Insights into the Potential of Biopolymeric Aerogels as an Advanced Soil-Fertilizer Delivery Systems
Abstract
:1. Introduction
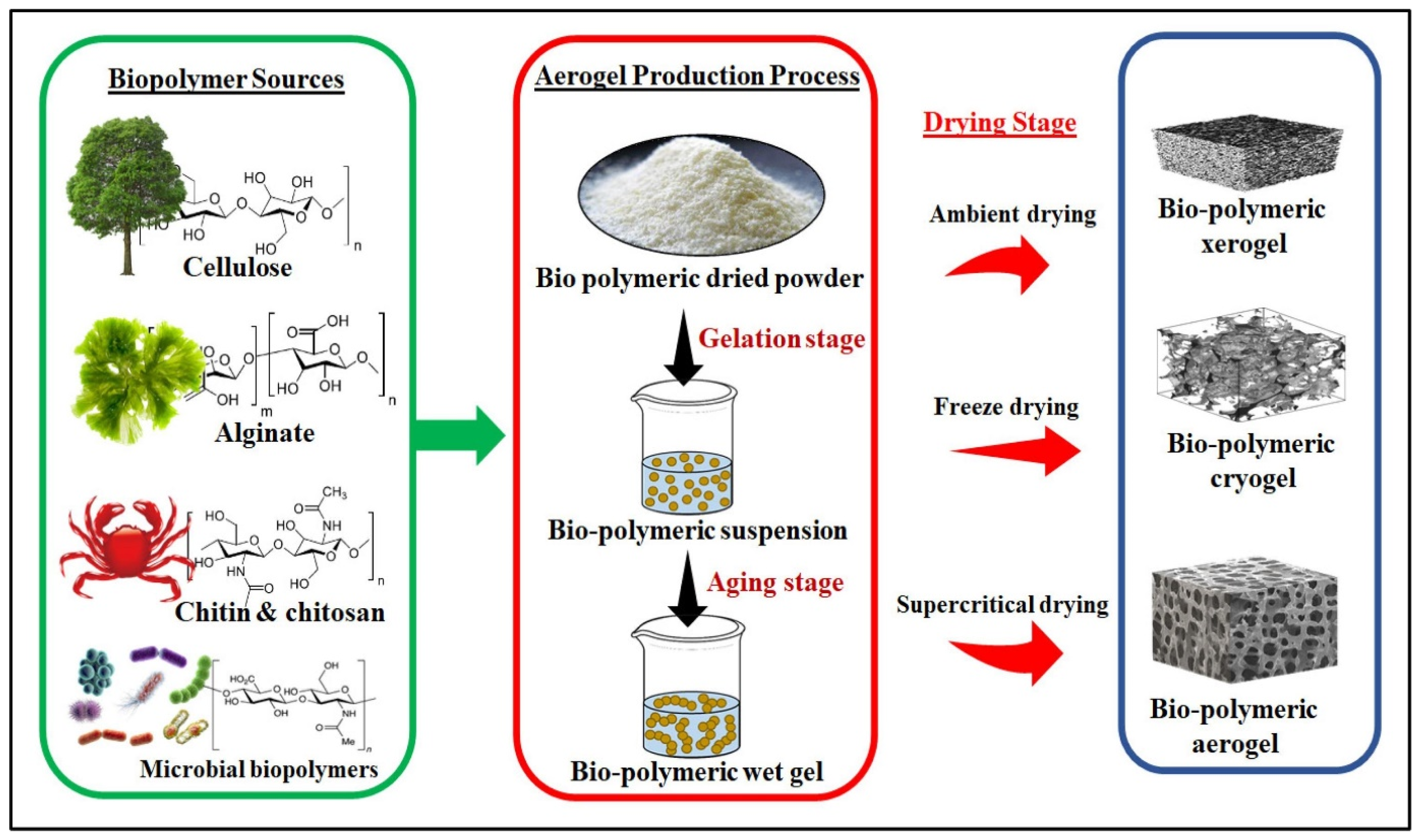
2. Properties of Biopolymeric Aerogels
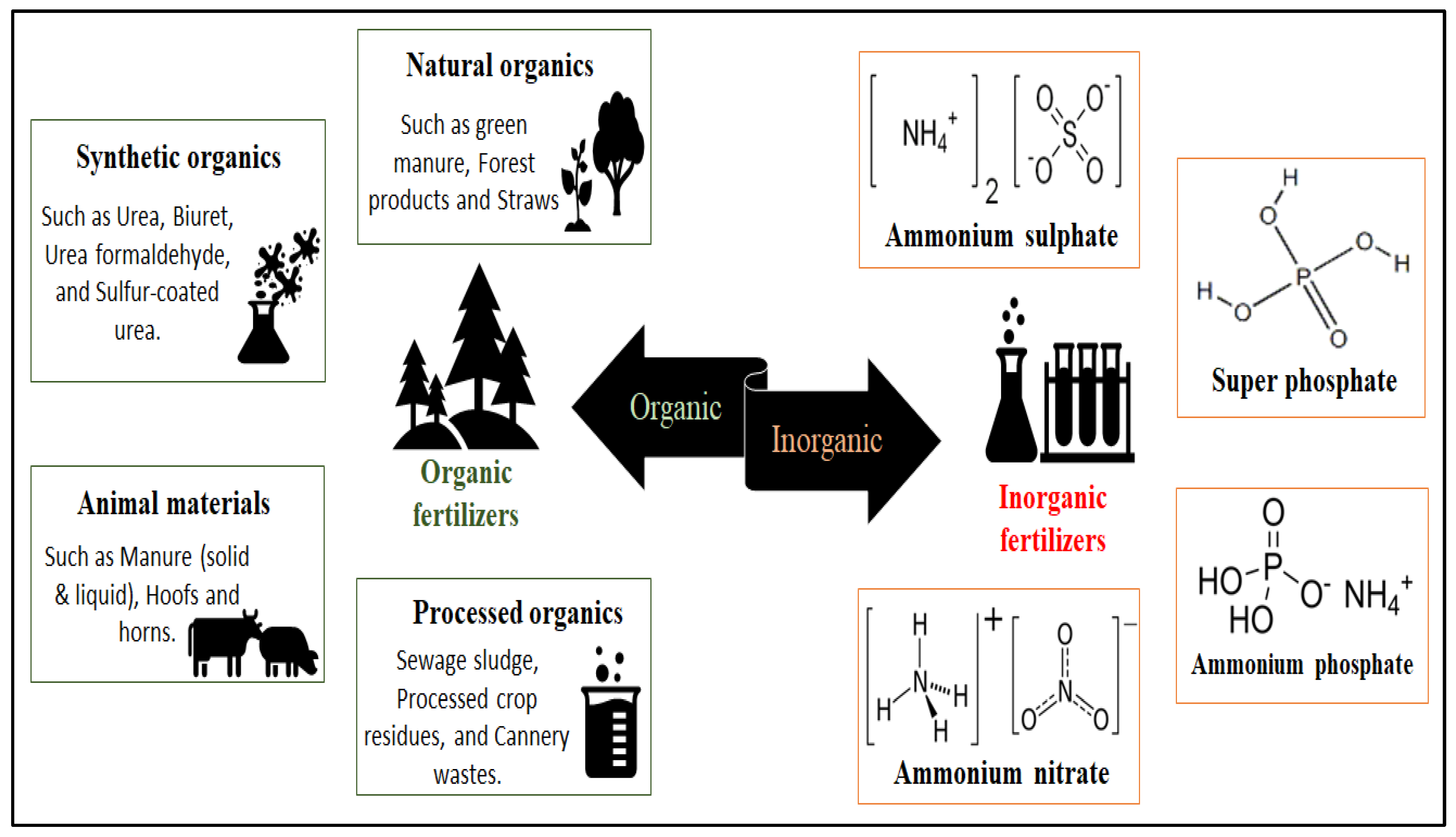
3. Soil Fertilizers: Properties and Issues
3.1. Types of Common Fertilizers
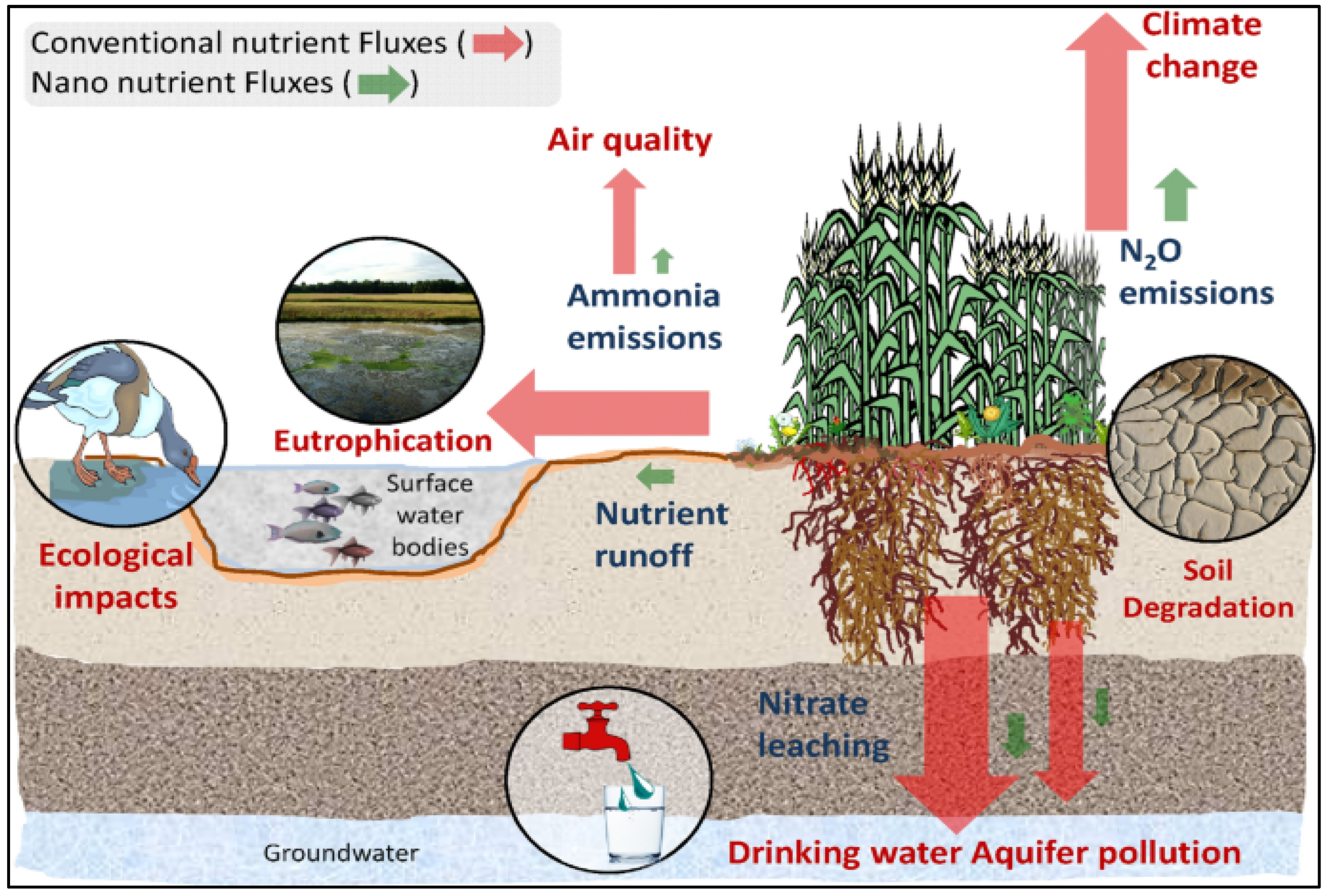
3.2. Environmental and Health Issues with Soil Fertilizers
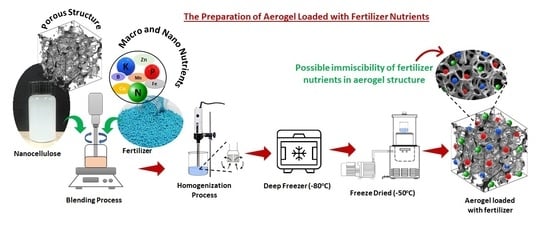
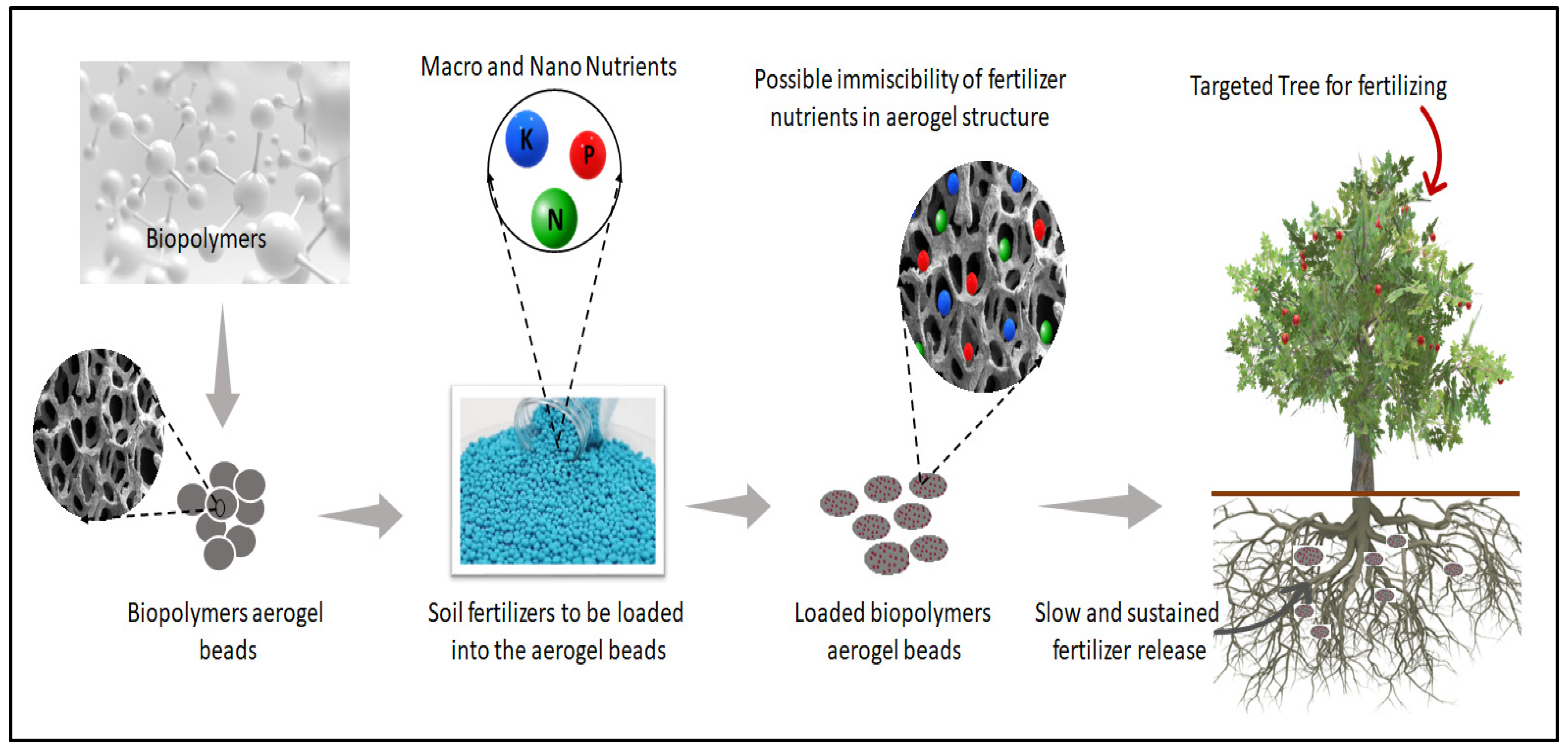
4. Biopolymer Aerogels in Soil-Fertilizer Delivery
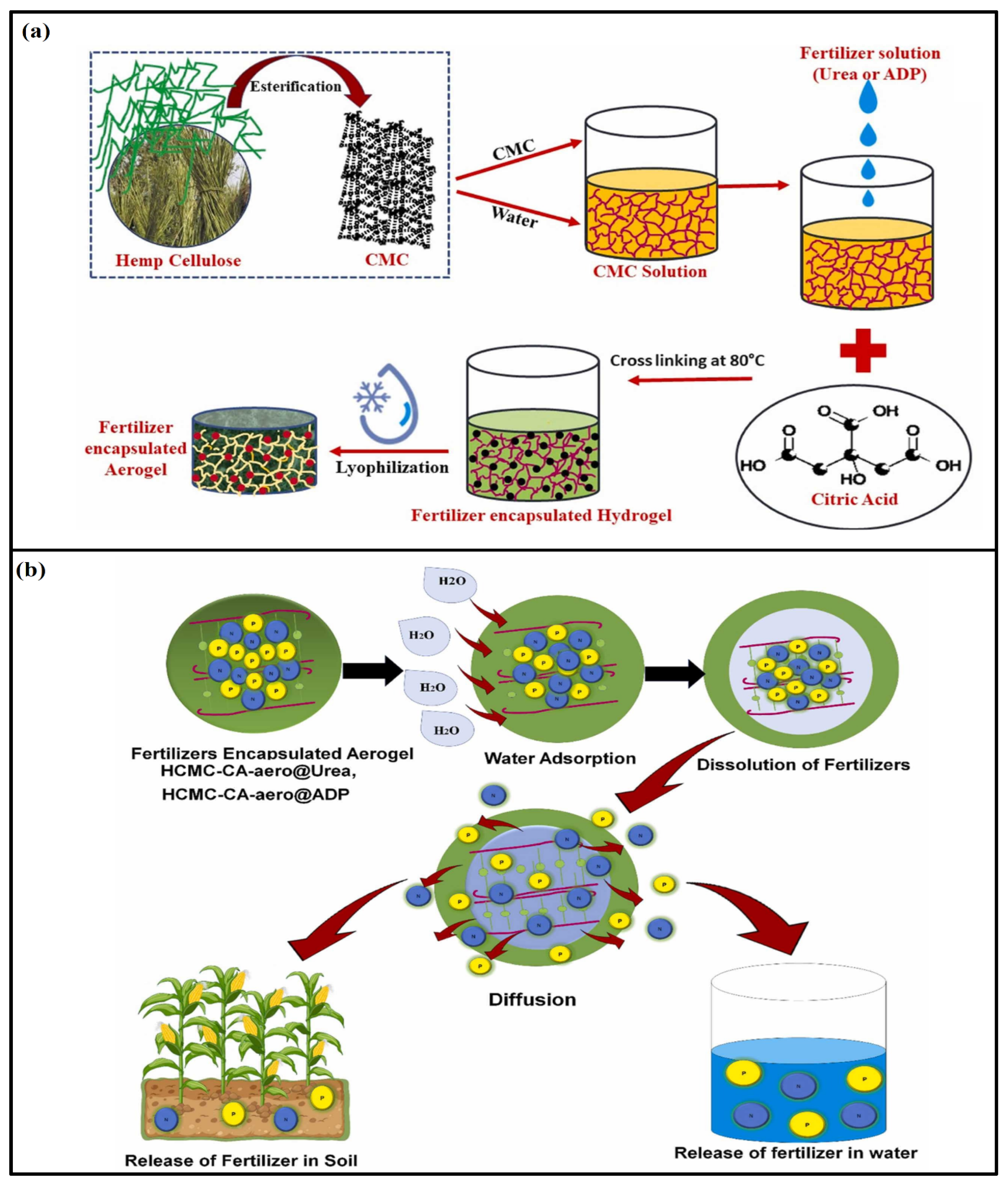
4.1. Cellulose-Based Aerogel in Soil-Fertilizer Delivery
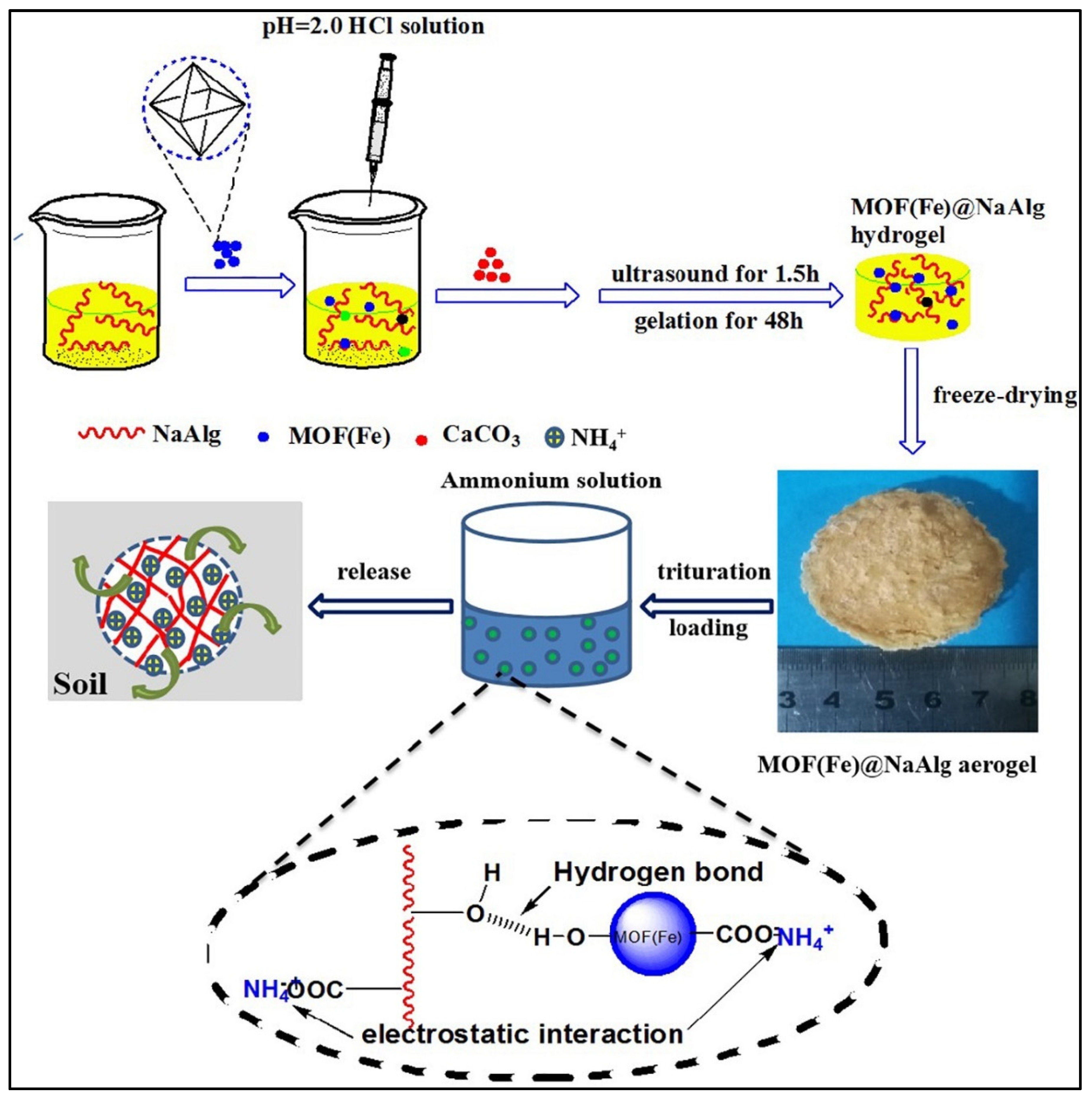
4.2. Alginate-Based Aerogel in Soil-Fertilizer Delivery
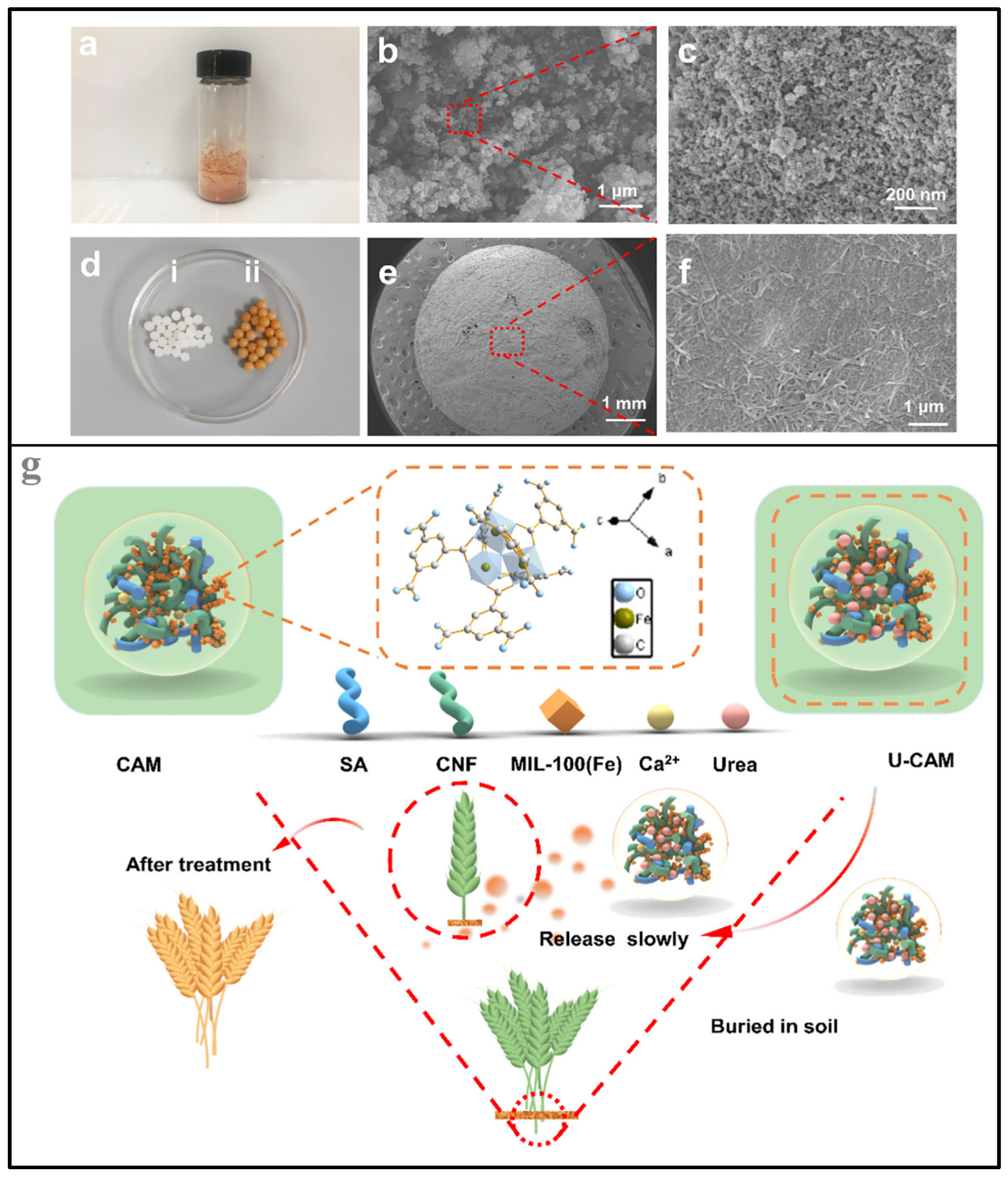
