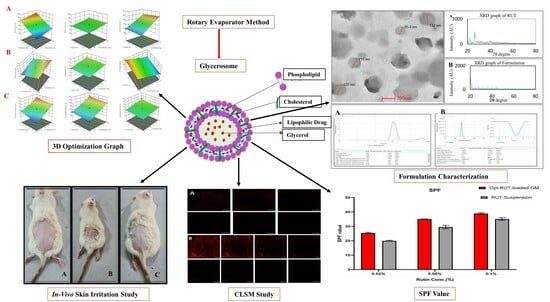
Quality by Design-Optimized Glycerosome-Enabled Nanosunscreen Gel of Rutin Hydrate
Abstract
:1. Introduction
2. Results and Discussion
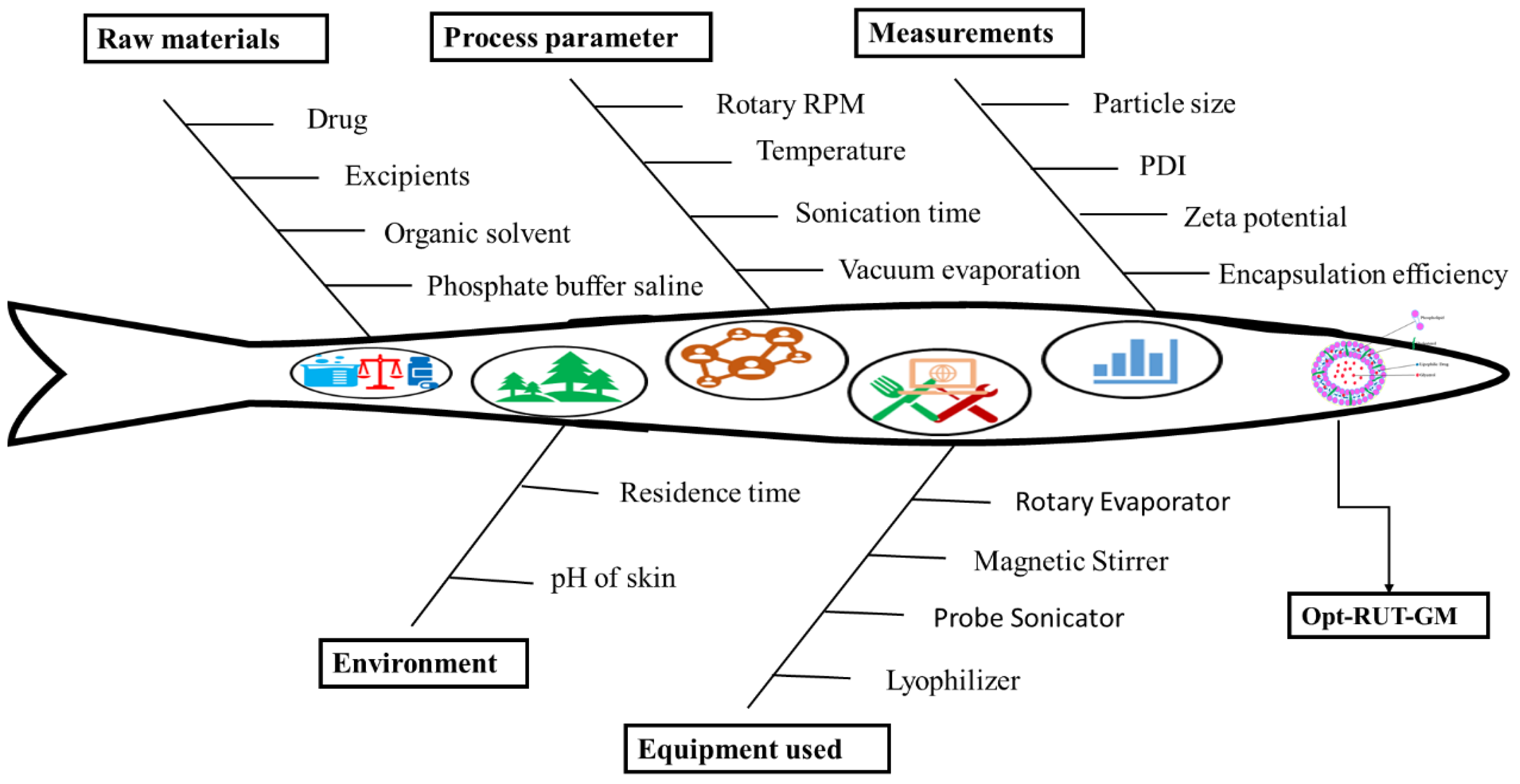
2.1. Using the Quality by Design Method to Develop a Robust Formulation
2.2. Model Fitting and Optimization Using Box Behnken Design
2.3. Optimization of RUT-Loaded GM by Statistical Design
2.3.1. Analysis of Response Surfaces
Impact of Independent Factor on Vesicle Size (Y1)
Influence of Independent Factor on PDI
Influence of Independent Factor on EE%
2.3.2. Experimental Design Validation
2.3.3. Optimized Level of Formulation by Point Prediction
2.4. Characterization of Opt-RUT-Loaded-GMs
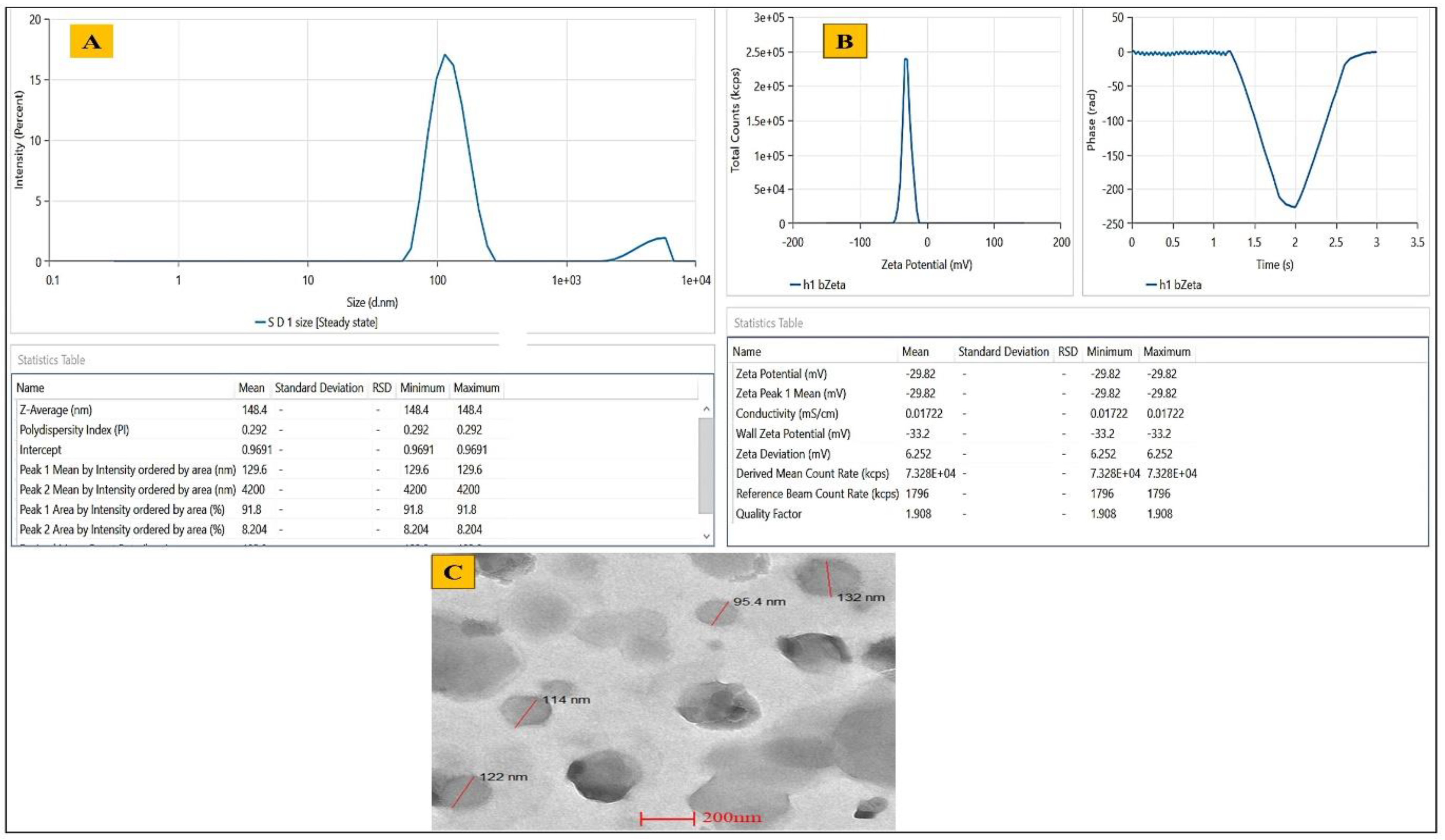
2.4.1. Determination of Vesicles Size, PDI, Zeta Potential and EE%
2.4.2. Determination of Entrapment Efficiency
2.4.3. Morphological Evaluation
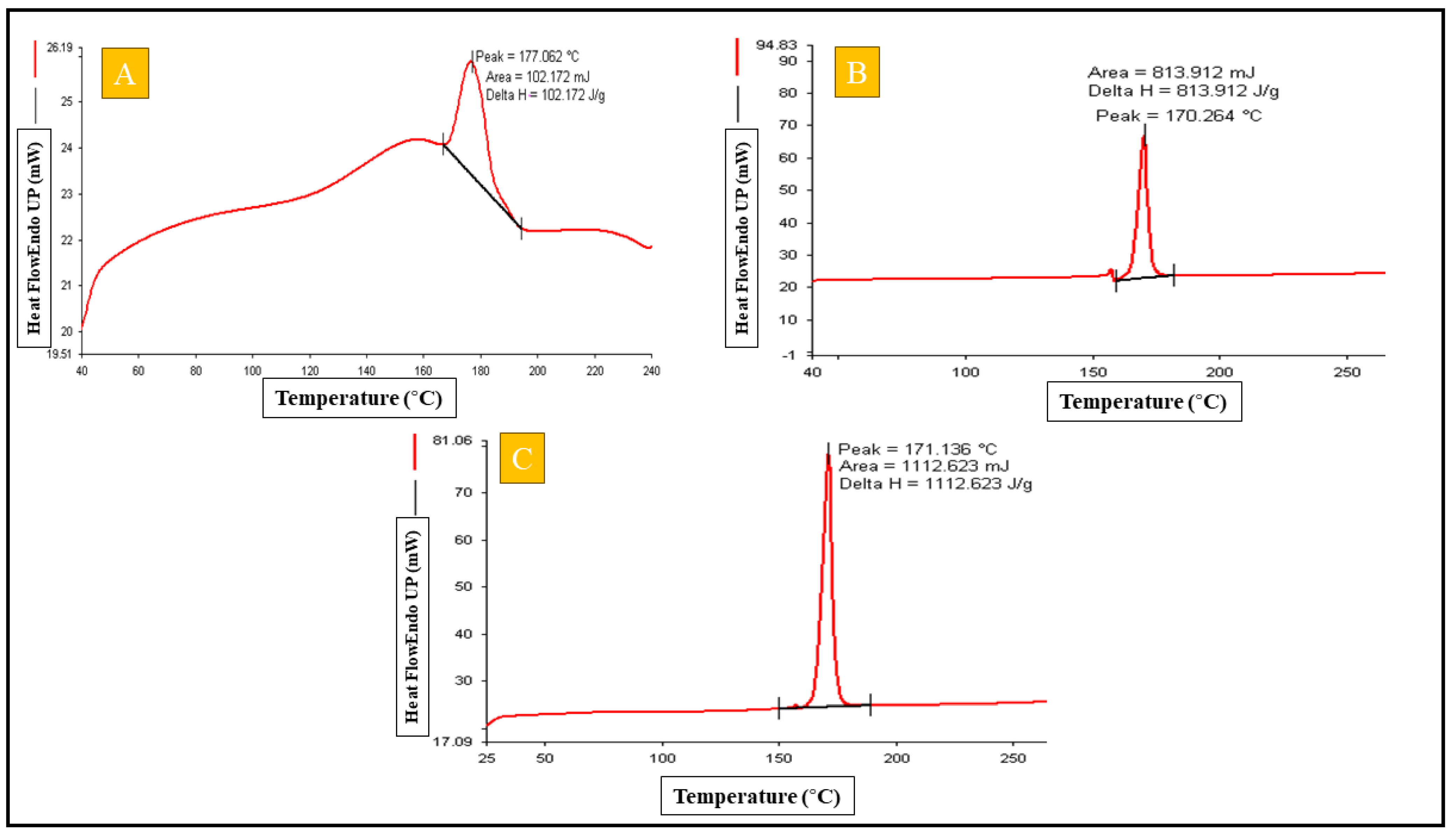
2.4.4. Differential Scanning Calorimetry
2.4.5. Compatibility Study Using Fourier Transform Infrared
2.4.6. Powder X-ray Diffraction Analysis
2.4.7. Antioxidant Analysis by 2,2-Diphenyl-1-picrylhydrazyl Assay Method
2.5. Characterization of Opt-RUT-Loaded-GMs Gel
2.6. In Vitro Drug Release Study
2.7. Sun Protection Factor (SPF) Evaluation
2.8. Confocal Laser Scanning Microscopy
2.9. Ex Vivo Dermatokinetic Study
2.10. Skin Irritation Study
2.11. Accelerated Predictive Stability Study
3. Conclusions
4. Material and Methods
4.1. Materials
4.2. Methods
4.2.1. Quality by Design (QbD) Identification of Component
4.2.2. Preparation of RUT-Glycerosomes (RUT-GMs)
4.2.3. Optimization of RUT-Loaded-GMs
4.2.4. Characterization of Opt-RUT-Loaded-GMs
Vesicle Size, PDI and Zeta Potential
Entrapment Efficiency
Differential Scanning Calorimetery
Fourier Transform Infrared Compatibility Study
X-ray Diffraction
Surface Morphology of Opt-RUT-Loaded-GMs
Determination of Antioxidant Activity by 2,2-Diphenyl-1-picrylhydrazyl Assay
4.2.5. Preparation of Opt-RUT-Loaded-GMs Carbopol® Gel
4.2.6. Characterization of Opt-RUT-Loaded-GMs Gel
pH Evaluation
Homogeneity and Viscosity
Spreadability
Extrudability
Texture Analysis
4.2.7. In Vitro Release
4.2.8. Sun Protection Factor Value Determination
- SPF = CF × (λ) × I (λ) × absorbance (λ)
