1. Introduction
Organ transplantation remains the sole treatment option for individuals with end-stage organ failure [
1]. However, the scarcity of available donor organs, coupled with logistical challenges arising from the limited ex vivo organ survival time, results in a significant number of patients dying before they can receive a transplant [
2,
3]. The pressing issue of organ shortage has propelled research in the field of 3D bioprinting, offering a promising solution to alleviate the crisis [
4].
Three-dimensional bioprinting is an additive manufacturing technology that uses a digital file as a blueprint to print an object, voxel by voxel and layer by layer. Although tissue engineering was introduced by Langer and Vacanti approximately 30 years ago [
5], the technology for 3D printing large organs is still in its developmental stages and has not reached maturity [
6]. A notable accomplishment in 3D bioprinting is the successful fabrication of a functioning miniature heart at a scale of 15 mm [
7]. The fabrication of the miniature heart was achieved through a four-hour long process, which underscores both the progress made in bioprinting complex organs and the challenges that remain in scaling up the technology for larger organs. The storage of large organs post-3D bioprinting parallels the challenges currently faced in preserving donor organs for transplantation. Curiously, this specific challenge has not received any attention from researchers focused on large organ production via 3D bioprinting.
To develop effective 3D bioprinting technologies for the fabrication of large organs, it is important to understand the limitations in current 3D bioprinting methods. A commonly used 3D bioprinting technology is extrusion printing [
8], in which a bioink is extruded through a nozzle and deposited voxel by voxel, layer by layer, to construct the desired 3D structure [
9]. One of the pivotal elements in 3D bioprinting is the composition of the bioink, which comprises a combination of cells, biomaterials, and bioactive molecules. Aqueous solutions of sodium alginate are commonly used as bioprinting inks [
8]. This is due to alginate’s exceptional biocompatibility, biosafety, and cost-effectiveness [
10]. Alginate is extracted from the cell walls of brown algae in the form of alginic acid. Sodium alginate, derived from alginic acid, can form hydrogels by substituting the sodium ions from the guluronic acid residues with different divalent cations, such as
, which crosslink the polymer chains via the “egg-box” model [
11]. The crosslinking of alginate is irreversible.
In 3D bioprinting, the viscosity of the ink plays a crucial role. The low viscosity of alginate before crosslinking enables easy extrusion through a small nozzle, facilitating high precision and resolution during printing. Moreover, the use of low-viscosity ink is vital for cell viability within the extruded ink. Studies have indicated that cells do not survive extrusion with high-viscosity inks [
12]. However, maintaining the structural rigidity of alginate-based 3D printed objects requires crosslinking. This presents a challenge in determining the optimal timing for crosslinking: if performed before printing, the alginate becomes too viscous to be extruded through the nozzle. Delaying crosslinking for too long after deposition on the print plate can result in the collapse of the non-crosslinked alginate structure. Also, even in its crosslinked form, the 3D printed alginate structure is not sufficiently rigid to support the weight of a large organ during the layer-by-layer deposition process, prior to the completion of the structure. This limitation poses a significant challenge in the 3D bioprinting of large-scale structures. In the bioprinting of large organs, living cells are included within the bioprinting ink. However, a significant challenge arises in ensuring the survival of these 3D bioprinted cells throughout the lengthy printing process. As previously mentioned, the 3D bioprinting of a miniature heart typically takes around four hours. Unfortunately, cells cannot remain viable outside their optimal growth environment for such prolonged durations. Consequently, it is likely that the cells within the initially printed layers may not survive until the completion of the printing process. This cell survival challenge presents a critical hurdle in the successful bioprinting of large organs.
Our research group has made significant advancements in addressing the challenges associated with 3D bioprinting of large structures through the development of the “Temperature-Controlled-Cryoprinting” (TCC) technology [
13,
14,
15]. We utilized low-viscosity alginate solutions as the 3D printing ink, which facilitated precise ink deposition and improved cell survival [
12]. The key innovation in the TCC technology resides in the precise temperature control during the freezing of each deposited voxel as it is deposited [
13,
15].
