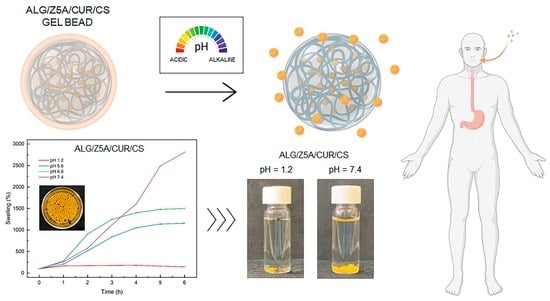
Synthesis and Characterization of Alginate Gel Beads with Embedded Zeolite Structures as Carriers of Hydrophobic Curcumin
Abstract
:1. Introduction
2. Results and Discussion
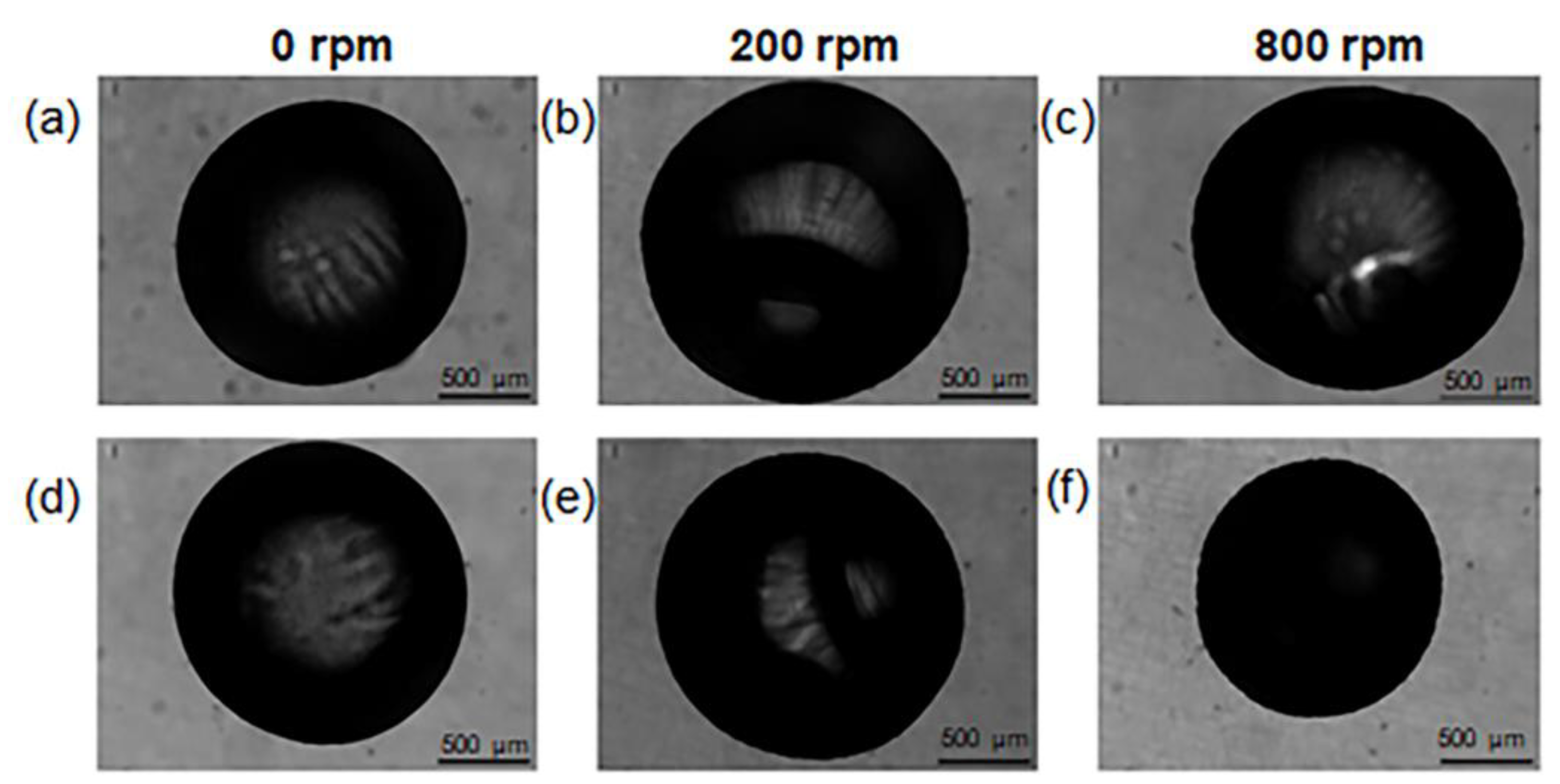
2.1. Optimization of Process Parameters
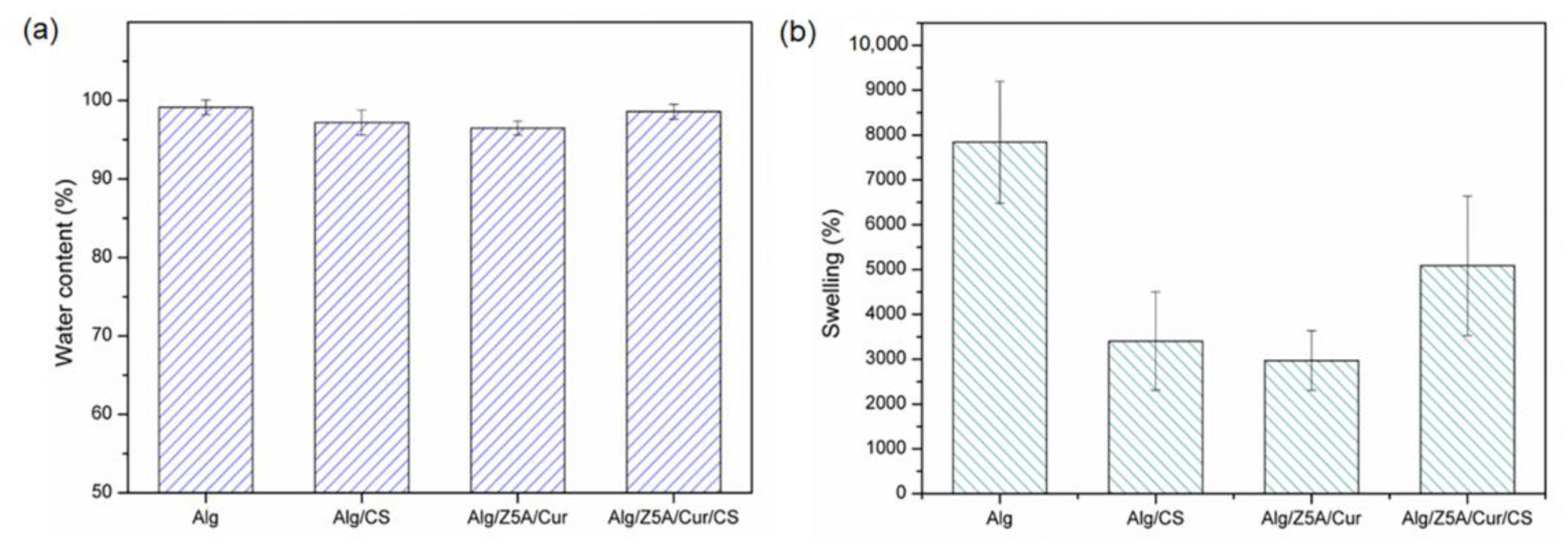
2.2. Morphological and Swelling Properties
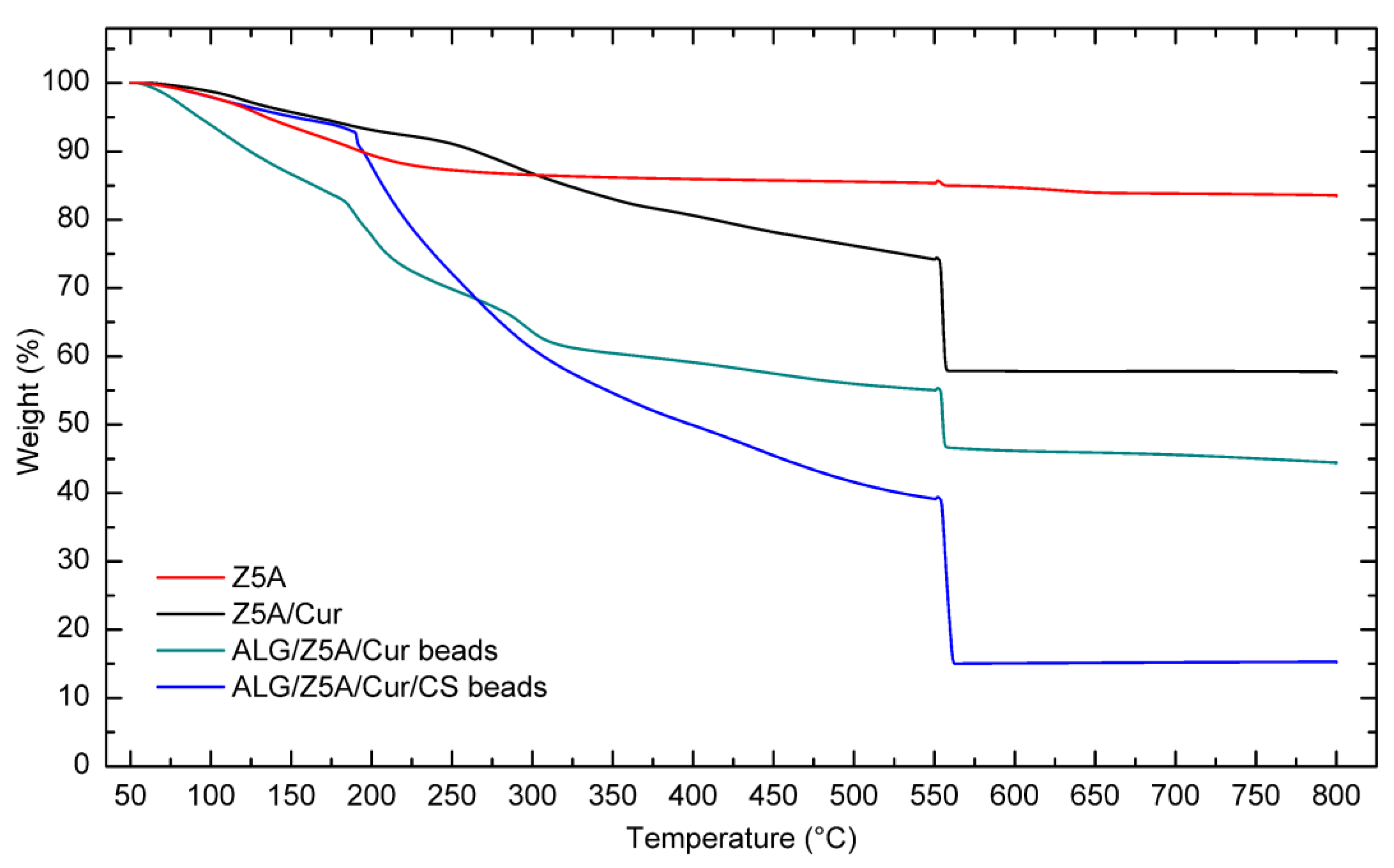
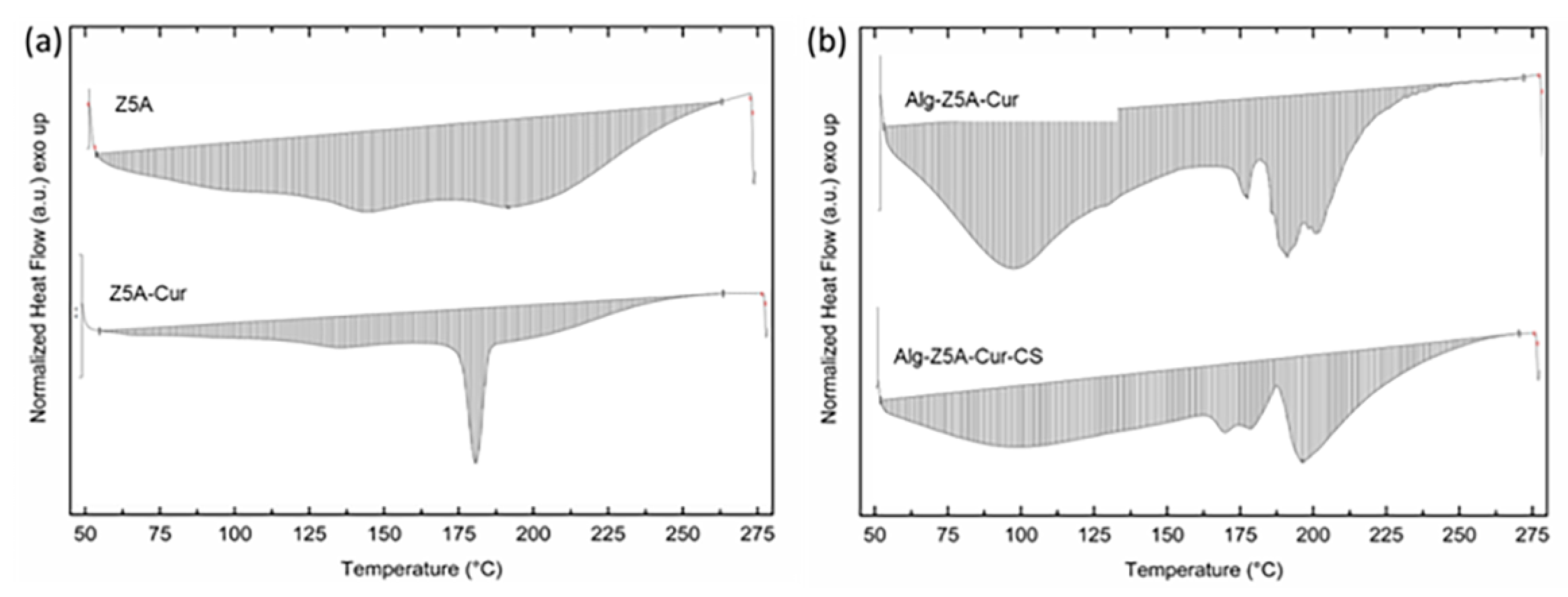
2.3. Thermal Analysis
2.4. FTIR Analysis
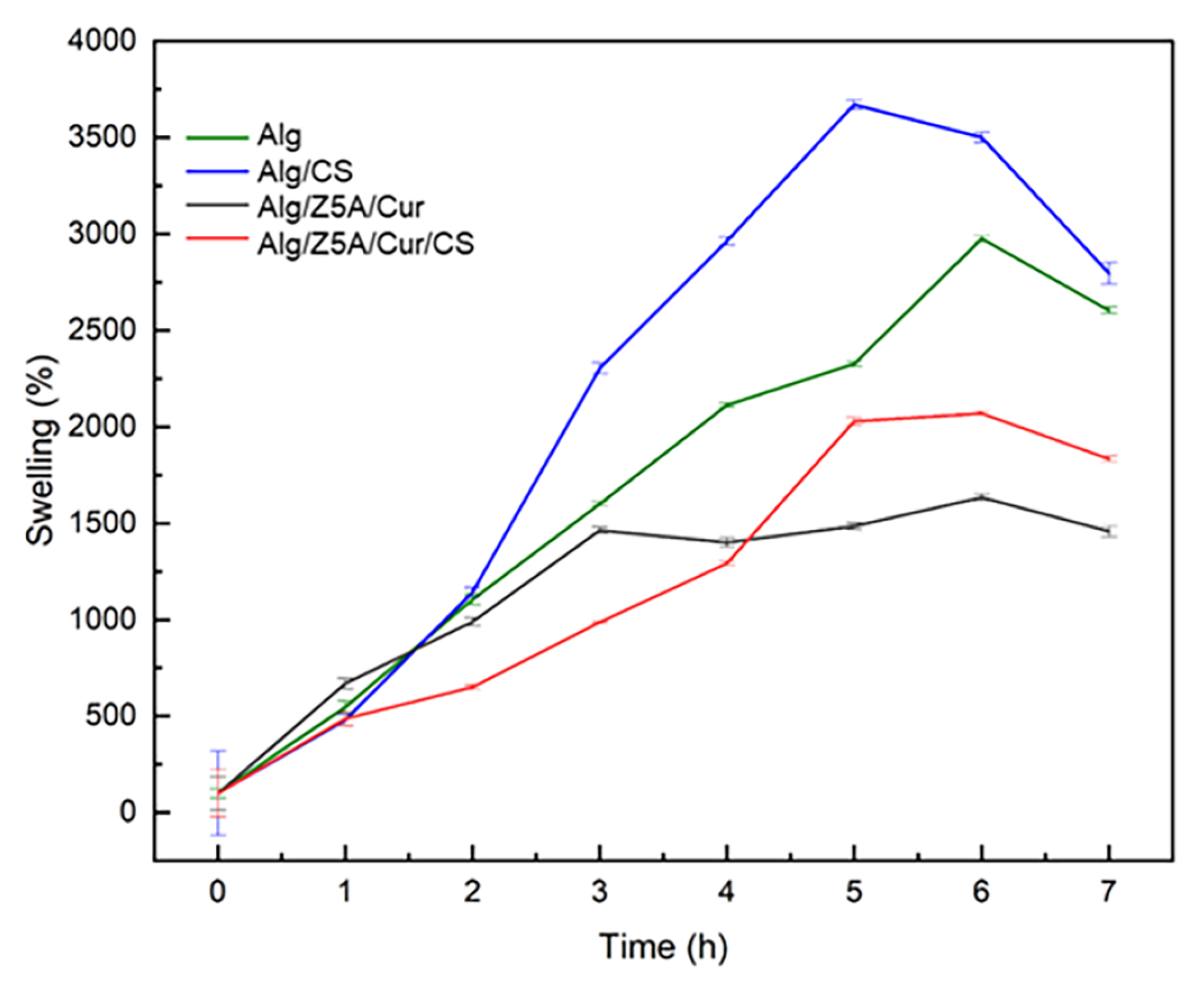
2.5. Swelling Kinetics and Degradation Tests
3. Conclusions
4. Materials and Methods
4.1. Materials
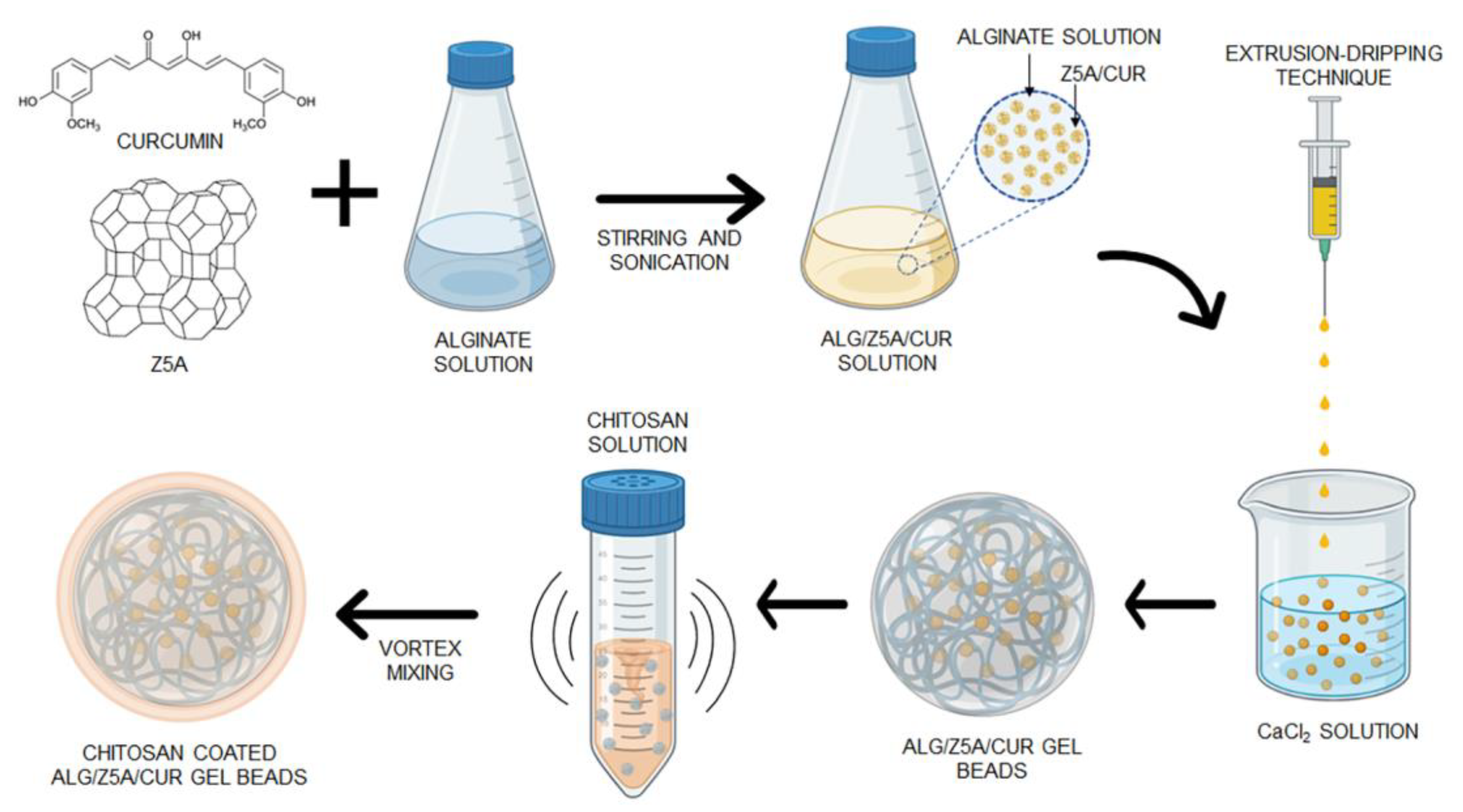
4.2. Fabrication of Alginate Gel Beads
4.3. Preparation of Chitosan Coated Alg/Z5A/Cur Gel Beads
4.4. Surface Morphology and Dimensional Analysis
4.5. Thermal Characterization
4.6. FTIR Analysis
4.7. Swelling Properties
4.8. Swelling Kinetics and Degradation Test
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef]
- Liu, L.; Wu, D.; Tu, H.; Cao, M.; Li, M.; Peng, L.; Yang, J. Applications of Hydrogels in Drug Delivery for Oral and Maxillofacial Diseases. Gels 2023, 9, 146. [Google Scholar] [CrossRef] [PubMed]
- Ciarleglio, G.; Toto, E.; Santonicola, M.G. Conductive and Thermo-Responsive Composite Hydrogels with Poly(N-isopropylacrylamide) and Carbon Nanotubes Fabricated by Two-Step Photopolymerization. Polymers 2023, 15, 1022. [Google Scholar] [CrossRef]
- Yin, Z.C.; Wang, Y.L.; Wang, K. A pH-responsive composite hydrogel beads based on agar and alginate for oral drug delivery. J. Drug Deliv. Sci. Technol. 2018, 43, 12–18. [Google Scholar] [CrossRef]