- CF = Correction factor (10)
- EE = Erythmogenic effect of radiation with wavelength λ
- I = Solar Ray Simulation Spectrum
- Abs = spectrophotometric absorbance values at wavelength λ.
4.2.9. Confocal Laser Scanning Microscopy
4.2.10. Ex Vivo Dermatokinetics
4.2.11. In Vivo Study
Skin Irritation Study
4.2.12. Accelerated Stability Study According to ICH Q1A (R2) Guidelines
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdellatif, M.M.; Khalil, I.A.; Khalil, M.A.F. Sertaconazole Nitrate Loaded Nanovesicular Systems for Targeting Skin Fungal Infection: In-Vitro, Ex-Vivo and in-Vivo Evaluation. Int. J. Pharm. 2017, 527, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Advaita, N.; Lestari, R.G.; Aidid, K.U.; Sasongko, H. In Vitro Evaluation of Sun Protection Factor of Vasconcellea Pubescens Fruit Extract. Int. Conf. Pharm. Res. Pract. 2015, 122–125. [Google Scholar]
- Ahad, A.; Al-Saleh, A.A.; Al-Mohizea, A.M.; Al-Jenoobi, F.I.; Raish, M.; Yassin, A.E.B.; Alam, M.A. Formulation and Characterization of Phospholipon 90 G and Tween 80 Based Transfersomes for Transdermal Delivery of Eprosartan Mesylate. Pharm. Dev. Technol. 2018, 23, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Aqil, M.; Imam, S.S.; Ahad, A.; Parveen, A.; Qadir, A.; Ali, M.H.; Akhtar, M. Formulation and Evaluation of Embelin Loaded Nanoliposomes: Optimization, in Vitro and Ex Vivo Evaluation. J. Drug Deliv. Sci. Technol. 2022, 72, 103414. [Google Scholar] [CrossRef]
- Arooj, A.; Rehman, A.U.; Iqbal, M.; Naz, I.; Alhodaib, A.; Ahmed, N. Development of Adapalene Loaded Liposome Based Gel for Acne. Gels 2023, 9, 135. [Google Scholar] [CrossRef]
- Aydin, N.; Turkez, H.; Tozlu, O.O.; Arslan, M.E.; Yavuz, M.; Sonmez, E.; Ozpolat, O.F.; Cacciatore, I.; Di Stefano, A.; Mardinoglu, A. Ameliorative Effects by Hexagonal Boron Nitride Nanoparticles against Beta Amyloid Induced Neurotoxicity. Nanomaterials 2022, 12, 2690. [Google Scholar] [CrossRef]
- Beg, S.; Hasnain, M.S.; Rahman, M.; Swain, S. Introduction to Quality by Design (QbD): Fundamentals, Principles, and Applications. In Pharmaceutical Quality by Design; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–17. [Google Scholar]