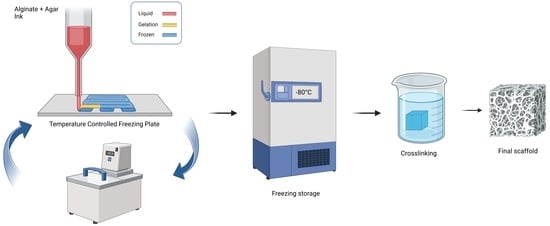
Figure 1 provides an illustration of the TCC process [
16]. It shows that as each layer is printed and frozen, the platform is submerged in a cryogenic fluid bath maintained at a controlled temperature below freezing. In the TCC process, the distance between the last printed and frozen layer and the cryogenic bath upper surface is carefully regulated to enable freezing with a controlled cooling rate for the subsequent printed layer.
Freezing of each layer as it is deposited imparts immediate rigidity to the structure as soon as it is printed. Consequently, it enables the manufacturing of complex and large structures that would otherwise be challenging to achieve. For instance, the creation of structures with internal cavities becomes feasible through this method [
17]. Freezing of a gel with temperature-controlled cryoprinting, has several other advantages in addition to providing rigidity to the printed object. Conventional 3D printing generates isotropic structures at the microscale. Temperature-controlled freezing can be used to control the size, structure, and direction of the ice crystals that form [
6,
7,
8]. This can be used to generate controlled anisotropic microstructures, anisotropic rigidity, and material properties of the 3D cryoprinted structure [
3,
11,
12].
The accurate control of the cooling rate during the freezing of each layer provides another advantage. It allows the freezing of every deposited cell with optimal cryopreservation thermal parameters during the entire 3D bioprinting process [
14,
18]. With TCC, each cell in the entire structure is cryopreserved as soon as it is printed [
12]. Once the entire 3D structure is frozen using temperature-controlled cryoprinting (TCC), all the cells within the large structure are cryopreserved using optimal cryopreservation protocols. This ensures their viability and makes the structure ready for storage in a frozen state and transportation. This is particularly important in printing large biological organs with cells, because 3D printing is a slow process and cells printed in the early stage of the process can die by the time the entire organ is printed. Recent studies have demonstrated the capability of TCC technology to produce large, cryopreserved 3D printed structures with cells that successfully survive in the printing process and subsequent cryopreservation [
12]. In that study, the bioprinting ink used also incorporated a cryoprotective solution, specifically, dimethyl sulfoxide (Me
2SO). The inclusion of Me
2SO in the bioprinting ink played a crucial role in ensuring the long-term survival of cells within the 3D printed structure during cryopreservation at a temperature of −80 °C. This finding highlights the effectiveness of the TCC-enabled cryopreservation process and the potential for extended storage of 3D bioprinted structures with preserved cell viability.
In the temperature-controlled-cryoprinting (TCC) technique, the crosslinking of alginate with CaCl
2 can be performed through two methods: before freezing or after freezing, during thawing [
19]. This study specifically focuses on the technique where crosslinking occurs during the thawing process [
16]. In this approach, the entire object is 3D printed using temperature-controlled cryoprinting of each alginate voxel before any crosslinking takes place. The frozen structure (with or without cells) is maintained at a desired subfreezing preservation temperature [
12,
16]. During the crosslinking step, the frozen structure is immersed in a solution of a crosslinker, typically CaCl
2, at a controlled temperature, above freezing. The temperatures of the frozen object and the crosslinking bath are carefully chosen to ensure that as the frozen object melts, the diffusion of CaCl
2 into the melted region occurs simultaneously with the propagation of the change of phase interface [
16]. This allows for crosslinking to take place as the structure melts, thereby achieving structural rigidity [
16]. A schematic representation of all steps involved in this procedure is illustrated in
Figure 1 ([
12]).
The crosslinking technique described above has its limitations. The propagation of the change of phase interface and the diffusion of the CaCl
2 must occur simultaneously to ensure that the structure’s rigidity, obtained through freezing, is immediately replaced by the rigidity provided by alginate crosslinking. The melting process is affected by the lower diffusivity of CaCl
2 through the crosslinked alginate compared to thermal diffusivity. It would be beneficial to discover methods to enhance the diffusivity of CaCl
2 through the crosslinked alginate, thereby reducing the time required for this manufacturing step. Additionally, this technology shares a common drawback with all alginate-based 3D printing processes, which is the shrinkage of the printed structure. This shrinkage leads to a decrease in form fidelity [
16]. This study was specifically conducted to address the two beforementioned limitations: (a) the low diffusivity of CaCl
2 through crosslinked alginate during the melting-associated crosslinking process and (b) the commonly observed low form fidelity in alginate-based 3D printing.