- Khadka, B.; Lee, B.; Kim, K.-T. Drug Delivery Systems for Personal Healthcare by Smart Wearable Patch System. Biomolecules 2023, 13, 929. [Google Scholar] [CrossRef] [PubMed]
- Paciello, A.; Santonicola, M.G. A supramolecular two-photon-active hydrogel platform for direct bioconjugation under near-infrared radiation. J. Mater. Chem. B 2015, 3, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Tofanica, B.-M.; Belosinschi, D.; Volf, I. Gels, Aerogels and Hydrogels: A Challenge for the Cellulose-Based Product Industries. Gels 2022, 8, 497. [Google Scholar] [CrossRef]
- Paciello, A.; Santonicola, M.G. Supramolecular polycationic hydrogels with high swelling capacity prepared by partial methacrylation of polyethyleneimine. RSC Adv. 2015, 5, 88866–88875. [Google Scholar] [CrossRef]
- Trombino, S.; Sole, R.; Curcio, F.; Cassano, R. Polymeric Based Hydrogel Membranes for Biomedical Applications. Membranes 2023, 13, 576. [Google Scholar] [CrossRef]
- Chen, J.; Chen, D.; Chen, J.; Shen, T.; Jin, T.; Zeng, B.; Li, L.; Yang, C.; Mu, Z.; Deng, H.; et al. An all-in-one CO gas therapy-based hydrogel dressing with sustained insulin release, anti-oxidative stress, antibacterial, and anti-inflammatory capabilities for infected diabetic wounds. Acta Biomater. 2022, 146, 49–65. [Google Scholar] [CrossRef]
- Chen, J.; Mu, Z.; Chen, D.; Huang, C.; Jin, T.; Li, L.; Zeng, Y.; Zhou, Q.; Zhang, Y.; Mao, H.; et al. H2S-releasing versatile hydrogel dressing with potent antimicrobial, anti-inflammatory, epithelialization and angiogenic capabilities for diabetic wound healing. Chem. Eng. J. 2023, 469, 143985. [Google Scholar] [CrossRef]