- Blancett, C.D.; Fetterer, D.P.; Koistinen, K.A.; Morazzani, E.M.; Monninger, M.K.; Piper, A.E.; Kuehl, K.A.; Kearney, B.J.; Norris, S.L.; Rossi, C.A.; et al. Accurate Virus Quantitation Using a Scanning Transmission Electron Microscopy (STEM) Detector in a Scanning Electron Microscope. J. Virol. Methods 2017, 248, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Brar, V.; Kaur, G. Thiolated Okra Chitosan Nanoparticles: Preparation and Optimisation as Intranasal Drug Delivery Agents. J. Microencapsul. 2020, 37, 624–639. [Google Scholar] [CrossRef]
- Briuglia, M.L.; Rotella, C.; McFarlane, A.; Lamprou, D.A. Influence of Cholesterol on Liposome Stability and on in Vitro Drug Release. Drug Deliv. Transl. Res. 2015, 5, 231–242. [Google Scholar] [CrossRef]
- Dantas, M.G.B.; Reis, S.A.G.B.; Damasceno, C.M.D.; Rolim, L.A.; Rolim-Neto, P.J.; Carvalho, F.O.; Quintans-Junior, L.J.; da Silva Almeida, J.R.G. Development and Evaluation of Stability of a Gel Formulation Containing the Monoterpene Borneol. Sci. World J. 2016, 2016, 7394685. [Google Scholar] [CrossRef]
- Zaru, M.; Manca, M.L.; Fadda, M.; Orsini, G. Glycerosomes and Use Thereof in Pharmaceutical and Cosmetic Preparations for Topical Applications. U.S. Patent 8,778,367 B2, 15 July 2014. [Google Scholar]
- Fatima, Q.; Ahmed, N.; Siddiqui, B.; Rehman, A.U.; Haq, I.U.; Khan, G.M.; Elaissari, A. Enhanced Antimicrobial Activity of Silver Sulfadiazine Cosmetotherapeutic Nanolotion for Burn Infections. Cosmetics 2022, 9, 93. [Google Scholar] [CrossRef]
- Ghazwani, M.; Hani, U.; Alam, A.; Alqarni, M.H. Quality-by-Design-Assisted Optimization of Carvacrol Oil-Loaded Niosomal Gel for Anti-Inflammatory Efficacy by Topical Route. Gels 2023, 9, 401. [Google Scholar] [CrossRef] [PubMed]
- Gollavilli, H.; Hegde, A.R.; Managuli, R.S.; Bhaskar, K.V.; Dengale, S.J.; Reddy, M.S.; Kalthur, G.; Mutalik, S. Naringin Nano-Ethosomal Novel Sunscreen Creams: Development and Performance Evaluation. Colloids Surf. B Biointerfaces 2020, 193, 111122. [Google Scholar] [CrossRef]
- Hooresfand, Z.; Ghanbarzadeh, S.; Hamishehkar, H. Preparation and Characterization of Rutin-Loaded Nanophytosomes. Pharm. Sci. 2015, 21, 145–151. [Google Scholar] [CrossRef]