4.3. Other Biopolymer Aerogels in Soil-Fertilizer Delivery
5. Future Directions and Challenges of Biopolymer Aerogels in Soil-Fertilizer Delivery
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Itelima, J.; Bang, W.; Onyimba, I.; Sila, M.; Egbere, O. Bio-fertilizers as key player in enhancing soil fertility and crop productivity: A review. Plant Sci. Biotechnol. 2018, 6, 73–83. [Google Scholar]
- Shaji, H.; Chandran, V.; Mathew, L. Organic fertilizers as a route to controlled release of nutrients. In Controlled Release Fertilizers for Sustainable Agriculture; Elsevier: Amsterdam, The Netherlands, 2021; pp. 231–245. [Google Scholar]
- Elemike, E.E.; Uzoh, I.M.; Onwudiwe, D.C.; Babalola, O.O. The role of nanotechnology in the fortification of plant nutrients and improvement of crop production. Appl. Sci. 2019, 9, 499. [Google Scholar] [CrossRef]
- Majeed, A.; Muhammad, Z.; Ahmad, H. Plant growth promoting bacteria: Role in soil improvement, abiotic and biotic stress management of crops. Plant Cell Rep. 2018, 37, 1599–1609. [Google Scholar] [CrossRef] [PubMed]
- Liliane, T.N.; Charles, M.S. Factors affecting yield of crops. In Agronomy—Climate Change & Food Security; IntechOpen: London, UK, 2020; Volume 9. [Google Scholar]
- Hammad, H.M.; Khaliq, A.; Abbas, F.; Farhad, W.; Fahad, S.; Aslam, M.; Shah, G.M.; Nasim, W.; Mubeen, M.; Bakhat, H.F. Comparative effects of organic and inorganic fertilizers on soil organic carbon and wheat productivity under arid region. Commun. Soil Sci. Plant Anal. 2020, 51, 1406–1422. [Google Scholar] [CrossRef]
- Ostadi, A.; Javanmard, A.; Machiani, M.A.; Morshedloo, M.R.; Nouraein, M.; Rasouli, F.; Maggi, F. Effect of different fertilizer sources and harvesting time on the growth characteristics, nutrient uptakes, essential oil productivity and composition of Mentha × piperita L. Ind. Crops Prod. 2020, 148, 112290. [Google Scholar] [CrossRef]
- Zhang, X.; Qu, J.; Li, H.; La, S.; Tian, Y.; Gao, L. Biochar addition combined with daily fertigation improves overall soil quality and enhances water-fertilizer productivity of cucumber in alkaline soils of a semi-arid region. Geoderma 2020, 363, 114170. [Google Scholar] [CrossRef]
- Aier, I.; Ariina, M.S.; Bier, K.; Awomi, O.S. Vertical Farming: The Future Food Production System. 2023. Available online: https://justagriculture.in/files/newsletter/2021/july/96.%20Vertical%20Farming-%20The%20Future%20Food%20Production%20System.pdf (accessed on 12 July 2021).
