Carbonization of Corn Leaf Waste for Na-Ion Storage Application Using Water-Soluble Carboxymethyl Cellulose Binder
Abstract
:1. Introduction
2. Results
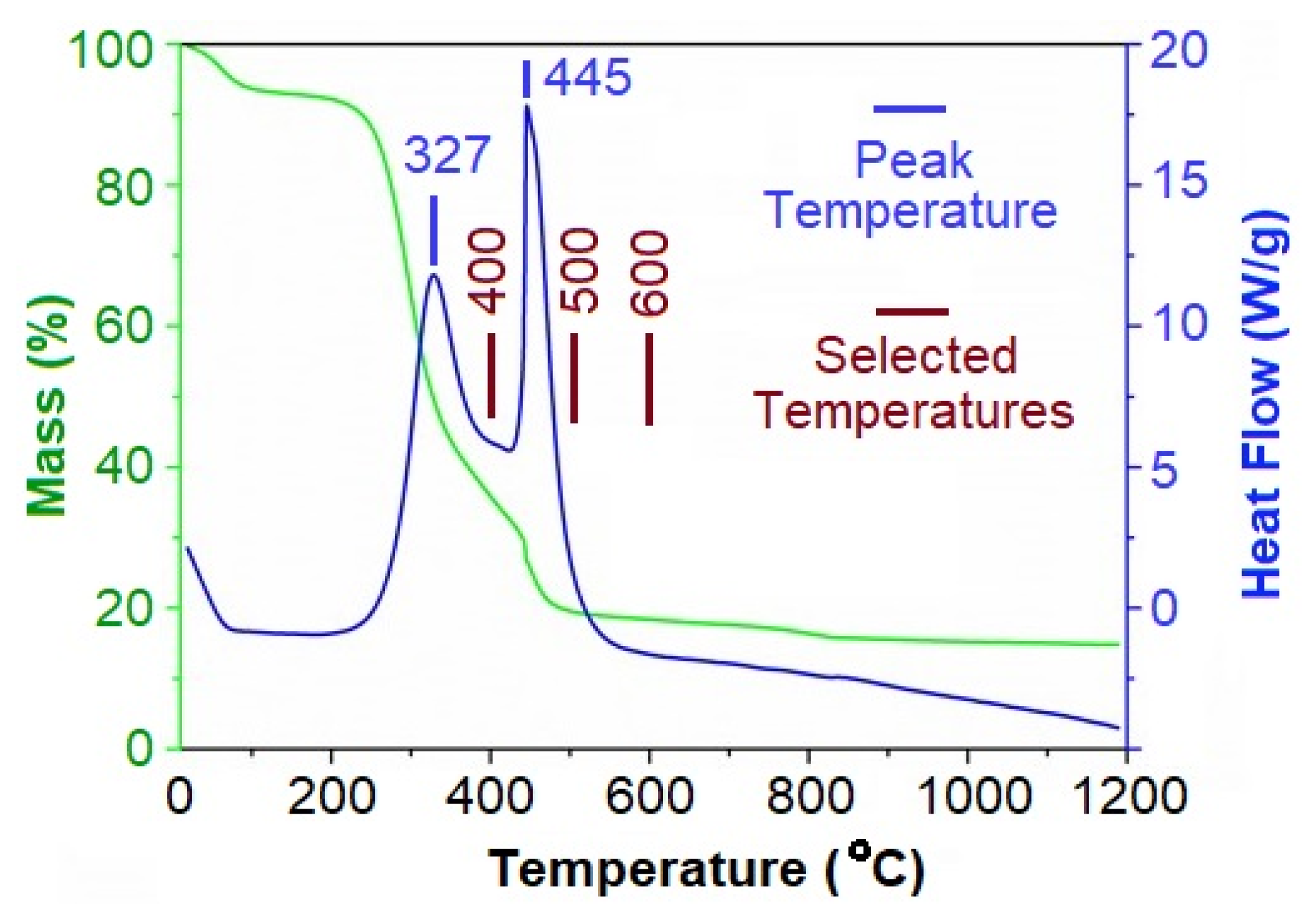
2.1. Thermal Analysis
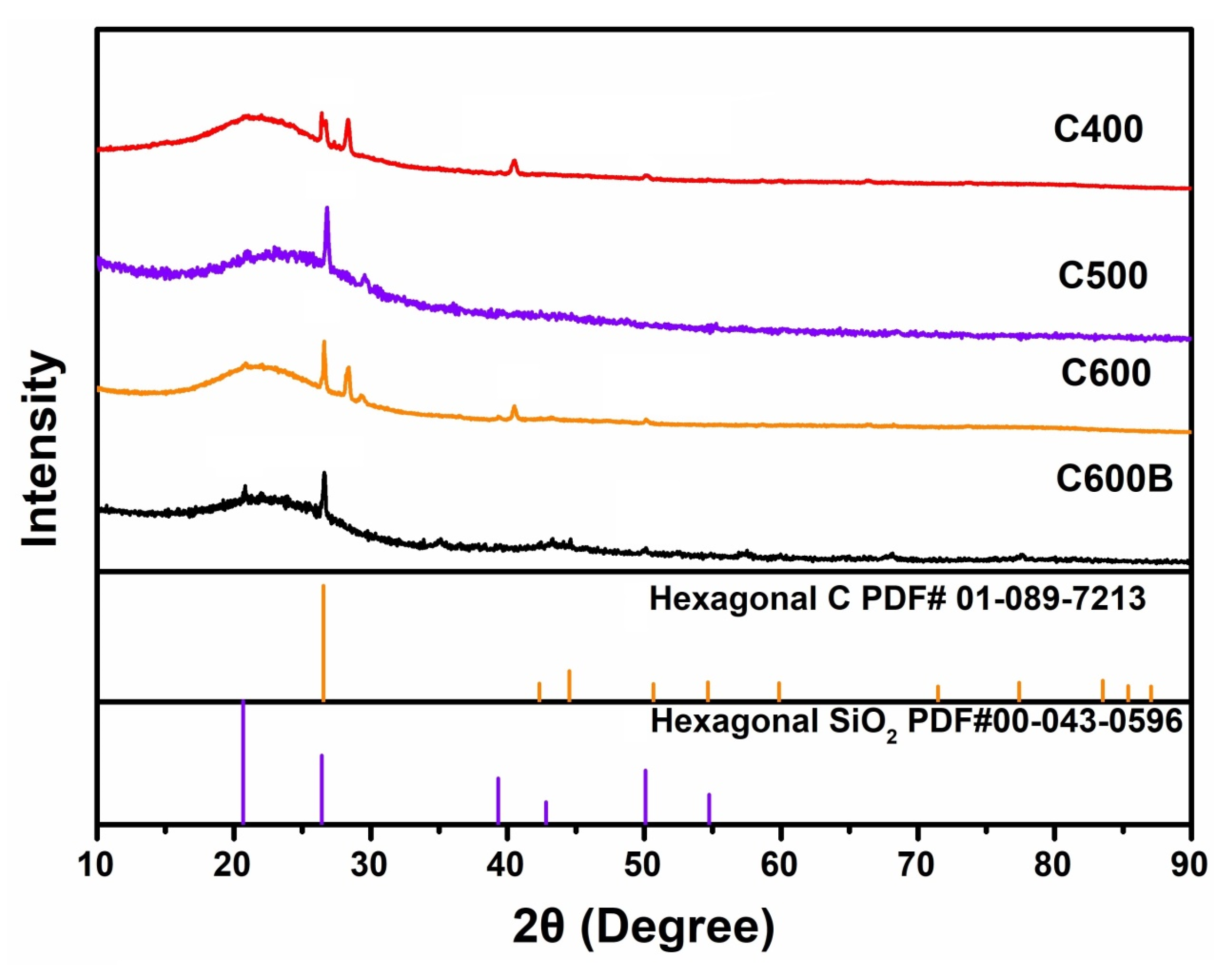
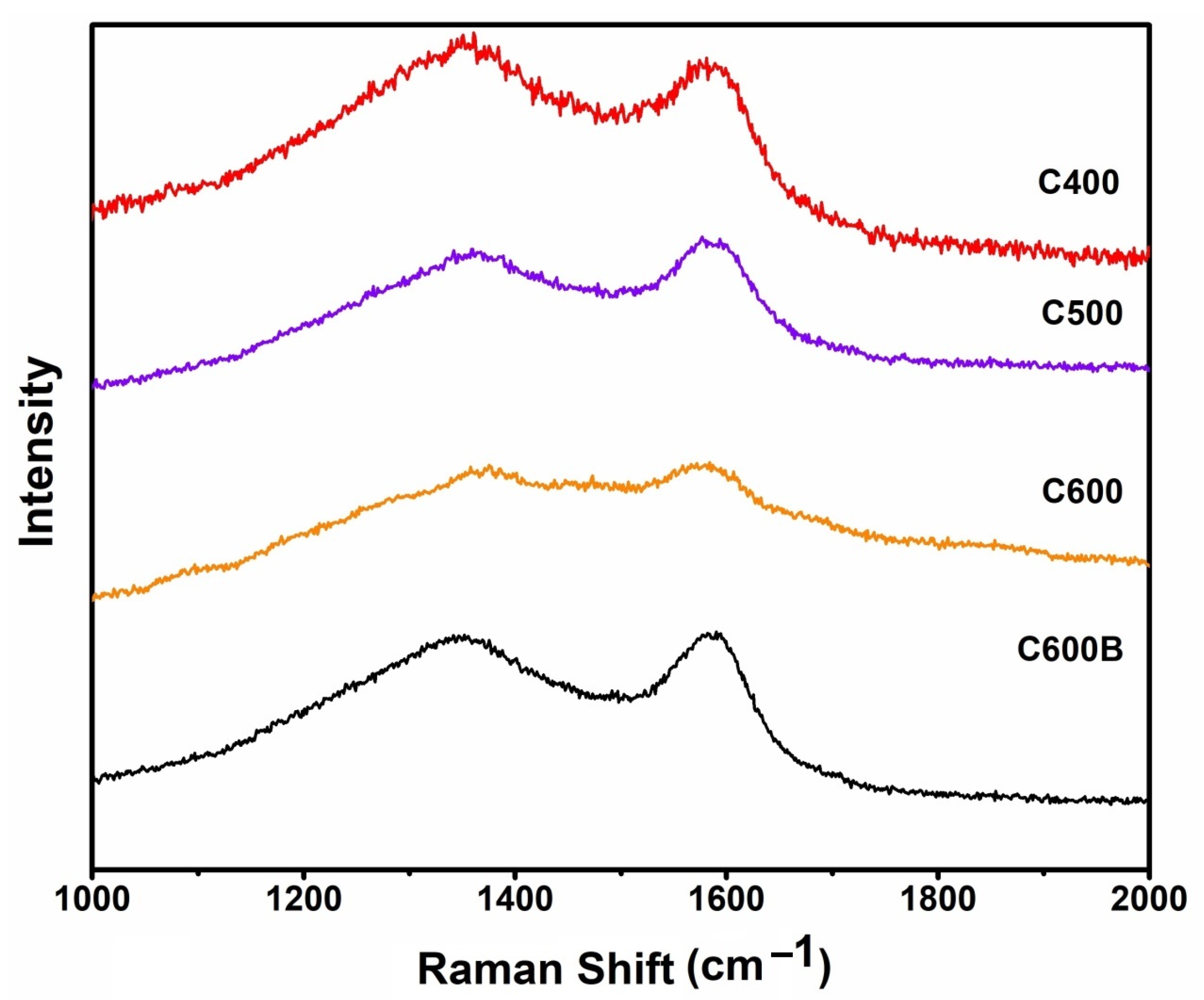
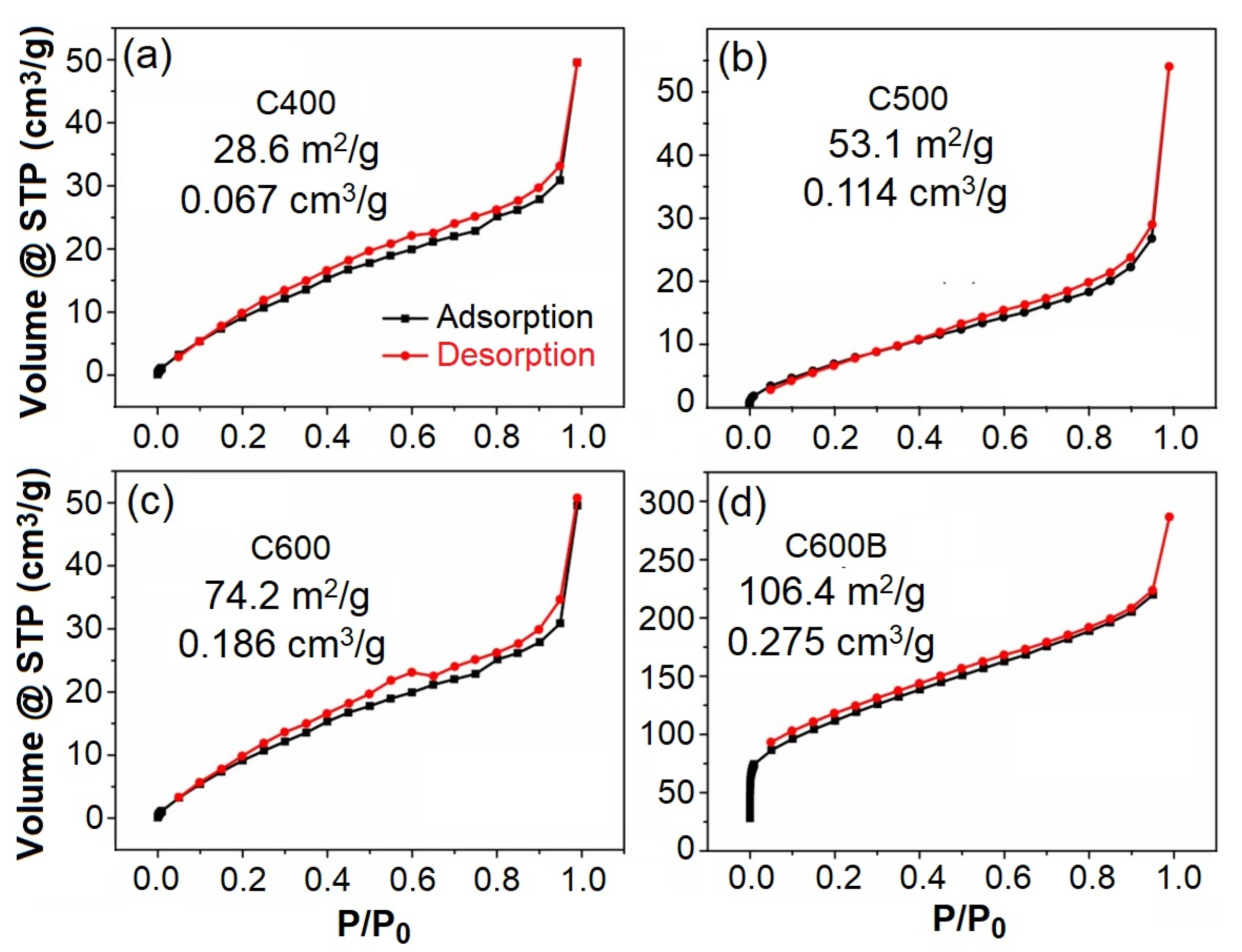
2.2. Structural and Surface Characterizations
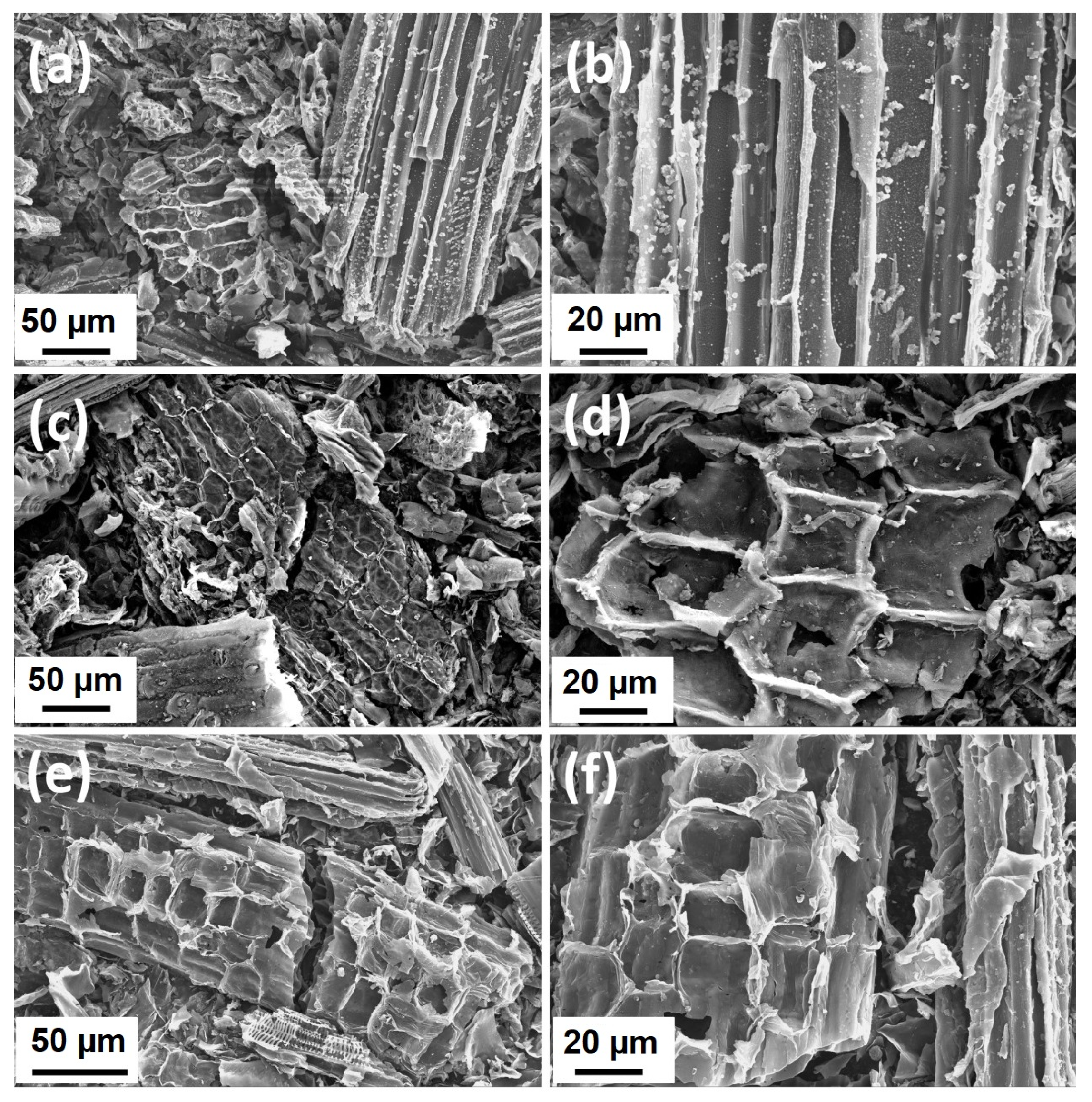
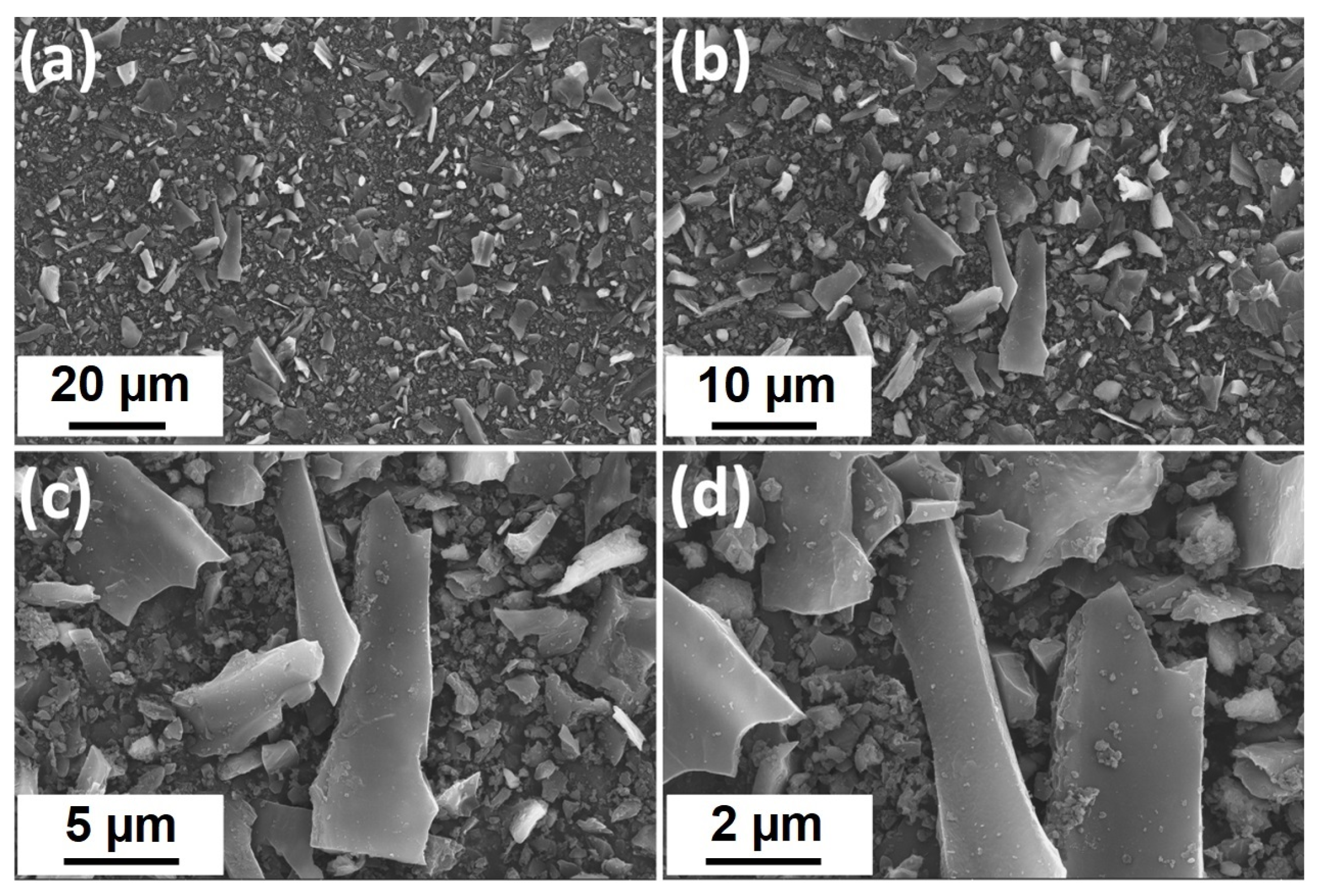
2.3. Morphological Characteristics
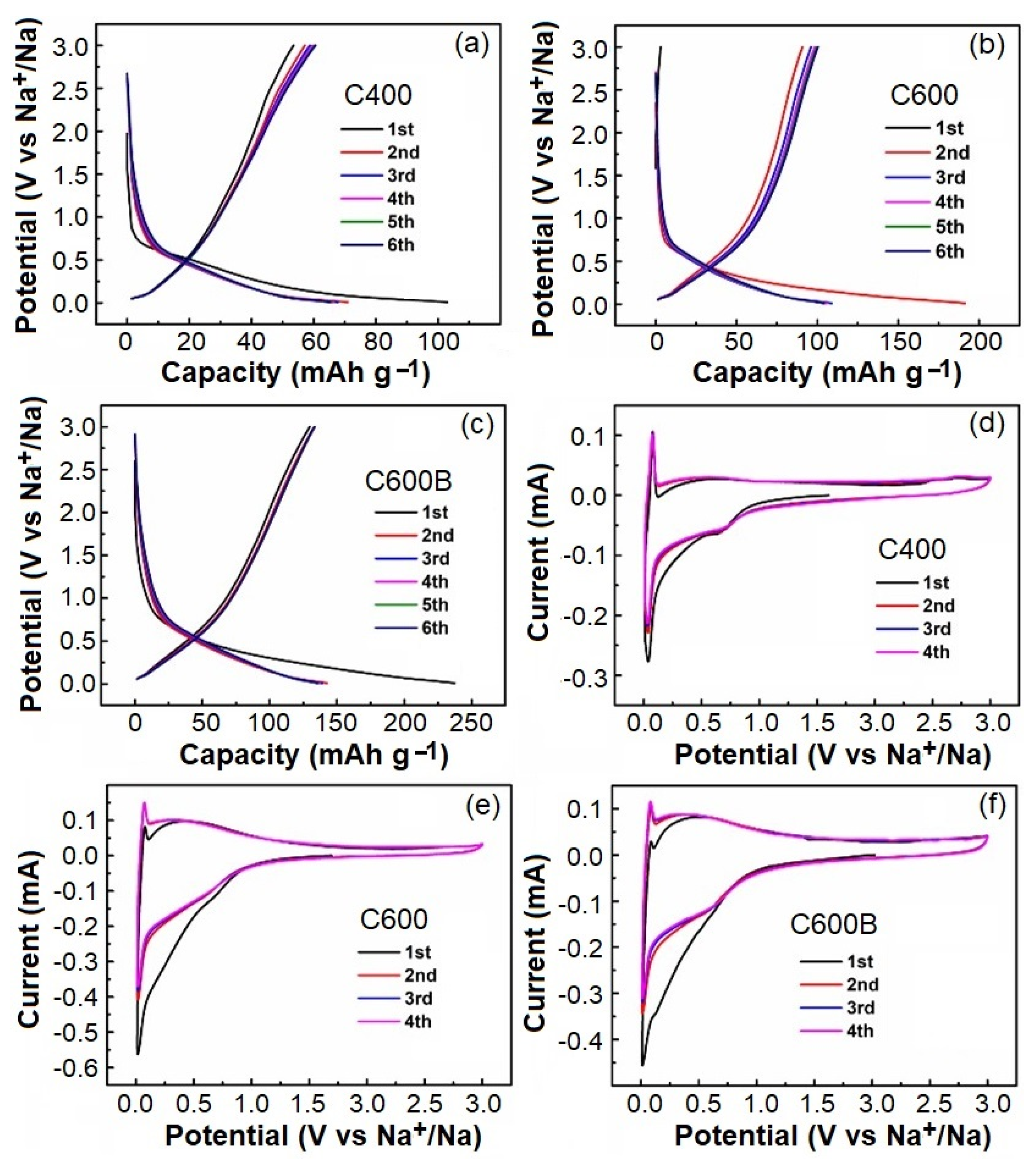
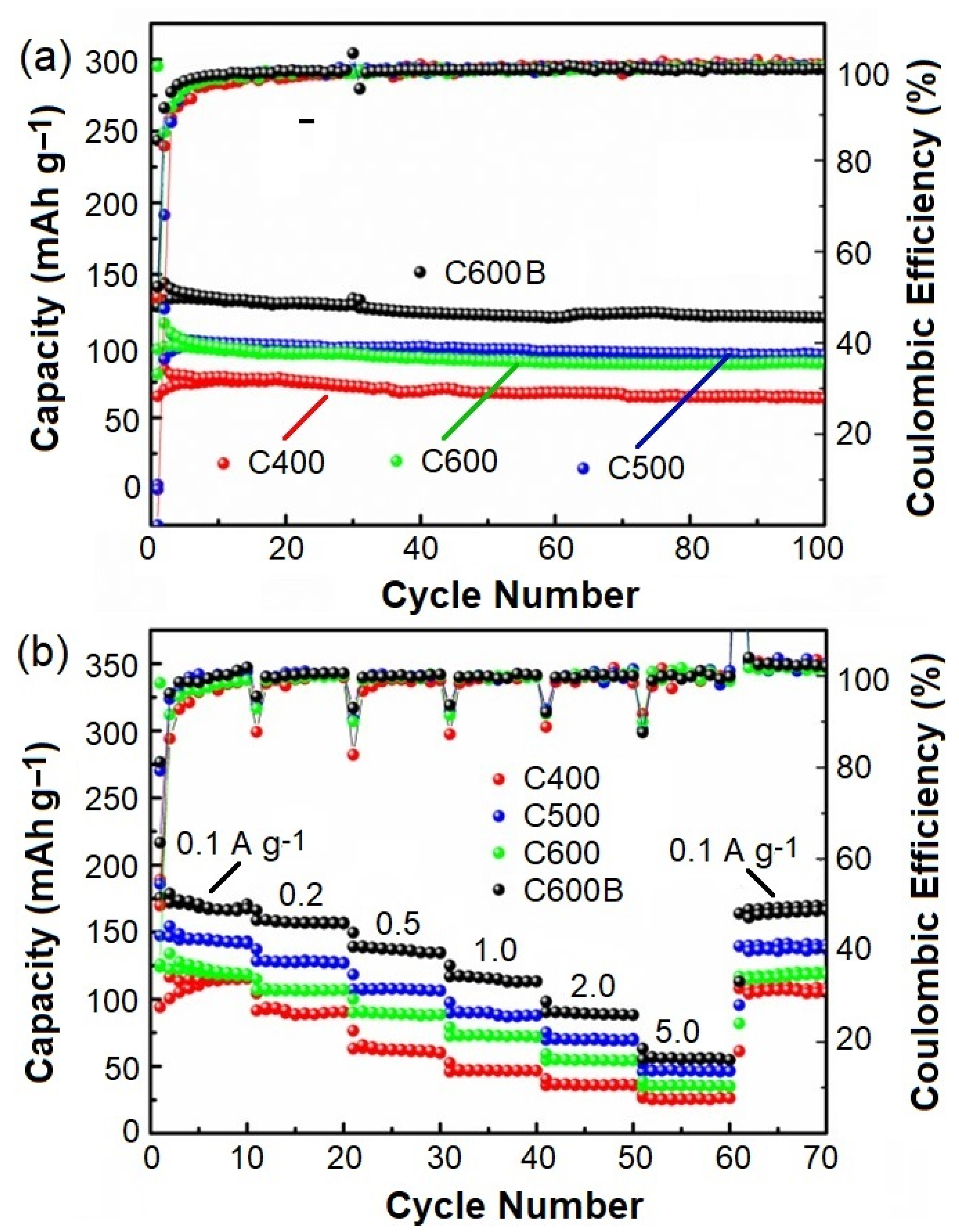
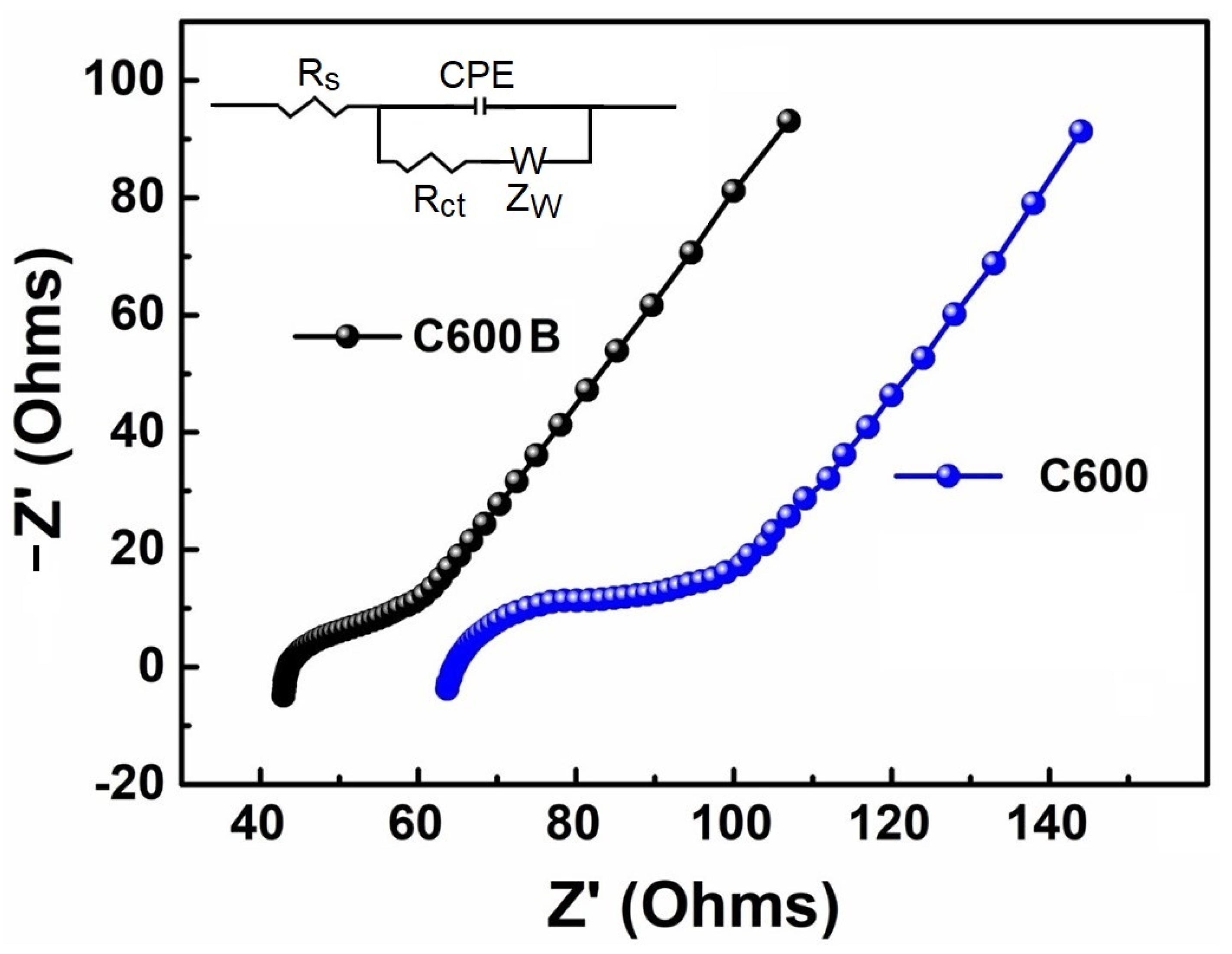
2.4. Electrochemical Performance
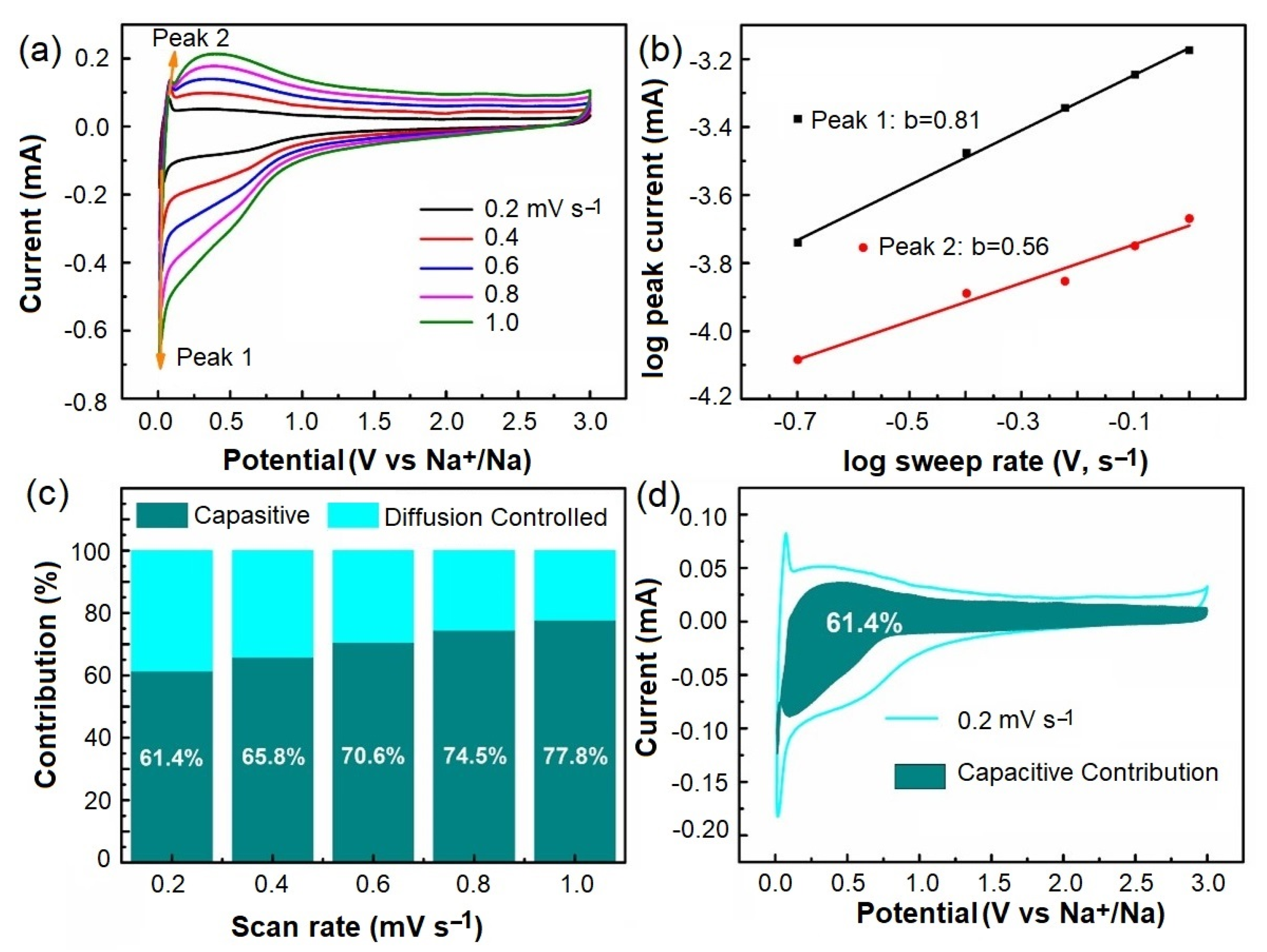
2.5. Pseudocapacitive Performance
3. Discussion
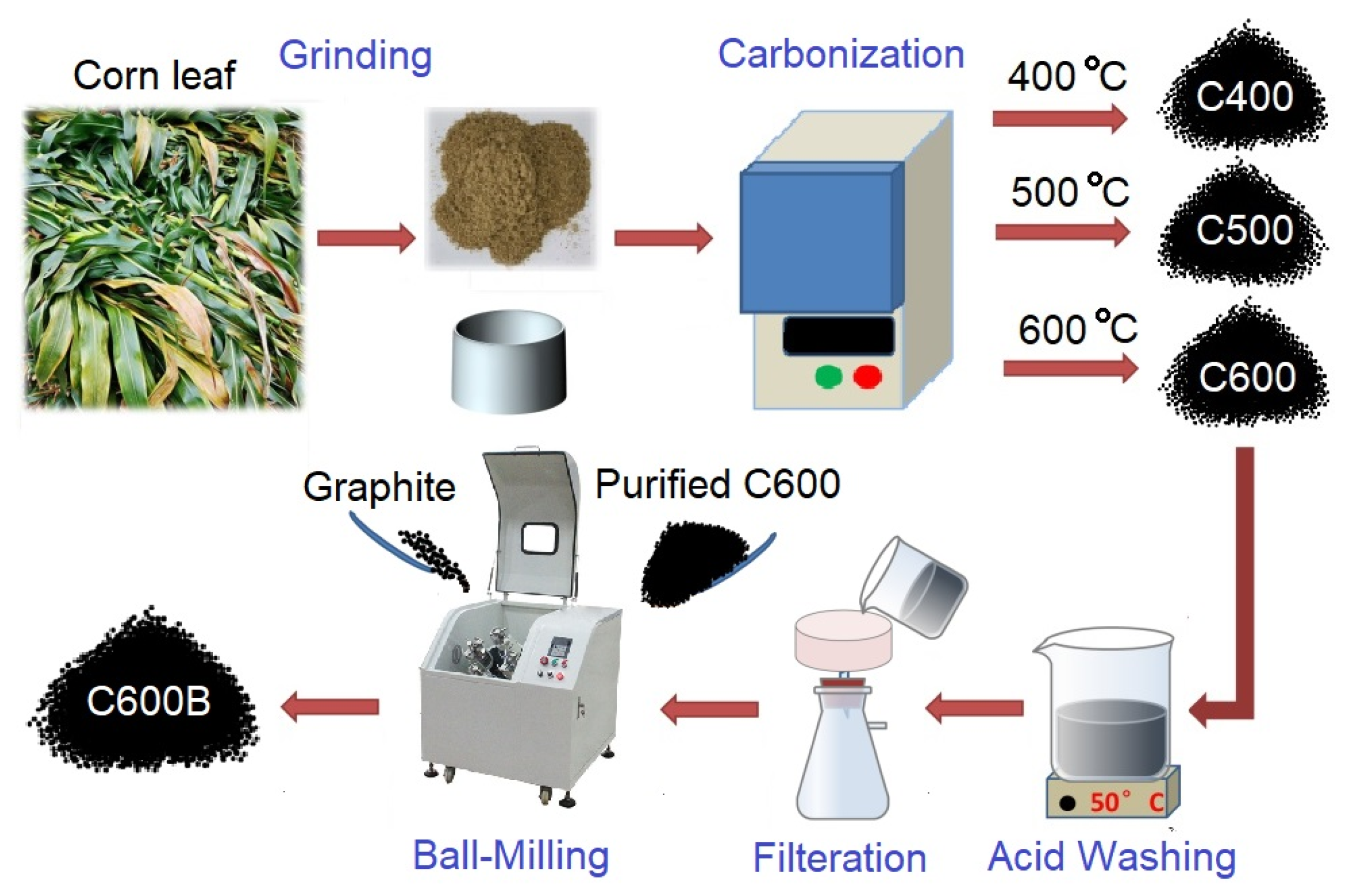
4. Materials and Methods
4.1. Thermal Analysis
4.2. Carbonization Trials
4.3. Ball-Milling Acid-Washing
4.4. Structural and Microstructural Characterization
4.5. Electrochemical Measurements
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mukoro, V.; Sharmina, M.; Schmid, A.G. A review of business models for access to affordable and clean energy in Africa: Do they deliver social, economic, and environmental value? Energy Res. Social Sci. 2022, 88, 102530. [Google Scholar] [CrossRef]
- Kamali, A.R.; Zhao, H. Electrochemical conversion of natural graphite minerals into carbon nanostructures incorporated with Fe3Si for Li-ion storage application. J. Alloys Compd. 2023, 949, 169819. [Google Scholar] [CrossRef]
- Yu, T.; Li, G.; Duan, Y.; Wu, Y.; Zhang, T.; Zhao, X.; Luo, M.; Liu, Y. The research and industrialization progress and prospects of sodium ion battery. J. Alloys Compd. 2023, 958, 170486. [Google Scholar] [CrossRef]
- Mu, T.; Wang, Z.; Yao, N.; Zhang, M.; Bai, M.; Wang, Z.; Wang, X.; Cai, X.; Ma, Y. Technological penetration and carbon-neutral evaluation of rechargeable battery systems for large-scale energy storage. J. Energy Storage 2023, 69, 107917. [Google Scholar] [CrossRef]
- Huang, J.; Dong, X.; Chen, J.; Zeng, A. The slow-release effect of recycling on rapid demand growth of critical metals from EV batteries up to 2050: Evidence from China. Resour. Policy 2023, 82, 103504. [Google Scholar] [CrossRef]
