Using the Water Absorption Ability of Dried Hydrogels to Form Hydrogel-Supported Lipid Bilayers
Abstract
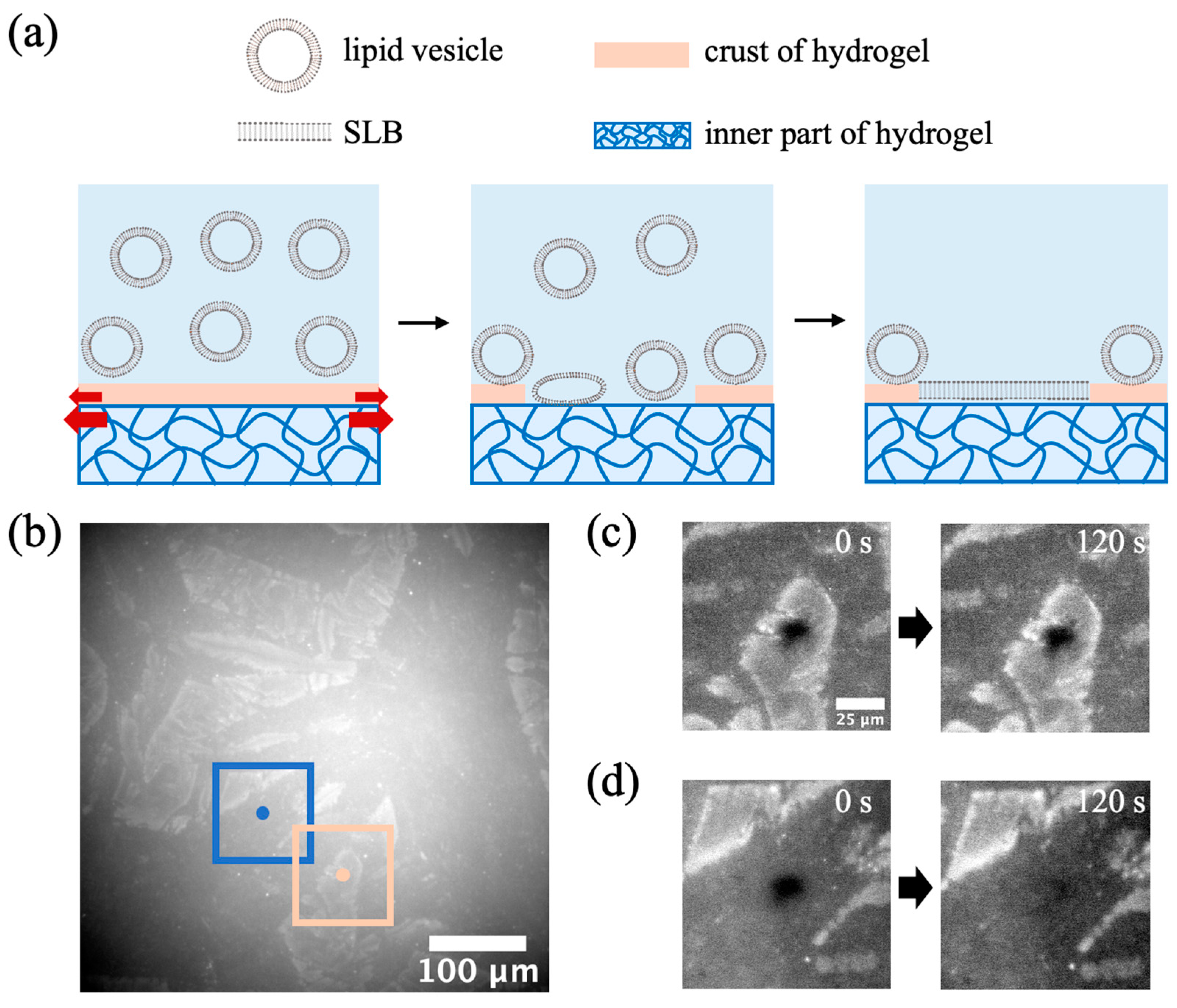
:1. Introduction
2. Results and Discussion
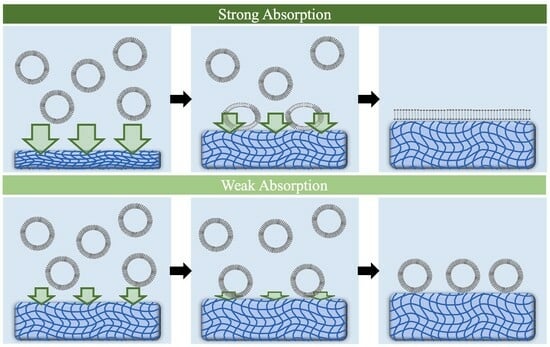
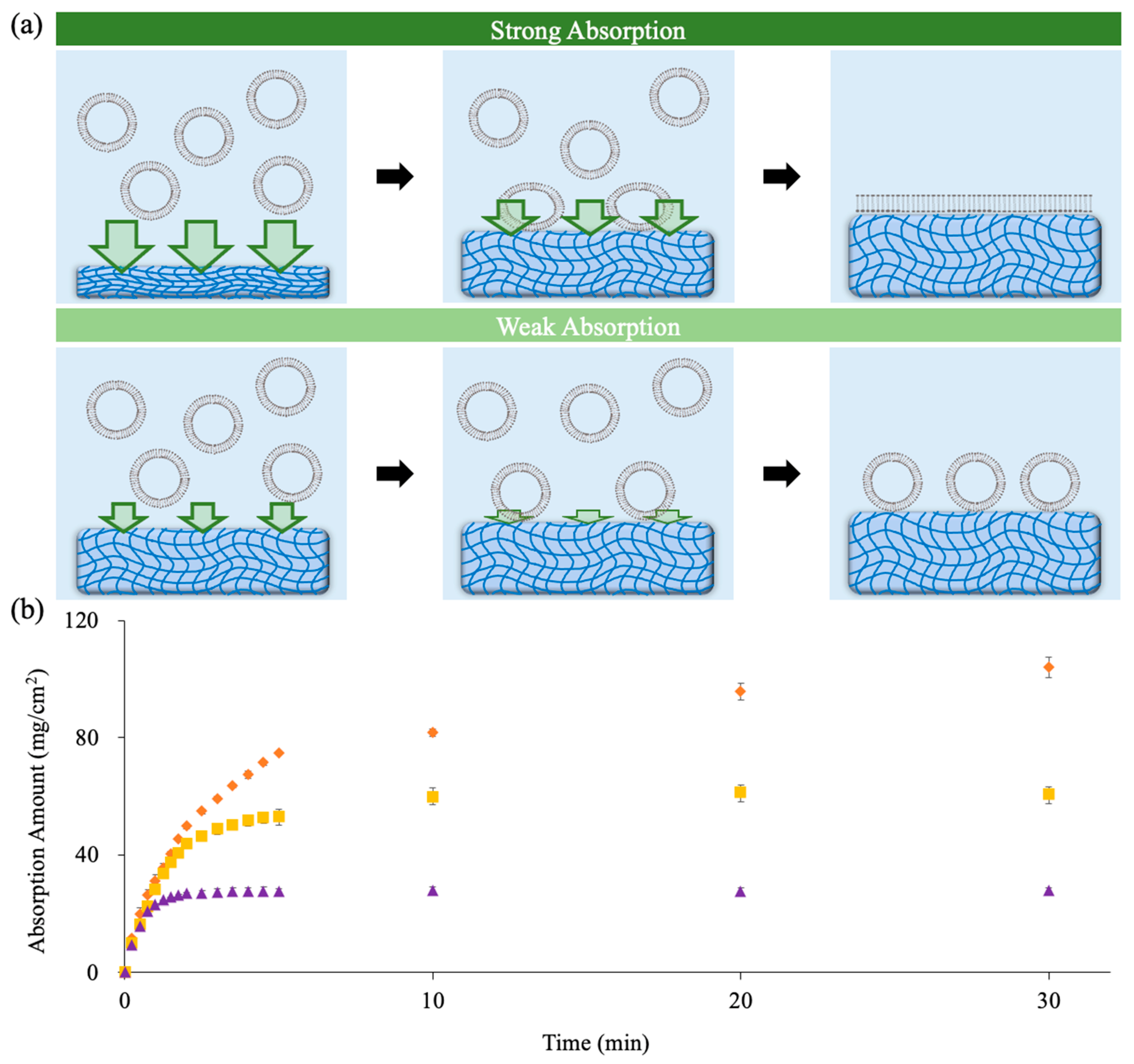
2.1. Adjusting Water Absorption Ability to Induce Vesicle Rupture
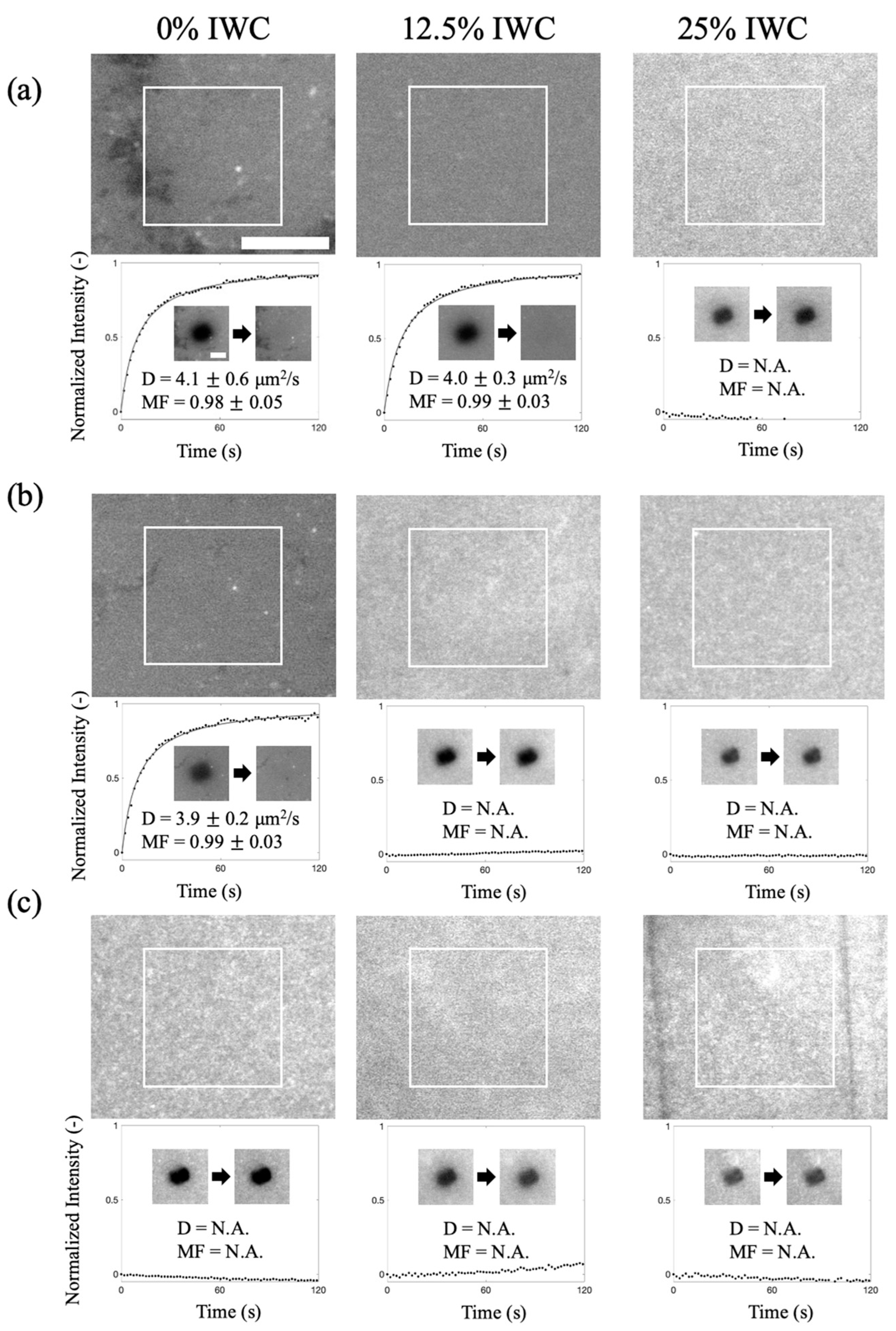
2.2. Formation of SLBs on Polyacrylamide Hydrogel with Various Absorption Abilities
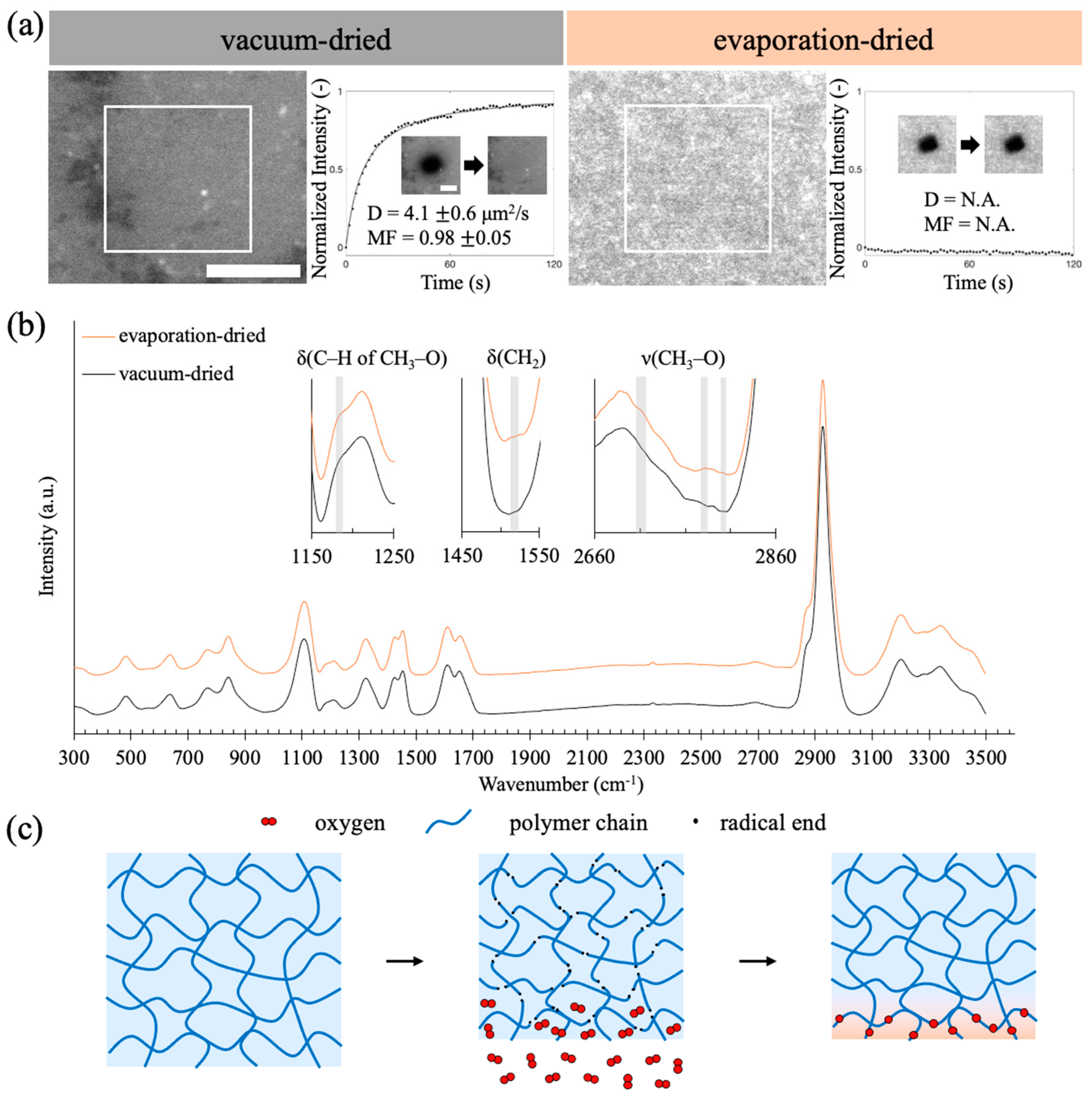
2.3. Exposure to Oxygen during Shrinkage Hinders SLB Formation on the Hydrogel Surface
2.4. Severe Expansion in the Horizontal Plane of the Evaporation-Dried Hydrogel Produces a Crack Structure
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Dried PAAm Hydrogels
4.3. Calculation of the Absorption Flux of Dried Hydrogels
4.4. Formation of Supported Lipid Membranes on PAAm
4.5. Fluorescence Microscopy and FRAP
4.6. Raman Measurement
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Richter, R.P.; Bérat, R.; Brisson, A.R. Formation of Solid-Supported Lipid Bilayers: An Integrated View. Langmuir 2006, 22, 3497–3505. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zheng, J.; Li, L.; Jiang, S. Strong Resistance of Phosphorylcholine Self-Assembled Monolayers to Protein Adsorption: Insights into Nonfouling Properties of Zwitterionic Materials. J. Am. Chem. Soc. 2005, 127, 14473–14478. [Google Scholar] [CrossRef] [PubMed]
