Autoclavable Albumin-Based Cryogels with Uncompromising Properties
Abstract
:1. Introduction
2. Results and Discussion
Characterization of BSAMA and BSAMAA Cryogels
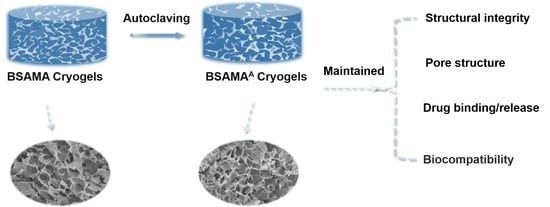
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Fabrication of BSAMA Cryogels
4.3. Sterilization of BSAMA Cryogels
4.4. Micro-Structural Imaging of BSAMA Cryogels
4.5. Physical Characterization of BSAMA Cryogels
4.6. Fourier Transform Infrared Spectroscopy (FTIR)
4.7. Enzymatic Degradation
4.8. Analysis of Bacterial Adhesion on BSAMA Cryogels
4.9. Drug Loading and Drug Release
4.10. In Vitro Biocompatibility of BSAMA Cryogels
4.10.1. Cell Seeding
4.10.2. CCK-8
4.10.3. Live/Dead
4.11. Hemocompatibility of BSAMA Cryogels
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bento, C.S.; Gaspar, M.C.; Coimbra, P.; de Sousa, H.C.; Braga, M.E. A review of conventional and emerging technologies for hydrogels sterilization. Int. J. Pharm. 2023, 634, 122671. [Google Scholar] [CrossRef] [PubMed]
- Pohan, G.; Mattiassi, S.; Yao, Y.; Zaw, A.M.; Anderson, D.E.J.; Cutiongco, M.F.A.; Hinds, M.T.; Yim, E.K.F. Effect of Ethylene Oxide Sterilization on Polyvinyl Alcohol Hydrogel Compared with Gamma Radiation. Tissue Eng. Part A 2020, 26, 1077–1090. [Google Scholar] [CrossRef] [PubMed]
- Kanjickal, D.; Lopina, S.; Evancho-Chapman, M.M.; Schmidt, S.; Inbaraj, J.J.; Cardon, T.B.; Lorigan, G.A. Electron spin resonance studies of the effects of sterilization on poly(ethylene glycol) hydrogels. J. Biomed. Mater. Res. Part A 2009, 88, 409–418. [Google Scholar] [CrossRef]
- Villard, P.; Rezaeeyazdi, M.; Colombani, T.; Joshi-Navare, K.; Rana, D.; Memic, A.; Bencherif, S.A. Autoclavable and Injectable Cryogels for Biomedical Applications. Adv. Healthc. Mater. 2019, 8, e1900679. [Google Scholar] [CrossRef] [PubMed]
- Memic, A.; Colombani, T.; Eggermont, L.J.; Rezaeeyazdi, M.; Steingold, J.; Rogers, Z.J.; Navare, K.J.; Mohammed, H.S.; Bencherif, S.A. Latest Advances in Cryogel Technology for Biomedical Applications. Adv. Ther. 2019, 2, 1800114. [Google Scholar] [CrossRef]
- Xu, M.; Mehwish, N.; Lee, B.H. Facile Fabrication of Transparent and Opaque Albumin Methacryloyl Gels with Highly Improved Mechanical Properties and Controlled Pore Structures. Gels 2022, 8, 367. [Google Scholar] [CrossRef]
- Mehwish, N.; Chen, Y.; Zaeem, M.; Wang, Y.; Lee, B.H.; Deng, H. Novel biohybrid spongy scaffolds for fabrication of suturable intraoral graft substitutes. Int. J. Biol. Macromol. 2022, 214, 617–631. [Google Scholar] [CrossRef]
- Niu, X.; Lin, M.; Lee, B.H. An Engineered Protein-Based Building Block (Albumin Methacryloyl) for Fabrication of a 3D In Vitro Cryogel Model. Gels 2022, 8, 404. [Google Scholar] [CrossRef]
- Galante, R.; Pinto, T.J.A.; Colaco, R.; Serro, A.P. Sterilization of hydrogels for biomedical applications: A review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2472–2492. [Google Scholar] [CrossRef]
- Beard, M.C.; Cobb, L.H.; Grant, C.S.; Varadarajan, A.; Henry, T.; Swanson, E.A.; Kundu, S.; Priddy, L.B. Autoclaving of Poloxamer 407 hydrogel and its use as a drug delivery vehicle. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 338–3477. [Google Scholar] [CrossRef]