- Dimkpa, C.O.; Fugice, J.; Singh, U.; Lewis, T.D. Development of fertilizers for enhanced nitrogen use efficiency–Trends and perspectives. Sci. Total Environ. 2020, 731, 139113. [Google Scholar] [CrossRef]
- Abd El-Azeim, M.; Sherif, M.; Hussien, M.; Tantawy, I.; Bashandy, S. Impacts of nano-and non-nanofertilizers on potato quality and productivity. Acta Ecol. Sin. 2020, 40, 388–397. [Google Scholar] [CrossRef]
- Yadav, K.K.; Sarkar, S. Biofertilizers, impact on soil fertility and crop productivity under sustainable agriculture. Environ. Ecol. 2019, 37, 89–93. [Google Scholar]
- Guo, L.; Li, H.; Cao, X.; Cao, A.; Huang, M. Effect of agricultural subsidies on the use of chemical fertilizer. J. Environ. Manag. 2021, 299, 113621. [Google Scholar] [CrossRef]
- Walker, W.J.; Branham, B. Environmental impacts of turfgrass fertilization. In Golf Course Management & Construction; CRC Press: Boca Raton, FL, USA, 2020; pp. 105–219. [Google Scholar]
- Tyagi, J.; Ahmad, S.; Malik, M. Nitrogenous fertilizers: Impact on environment sustainability, mitigation strategies, and challenges. Int. J. Environ. Sci. Technol. 2022, 19, 11649–11672. [Google Scholar] [CrossRef]
- Vyavahare, G.; Lee, Y.; Seok, Y.J.; Kim, H.; Sung, J.; Park, J.H. Monitoring of Soil Nutrient Levels by An EC Sensor during Spring Onion (Allium fistulosum) Cultivation under Different Fertilizer Treatment. 2023. Available online: https://assets.researchsquare.com/files/rs-2661677/v1/3c44c1ba-0178-460e-86d0-060198294213.pdf?c=1679320813 (accessed on 12 July 2021).
- Han, W.; Sun, J.; Zhang, K.; Mao, L.; Gao, L.; Hou, X.; Cui, N.; Kang, W.; Gong, D. Optimizing drip fertigation management based on yield, quality, water and fertilizer use efficiency of wine grape in North China. Agric. Water Manag. 2023, 280, 108188. [Google Scholar] [CrossRef]
- Radočaj, D.; Jurišić, M.; Gašparović, M. The role of remote sensing data and methods in a modern approach to fertilization in precision agriculture. Remote Sens. 2022, 14, 778. [Google Scholar] [CrossRef]
- Beig, B.; Niazi, M.B.K.; Sher, F.; Jahan, Z.; Malik, U.S.; Khan, M.D.; Américo-Pinheiro, J.H.P.; Vo, D.-V.N. Nanotechnology-based controlled release of sustainable fertilizers. A review. Environ. Chem. Lett. 2022, 20, 2709–2726. [Google Scholar] [CrossRef]
- Yao, Z.; Zhang, W.; Wang, X.; Zhang, L.; Zhang, W.; Liu, D.; Chen, X. Agronomic, environmental, and ecosystem economic benefits of controlled-release nitrogen fertilizers for maize production in Southwest China. J. Clean. Prod. 2021, 312, 127611. [Google Scholar] [CrossRef]
- Munoz, M.C. Industrial Ecology Approach to Management of Fly Ash from Fluidized Bed Combustion: Production of Slow-Release Fertilizer and Soil Conditioner. Ph.D. Thesis, University of Puerto Rico, Mayaguez, Puerto Rico, 2006. [Google Scholar]
- Azeem, B.; KuShaari, K.; Man, Z.B.; Basit, A.; Thanh, T.H. Review on materials & methods to produce controlled release coated urea fertilizer. J. Control. Release 2014, 181, 11–21. [Google Scholar]
- Tian, H.; Liu, Z.; Zhang, M.; Guo, Y.; Zheng, L.; Li, Y.C. Biobased polyurethane, epoxy resin, and polyolefin wax composite coating for controlled-release fertilizer. ACS Appl. Mater. Interfaces 2019, 11, 5380–5392. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Adnan, A.; Yahya, E.B.; Olaiya, N.; Safrida, S.; Hossain, M.S.; Balakrishnan, V.; Gopakumar, D.A.; Abdullah, C.; Oyekanmi, A. A review on plant cellulose nanofibre-based aerogels for biomedical applications. Polymers 2020, 12, 1759. [Google Scholar] [CrossRef]
- Abdullah; Zou, Y.; Farooq, S.; Walayat, N.; Zhang, H.; Faieta, M.; Pittia, P.; Huang, Q. Bio-aerogels: Fabrication, properties and food applications. Crit. Rev. Food Sci. Nutr. 2022, 1–23. [Google Scholar] [CrossRef]
- Rege, A.; Ratke, L.; Külcü, İ.D.; Gurikov, P. Stiffening of biopolymer aerogel networks upon wetting: A model-based study. J. Non-Cryst. Solids 2020, 531, 119859. [Google Scholar] [CrossRef]
- Schroeter, B.; Jung, I.; Bauer, K.; Gurikov, P.; Smirnova, I. Hydrophobic modification of biopolymer aerogels by cold plasma coating. Polymers 2021, 13, 3000. [Google Scholar] [CrossRef]
- Iskandar, M.; Yahya, E.B.; Abdul Khalil, H.; Rahman, A.; Ismail, M. Recent progress in modification strategies of nanocellulose-based aerogels for oil absorption application. Polymers 2022, 14, 849. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Malfait, W.J.; Guerrero-Alburquerque, N.; Koebel, M.M.; Nyström, G. Biopolymer aerogels and foams: Chemistry, properties, and applications. Angew. Chem. Int. Ed. 2018, 57, 7580–7608. [Google Scholar] [CrossRef]
- Subrahmanyam, R.; Gurikov, P.; Dieringer, P.; Sun, M.; Smirnova, I. On the road to biopolymer aerogels—Dealing with the solvent. Gels 2015, 1, 291–313. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; García-González, C.A.; Del Gaudio, P.; Portugal, A.; Mahmoudi, M. Synthesis and biomedical applications of aerogels: Possibilities and challenges. Adv. Colloid Interface Sci. 2016, 236, 1–27. [Google Scholar] [CrossRef]
- Huo, Y.; Liu, Y.; Xia, M.; Du, H.; Lin, Z.; Li, B.; Liu, H. Nanocellulose-based composite materials used in drug delivery systems. Polymers 2022, 14, 2648. [Google Scholar] [CrossRef]
- Wei, S.; Ching, Y.C.; Chuah, C.H. Synthesis of chitosan aerogels as promising carriers for drug delivery: A review. Carbohydr. Polym. 2020, 231, 115744. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Y.; Zhang, L.; Xu, Z.; Dai, H.; Wu, W. Nanocellulose/gelatin composite cryogels for controlled drug release. ACS Sustain. Chem. Eng. 2019, 7, 6381–6389. [Google Scholar] [CrossRef]
- De Marco, I.; Iannone, R.; Miranda, S.; Riemma, S. An environmental study on starch aerogel for drug delivery applications: Effect of plant scale-up. Int. J. Life Cycle Assess. 2018, 23, 1228–1239. [Google Scholar] [CrossRef]
- Gonçalves, V.S.; Gurikov, P.; Poejo, J.; Matias, A.A.; Heinrich, S.; Duarte, C.M.; Smirnova, I. Alginate-based hybrid aerogel microparticles for mucosal drug delivery. Eur. J. Pharm. Biopharm. 2016, 107, 160–170. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Yahya, E.B.; Jummaat, F.; Adnan, A.; Olaiya, N.; Rizal, S.; Abdullah, C.; Pasquini, D.; Thomas, S. Biopolymers based aerogels: A review on revolutionary solutions for smart therapeutics delivery. Prog. Mater. Sci. 2022, 131, 101014. [Google Scholar] [CrossRef]