- Kiser, P.F.; Wilson, G.; Needham, D. Lipid-coated microgels for the triggered release of doxorubicin. J. Control. Release 2000, 68, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, R. When Microfluidic Devices Go Bad. Anal. Chem. 2005, 77, 429A–432A. [Google Scholar] [CrossRef]
- Belling, J.N.; Heidenreich, L.K.; Park, J.H.; Kawakami, L.M.; Takahashi, J.; Frost, I.M.; Gong, Y.; Young, T.D.; Jackman, J.A.; Jonas, S.J.; et al. Lipid-Bicelle-Coated Microfluidics for Intracellular Delivery with Reduced Fouling. ACS Appl. Mater. Interfaces 2020, 12, 45744–45752. [Google Scholar] [CrossRef]
- Dressaire, E.; Sauret, A. Clogging of microfluidic systems. Soft Matter 2017, 13, 37–48. [Google Scholar] [CrossRef]
- Yang, T.; Jung, S.-Y.; Mao, H.; Cremer, P.S. Fabrication of Phospholipid Bilayer-Coated Microchannels for On-Chip Immunoassays. Anal. Chem. 2001, 73, 165–169. [Google Scholar] [CrossRef]
- McKeating, K.S.; Hinman, S.S.; Rais, N.A.; Zhou, Z.; Cheng, Q. Antifouling Lipid Membranes over Protein A for Orientation-Controlled Immunosensing in Undiluted Serum and Plasma. ACS Sens. 2019, 4, 1774–1782. [Google Scholar] [CrossRef]
- Castellana, E.T.; Cremer, P.S. Solid supported lipid bilayers: From biophysical studies to sensor design. Surf. Sci. Rep. 2006, 61, 429–444. [Google Scholar] [CrossRef]
- Hardy, G.J.; Nayak, R.; Zauscher, S. Model cell membranes: Techniques to form complex biomimetic supported lipid bilayers via vesicle fusion. Curr. Opin. Colloid Interface Sci. 2013, 18, 448–458. [Google Scholar] [CrossRef]