This study aimed to investigate the hypothesis that incorporation agar into the alginate ink could potentially enhance the diffusion of the CaCl
2 during the melting/crosslinking step, resulting in better maintenance of shape fidelity. A recent observation on the freezing and melting of agar gel is particularly relevant [
20]. According to the study, the ice crystals formed during the freezing of an agarose gel disrupt the structural integrity of the gel. Upon melting, the gel structure transforms into alginate flakes and free water.
A rough comparison between the diffusion coefficient of CaCl
2 in an aqueous solution [
21] and in a 2% agar gel [
22] reveals that the diffusion in the aqueous solution is higher than in the gel by a factor of about 1.5. An order-of-magnitude comparison between the diffusion coefficient of CaCl
2 in aqueous solutions and the diffusivity of
crosslinked alginate [
23] demonstrates that the diffusivity in an aqueous solution is at least one order of magnitude greater than that in the crosslinked alginate. It is important to note that these values are not precise and depend on various factors, particularly the concentrations of agar and alginate. Nevertheless, these findings suggest that the addition of agar to an alginate ink should increase the diffusivity of CaCl
2 through the crosslinked alginate after the melting of the frozen agar gel.
The practice of adding agar to alginate to enhance the rigidity and printing fidelity of alginate in casting and 3D printing is well documented. In fact, the use of a combination of agar and alginate for casting dental imprints has been known since 1937. Extensive research in the field of dentistry has demonstrated that agar produces imprints that closely resemble the source, while the combination of agar and alginate results in a gel that is easier to handle, possesses mechanical strength like alginate, and reliably captures impressions, as evidenced in [
24]. Agar has been employed in conjunction with alginate in various other applications. For instance, agar was incorporated into alginate hydrogel beads for oral drug delivery, aiming to enhance the mechanical strength of the beads [
25]. The combination of agar and alginate has also been utilized in the production of packaging films [
26]. Furthermore, this combination has already found utility in 3D bioprinting, where it increases ink viscosity and imparts rigidity to the printed objects [
27,
28,
29]. Based on these previous applications, we envisioned that an ink composed of agar and alginate could retain the fidelity of the print, even when using the TCC (melting/crosslinking) method.
Agar is a gel-like substance derived from the cell walls of red algae. Unlike alginate, which forms gels through chemical crosslinking, agar gels undergo physical changes, specifically hydrogen bonding crosslinking. Agar transforms into a gel as it cools from 85 °C to about 32–42 °C. Notably, the gelation of agar is reversible, unlike the irreversible crosslinking experienced by alginate.
In the envisioned TCC process using an ink of agar and alginate, the following sequence of events takes place when a solution of agar and alginate is deposited on the printing surface. As the printed voxel cools to approximately 30 °C, the agar first undergoes gelation by forming a network through hydrogen bonding crosslinking of agar molecular chains. At this stage, the alginate is not yet crosslinked but is trapped within the agar gel network, with the structure of the print being fixed by the agar gel. During TCC, the deposited voxel freezes completely, encompassing the gelled agar and the liquid alginate held in place by the agar network. As the frozen object begins to thaw in a solution of CaCl
2, as described in [
16], the molten agar loses its structural integrity and assumes the diffusivity characteristics of water instead of that of a gel, as previously discussed. Consequently, the diffusivity of CaCl
2 through the crosslinked alginate with liquid agar becomes higher compared to the diffusivity in a crosslinked alginate solution without agar. This results in faster and more controlled crosslinking. The fidelity of the printed structure is initially maintained during TCC through the gelation of agar, followed by freezing, and ultimately through the crosslinking of the alginate.
In this paper, we will explore the effects of the agar/alginate ink composition on the TCC process regarding deformation, diffusivity, stability in an environment that mimics the human body, microscale structure, and printability.