- Iqubal, M.K.; Iqubal, A.; Imtiyaz, K.; Rizvi, M.M.A.; Gupta, M.M.; Ali, J.; Baboota, S. Combinatorial Lipid-Nanosystem for Dermal Delivery of 5-Fluorouracil and Resveratrol against Skin Cancer: Delineation of Improved Dermatokinetics and Epidermal Drug Deposition Enhancement Analysis. Eur. J. Pharm. Biopharm. 2021, 163, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Nagpal, M.; Singh, M.; Singh, T.G.; Aggarwal, G.; Dhingra, G.A. Improved Antibacterial Activity of Topical Gel-Based on Nanosponge Carrier of Cinnamon Oil. BioImpacts 2021, 11, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Kausar, H.; Mujeeb, M.; Ahad, A.; Moolakkadath, T.; Aqil, M.; Ahmad, A.; Akhter, M.H. Optimization of Ethosomes for Topical Thymoquinone Delivery for the Treatment of Skin Acne. J. Drug Deliv. Sci. Technol. 2019, 49, 177–187. [Google Scholar] [CrossRef]
- Khan, A.W.; Kotta, S.; Ansari, S.H.; Sharma, R.K.; Kumar, A.; Ali, J. Formulation Development, Optimization and Evaluation of Aloe Vera Gel for Wound Healing. Pharmacogn. Mag. 2013, 9, S6–S10. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Dang, S.; Nigam, K.; Ali, J.; Baboota, S. Selegiline Nanoformulation in Attenuation of Oxidative Stress and Upregulation of Dopamine in the Brain for the Treatment of Parkinson’s Disease. Rejuvenation Res. 2018, 21, 464–476. [Google Scholar] [CrossRef]
- Md, S.; Alhakamy, N.A.; Aldawsari, H.M.; Husain, M.; Khan, N.; Alfaleh, M.A.; Asfour, H.Z.; Riadi, Y.; Bilgrami, A.L.; Akhter, M.H. Plumbagin-Loaded Glycerosome Gel as Topical Delivery System for Skin Cancer Therapy. Polymers 2021, 13, 923. [Google Scholar] [CrossRef]
- Mohapatra, S.; Mirza, M.A.; Ahmad, S.; Farooq, U.; Ansari, M.J.; Kohli, K.; Iqbal, Z. Quality by Design Assisted Optimization and Risk Assessment of Black Cohosh Loaded Ethosomal Gel for Menopause: Investigating Different Formulation and Process Variables. Pharmaceutics 2023, 15, 465. [Google Scholar] [CrossRef]
- Moolakkadath, T.; Aqil, M.; Ahad, A.; Imam, S.S.; Praveen, A.; Sultana, Y.; Mujeeb, M. Preparation and Optimization of Fisetin Loaded Glycerol Based Soft Nanovesicles by Box-Behnken Design. Int. J. Pharm. 2020, 578, 119125. [Google Scholar] [CrossRef]
- Naguib, M.J.; Salah, S.; Halim, S.A.A.; Badr-Eldin, S.M. Investigating the Potential of Utilizing Glycerosomes as a Novel Vesicular Platform for Enhancing Intranasal Delivery of Lacidipine. Int. J. Pharm. 2020, 582, 119302. [Google Scholar] [CrossRef]