- Cascone, S.; Lamberti, G. Hydrogel-based commercial products for biomedical applications: A review. Int. J. Pharm. 2020, 573, 118803. [Google Scholar] [CrossRef] [PubMed]
- Vedadghavami, A.; Minooei, F.; Mohammadi, M.H.; Khetani, S.; Rezaei Kolahchi, A.; Mashayekhan, S.; Sanati-Nezhad, A. Manufacturing of hydrogel biomaterials with controlled mechanical properties for tissue engineering applications. Acta Biomater. 2017, 62, 42–63. [Google Scholar] [CrossRef] [PubMed]
- Schönherr, H.; Johnson, J.M.; Lenz, P.; Frank, C.W.; Boxer, S.G. Vesicle Adsorption and Lipid Bilayer Formation on Glass Studied by Atomic Force Microscopy. Langmuir 2004, 20, 11600–11606. [Google Scholar] [CrossRef] [PubMed]
- Jass, J.; Tjärnhage, T.; Puu, G. From Liposomes to Supported, Planar Bilayer Structures on Hydrophilic and Hydrophobic Surfaces: An Atomic Force Microscopy Study. Biophys. J. 2000, 79, 3153–3163. [Google Scholar] [CrossRef] [PubMed]
- Reimhult, E.; Höök, F.; Kasemo, B. Intact Vesicle Adsorption and Supported Biomembrane Formation from Vesicles in Solution: Influence of Surface Chemistry, Vesicle Size, Temperature, and Osmotic Pressure. Langmuir 2003, 19, 1681–1691. [Google Scholar] [CrossRef]
- Reimhult, E.; Höök, F.; Kasemo, B. Vesicle adsorption on SiO2 and TiO2: Dependence on vesicle size. J. Chem. Phys. 2002, 117, 7401–7404. [Google Scholar] [CrossRef]