- Qamar, S.; Karim, S.; Aslam, S.; Jahangeer, M.; Nelofer, R.; Nadeem, A.A.; Qamar, S.A.; Jesionowski, T.; Bilal, M. Alginate-Based Bio-Nanohybrids with Unique Properties for Biomedical Applications. Starch Stärke 2022, 2200100, 2200100. [Google Scholar] [CrossRef]
- Wang, L.; Gang, X.; Xiao, Y.; Ren, Y.; Wang, J.; Niu, B.; Li, W. Sodium Alginate/carboxymethyl chitosan-CuO hydrogel beads as a pH-sensitive carrier for the controlled release of curcumin. Eur. Polym. J. 2023, 192, 112069. [Google Scholar] [CrossRef]
- Mohammadi, R.; Saboury, A.; Javanbakht, S.; Foroutan, R.; Shaabani, A. Carboxymethylcellulose/polyacrylic acid/starch-modified Fe3O4 interpenetrating magnetic nanocomposite hydrogel beads as pH-sensitive carrier for oral anticancer drug delivery system. Eur. Polym. J. 2021, 153, 110500. [Google Scholar] [CrossRef]
- Xu, W.; Huang, L.; Jin, W.; Ge, P.; Shah, B.R.; Zhu, D.; Jing, J. Encapsulation and release behavior of curcumin based on nanoemulsions-filled alginate hydrogel beads. Int. J. Biol. Macromol. 2019, 134, 210–215. [Google Scholar] [CrossRef]
- Ciarleglio, G.; Vella, S.; Toto, E.; Santonicola, M.G. Emulsion-based multi-responsive microspheres for the delivery of lipophilic Ozoile. Ceram. Int. 2023, 49, 24517–24524. [Google Scholar] [CrossRef]
- Li, J.; Jin, H.; Razzak, M.A.; Kim, E.J.; Choi, S.S. Crosslinker-free Bovine Serum Albumin-loaded Chitosan/alginate Nanocomplex for pH-responsive Bursting Release of Oral-administered Protein. Biotechnol. Bioprocess Eng. 2022, 27, 40–50. [Google Scholar] [CrossRef]
- Reddy, O.S.; Subha, M.; Jithendra, T.; Madhavi, C.; Rao, K.C. Curcumin encapsulated dual cross linked sodium alginate/montmorillonite polymeric composite beads for controlled drug delivery. J. Pharm. Anal. 2021, 11, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Musielak, E.; Feliczak-Guzik, A.; Jaroniec, M.; Nowak, I. Modification and Functionalization of Zeolites for Curcumin Uptake. Materials 2022, 15, 6316. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elsatar, A.; Farag, M.; Youssef, H.; Salih, S.; Mounier, M.; El-Meliegy, E. Different zeolite systems for colon cancer therapy: Monitoring of ion release, cytotoxicity and drug release behavior. Prog. Biomater. 2019, 8, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Yang, Z.; Shi, H.; Turki Jalil, A.; Mahmood Saleh, M.; Mi, W. Potentiation of Curcumin-loaded zeolite Y nanoparticles/PCL-gelatin electrospun nanofibers for postsurgical glioblastoma treatment. J. Drug Deliv. Sci. Technol. 2023, 80, 104105. [Google Scholar] [CrossRef]
- Frank, L.A.; Onzi, G.R.; Morawski, A.S.; Pohlmann, A.R.; Guterres, S.S.; Contri, R.V. Chitosan as a coating material for nanoparticles intended for biomedical applications. React. Funct. Polym. 2020, 147, 104459. [Google Scholar] [CrossRef]
- Li, J.; Hwang, I.-C.; Chen, X.; Park, H.J. Effects of chitosan coating on curcumin loaded nano-emulsion: Study on stability and in vitro digestibility. Food Hydrocoll. 2016, 60, 138–147. [Google Scholar] [CrossRef]
- Dawoud, M. Chitosan coated solid lipid nanoparticles as promising carriers for docetaxel. J. Drug Deliv. Sci. Technol. 2021, 62, 102409. [Google Scholar] [CrossRef]
- Rastogi, R.; Sultana, Y.; Aqil, M.; Ali, A.; Kumar, S.; Chuttani, K.; Mishra, A. Alginate microspheres of isoniazid for oral sustained drug delivery. Int. J. Pharm. 2007, 334, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.M.; Mitchell, M.S.; Mohan, R.S. Isolation of curcumin from turmeric. J. Chem. Educ. 2000, 77, 359. [Google Scholar] [CrossRef]