- Yahya, E.B.; Jummaat, F.; Amirul, A.; Adnan, A.; Olaiya, N.; Abdullah, C.; Rizal, S.; Mohamad Haafiz, M.; Abdul Khalil, H.P.S. A review on revolutionary natural biopolymer-based aerogels for antibacterial delivery. Antibiotics 2020, 9, 648. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, A.; Baldino, L.; Misol, A.; Cardea, S.; Del Valle, E.M.M. Role of rheological properties on physical chitosan aerogels obtained by supercritical drying. Carbohydr. Polym. 2020, 233, 115850. [Google Scholar] [CrossRef] [PubMed]
- Abdul Khalil, H.P.S.; Yahya, E.B.; Tajarudin, H.A.; Surya, I.; Muhammad, S.; Fazita, M.N. Enhancing the properties of industrial waste nanocellulose bioaerogels using turmeric nano particles. Ind. Crops Prod. 2023, 197, 116500. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Y.; Wang, X.; Liu, S.; Yao, Y. Characterization of the nano-cellulose aerogel from mixing CNF and CNC with different ratio. Mater. Lett. 2018, 229, 103–106. [Google Scholar] [CrossRef]
- Cao, M.; Liu, B.-W.; Zhang, L.; Peng, Z.-C.; Zhang, Y.-Y.; Wang, H.; Zhao, H.-B.; Wang, Y.-Z. Fully biomass-based aerogels with ultrahigh mechanical modulus, enhanced flame retardancy, and great thermal insulation applications. Compos. Part B Eng. 2021, 225, 109309. [Google Scholar] [CrossRef]
- Rodríguez-Dorado, R.; López-Iglesias, C.; García-González, C.A.; Auriemma, G.; Aquino, R.P.; Del Gaudio, P. Design of aerogels, cryogels and xerogels of alginate: Effect of molecular weight, gelation conditions and drying method on particles’ micromeritics. Molecules 2019, 24, 1049. [Google Scholar] [CrossRef]
- Anderson, A.M.; Wattley, C.W.; Carroll, M.K. Silica aerogels prepared via rapid supercritical extraction: Effect of process variables on aerogel properties. J. Non-Cryst. Solids 2009, 355, 101–108. [Google Scholar] [CrossRef]
- Chandrasekaran, R.; Hillgärtner, M.; Ganesan, K.; Milow, B.; Itskov, M.; Rege, A. Computational design of biopolymer aerogels and predictive modelling of their nanostructure and mechanical behaviour. Sci. Rep. 2021, 11, 10198. [Google Scholar] [CrossRef]
- Rostamitabar, M.; Ghahramani, A.; Seide, G.; Jockenhoevel, S.; Ghazanfari, S. Drug loaded cellulose–chitosan aerogel microfibers for wound dressing applications. Cellulose 2022, 29, 6261–6281. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, X. Thermal conductivity analysis of high porosity structures with open and closed pores. Int. J. Heat Mass Transf. 2022, 183, 122089. [Google Scholar] [CrossRef]
- Setyawan, H.; Fauziyah, M.A.; Tomo, H.S.S.; Widiyastuti, W.; Nurtono, T. Fabrication of Hydrophobic Cellulose Aerogels from Renewable Biomass Coir Fibers for Oil Spillage Clean-Up. J. Polym. Environ. 2022, 30, 5228–5238. [Google Scholar] [CrossRef]
- Zhu, W.-B.; Li, Y.-Q.; Wang, J.; Wang, Y.-Y.; Huang, P.; Hu, N.; Liao, K.; Fu, S.-Y. High-performance fiber-film hybrid-structured wearable strain sensor from a highly robust and conductive carbonized bamboo aerogel. ACS Appl. Bio Mater. 2020, 3, 8748–8756. [Google Scholar] [CrossRef]
- Lencina, M.S.; Piqueras, C.M.; Vega, D.A.; Villar, M.A.; Del Barrio, M.C. Environmentally friendly starch/alginate aerogels for copper adsorption from aqueous media. A microstructural and kinetic study. J. Environ. Sci. Health Part A 2023, 58, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Polman, E.M.; Gruter, G.-J.M.; Parsons, J.R.; Tietema, A. Comparison of the aerobic biodegradation of biopolymers and the corresponding bioplastics: A review. Sci. Total Environ. 2021, 753, 141953. [Google Scholar] [CrossRef] [PubMed]
- Firmanda, A.; Fahma, F.; Syamsu, K.; Sari, Y.W.; Suryanegara, L.; Wood, K.; Saito, Y. Factors influencing the biodegradability of agro-biopolymer based slow or controlled release fertilizer. J. Polym. Environ. 2023, 31, 1706–1724. [Google Scholar] [CrossRef]
- Shi, B.; Topolkaraev, V.; Wang, J. Biopolymers, processing, and biodegradation. In Renewable and Sustainable Polymers; ACS Publications: Washington, DC, USA, 2011; pp. 117–132. [Google Scholar]
- Abdul Khalil, H.P.S.; Yahya, E.B.; Tajarudin, H.A.; Balakrishnan, V.; Nasution, H. Insights into the role of biopolymer-based xerogels in biomedical applications. Gels 2022, 8, 334. [Google Scholar] [CrossRef]
- Yahya, E.B.; Alzalouk, M.M.; Alfallous, K.A.; Abogmaza, A.F. Antibacterial cellulose-based aerogels for wound healing application: A review. Biomed. Res. Ther. 2020, 7, 4032–4040. [Google Scholar] [CrossRef]
- Ubeyitogullari, A.; Ahmadzadeh, S.; Kandhola, G.; Kim, J.W. Polysaccharide-based porous biopolymers for enhanced bioaccessibility and bioavailability of bioactive food compounds: Challenges, advances, and opportunities. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4610–4639. [Google Scholar] [CrossRef]
- Smirnova, I.; Gurikov, P. Aerogel production: Current status, research directions, and future opportunities. J. Supercrit. Fluids 2018, 134, 228–233. [Google Scholar] [CrossRef]
- Yahya, E.B.; Abdul Khalil, H.P.S.; Ahmad, M.I.; Rizal, S.; Muhammad, S. Cleaner approach of preparing antibacterial bioaerogel scaffolds using oil palm waste nanocellulose. Ind. Crops Prod. 2023, 191, 115897. [Google Scholar] [CrossRef]
- Rizal, S.; Yahya, E.B.; Abdul Khalil, H.P.S.; Abdullah, C.; Marwan, M.; Ikramullah, I.; Muksin, U. Preparation and characterization of nanocellulose/chitosan aerogel scaffolds using chemical-free approach. Gels 2021, 7, 246. [Google Scholar] [CrossRef] [PubMed]
- Veronovski, A.; Knez, Ž.; Novak, Z. Preparation of multi-membrane alginate aerogels used for drug delivery. J. Supercrit. Fluids 2013, 79, 209–215. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Q.; Zhan, X.; Chen, F. A multifunctional gelatin-based aerogel with superior pollutants adsorption, oil/water separation and photocatalytic properties. Chem. Eng. J. 2019, 358, 1539–1551. [Google Scholar] [CrossRef]
- García-González, C.A.; Smirnova, I. Use of supercritical fluid technology for the production of tailor-made aerogel particles for delivery systems. J. Supercrit. Fluids 2013, 79, 152–158. [Google Scholar] [CrossRef]
- Gong, X.; Wang, Y.; Zeng, H.; Betti, M.; Chen, L. Highly porous, hydrophobic, and compressible cellulose nanocrystals/poly (vinyl alcohol) aerogels as recyclable absorbents for oil–water separation. ACS Sustain. Chem. Eng. 2019, 7, 11118–11128. [Google Scholar] [CrossRef]