- Nikita, M.A.; Sultana, Y. A Grafted Copolymer-Based Nanomicelles for Topical Ocular Delivery of Everolimus: Formulation, Characterization, Ex-Vivo Permeation, in-Vitro Ocular Toxicity, and Stability Study. Eur. J. Pharm. Sci. 2021, 159, 105735. [Google Scholar] [CrossRef]
- Paul, D.; Jacob, M.; Agrawal, N. Study of Additive Effect of Dexmedetomidine Added to Epidural Ropivacaine for Orthopedic Lower Limb Procedures. Int. J. Biomed. Res. 2017, 8, 8. [Google Scholar] [CrossRef]
- Pawar, A.Y.; Jadhav, K.R.; Naikwade, K.; Mahajan, T.P. Formulation & Evaluation of Naringin Nanoethosome by Cold Method. J. Pharm. Res. Int. 2021, 33, 139–152. [Google Scholar] [CrossRef]
- Pleguezuelos-Villa, M.; Diez-Sales, O.; Manca, M.L.; Manconi, M.; Sauri, A.R.; Escribano-Ferrer, E.; Nácher, A. Mangiferin Glycethosomes as a New Potential Adjuvant for the Treatment of Psoriasis. Int. J. Pharm. 2020, 573, 118844. [Google Scholar] [CrossRef]
- Rapalli, V.K.; Sharma, S.; Roy, A.; Singhvi, G. Design and Dermatokinetic Evaluation of Apremilast Loaded Nanostructured Lipid Carriers Embedded Gel for Topical Delivery: A Potential Approach for Improved Permeation and Prolong Skin Deposition. Colloids Surf. B Biointerfaces 2021, 206, 111945. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.F.; Kharshoum, R.M.; Sayed, O.M.; Lekaa, F.; Hakim, A. Formulation Design and Optimization of Novel Soft Glycerosomes for Enhanced Topical Delivery of Celecoxib and Cupferron by Box–Behnken Statistical Design. Drug Dev. Ind. Pharm. 2018, 44, 1871–1884. [Google Scholar] [CrossRef] [PubMed]
- Santadkha, T.; Skolpap, W.; Thitapakorn, V. Diffusion Modeling and In Vitro Release Kinetics Studies of Curcumin−Loaded Superparamagnetic Nanomicelles in Cancer Drug Delivery System. J. Pharm. Sci. 2022, 111, 1690–1699. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, K.; Chowdhury, R.; Bhattacharjee, C. Isolation and Structural Elucidation of Flavonoids from Aquatic Fern Azolla Microphylla and Evaluation of Free Radical Scavenging Activity. Int. J. Pharm. Pharm. Sci. 2013, 5 (Suppl. S3), 743–749. [Google Scholar]