- Oleson, T.A.; Sahai, N. Interaction energies between oxide surfaces and multiple phosphatidylcholine bilayers from extended-DLVO theory. J. Colloid Interface Sci. 2010, 352, 316–326. [Google Scholar] [CrossRef]
- Anderson, T.H.; Min, Y.; Weirich, K.L.; Zeng, H.; Fygenson, D.; Israelachvili, J.N. Formation of Supported Bilayers on Silica Substrates. Langmuir 2009, 25, 6997–7005. [Google Scholar] [CrossRef]
- Cremer, P.S.; Boxer, S.G. Formation and Spreading of Lipid Bilayers on Planar Glass Supports. J. Phys. Chem. B 1999, 103, 2554–2559. [Google Scholar] [CrossRef]
- Keller, C.A.; Kasemo, B. Surface Specific Kinetics of Lipid Vesicle Adsorption Measured with a Quartz Crystal Microbalance. Biophys. J. 1998, 75, 1397–1402. [Google Scholar] [CrossRef]
- Keller, C.A.; Glasmästar, K.; Zhdanov, V.P.; Kasemo, B. Formation of Supported Membranes from Vesicles. Phys. Rev. Lett. 2000, 84, 5443–5446. [Google Scholar] [CrossRef] [PubMed]
- Leonenko, Z.V.; Carnini, A.; Cramb, D.T. Supported planar bilayer formation by vesicle fusion: The interaction of phospholipid vesicles with surfaces and the effect of gramicidin on bilayer properties using atomic force microscopy. Biochim. Biophys. Acta (BBA) Biomembr. 2000, 1509, 131–147. [Google Scholar] [CrossRef]
- Hamai, C.; Yang, T.; Kataoka, S.; Cremer, P.S.; Musser, S.M. Effect of Average Phospholipid Curvature on Supported Bilayer Formation on Glass by Vesicle Fusion. Biophys. J. 2006, 90, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- Chiang, P.