- De Marco, I.; Reverchon, E. Starch aerogel loaded with poorly water-soluble vitamins through supercritical CO2 adsorption. Chem. Eng. Res. Des. 2017, 119, 221–230. [Google Scholar] [CrossRef]
- Alnaief, M.; Obaidat, R.; Mashaqbeh, H. Effect of processing parameters on preparation of carrageenan aerogel microparticles. Carbohydr. Polym. 2018, 180, 264–275. [Google Scholar] [CrossRef]
- Joshi, S.K.; Gauraha, A.K. Global biofertilizer market: Emerging trends and opportunities. Trends Appl. Microbiol. Sustain. Econ. 2022, 689–697. [Google Scholar]
- Schnitkey, G.; Paulson, N.; Zulauf, C.; Swanson, K.; Colussi, J.; Baltz, J. Nitrogen fertilizer prices and supply in light of the Ukraine-Russia conflict. Farm Doc Dly. 2022, 12, 45. [Google Scholar]
- de Janvry, A.; Sadoulet, E. Using agriculture for development: Supply- and demand-side approaches. World Dev. 2020, 133, 105003. [Google Scholar] [CrossRef]
- Fan, X.; Li, Y.; Alva, A. Effects of temperature and soil type on ammonia volatilization from slow-release nitrogen fertilizers. Commun. Soil Sci. Plant Anal. 2011, 42, 1111–1122. [Google Scholar] [CrossRef]
- Chien, S.H.; Teixeira, L.A.; Cantarella, H.; Rehm, G.W.; Grant, C.A.; Gearhart, M.M. Agronomic effectiveness of granular nitrogen/phosphorus fertilizers containing elemental sulfur with and without ammonium sulfate: A review. Agron. J. 2016, 108, 1203–1213. [Google Scholar] [CrossRef]
- Alameen, A.A.; Al-Gaadi, K.A.; Tola, E. Development and performance evaluation of a control system for variable rate granular fertilizer application. Comput. Electron. Agric. 2019, 160, 31–39. [Google Scholar] [CrossRef]
- Sancho, I.; Licon, E.; Valderrama, C.; de Arespacochaga, N.; López-Palau, S.; Cortina, J. Recovery of ammonia from domestic wastewater effluents as liquid fertilizers by integration of natural zeolites and hollow fibre membrane contactors. Sci. Total Environ. 2017, 584, 244–251. [Google Scholar] [CrossRef]
- Lu, J.; Cheng, M.; Zhao, C.; Li, B.; Peng, H.; Zhang, Y.; Shao, Q.; Hassan, M. Application of lignin in preparation of slow-release fertilizer: Current status and future perspectives. Ind. Crops Prod. 2022, 176, 114267. [Google Scholar] [CrossRef]
- Fertahi, S.; Ilsouk, M.; Zeroual, Y.; Oukarroum, A.; Barakat, A. Recent trends in organic coating based on biopolymers and biomass for controlled and slow release fertilizers. J. Control. Release 2021, 330, 341–361. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.B.; Ali, A.; Prasad, M.; Yadav, A.; Shrivastav, P.; Goyal, D.; Dantu, P.K. Role of organic fertilizers in improving soil fertility. In Contaminants in Agriculture: Sources, Impacts and Management; Springer: Berlin/Heidelberg, Germany, 2020; pp. 61–77. [Google Scholar]
- Durán-Lara, E.F.; Valderrama, A.; Marican, A. Natural organic compounds for application in organic farming. Agriculture 2020, 10, 41. [Google Scholar] [CrossRef]
- Singh, M. Organic farming for sustainable agriculture. Indian J. Org. Farming 2021, 1, 1–8. [Google Scholar]
- Hsu, C.-M.; Lai, H.-Y. Comprehensive Assessment of the Influence of Applying Two Kinds of Chicken-Manure-Processed Organic Fertilizers on Soil Properties, Mineralization of Nitrogen, and Yields of Three Crops. Agronomy 2022, 12, 2355. [Google Scholar] [CrossRef]
- Stadler, C.; Von Tucher, S.; Schmidhalter, U.; Gutser, R.; Heuwinkel, H. Nitrogen release from plant-derived and industrially processed organic fertilizers used in organic horticulture. J. Plant Nutr. Soil Sci. 2006, 169, 549–556. [Google Scholar] [CrossRef]
- Lazcano, C.; Zhu-Barker, X.; Decock, C. Effects of organic fertilizers on the soil microorganisms responsible for N2O emissions: A review. Microorganisms 2021, 9, 983. [Google Scholar] [CrossRef] [PubMed]
- Möller, K.; Schultheiß, U. Chemical characterization of commercial organic fertilizers. Arch. Agron. Soil Sci. 2015, 61, 989–1012. [Google Scholar] [CrossRef]
- Jiaying, M.; Tingting, C.; Jie, L.; Weimeng, F.; Baohua, F.; Guangyan, L.; Hubo, L.; Juncai, L.; Zhihai, W.; Longxing, T. Functions of nitrogen, phosphorus and potassium in energy status and their influences on rice growth and development. Rice Sci. 2022, 29, 166–178. [Google Scholar] [CrossRef]
- Walling, E.; Vaneeckhaute, C. Greenhouse gas emissions from inorganic and organic fertilizer production and use: A review of emission factors and their variability. J. Environ. Manag. 2020, 276, 111211. [Google Scholar] [CrossRef]
- Ricker-Gilbert, J. Inorganic fertiliser use among smallholder farmers in sub-Saharan Africa: Implications for input subsidy policies. In The role of Smallholder Farms in Food and Nutrition Security; Springer: Berlin/Heidelberg, Germany, 2020; pp. 81–98. [Google Scholar]
- Guo, Y.; Chen, Y.; Searchinger, T.D.; Zhou, M.; Pan, D.; Yang, J.; Wu, L.; Cui, Z.; Zhang, W.; Zhang, F. Air quality, nitrogen use efficiency and food security in China are improved by cost-effective agricultural nitrogen management. Nat. Food 2020, 1, 648–658. [Google Scholar] [CrossRef]
- Aryal, J.P.; Sapkota, T.B.; Krupnik, T.J.; Rahut, D.B.; Jat, M.L.; Stirling, C.M. Factors affecting farmers’ use of organic and inorganic fertilizers in South Asia. Environ. Sci. Pollut. Res. 2021, 28, 51480–51496. [Google Scholar] [CrossRef]
- Jaja, E.T.; Barber, L.I. Organic and inorganic fertilizers in food production system in Nigeria. Nature 2017, 7, e18. [Google Scholar]
- Vejan, P.; Khadiran, T.; Abdullah, R.; Ahmad, N. Controlled release fertilizer: A review on developments, applications and potential in agriculture. J. Control. Release 2021, 339, 321–334. [Google Scholar] [CrossRef]
- Negassa, W.; Getaneh, F.; Deressa, A.; Dinsa, B. Integrated use of organic and inorganic fertilizers for maize production. In Proceedings of the Utilization of Diversity in Land Use Systems: Sustainable and Organic Approaches to Meet Human Needs. Conference Tropentag, Witzenhausen, Germany, 9–11 October 2007; pp. 9–12. [Google Scholar]
- Bhatt, M.K.; Labanya, R.; Joshi, H.C. Influence of long-term chemical fertilizers and organic manures on soil fertility—A review. Univers. J. Agric. Res. 2019, 7, 177–188. [Google Scholar]