- Sri, K.V.; Kondaiah, A.; Ratna, J.V.; Annapurna, A. Preparation and Characterization of Quercetin and Rutin Cyclodextrin Inclusion Complexes. Drug Dev. Ind. Pharm. 2007, 33, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, N.; Srivastava, S.K.; Arora, S.; Omar, Y.; Ijaz, Z.M.; AL-Ghadhban, A.; Deshmukh, S.K.; Carter, J.E.; Singh, A.P.; Singh, S. Comparative Analysis of the Relative Potential of Silver, Zinc-Oxide and Titanium-Dioxide Nanoparticles against UVB-Induced DNA Damage for the Prevention of Skin Carcinogenesis. Cancer Lett. 2016, 383, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Waghule, T.; Dabholkar, N.; Gorantla, S.; Rapalli, V.K.; Saha, R.N.; Singhvi, G. Quality by Design (QbD) in the Formulation and Optimization of Liquid Crystalline Nanoparticles (LCNPs): A Risk Based Industrial Approach. Biomed. Pharmacother. 2021, 141, 111940. [Google Scholar] [CrossRef] [PubMed]
- Waheed, A.; Zameer, S.; Sultana, N.; Ali, A.; Aqil, M.; Sultana, Y.; Iqbal, Z. Engineering of QbD Driven and Ultrasonically Shaped Lyotropic Liquid Crystalline Nanoparticles for Apigenin in the Management of Skin Cancer. Eur. J. Pharm. Biopharm. 2022, 180, 269–280. [Google Scholar] [CrossRef]
- Westh, P. Unilamellar DMPC Vesicles in Aqueous Glycerol: Preferential Interactions and Thermochemistry. Biophys. J. 2003, 84, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Guo, J.; Yuan, J. In Vitro Antioxidant Properties of Rutin. LWT 2008, 41, 1060–1066. [Google Scholar] [CrossRef]





| QTPP | Target | Justification |
|---|---|---|
| Drug delivery system | Glycerosome | Improved skin permeability is preferred by glycerosomes. |
| Release type | Sustained release | Slow drug release is necessary to ensure sufficient drug concentration for proper sunburn action. |
| Route of administration | Topical | In comparison to oral treatment, it provides localized action with minimum systemic side effects. |
| Dosage form | Hydrogel | Provides easier topical application and improved penetration through the skin |
| Appearance | white gel with a smooth texture | It is free from gritty particles; the gel is uniformly smooth as well as odorless and Colorless. |
| Stability | >3 months | Maintains the therapeutic efficacy of the formulation. |
| CQAs | Target | Justification |
| Phospholipid 90G | Optimal vesicle size | Phospholipid 90G is a complex of phospholipids that contributes to the development of the phospholipid bilayer and also affects the size of the vesicles. [5] |