- Takei, T.; Danjo, S.; Sakoguchi, S.; Tanaka, S.; Yoshinaga, T.; Nishimata, H.; Yoshida, M. Autoclavable physically-crosslinked chitosan cryogel as a wound dressing. J. Biosci. Bioeng. 2018, 125, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Memic, A.; Rezaeeyazdi, M.; Villard, P.; Rogers, Z.J.; Abdullah, T.; Colombani, T.; Bencherif, S.A. Effect of Polymer Concentration on Autoclaved Cryogel Properties. Macromol. Mater. Eng. 2020, 305, 1900824. [Google Scholar] [CrossRef]
- Kong, F.; Mehwish, N.; Lee, B.H. Emerging albumin hydrogels as personalized biomaterials. Acta Biomater. 2023, 157, 67–90. [Google Scholar] [CrossRef] [PubMed]
- Eigel, D.; Werner, C.; Newland, B. Cryogel biomaterials for neuroscience applications. Neurochem. Int. 2021, 147, 105012. [Google Scholar] [CrossRef]
- Yuan, L.; Li, X.; Ge, L.; Jia, X.; Lei, J.; Mu, C.; Li, D. Emulsion Template Method for the Fabrication of Gelatin-Based Scaffold with a Controllable Pore Structure. ACS Appl. Mater. Interfaces 2019, 11, 269–277. [Google Scholar] [CrossRef]
- Oh, S.H.; Park, I.K.; Kim, J.M.; Lee, J.H. In vitro and in vivo characteristics of PCL scaffolds with pore size gradient fabricated by a centrifugation method. Biomaterials 2007, 28, 1664–1671. [Google Scholar] [CrossRef]
- Cotner, S.N.; Es-haghi, S.S. Unimpaired highly extensible tough chemically crosslinked hydrogel after experiencing freeze/thaw and boiling processes. Polym. Eng. Sci. 2022, 63, 402–412. [Google Scholar] [CrossRef]
- Chen, Y.; Zhai, M.J.; Mehwish, N.; Xu, M.D.; Wang, Y.; Gong, Y.X.; Ren, M.M.; Deng, H.; Lee, B.H. Comparison of globular albumin methacryloyl and random-coil gelatin methacryloyl: Preparation, hydrogel properties, cell behaviors, and mineralization. Int. J. Biol. Macromol. 2022, 204, 692–708. [Google Scholar] [CrossRef]
- Tamayol, A.; Najafabadi, A.H.; Aliakbarian, B.; Arab-Tehrany, E.; Akbari, M.; Annabi, N.; Juncker, D.; Khademhosseini, A. Hydrogel Templates for Rapid Manufacturing of Bioactive Fibers and 3D Constructs. Adv. Healthc. Mater. 2015, 4, 2146–2153. [Google Scholar] [CrossRef]
- Rizwan, M.; Chan, S.W.; Comeau, P.A.; Willett, T.L.; Yim, E.K.F. Effect of sterilization treatment on mechanical properties, biodegradation, bioactivity and printability of GelMA hydrogels. Biomed. Mater. 2020, 15, 065017. [Google Scholar] [CrossRef]
- Hnaien, M.; Hassen, W.M.; Abdelghani, A.; Cotte, S.; Leonard, D.; Bessueille, F.; Jaffrezic-Renault, N. A conductometric biosensor for the estimation of the number of cleaving sites in peptides and proteins. Electrochem. Commun. 2009, 11, 165–168. [Google Scholar] [CrossRef]
- Iqbal, H.; Yang, T.; Li, T.; Zhang, M.; Ke, H.; Ding, D.; Deng, Y.; Chen, H. Serum protein-based nanoparticles for cancer diagnosis and treatment. J. Control. Release 2021, 329, 997–1022. [Google Scholar] [CrossRef] [PubMed]
- Solanki, R.; Rostamabadi, H.; Patel, S.; Jafari, S.M. Anticancer nano-delivery systems based on bovine serum albumin nanoparticles: A critical review. Int. J. Biol. Macromol. 2021, 193, 528–540. [Google Scholar] [CrossRef] [PubMed]
- Ying, M.; Li, Q.; Wu, J.; Jiang, Y.; Xu, Z.; Ma, M.; Xu, G. CuS@BSA-NB2 Nanoparticles for HER2-Targeted Photothermal Therapy, Frontiers in Pharmacology. Front. Pharmacol. 2022, 12, 779591. [Google Scholar] [CrossRef] [PubMed]