- He, D.; Liu, Y.; Fan, Y.; Dun, C.; Qiao, Y.; Chou, S. Two-dimensional calcium terephthalate as a low-cost, high-performance anode for sodium-ion batteries. Chem. Commun. 2022, 58, 4048–4051. [Google Scholar] [CrossRef]
- Yang, L.; Lei, Y.; Liang, X.; Qu, L.; Xu, K.; Hua, Y.; Feng, J. SnO2 nanoparticles composited with biomass N-doped carbon microspheres as low cost, environmentally friendly and high-performance anode material for sodium-ion and lithium-ion batteries. J. Power Sources 2022, 547, 232032. [Google Scholar] [CrossRef]
- Zhao, H.; Rezaei, A.; Kamali, A.R. Electrolytic conversion of natural graphite into carbon nanostructures with enhanced electrical conductivity and Na-ion storage performance. J. Electrochem. Soc. 2022, 169, 054512. [Google Scholar] [CrossRef]
- Darjazi, H.; Bottoni, L.; Moazami, H.R.; Rezvani, S.J.; Balducci, L.; Sbrascini, L.; Staffolani, A.; Tombesi, A.; Nobili, F. From waste to resources: Transforming olive leaves to hard carbon as sustainable and versatile electrode material for Li/Na-ion batteries and supercapacitors. Mater. Today Sust. 2023, 21, 100313. [Google Scholar] [CrossRef]
- Chen, Y.; Li, F.; Guo, Z.; Song, Z.; Lin, Y.; Lin, W.; Zheng, L.; Huang, Z.; Hong, Z.; Titirici, M.M. Sustainable and scalable fabrication of high-performance hard carbon anode for Na-ion battery. J. Power Sources 2023, 557, 232534. [Google Scholar] [CrossRef]
- Dou, X.; Hasa, I.; Hekmatfar, M.; Diemant, T.; Behm, R.J.; Buchholz, D.L.; Passerini, S. Pectin, Hemicellulose, or Lignin? Impact of the Biowaste Source on the Performance of Hard Carbons for Sodium-Ion Batteries. Chem. Sus. Chem. 2017, 10, 2668–2676. [Google Scholar] [CrossRef] [PubMed]
- Nita, C.; Zhang, B.; Dentzer, J.; Ghimbeu, C.M. Hard carbon derived from coconut shells, walnut shells, and corn silk biomass waste exhibiting high capacity for Na-ion batteries. J. Energy Chem. 2021, 58, 207–218. [Google Scholar] [CrossRef]
- Zhu, L.; Han, T.; Lin, X.; Chen, Z.; Hu, C.; Liu, J. In-situ growing nanowires on biomass corn pods as free-standing electrodes with low surface reaction barrier for Li-, Al-, and Na-ion batteries. Appl. Surf. Sci. 2023, 608, 155223. [Google Scholar] [CrossRef]