| Cholesterol | Fluidity and rigidity | Influence of Cholesterol on vesicle size and membrane fluidity. The formation of the phospholipid bilayer involves these components. [6] |
| Glycerol | Greater flexibility | The glycerol in these vesicles increases the deformability index, resulting in improved therapeutic skin penetration and permeability. [7] |
| Hydration media | Optimal glycerosome stability (absence of aggregation) | The lipid film is hydrated as part of the process of creating the phospholipid bilayer. [8] |
| API | The lipophilic API has been successfully incorporated into the glycerosomal wall. | The size of the vesicles may change depending on the nature of API included in the glycerosomes product [5]. |
| Particle size | Less than 300 nm | To demonstrate enhanced drug penetration at the targeted skin layers with controlled drug release pattern. |
| Polydispersity index | Less than 0.5 | Better homogeneity will result in more effective drug entrapment and release. |
| Entrapment Efficiency | More than 80% | Ensures high drug loading, and improved therapeutic results. |
| Rutin Conventional Gel | Opt-RUT-Loaded-GMs Gel | |||
|---|---|---|---|---|
| Dermatokinetic Parameters | Epidermis | Dermis | Epidermis | Dermis |
| TSkin max (h) | 2 | 1.5 | 2 | 2 |
| CSkin max (µg/cm2) | 64.8814 | 32.4635 | 118.302 | 52.7329 |
| AUC0–8 (µg/cm2 h) | 248.3277 | 116.900 | 438.7941 | 197.4062 |
| Ke (h−1) | 0.15524 | 0.145283 | 0.129488 | 0.143148 |
| Rats | Negative Control | Positive Control | RUT-Loaded-GM Gel | |||
|---|---|---|---|---|---|---|
| S no. | Erythema | Edema | Erythema | Edema | Erythema | Edema |
| 1 | 0 | 0 | 2 | 1 | 0 | 0 |
| 2 | 0 | 0 | 2 | 1 | 0 | 0 |
| 3 | 0 | 0 | 3 | 2 | 1 | 0 |
| - | 0 | 0 | 2.33 ± 0.57 | 1.33 ± 0.57 | 0.33 ± 0.57 | 0 |
| Storage Time | Appearance | Separation | pH ± SD | Vesicle Size (nm ± SD) | EE (% ±SD) | PDI (±SD) |
|---|---|---|---|---|---|---|
| Storage condition (40 °C ± 2 °C) | ||||||
| 0 day | Clear | No | 5.7 ± 0.1 | 148.067 ± 0.75 | 83.25 ± 0.31 | 0.290 ± 0.004 |
| 1 week | Clear | No | 5.63 ± 0.20 | 150.36 ± 1.33 | 84.14 ± 0.88 | 0.29 ± 0.001 |
| 2 weeks | Clear | No | 5.94 ± 0.17 | 156.4 ± 0.79 | 83.34 ± 0.26 | 0.32 ± 0.01 |