- Ahali Abadeh, Z.; Saviano, G.; Ballirano, P.; Santonicola, M.G. Curcumin-loaded zeolite as anticancer drug carrier: Effect of curcumin adsorption on zeolite structure. Pure Appl. Chem. 2020, 92, 461–471. [Google Scholar] [CrossRef]
- AL BALUSHI, K.S.A.; Geetha, D.; AL GHARIBI, A.S.R.K.; ADEEB, M.A.S.; AL HUDAIFI, A.S.M.; AL SHABIBI, S.S.K. Extraction of Bio Polymers from Crustacean Shells and its Application in Refinery Wastewater Treatment. Walailak J. Sci. & Tech. 2021, 18, 11543. [Google Scholar] [CrossRef]
- Queiroz, M.F.; Teodosio Melo, K.R.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O. Does the use of chitosan contribute to oxalate kidney stone formation? Mar. Drugs 2014, 13, 141–158. [Google Scholar] [CrossRef]
- Uyen, N.T.T.; Hamid, Z.A.A.; Ahmad, N.B. Synthesis and characterization of curcumin loaded alginate microspheres for drug delivery. J. Drug Deliv. Sci. Technol. 2020, 58, 101796. [Google Scholar] [CrossRef]
- Al Juhaiman, L.A. Curcumin extract as a green inhibitor of leaching from aluminum cookware at quasi-cooking conditions. Green Sustainable Chem. 2016, 6, 57–70. [Google Scholar] [CrossRef]
- Ho, T.H.; Dao, T.P.T.; Nguyen, T.A.; Le, D.D.; Dang, M.C. Cross-flow membrane emulsification technique for fabrication of drug-loaded particles. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 045008. [Google Scholar] [CrossRef]
- Jacas-Rodríguez, A.; Rodríguez-Pascual, P.; Franco-Manzano, D.; Contreras, L.; Polop, C.; Rodriguez, M. Mixed matrix membranes prepared from polysulfone and linde type A zeolite. Sci. Eng. Compos. Mater. 2020, 27, 236–244. [Google Scholar] [CrossRef]
