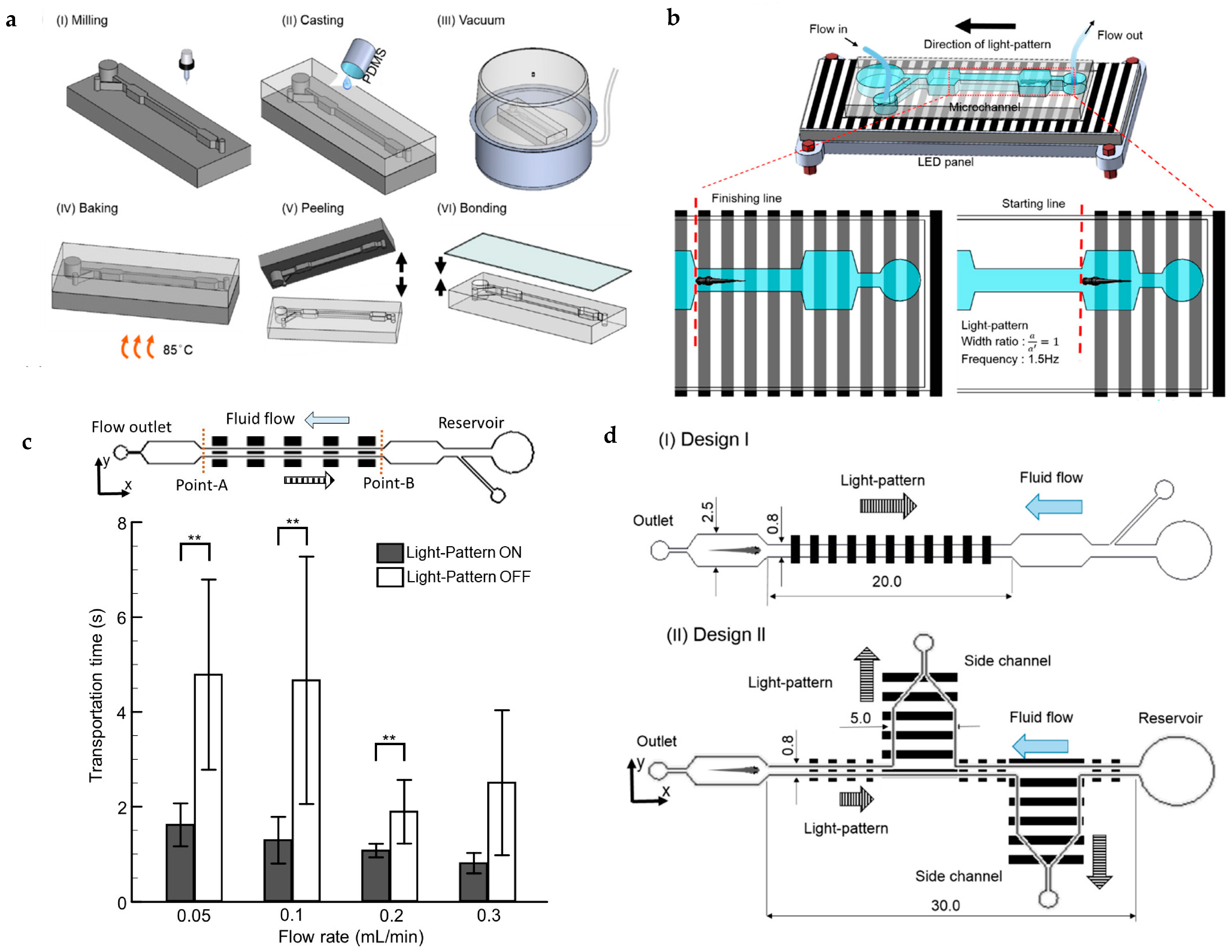
| Flow velocity and visual cues | A microfluidic platform for optimizing the transportation time of zebrafish larvae utilizing a combination of flow velocity and visual stimuli. The visual stimulation was achieved using computer-animated moving gratings. | [81] |
| Visual cues | Visual moving gratings generated using a custom-made GUI to transport the larvae with optimized grating parameters. | [82] |
| Sound and visual cues | A microfluidic platform for the sorting/trapping of hatched zebrafish larvae using a non-invasive method based on light cues and acoustic actuation. | [83] |
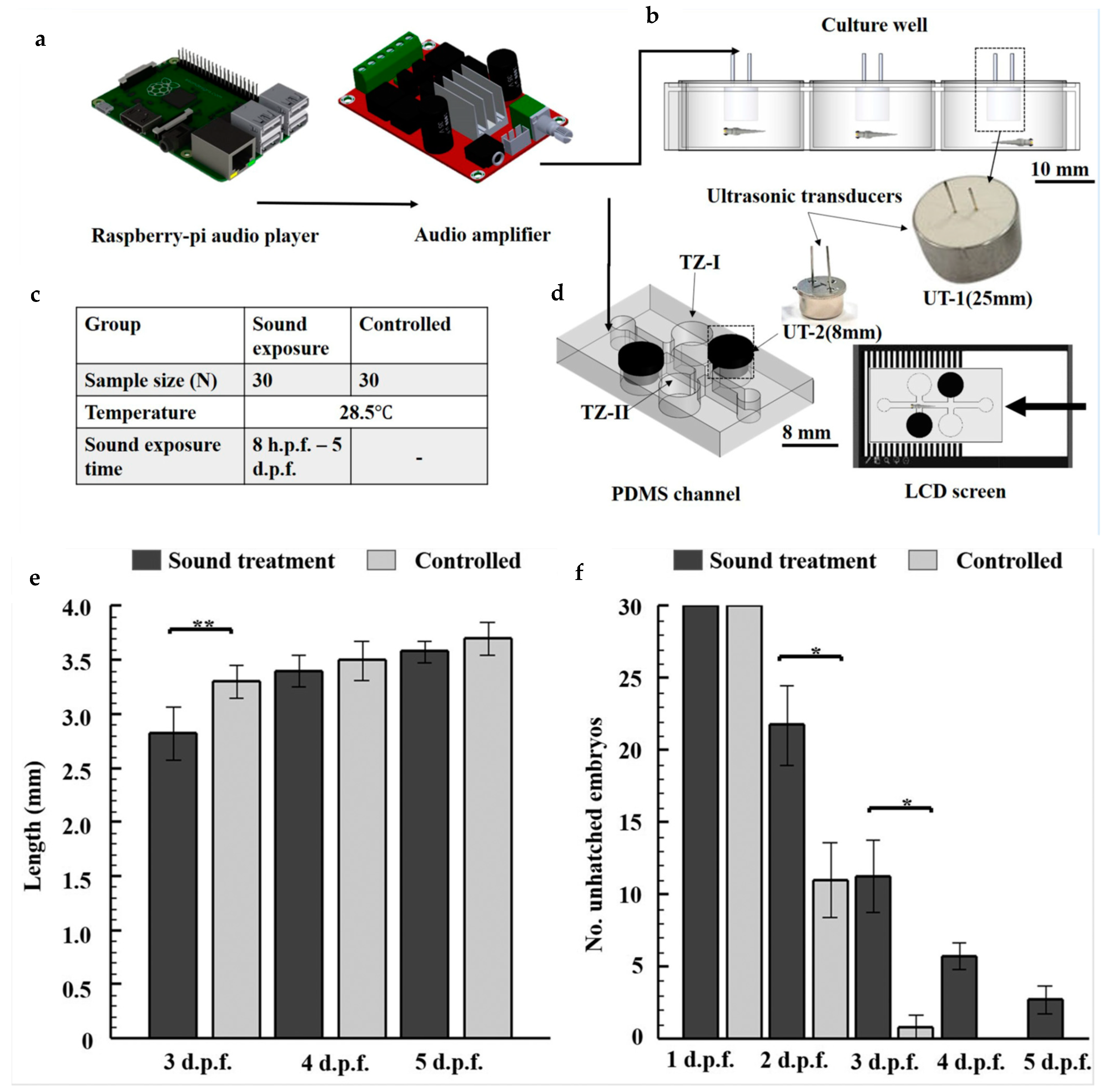
| Sound | Microfluidic platform to assess spatial memory in 4–6 dpf zebrafish larvae using acoustic stimuli and quantify their startle responses to evaluate memory acquisition. | [84] |
| Microinjection Techniques | ||
| Methodology | Feature | Reference |
| Flow-based orientation control | Orientation control of single larva by controlling the flow through set of syringe pumps and then aspirated through a pipette (with a connected syringe filled with certain amount of air to avoid damage to larva body) using a nonlinear aspiration model. | [62] |
| Orientation control using motorized stage | A k-means clustering algorithm automates larva detection and positioning for heart microinjection, using 2D rotation control and a rolling model to determine injection depth. | [63] |