| 1 month | Clear | No | 5.99 ± 0.11 | 163.67 ± 1.86 | 82.14 ± 0.54 | 0.319± 0.003 |
| 2 months | Turbid | No | 6.26 ± 0.06 | 172.8 ± 1.11 | 80.82 ± 0.7 | 0.382 ± 0.064 |
| 3 months | Turbid | Yes | 6.39 ± 0.13 | 184.1 ± 1.014 | 79.17 ± 0.36 | 0.35 ± 0.008 |
| Storage condition (40 ± 2 °C/75 ± 5% RH) | ||||||
| 0 day | Clear | No | 5.76 ± 0.152 | 147.22 ± 1.03 | 82.90 ± 0.655 | 0.289 ± 0.004 |
| 1 week | Clear | No | 5.69 ± 0.10 | 155.64 ± 0.89 | 81.24 ± 1.009 | 0.362 ± 0.014 |
| 2 weeks | Clear | No | 6.16 ± 0.254 | 166.62 ± 0.79 | 81.82 ± 0.796 | 0.389 ± 0.009 |
| 1 month | Turbid | Yes | 6.49 ± 0.26 | 188.51 ± 0.89 | 78.58 ± 0.94 | 0.45 ± 0.01 |
| 2 months | Milky | Yes | 6.39 ± 0.142 | 226.33 ± 2.28 | 73.54 ± 1.36 | 0.55 ± 0.01 |
| 3 months | Milky | Yes | 6.56 ± 0.210 | 251.26 ± 2.31 | 69.06 ± 0.448 | 0.64 ± 0.012 |
| Evaluation Parameters | Initial | 1 month | 3 months | |||
| 4 ± 2 °C | 40 ± 2 °C/75 ± 5% RH | 4 ± 2 °C | 40 ± 2 °C/75 ± 5% RH | |||
| Color | Whitish | Whitish | Whitish | Whitish | Whitish | |
| Appearance | Translucent | Translucent | Translucent | Translucent | Translucent | |
| Phase Separation | None | None | None | None | None | |
| Clarity | *** | *** | *** | *** | ** | |
| pH | 5.65 ± 0.28 | 5.71 ± 0.16 | 5.8 ± 0.34 | 5.73 ± 0.12 | 5.87 ± 0.43 | |
| Homogeneity | *** | *** | *** | *** | *** | |
| Washability | Washable | Washable | Washable | Washable | Washable | |
| Odor | None | None | None | None | None | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, M.S.; Sultana, N.; Rashid, M.A.; Alhamhoom, Y.; Ali, A.; Waheed, A.; Ansari, M.S.; Aqil, M.; Mujeeb, M. Quality by Design-Optimized Glycerosome-Enabled Nanosunscreen Gel of Rutin Hydrate. Gels 2023, 9, 752. https://doi.org/10.3390/gels9090752
Alam MS, Sultana N, Rashid MA, Alhamhoom Y, Ali A, Waheed A, Ansari MS, Aqil M, Mujeeb M. Quality by Design-Optimized Glycerosome-Enabled Nanosunscreen Gel of Rutin Hydrate. Gels. 2023; 9(9):752. https://doi.org/10.3390/gels9090752
Chicago/Turabian StyleAlam, Md. Shabbir, Niha Sultana, Md. Abdur Rashid, Yahya Alhamhoom, Asad Ali, Ayesha Waheed, Mo. Suheb Ansari, Mohd. Aqil, and Mohd Mujeeb. 2023. "Quality by Design-Optimized Glycerosome-Enabled Nanosunscreen Gel of Rutin Hydrate" Gels 9, no. 9: 752. https://doi.org/10.3390/gels9090752