- Darandale, S.; Vavia, P. Cyclodextrin-based nanosponges of curcumin: Formulation and physicochemical characterization. J. Incl. Phenom. Macrocycl. Chem. 2013, 75, 315–322. [Google Scholar] [CrossRef]
- Seyed Dorraji, M.; Rasoulifard, M.; Madadi, S.; Doosti, M.; Chiti, H.; Mousavi, S. Synthesis and evaluation of the efficiency of antibacterial hydrogel beads based on the sodium alginate–ferula gum for delayed release of quercetin. Polym. Bull. 2021, 78, 3667–3685. [Google Scholar] [CrossRef]
- Shi, J.; Alves, N.M.; Mano, J.F. Chitosan coated alginate beads containing poly (N-isopropylacrylamide) for dual-stimuli-responsive drug release. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 84, 595–603. [Google Scholar] [CrossRef]
- Guo, X.; Li, Y.; Chen, N.; Wang, X.; Xie, Q. Construction of sustainable release antimicrobial microspheres loaded with potassium diformate. J. Inorg. Mater. 2021, 36, 181–187. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, X.; Li, Y.; Fu, B.; Yang, Y.; Chen, N.; Wang, X.; Xie, Q. Fabrication of KDF-loaded chitosan-oligosaccharide-encapsulated konjac glucomannan/sodium alginate/zeolite P microspheres with sustained-release antimicrobial activity. J. Mol. Struct. 2022, 1250, 131682. [Google Scholar] [CrossRef]
- Po, H.N.; Senozan, N. The Henderson-Hasselbalch equation: Its history and limitations. J. Chem. Educ. 2001, 78, 1499. [Google Scholar] [CrossRef]
- Rayment, P.; Wright, P.; Hoad, C.; Ciampi, E.; Haydock, D.; Gowland, P.; Butler, M.F. Investigation of alginate beads for gastro-intestinal functionality, Part 1: In vitro characterisation. Food Hydrocolloids 2009, 23, 816–822. [Google Scholar] [CrossRef]