- Lim, S.; Kim, S.; Ahn, K.H.; Lee, S.J. The effect of binders on the rheological properties and the microstructure formation of lithium-ion battery anode slurries. J. Power Sources 2015, 299, 221–230. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, S.H.; Ahn, K.H. Role of carboxymethyl cellulose binder and its effect on the preparation process of anode slurries for Li-ion batteries. Colloids Surf. A 2023, 664, 131130. [Google Scholar] [CrossRef]
- Huang, L.H.; Li, C.C. Effects of interactions between binders and different-sized silicons on dispersion homogeneity of anodes and electrochemistry of lithium-silicon batteries. J. Power Sources 2019, 409, 38–47. [Google Scholar] [CrossRef]
- Amer, M.W.; Alhesan, J.S.A.; Ibrahim, S.; Qussay, G.; Marshall, M.; Al-Ayed, O.S. Potential use of corn leaf waste for biofuel production in Jordan (physio-chemical study). Energy 2021, 214, 118863. [Google Scholar] [CrossRef]
- Belkacem, I.B.; Bouhafsoun, A.; Jamaladdeen, R.; Coudour, B.; Roudaut, C.; Garo, J.P.; Wang, H.Y.; Djabeur, A.; Lemée, L. Impact of biochemical composition on the pyrolysis products of cork oak (Quercus suber) samples. Bioresour. Technol. Rep. 2023, 21, 101353. [Google Scholar] [CrossRef]
- Kamali, A.R.; Divitini, G.; Ducati, C.; Fray, D.J. Transformation of molten SnCl2 to SnO2 nano-single crystals. Ceram. Int. 2014, 60, 8533–8538. [Google Scholar] [CrossRef]
- Acikalin, K. Thermogravimetric analysis of walnut shell as pyrolysis feedstock. J. Therm. Anal. Calorim. 2011, 105, 145–150. [Google Scholar] [CrossRef]
- Fan, Y.; Fowler, G.; Norris, C. Potential of a pyrolytic coconut shell as a sustainable biofiller for styrene–butadiene rubber. Ind. Eng. Chem. Res. 2017, 56, 4779–4791. [Google Scholar] [CrossRef]
- Sullivan, A.L.; Ball, R. Thermal decomposition and combustion chemistry of cellulosic biomass. Atmos. Environ. 2012, 47, 133–141. [Google Scholar] [CrossRef]