-C.; Tanady, K.; Huang, L.-T.; Chao, L. Rupturing giant plasma membrane vesicles to form micron-sized supported cell plasma membranes with native transmembrane proteins. Sci. Rep. 2017, 7, 15139. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-Y.; Chen, W.-L.; Ober, C.K.; Daniel, S. Biologically Complex Planar Cell Plasma Membranes Supported on Polyelectrolyte Cushions Enhance Transmembrane Protein Mobility and Retain Native Orientation. Langmuir 2018, 34, 1061–1072. [Google Scholar] [CrossRef]
- Smith, E.A.; Coym, J.W.; Cowell, S.M.; Tokimoto, T.; Hruby, V.J.; Yamamura, H.I.; Wirth, M.J. Lipid Bilayers on Polyacrylamide Brushes for Inclusion of Membrane Proteins. Langmuir 2005, 21, 9644–9650. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, Z.; Xiao, J.; Yang, S.; Wang, Y.J.; Tong, P. Studies of Phospholipid Vesicle Deposition/Transformation on a Polymer Surface by Dissipative Quartz Crystal Microbalance and Atomic Force Microscopy. J. Phys. Chem. B 2009, 113, 14925–14933. [Google Scholar] [CrossRef]
- Shoaib, T.; Nalam, P.C.; He, Y.; Chen, Y.; Espinosa-Marzal, R.M. Assembly, Morphology, Diffusivity, and Indentation of Hydrogel-Supported Lipid Bilayers. Langmuir 2017, 33, 7105–7117. [Google Scholar] [CrossRef]
- Pincet, F.; Adrien, V.; Yang, R.; Delacotte, J.; Rothman, J.E.; Urbach, W.; Tareste, D. FRAP to Characterize Molecular Diffusion and Interaction in Various Membrane Environments. PLoS ONE 2016, 11, e0158457. [Google Scholar] [CrossRef]