- Sadeghi, D.; Solouk, A.; Samadikuchaksaraei, A.; Seifalian, A.M. Preparation of internally-crosslinked alginate microspheres: Optimization of process parameters and study of pH-responsive behaviors. Carbohydr. Polym. 2021, 255, 117336. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.K.; Doolaanea, A.A.; Al-Mahmood, S.M.A.; Kennedy, J.F.; Chatterjee, B.; Bera, H. Electro-hydrodynamic assisted synthesis of lecithin-stabilized peppermint oil-loaded alginate microbeads for intestinal drug delivery. Int. J. Biol. Macromol. 2021, 185, 861–875. [Google Scholar] [CrossRef] [PubMed]
- Pilipenko, N.; Goncalves, O.H.; Bona, E.; Fernandes, I.P.; Pinto, J.A.; Sorita, G.D.; Leimann, F.V.; Barreiro, M.F. Tailoring swelling of alginate-gelatin hydrogel microspheres by crosslinking with calcium chloride combined with transglutaminase. Carbohydr. Polym. 2019, 223, 115035. [Google Scholar] [CrossRef] [PubMed]





| Alginate Solution (wt%) | CaCl2 Crosslinking Solution (wt%) | Needle Gauge (G) | Stirring Rate (rpm) | Collecting Distance (cm) | Mean Diameter (mm) |
|---|---|---|---|---|---|
| 1 | 10 | 30 | 0 | 7 | 1.68 ± 0.04 |
| 10 | 30 | 0 | 10 | 1.80 ± 0.31 | |
| 10 | 30 | 0 | 11.5 | 1.63 ± 0.05 | |
| 2 | 5 | 27 | 0 | 1.6 | 2.35 ± 0.30 |
| 10 | 27 | 0 | 1.6 | 1.95 ± 0.30 | |
| 10 | 30 | 0 | 1.6 | 1.56 ± 0.10 | |
| 10 | 30 | 0 | 7 | 1.54 ± 0.05 | |
| 10 | 30 | 0 | 10 | 1.68 ± 0.12 | |
| 10 | 30 | 0 | 11.5 | 1.66 ± 0.10 | |
| 10 | 30 | 200 | 1.6 | 1.59 ± 0.09 | |
| 10 | 30 | 200 | 7 | 1.67 ± 0.11 | |
| 10 | 30 | 200 | 10 | 1.68 ± 0.10 | |
| 10 | 30 | 200 | 11.5 | 1.66 ± 0.10 | |
| 20 | 30 | 0 | 7 | 1.59 ± 0.05 | |
| 4 | 5 | 23 | 0 | 1.6 | 2.42 ± 0.20 |
| 10 | 27 | 0 | 1.6 | 2.42 ± 0.30 | |
| 10 | 27 | 0 | 1.6 | 2.47 ± 0.07 |
| 0 rpm | 200 rpm | 800 rpm | |
|---|---|---|---|