- Paredes, C.A.M.; Linzán, I.R.; Saquete, M.D.; Luque, R.; Osman, S.M.; Botella, N.B.; Manuel, R.D.J. Silica-derived materials from agro-industrial waste biomass: Characterization and comparative studies. Environ. Res. 2023, 231, 116002. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.S.; Dong, X.L.; Wang, B.Y.; Xia, J.L.; Li, W.C. Revealing the sodium storage behavior of biomass-derived hard carbon by using pure lignin and cellulose as model precursors. Renew. Energy 2022, 189, 630–638. [Google Scholar] [CrossRef]
- He, Z.K.; Sun, Q.; Xie, K.; Lu, P.; Shi, Z.; Kamali, A.R. Reactive molten salt synthesis of natural graphite flakes decorated with SnO2 nanorods as high performance, low cost anode material for lithium ion batteries. J. Alloys Compd. 2019, 792, 1213–1222. [Google Scholar] [CrossRef]
- Kamali, A.R.; Yang, J. Effect of molten salts on the structure, morphology and electrical conductivity of PET-derived carbon nanostructures. Polym. Degrad. Stab. 2020, 177, 109184. [Google Scholar] [CrossRef]
- Wei, S.; Kamali, A.R. Waste plastic derived Co3Fe7/CoFe2O4@carbon magnetic nanostructures for efficient dye adsorption. J. Alloys Compd. 2021, 886, 161201. [Google Scholar] [CrossRef]
- Kamali, A.R. Nanocatalytic conversion of CO2 into nanodiamonds. Carbon 2017, 123, 205–215. [Google Scholar] [CrossRef]
- Baharudin, K.B.; Abdullah, N.; Yap, Y.H.T.; Derawi, D. Renewable diesel via solventless and hydrogen-free catalytic deoxygenation of palm fatty acid distillate. J. Cleaner Prod. 2020, 274, 122850. [Google Scholar] [CrossRef]
- Zhu, W.; Kamali, A.R. Molten Salt-Assisted Catalytic Preparation of MoS2/α-MoO3/Graphene as High-Performance Anode of Li-Ion Battery. Catalysts 2023, 13, 499. [Google Scholar] [CrossRef]
- Li, G.; Xu, L.; Hao, Q.; Wang, M.; Qian, Y. Synthesis, characterization and application of carbon nanocages as anode materials for high-performance lithium-ion batteries. RSC Adv. 2012, 2, 284–291. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, H.; Jiang, B.; Zhao, Y.; Meng, X.; Ma, T. Hierarchical porous architectures derived from low-cost biomass equisetum arvense as a promising anode material for lithium-ion batteries. J. Mol. Struct. 2021, 1221, 128794. [Google Scholar] [CrossRef]