3. Conclusions
In conclusion, the utilization of SA/AG ink in temperature-controlled cryoprinting (TCC) has led to the successful fabrication of constructs with good shape fidelity, shape retaining ability, and high porosity. The SA/AG ink undergoes key phenomena during the TCC process that contribute to important outcomes. Firstly, the gelation of agar before freezing helps maintain the shape of the printed structure until freezing occurs. This ensures the preservation of the desired structure with high accuracy. During the melting stage, the agar loses its water-holding ability, thereby increasing the diffusivity of the crosslinker as the structure melts. This enhanced diffusivity facilitates a more effective and faster crosslinking process. The scaffolds produced using the SA/AG ink demonstrate remarkable structural stability during crosslinking and exposure to liquid, making them highly suitable for in vivo applications. Electron microscopy analysis confirms the presence of high porosity within the SA/AG-based TCC scaffolds, which explains the increased diffusivity observed after freezing agar-containing ink. The freezing process employed during cryoprinting plays a crucial role in enhancing the porosity of the scaffolds. In summary, the combination of Agar and Sodium Alginate in the ink formulation represents an effective approach for temperature-controlled cryoprinting. This formulation enables the fabrication of scaffolds with desirable properties, including superior shape fidelity, excellent structure retention, and high porosity. These findings open new possibilities for advanced tissue engineering and regenerative medicine applications.
Recently, our team demonstrated the feasibility of utilizing TCC technology to 3D cryoprinting viable Vero cells. This innovative approach employs an ink composed of a blend of alginate, collagen, and cells. Significantly, these cells demonstrate survival and growth after undergoing thawing and crosslinking processes, even within structures as complex as seven printed layers [
12]. The primary objective of developing this technology was to manufacture 3D cell structures for investigating viral contamination, providing an alternative to prevalent 2D cell structure methodologies in this field.
Preliminary findings within our research group suggest that this technology offers versatility in layer count, effectively eliminating the previous dimensional constraints inherent in 3D printing of biological constructs. By combining the technology outlined in [
12] with the discoveries elucidated in this paper, the prospect of bioprinting substantial organs while retaining structural precision and cellular vitality becomes attainable. A comprehensive review of 3D bioprinting research on the liver can be found in [
36]. At present, our research efforts are focused on addressing the obstacles outlined in [
36] concerning the 3D bioprinting of a liver. We aim to tackle these challenges by leveraging TCC technology, employing an ink formulation comprising alginate, gelatin, agar, and live cells. Our ultimate objective is to achieve the 3D bioprinting of an entire liver lobe, which will provide a foundational framework for investigating the effects of various pharmaceuticals on the human liver.
4. Materials and Methods
4.1. Agar/Alginate Solution
To prepare the gel solution, Agar (Sigma-Aldrich, St. Louis, MO, USA) and sodium alginate (Sigma-Aldrich, St. Louis, MO, USA) were dissolved in deionized (DI) water. The DI water was heated up to 100 °C using a heating plate (Corning Inc., Somerville, MA, USA) to dissolve the agar and sodium alginate powders. Once all the powder was dissolved, the solution was cooled to 40 °C. The study involved three compositions: 2% Sodium Alginate/2% Agar w/w (2% AG/2% SA), 2% Sodium Alginate/1% Agar w/w (1% SA/2% AG), and 2% Sodium Alginate w/w (2% SA).
4.2. Crosslinking Bath
A 2% calcium chloride (CaCl2) bath was prepared for crosslinking by dissolving 8 g of CaCl2 powder (Fisher Scientific, Hampton, NH, USA) in 400 mL of DI water. All frozen samples were stored in a −80 °C freezer prior to crosslinking. To initiate crosslinking, the samples were taken out from the freezer and fully submerged in the crosslinking bath at room temperature (23 °C) for 15 min. The crosslinking and thawing processes were continuously monitored, and significant events were recorded with corresponding timestamps.
4.3. Electron Microscope
Electron microscopy was performed on all the processed agar/alginate compositions. The sample being examined was initially rapidly frozen by immersing it in liquid nitrogen and then subjected to freeze drying using a 4.5 L −50 °C freeze dryer (Labconco FreeZone, Kansas City, MO, USA) for a duration of 2 days. The resulting freeze-dried sample was observed using a Hitachi TM-4000 scanning electron microscope (Hitachi, Chiyoda City, Tokyo, Japan) at various magnifications.