| ΔD | 0.04 ± 0.07 | −0.02 ± 0.10 | −0.10 ± 0.20 |
| ΔWt | 0.9 ± 0.1 | 1.1 ± 0.2 | 1.2 ± 0.2 |
| Swollen | Dry | |||
|---|---|---|---|---|
| Beads Type | Ds (mm) | SFs | Dd (mm) | SFd |
| Alg | 1.54 ± 0.05 | 0.02 ± 0.03 | 0.50 ± 0.08 | 0.06 ± 0.04 |
| Alg/CS | 1.51 ± 0.02 | 0.03 ± 0.02 | 0.48 ± 0.08 | 0.06 ± 0.08 |
| Alg/Z5A/Cur | 1.71 ± 0.08 | 0.03 ± 0.02 | 0.82 ± 0.12 | 0.03 ± 0.05 |
| Alg/Z5A/Cur/CS | 1.67 ± 0.05 | 0.03 ± 0.02 | 0.52 ± 0.03 | 0.03 ± 0.03 |
| Sample Type | Weight Loss at 800 °C (%) | Residue at 800 °C (%) |
|---|---|---|
| ZA5 | 15.80 | 82.41 |
| ZA5/Cur | 41.52 | 56.86 |
| Alg/Z5A/Cur beads | 41.89 | 37.49 |
| Alg/Z5A/Cur/CS beads | 83.19 | 15.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciarleglio, G.; Cinti, F.; Toto, E.; Santonicola, M.G. Synthesis and Characterization of Alginate Gel Beads with Embedded Zeolite Structures as Carriers of Hydrophobic Curcumin. Gels 2023, 9, 714. https://doi.org/10.3390/gels9090714
Ciarleglio G, Cinti F, Toto E, Santonicola MG. Synthesis and Characterization of Alginate Gel Beads with Embedded Zeolite Structures as Carriers of Hydrophobic Curcumin. Gels. 2023; 9(9):714. https://doi.org/10.3390/gels9090714
Chicago/Turabian StyleCiarleglio, Gianluca, Federica Cinti, Elisa Toto, and Maria Gabriella Santonicola. 2023. "Synthesis and Characterization of Alginate Gel Beads with Embedded Zeolite Structures as Carriers of Hydrophobic Curcumin" Gels 9, no. 9: 714. https://doi.org/10.3390/gels9090714
APA StyleCiarleglio, G., Cinti, F., Toto, E., & Santonicola, M. G. (2023). Synthesis and Characterization of Alginate Gel Beads with Embedded Zeolite Structures as Carriers of Hydrophobic Curcumin. Gels, 9(9), 714. https://doi.org/10.3390/gels9090714