- Hovis, J.S.; Boxer, S.G. Patterning and Composition Arrays of Supported Lipid Bilayers by Microcontact Printing. Langmuir 2001, 17, 3400–3405. [Google Scholar] [CrossRef]
- Attwood, S.J.; Choi, Y.; Leonenko, Z. Preparation of DOPC and DPPC Supported Planar Lipid Bilayers for Atomic Force Microscopy and Atomic Force Spectroscopy. Int. J. Mol. Sci. 2013, 14, 3514–3539. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Sharma, N. Mechanistic implications of plastic degradation. Polym. Degrad. Stab. 2008, 93, 561–584. [Google Scholar] [CrossRef]
- Platzer, N. Encyclopedia of Polymer Science and Engineering, H.F. Mark, N.M.; Bikales, C.G. Overberger, and G. Menges, Wiley-Interscience, New York, 1985, 720 pp. J. Polym. Sci. Part C Polym. Lett. 1986, 4, 630–696. [Google Scholar] [CrossRef]
- Ceretti, D.V.A.; Edeleva, M.; Cardon, L.; D’hooge, D.R. Molecular Pathways for Polymer Degradation during Conventional Processing, Additive Manufacturing, and Mechanical Recycling. Molecules 2023, 28, 2344. [Google Scholar] [CrossRef]
- Caulfield, M.J.; Qiao, G.G.; Solomon, D.H. Some Aspects of the Properties and Degradation of Polyacrylamides. Chem. Rev. 2002, 102, 3067–3084. [Google Scholar] [CrossRef]
- Turrà, N.; Neuenschwander, U.; Hermans, I. Molecule-Induced Peroxide Homolysis. ChemPhysChem 2013, 14, 1666–1669. [Google Scholar] [CrossRef]