4.4. Experimental Systems
This paper presents the findings of multiple experimental studies focused on the TCC (temperature-controlled cryoprinting) process applied to agar/alginate solutions. Specifically, we investigate the deformation behavior, diffusivity, stability in a simulated human body environment, microscale structure, and printability of these solutions. Subsequently, we provide a detailed description of the devices employed in these experiments, both for the fabrication and testing of the samples. Two configurations were used in the study: (a) casting and (b) 3D printing.
4.5. Casts
A study with casting was designed to investigate the deformation behavior of samples composed of various agar and alginate compositions treated with freezing and immersion crosslinking. The samples were cast using the mold depicted in
Figure 1, which features a top surface measuring 3.7 cm × 3.7 cm with rounded edges. To ensure consistent results, different agar/alginate inks with the same volume (3 mL) were injected into the mold container, ensuring an identical original cast for each sample. The ink solution was prepared at 100 °C and then cooled to 40 °C before casting. Once cast, all samples were cooled to room temperature to allow agar gelation and subsequently transferred to a −80 °C freezer for freezing. After freezing, the samples were collected from the mold container and immersed in a CaCl
2 bath at room temperature for 15 min to initiate crosslinking. The process of thawing and crosslinking was closely monitored and recorded, aiming to gain insights into the underlying mechanisms.
4.6. Stability of Frozen/Crosslinked Cast Structures
To assess the structural integrity of tissue-engineered structures over extended exposure to a physiological environment, an experiment was conducted involving cast structures fabricated using different compositions of agar/alginate. The experimental setup mirrored the beforementioned post-crosslinking deformation study, utilizing the same casting geometry.
For each ink composition, two identical casts were prepared. One cast was stored in a sealed zip-lock bag at a temperature of 4 °C in a refrigerator, ensuring minimal evaporation. The second cast was immersed in a 1X phosphate-buffered saline (PBS) solution (Fisher Scientific, Hampton, NH, USA) at body temperature and placed in an incubator to simulate the conditions of the human body. All samples were left undisturbed for a duration of 24 h, after which their morphology was examined to evaluate any changes or degradation in their structure. This experiment aimed to determine the cast structures’ ability to retain their integrity in a liquid environment, simulating long-term exposure to the human body.
4.7. TCC Printing System
A temperature-controlled 3D cryoprinting (TCC) system was developed and constructed for this study. The printing component of the system was modified from an Ender 3 3D Printer, manufactured by Shenzhen Creality in Shenzhen, China. To enable cryoprinting, a freezing printing surface was designed and integrated into the printer. This surface was connected to a cooling bath supplied by Polyscience Inc., located in Phoenix, AZ. A cooling mixture comprising 50% ethanol and 50% water was employed in the bath, maintaining a temperature of −10 °C for the printing process. During printing, the syringe utilized for extrusion was equipped with a flexible heater from Omega, based in Norwalk, New Jersey. This heater was employed to sustain the syringe’s temperature at 40 °C, ensuring appropriate material flow and preventing unwanted gelation. Following the completion of the printing process, the frozen samples were carefully transferred to a −80 °C freezer manufactured by K2 Scientific, situated in Charlotte, NC, USA, for storage purposes.
4.8. Compression Test
Compression tests were carried out using a TA. XT plus 100 texture analyzer (Stable Microsystems Ltd., TA-XT2i, Godalming, UK). The compression tests utilized a 5 mm diameter cylindrical probe. During the tests, the samples were compressed by 2 mm at a speed of 0.2 mm/s for a duration of 10 s. To ensure accuracy and reliability, three repetitions of the compression test were performed for each group configuration or sample condition. This approach allowed for consistent and reproducible measurements during the compression analysis.
4.9. Statistical Analysis
In the shape retainability test and compression test analyses, statistical analysis was conducted using a one-way analysis of variance (ANOVA). This test allowed for the comparison of means across multiple groups or conditions. To determine significant differences between the groups, a post hoc analysis known as Duncan’s multiple range test (DMRT) was employed. The DMRT helps identify specific pairs of groups that exhibit significant differences while maintaining a 95% confidence level. The statistical analysis, including ANOVA and DMRT, was performed using SigmaPlot 15.0 software.