- Cao, Y.; Xiao, L.; Sushko, M.L.; Wang, W.; Schwenzer, B.; Xiao, J.; Nie, Z.; Saraf, L.V.; Yang, Z.; Liu, J. Sodium ion insertion in hollow carbon nanowires for battery applications. Nano Lett. 2012, 12, 3783–3787. [Google Scholar] [CrossRef]
- Chou, S.L.; Wang, J.Z.; Liu, H.K.; Dou, S.X. Rapid synthesis of Li4Ti5O12 microspheres as anode materials and its binder effect for lithium-ion battery. J. Phys. Chem. C 2011, 115, 16220–16227. [Google Scholar] [CrossRef]
- Marssi, M.E.; Janot, R. Pyrolysis temperature dependence of sodium storage mechanism in non-graphitizing carbons. Carbon 2023, 208, 216–226. [Google Scholar]
- Kamali, A.R.; Li, S. Molten salt-assisted valorization of waste PET plastics into nanostructured SnO2@terephthalic acid with excellent Li-ion storage performance. Appl. Energy 2023, 334, 120692. [Google Scholar] [CrossRef]
- Thalji, M.R.; Ali, G.A.M.; Liu, P.; Zhong, Y.L. W18O49 nanowires-graphene nanocomposite for asymmetric supercapacitors employing AlCl3 aqueous electrolyte. Chem. Eng. J. 2021, 409, 128216. [Google Scholar] [CrossRef]
- Liu, H.; Li, C.; Zhang, H.P.; Fu, L.J.; Wu, Y.P.; Wu, H.Q. Kinetic study on LiFePO4/C nanocomposites synthesized by solid state technique. J. Power Sources 2006, 159, 717–720. [Google Scholar] [CrossRef]
- He, Y.B.; Ling, G.W.; Tang, Z.Y.; Song, Q.S.; Yang, Q.H.; Chen, W.; Lv, W.; Su, Y.J.; Xu, Q. Safety properties of liquid state soft pack high power batteries with carbon-coated LiFePO4/graphite electrodes. J. Solid. State Electrochem. 2010, 14, 751–756. [Google Scholar] [CrossRef]
- Li, C.; Zhang, X.; Wang, K.; Sun, X.; Xu, Y.; Su, F.; Chen, C.M.; Liu, F.; Wu, Z.S.; Ma, Y. Nitrogen-enriched graphene framework from a large-scale magnesiothermic conversion of CO2 with synergistic kinetics for high-power lithium-ion capacitors. NPG Asia Mater. 2021, 13, 59. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y.; Dunn, B. Where do batteries end and Supercapacitors begin? Science 2014, 343, 1210–1211. [Google Scholar] [CrossRef]
- Bobyleva, Z.V.; Drozhzhin, O.A.; Dosaev, K.A.; Kamiyama, A.; Ryazantsev, S.V.; Komaba, S.; Antipov, E.V. Unveiling pseudocapacitive behavior of hard carbon anode materials for sodium-ion batteries. Electrochim. Acta 2020, 354, 136647. [Google Scholar] [CrossRef]