- Arifin, D.Y.; Lee, L.Y.; Wang, C.H. Mathematical modeling and simulation of drug release from microspheres: Implications to drug delivery systems. Adv. Drug Deliv. Rev. 2006, 58, 1274–1325. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, T.L.; Little, D. Synthetic scaffolds for musculoskeletal tissue engineering: Cellular responses to fiber parameters. npj Regen. Med. 2019, 4, 15. [Google Scholar] [CrossRef] [PubMed]
- Kuchinka, J.; Willems, C.; Telyshev, D.V.; Groth, T. Control of Blood Coagulation by Hemocompatible Material Surfaces-A Review. Bioengineering 2021, 8, 215. [Google Scholar] [CrossRef]
- Baron, R.I.; Duceac, I.A.; Morariu, S.; Bostanaru-Iliescu, A.C.; Coseri, S. Hemostatic Cryogels Based on Oxidized Pullulan/Dopamine with Potential Use as Wound Dressings. Gels 2022, 8, 726. [Google Scholar] [CrossRef]
- Li, X.; Kong, X.; Zhang, Z.; Nan, K.; Li, L.; Wang, X.; Chen, H. Cytotoxicity and biocompatibility evaluation of N,O-carboxymethyl chitosan/oxidized alginate hydrogel for drug delivery application. Int. J. Biol. Macromol. 2012, 50, 1299–1305. [Google Scholar] [CrossRef]
- Demirci, S.; Sahiner, M.; Ari, B.; Sunol, A.K.; Sahiner, N. Chondroitin Sulfate-Based Cryogels for Biomedical Applications. Gels 2021, 7, 127. [Google Scholar] [CrossRef]
- Chen, L.; Glass, J.J.; De Rose, R.; Sperling, C.; Kent, S.J.; Houston, Z.H.; Fletcher, N.L.; Rolfe, B.E.; Thurecht, K.J. Influence of Charge on Hemocompatibility and Immunoreactivity of Polymeric Nanoparticles. ACS Appl. Bio Mater. 2018, 1, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Goyama, T.; Fujii, Y.; Muraoka, G.; Nakatani, T.; Ousaka, D.; Imai, Y.; Kuwada, N.; Tsuji, T.; Shuku, T.; Uchida, H.A.; et al. Comprehensive hemocompatibility analysis on the application of diamond-like carbon to ePTFE artificial vascular prosthesis. Sci. Rep. 2023, 13, 8386. [Google Scholar] [CrossRef] [PubMed]
- Boulais, L.; Jellali, R.; Pereira, U.; Leclerc, E.; Bencherif, S.A.; Legallais, C. Cryogel-Integrated Biochip for Liver Tissue Engineering. ACS Appl. Bio Mater. 2021, 4, 5617–5626. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Nho, Y.H.; Yun, S.K.; Hwang, Y.S. Use of ethanol extracts of Terminalia chebula to prevent periodontal disease induced by dental plaque bacteria. BMC Complement. Altern. Med. 2017, 17, 113. [Google Scholar] [CrossRef] [PubMed]
- Hao, K.; Cui, R.; Fang, L.; Ling, J.; Zhu, B. Lysine-Sarcosine PiPo Functionalized Surface with Excellent Hemocompatibility. ACS Appl. Mater. Interfaces 2023, 15, 29700–29712. [Google Scholar] [CrossRef] [PubMed]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, K.; Mehwish, N.; Xu, M.; Zhu, H.; Hu, J.; Lin, M.; Yu, L.; Lee, B.H. Autoclavable Albumin-Based Cryogels with Uncompromising Properties. Gels 2023, 9, 712. https://doi.org/10.3390/gels9090712
Duan K, Mehwish N, Xu M, Zhu H, Hu J, Lin M, Yu L, Lee BH. Autoclavable Albumin-Based Cryogels with Uncompromising Properties. Gels. 2023; 9(9):712. https://doi.org/10.3390/gels9090712
Chicago/Turabian StyleDuan, Kairui, Nabila Mehwish, Mengdie Xu, Hu Zhu, Jiajun Hu, Mian Lin, Lu Yu, and Bae Hoon Lee. 2023. "Autoclavable Albumin-Based Cryogels with Uncompromising Properties" Gels 9, no. 9: 712. https://doi.org/10.3390/gels9090712
APA StyleDuan, K., Mehwish, N., Xu, M., Zhu, H., Hu, J., Lin, M., Yu, L., & Lee, B. H. (2023). Autoclavable Albumin-Based Cryogels with Uncompromising Properties. Gels, 9(9), 712. https://doi.org/10.3390/gels9090712