- Holmström, A.; Sörvik, E. Thermal degradation of polyethylene in a nitrogen atmosphere of low oxygen content: I. Changes in molecular weight distribution. J. Chromatogr. A 1970, 53, 95–108. [Google Scholar] [CrossRef]
- Gupta, M.K.; Bansil, R. Laser Raman spectroscopy of polyacrylamide. J. Polym. Sci. Polym. Phys. Ed. 1981, 19, 353–360. [Google Scholar] [CrossRef]
- Murugan, R.; Mohan, S.; Bigotto, A. FTIR and Polarised Raman Spectra of Acrylamide and Polyacrylamide. J. Korean Phys. Soc. 1998, 32, 505–512. [Google Scholar]
- Baldock, C.; Rintoul, L.; Keevil, S.F.; Pope, J.M.; George, G.A. Fourier transform Raman spectroscopy of polyacrylamide gels (PAGs) for radiation dosimetry. Phys. Med. Biol. 1998, 43, 3617. [Google Scholar] [CrossRef]
- Kanazawa, Y.; Nukada, K. Vibrational Spectra of Methyl Ether. I. Assignment of the Spectra. Bull. Chem. Soc. Jpn. 1962, 35, 612–618. [Google Scholar] [CrossRef]
- Bowen, R.D.; Edwards, H.G.M.; Varnali, T. Influence of a methyl substituent on the Raman spectrum of but-3-enyl methyl ether. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 93, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Bowen, R.D.; Edwards, H.G.M.; Farwell, D.W.; Morgan, S.E. Raman spectroscopy of n-pentyl methyl ether and deuterium labelled analogues. J. Raman Spectrosc. 2010, 41, 1725–1734. [Google Scholar] [CrossRef]
- John Wiley & Sons, Inc. SpectraBase; SpectraBase Compound ID=8GdSZXpNlSa SpectraBase Spectrum ID=JKKdhN5Cimb. Available online: https://spectrabase.com/spectrum/JKKdhN5Cimb (accessed on 18 August 2023).