- Bobyleva, Z.V.; Drozhzhin, O.A.; Dosaev, K.A.; Kamiyama, A.; Ryazantsev, S.V.; Komaba, S.; Antipov, E.V.; Shi, Q.; Chen, K. Corrosion assisted the formation of unique structure transition metal oxides/carbon nanofibers with fast and high lithium storage. Electrochim. Acta 2021, 400, 139373. [Google Scholar]
- Ahmed, A.T.A.; Hou, B.; Inamdar, A.I.; Cha, S.N.; Kim, H.; Im, H. Morphology Engineering of Self-Assembled Nanostructured CuCo2O4 Anodes for Lithium-Ion Batteries. Energy Technol. 2019, 7, 1900295. [Google Scholar] [CrossRef]
- Lou, S.; Cheng, X.; Gao, J.; Li, Q.; Wang, L.; Cao, Y.; Ma, Y.; Zuo, P.; Gao, Y.; Du, C.; et al. Pseudocapacitive Li+ intercalation in porous Ti2Nb10O29 nanospheres enables ultra-fast lithium storage. Energy Storage Mater. 2018, 11, 57–66. [Google Scholar] [CrossRef]
- Duan, H.; Zhang, S.; Chen, Z.; Xu, A.; Zhan, S.; Wu, S. Self-Formed Channel Boosts Ultrafast Lithium Ion Storage in Fe3O4@Nitrogen-Doped Carbon Nanocapsule. ACS Appl. Mater. Interfaces 2020, 12, 527–537. [Google Scholar] [CrossRef]
- Li, R.; Kamali, A.R. Molten salt assisted conversion of corn lignocellulosic waste into carbon nanostructures with enhanced Li-ion storage performance. Chem. Eng. Sci. 2023, 265, 118222. [Google Scholar] [CrossRef]
- Khan, M.F.S.; Akbar, M.; Xu, Z.; Wang, H. A review on the role of pretreatment technologies in the hydrolysis of lignocellulosic biomass of corn stover. Biomass Bioenergy 2021, 155, 106276. [Google Scholar] [CrossRef]
- Silva, J.C.; Siqueira, A.J.N.; Maia, H.B.; Nunes, R.R. Vermicomposting corn waste under cultural and climatic conditions of the Brazilian Backwoods. Bioresour. Technol. Rep. 2021, 15, 100730. [Google Scholar] [CrossRef]
- Shukla, A.; Kumar, D.; Girdhar, M.; Malik, T.; Mohan, A. Strategies of pretreatment of feedstocks for optimized bioethanol production: Distinct and integrated approaches. Biotechnol. Biofuels Bioprod. 2023, 16, 44. [Google Scholar] [CrossRef] [PubMed]
- Iamsaard, K.; Weng, C.H.; Tzeng, J.H.; Anotai, J.; Jacobsone, A.R.; Lin, Y.T. Systematic optimization of biochars derived from corn wastes, pineapple leaf, and sugarcane bagasse for Cu(II) adsorption through response surface methodology. Bioresour. Technol. 2023, 382, 129131. [Google Scholar] [CrossRef]