| Sperm Retention | ||
| Methodology | Feature | Reference |
| Retention of progressively motile sperm using microscale confinements | New microfluidic concept with PDMS baffles inserted in the sidewalls to form microscale confinements to create a flow stagnation zone, thus retaining the sperm. | [85] |
| Activation of sperm using cilia-based micromixer | Artificial cilia-based micromixer through the precise regulation of hydrodynamics induced on zebrafish sperm for superior sperm activation providing a better activation rate compared to manual processes. | [86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nath, T.; Tan, H. Recent Advancements in Fish-on-Chip: A Comprehensive Review. Fluids 2025, 10, 88. https://doi.org/10.3390/fluids10040088
Nath T, Tan H. Recent Advancements in Fish-on-Chip: A Comprehensive Review. Fluids. 2025; 10(4):88. https://doi.org/10.3390/fluids10040088
Chicago/Turabian StyleNath, Tushar, and Hua Tan. 2025. "Recent Advancements in Fish-on-Chip: A Comprehensive Review" Fluids 10, no. 4: 88. https://doi.org/10.3390/fluids10040088
APA StyleNath, T., & Tan, H. (2025). Recent Advancements in Fish-on-Chip: A Comprehensive Review. Fluids, 10(4), 88. https://doi.org/10.3390/fluids10040088