- John Wiley & Sons, Inc. SpectraBase; SpectraBase Compound ID=D7XSL85JNeN SpectraBase Spectrum ID=EdJmIwFAdEt. Available online: https://spectrabase.com/spectrum/EdJmIwFAdEt (accessed on 18 August 2023).
- Al Hashmi, A.R.; Al Maamari, R.S.; Al Shabibi, I.S.; Mansoor, A.M.; Zaitoun, A.; Al Sharji, H.H. Rheology and mechanical degradation of high-molecular-weight partially hydrolyzed polyacrylamide during flow through capillaries. J. Pet. Sci. Eng. 2013, 105, 100–106. [Google Scholar] [CrossRef]
- Buchholz, B.A.; Zahn, J.M.; Kenward, M.; Slater, G.W.; Barron, A.E. Flow-induced chain scission as a physical route to narrowly distributed, high molar mass polymers. Polymer 2004, 45, 1223–1234. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Chao, L. Tunable Nucleation Time of Functional Sphingomyelinase–Lipid Features Studied by Membrane Array Statistic Tool. Langmuir 2013, 29, 13008–13017. [Google Scholar] [CrossRef]
| Thickness (µm) | IWC (-) | 0% | 12.5% | 25% |
|---|---|---|---|---|
| 235 | initial flux (mg/cm2·min) | 58 ± 6 | 41 ± 3 | 24 ± 5 |
| duration (min) | 0.72 ± 0.15 | 0.44 ± 0.14 | 0.01 ± 0.19 | |
| 120 | initial flux (mg/cm2·min) | 43 ± 2 | 36 ± 1 | 30 ± 1 |
| duration (min) | 0.66 ± 0.05 | 0.47 ± 0.05 | 0.24 ± 0.06 | |
| 60 | initial flux (mg/cm2·min) | 44 ± 1 | 39 ± 1 | 34 ± 1 |
| duration (min) | 0.45 ± 0.01 | 0.36 ± 0.01 | 0.27 ± 0.01 |
| Solution volume (mL) | 0.135 | 0.270 | 0.540 |
| Thickness (µm) | 60 ± 3 | 120 ± 1 | 235 ± 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chin, C.-L.; Huang, L.-J.; Lu, Z.-X.; Weng, W.-C.; Chao, L. Using the Water Absorption Ability of Dried Hydrogels to Form Hydrogel-Supported Lipid Bilayers. Gels 2023, 9, 751. https://doi.org/10.3390/gels9090751
Chin C-L, Huang L-J, Lu Z-X, Weng W-C, Chao L. Using the Water Absorption Ability of Dried Hydrogels to Form Hydrogel-Supported Lipid Bilayers. Gels. 2023; 9(9):751. https://doi.org/10.3390/gels9090751
Chicago/Turabian StyleChin, Che-Lun, Lu-Jan Huang, Zheng-Xian Lu, Wei-Chun Weng, and Ling Chao. 2023. "Using the Water Absorption Ability of Dried Hydrogels to Form Hydrogel-Supported Lipid Bilayers" Gels 9, no. 9: 751. https://doi.org/10.3390/gels9090751
APA StyleChin, C.-L., Huang, L.-J., Lu, Z.-X., Weng, W.-C., & Chao, L. (2023). Using the Water Absorption Ability of Dried Hydrogels to Form Hydrogel-Supported Lipid Bilayers. Gels, 9(9), 751. https://doi.org/10.3390/gels9090751