- Boateng, I.D.; Mustapha, A.; Kuehnel, L.; Daubert, C.R.; Kumar, R.; Agliata, J.; Flint-Garcia, S.; Wan, C.; Somavat, P. From purple corn waste (pericarp) to polyphenol-rich extract with higher bioactive contents and superior product qualities using two-step optimization techniques. Ind. Crops Prod. 2023, 200, 116871. [Google Scholar] [CrossRef]
- Grycova, B.; Pryszcz, A.; Lestinsky, P.; Chamradova, K. Influence of potassium hydroxide and method of carbonization treatment in garden and corn waste microwave pyrolysis. Biomass Bioenergy 2018, 118, 40–45. [Google Scholar] [CrossRef]
- Cong, L.; Tian, G.; Luo, D.; Ren, X.; Xiang, X. Hydrothermally assisted transformation of corn stalk wastes into high-performance hard carbon anode for sodium-ion batteries. J. Electroanal. Chem. 2020, 871, 114249. [Google Scholar] [CrossRef]
- Shortt, J.; Galettis, P.; Cheah, C.Y.; Davis, J.; Ludford-Menting, M.; Link, E.K.; Martin, J.H.; Koldej, R.; Ritchie, D. A phase 1 clinical trial of the repurposable acetyllysine mimetic, n-methyl-2-pyrrolidone (NMP), in relapsed or refractory multiple myeloma. Clin. Epigenetics 2023, 15, 15. [Google Scholar] [CrossRef]
- Occhiuzzi, J.; Politano, G.G.; D’Olimpio, G.; Politano, A. The Quest for Green Solvents for the Sustainable Production of Nanosheets of Two-Dimensional (2D) Materials, a Key Issue in the Roadmap for the Ecology Transition in the Flatland. Molecules 2023, 28, 1484. [Google Scholar] [CrossRef]
- Hong, S.U.; Wang, Y.; Soha, L.S.; Yong, W.F. Are green solvents truly green? Integrating life cycle assessment and techno-economic analysis for sustainable membrane fabrication. Green Chem. 2023, 25, 4501–4512. [Google Scholar] [CrossRef]
- Azaki, N.J.; Ahmad, A.; Hassan, N.H.; Abdah, M.A.A.M.; Ataollahi, M.S.N.; Lee, T.K. Poly(methyl methacrylate) Grafted Natural Rubber Binder for Anodes in Lithium-Ion Battery Applications. ACS Appl. Polym. Mater. 2023, 5, 4953–4965. [Google Scholar]
- Lopez, C.G.; Richtering, W. Oscillatory rheology of carboxymethyl cellulose gels: Influence of concentration and pH. Carbohydrate Polym. 2021, 267, 118117. [Google Scholar] [CrossRef]
- Enache, A.C.; Grecu, I.; Samoila, P.; Cojocaru, C.; Harabagiu, V. Magnetic Ionotropic Hydrogels Based on Carboxymethyl Cellulose for Aqueous Pollution Mitigation. Gels 2023, 9, 358. [Google Scholar] [CrossRef]
- Kim, B.; Song, Y.; Youn, B.; Lee, D. Dispersion Homogeneity of Silicon Anode Slurries with Various Binders for Li-Ion Battery Anode Coating. Polymers 2023, 15, 1152. [Google Scholar] [CrossRef] [PubMed]
| C | O | Si | K | Ca | Mg | Al | |
|---|---|---|---|---|---|---|---|
| C600 | 70.73 | 19.53 | 6.67 | 1.67 | 1.08 | 0.33 | - |
| C600B | 78.00 | 18.76 | 2.58 | - | - | - | 0.66 |
| Current Density (mA g−1)/Cycle Number | |||||||
|---|---|---|---|---|---|---|---|
| Sample | 100/10 | 200/20 | 500/30 | 1000/40 | 2000/50 | 5000/60 | 100/70 |
| C400C | 114.2 | 88.9 | 62.5 | 47.0 | 35.9 | 25.5 | 106.7 |
| C500C | 124.3 | 106.9 | 89.4 | 73.0 | 54.9 | 35.4 | 116.2 |
| C600C | 144.4 | 127.3 | 107.7 | 90.3 | 69.7 | 46.3 | 135.8 |
| C600CB | 170.7 | 157.0 | 137.0 | 115.6 | 89.3 | 55.6 | 164.3 |
| Rs (Ω) | Rct (Ω) | σ (Ω s−1/2) | DNa+ (cm2 s−1) | |
|---|---|---|---|---|
| C600 | 65.13 | 85.90 | 273.31 | 3.71 × 10−19 |
| C600B | 42.67 | 48.89 | 241.03 | 4.77 × 10−19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Kamali, A.R. Carbonization of Corn Leaf Waste for Na-Ion Storage Application Using Water-Soluble Carboxymethyl Cellulose Binder. Gels 2023, 9, 701. https://doi.org/10.3390/gels9090701
Li R, Kamali AR. Carbonization of Corn Leaf Waste for Na-Ion Storage Application Using Water-Soluble Carboxymethyl Cellulose Binder. Gels. 2023; 9(9):701. https://doi.org/10.3390/gels9090701
Chicago/Turabian StyleLi, Ruiping, and Ali Reza Kamali. 2023. "Carbonization of Corn Leaf Waste for Na-Ion Storage Application Using Water-Soluble Carboxymethyl Cellulose Binder" Gels 9, no. 9: 701. https://doi.org/10.3390/gels9090701
APA StyleLi, R., & Kamali, A. R. (2023). Carbonization of Corn Leaf Waste for Na-Ion Storage Application Using Water-Soluble Carboxymethyl Cellulose Binder. Gels, 9(9), 701. https://doi.org/10.3390/gels9090701