- Moe, K.; Moh, S.M.; Htwe, A.Z.; Kajihara, Y.; Yamakawa, T. Effects of integrated organic and inorganic fertilizers on yield and growth parameters of rice varieties. Rice Sci. 2019, 26, 309–318. [Google Scholar] [CrossRef]
- Roba, T.B. Review on: The effect of mixing organic and inorganic fertilizer on productivity and soil fertility. Open Access Libr. J. 2018, 5, 1. [Google Scholar] [CrossRef]
- Place, F.; Barrett, C.B.; Freeman, H.A.; Ramisch, J.J.; Vanlauwe, B. Prospects for integrated soil fertility management using organic and inorganic inputs: Evidence from smallholder African agricultural systems. Food Policy 2003, 28, 365–378. [Google Scholar] [CrossRef]
- Attallah, M.; Metwally, S.; Moussa, S.; Soliman, M.A. Environmental impact assessment of phosphate fertilizers and phosphogypsum waste: Elemental and radiological effects. Microchem. J. 2019, 146, 789–797. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Pal, D.B. Nutrients contamination and eutrophication in the river ecosystem. In Ecological Significance of River Ecosystems; Elsevier: Amsterdam, The Netherlands, 2022; pp. 203–216. [Google Scholar]
- Ashitha, A.; Rakhimol, K.; Mathew, J. Fate of the conventional fertilizers in environment. In Controlled Release Fertilizers for Sustainable Agriculture; Elsevier: Amsterdam, The Netherlands, 2021; pp. 25–39. [Google Scholar]
- Bhardwaj, A.K.; Arya, G.; Kumar, R.; Hamed, L.; Pirasteh-Anosheh, H.; Jasrotia, P.; Kashyap, P.L.; Singh, G.P. Switching to nanonutrients for sustaining agroecosystems and environment: The challenges and benefits in moving up from ionic to particle feeding. J. Nanobiotechnol. 2022, 20, 19. [Google Scholar] [CrossRef] [PubMed]
- Aydinalp, C.; Cresser, M.S. The effects of global climate change on agriculture. Am. Eurasian J. Agric. Environ. Sci. 2008, 3, 672–676. [Google Scholar]
- Iqbal, M.A. Nano-fertilizers for sustainable crop production under changing climate: A global perspective. Sustain. Crop Prod. 2019, 8, 1–13. [Google Scholar]
- Xie, S.; Yang, F.; Feng, H.; Yu, Z.; Liu, C.; Wei, C.; Liang, T. Organic fertilizer reduced carbon and nitrogen in runoff and buffered soil acidification in tea plantations: Evidence in nutrient contents and isotope fractionations. Sci. Total Environ. 2021, 762, 143059. [Google Scholar] [CrossRef]
- Craswell, E. Fertilizers and nitrate pollution of surface and ground water: An increasingly pervasive global problem. SN Appl. Sci. 2021, 3, 518. [Google Scholar]
- Van de Velde, K.; Kiekens, P. Biopolymers: Overview of several properties and consequences on their applications. Polym. Test. 2002, 21, 433–442. [Google Scholar] [CrossRef]
- Dhlamini, B.; Paumo, H.K.; Kamdem, B.P.; Katata-Seru, L.; Bahadur, I. Nano-engineering metal-based fertilizers using biopolymers: An innovative strategy for a more sustainable agriculture. J. Environ. Chem. Eng. 2022, 10, 107729. [Google Scholar] [CrossRef]
- dos Santos, B.R.; Bacalhau, F.B.; dos Santos Pereira, T.; Souza, C.F.; Faez, R. Chitosan-montmorillonite microspheres: A sustainable fertilizer delivery system. Carbohydr. Polym. 2015, 127, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Deuber, F.; Mousavi, S.; Federer, L.; Adlhart, C. Amphiphilic nanofiber-based aerogels for selective liquid absorption from electrospun biopolymers. Adv. Mater. Interfaces 2017, 4, 1700065. [Google Scholar] [CrossRef]
- Chang, I.; Lee, M.; Tran, A.T.P.; Lee, S.; Kwon, Y.-M.; Im, J.; Cho, G.-C. Review on biopolymer-based soil treatment (BPST) technology in geotechnical engineering practices. Transp. Geotech. 2020, 24, 100385. [Google Scholar] [CrossRef]
- Rashid, A.S.A.; Tabatabaei, S.; Horpibulsuk, S.; Mohd Yunus, N.Z.; Hassan, W.H.W. Shear strength improvement of lateritic soil stabilized by biopolymer based stabilizer. Geotech. Geol. Eng. 2019, 37, 5533–5541. [Google Scholar] [CrossRef]
- Latifi, N.; Horpibulsuk, S.; Meehan, C.L.; Abd Majid, M.Z.; Tahir, M.M.; Mohamad, E.T. Improvement of problematic soils with biopolymer—An environmentally friendly soil stabilizer. J. Mater. Civ. Eng. 2017, 29, 04016204. [Google Scholar] [CrossRef]
- Seo, S.; Lee, M.; Im, J.; Kwon, Y.-M.; Chung, M.-K.; Cho, G.-C.; Chang, I. Site application of biopolymer-based soil treatment (BPST) for slope surface protection: In-situ wet-spraying method and strengthening effect verification. Constr. Build. Mater. 2021, 307, 124983. [Google Scholar] [CrossRef]
- Mikula, K.; Izydorczyk, G.; Skrzypczak, D.; Mironiuk, M.; Moustakas, K.; Witek-Krowiak, A.; Chojnacka, K. Controlled release micronutrient fertilizers for precision agriculture—A review. Sci. Total Environ. 2020, 712, 136365. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, S.; Li, H.; Ma, J.; Wang, X.; Shi, H.; Wang, Z.; Zhang, F.; Niu, M.; Guo, Y. One-pot preparation of amphoteric cellulose polymers for simultaneous recovery of ammonium and dihydrogen phosphate from wastewater and reutilizing as slow-release fertilizer. Eur. Polym. J. 2022, 171, 111223. [Google Scholar] [CrossRef]
- Shi, W.; Ching, Y.C.; Chuah, C.H. Preparation of aerogel beads and microspheres based on chitosan and cellulose for drug delivery: A review. Int. J. Biol. Macromol. 2021, 170, 751–767. [Google Scholar] [CrossRef]
- Rostamitabar, M.; Subrahmanyam, R.; Gurikov, P.; Seide, G.; Jockenhoevel, S.; Ghazanfari, S. Cellulose aerogel micro fibers for drug delivery applications. Mater. Sci. Eng. C 2021, 127, 112196. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Li, A.; Zheng, T.; Lu, L.; Cao, Y. Hydrophobic and flexible cellulose aerogel as an efficient, green and reusable oil sorbent. RSC Adv. 2015, 5, 82027–82033. [Google Scholar] [CrossRef]
- Kaur, J.; Sharma, K.; Kaushik, A. Waste hemp-stalk derived nutrient encapsulated aerogels for slow release of fertilizers: A step towards sustainable agriculture. J. Environ. Chem. Eng. 2023, 11, 109582. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, J. Preparation and characterization of multifunctional slow release fertilizer coated with cellulose derivatives. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 774–781. [Google Scholar] [CrossRef]
- Liu, Z.; Couto, R.; Seifried, B.; Yépez, B.; Moquin, P.; Temelli, F. Supercritical adsorptive precipitation of coenzyme Q10 on sodium alginate aerogel. J. Supercrit. Fluids 2022, 189, 105701. [Google Scholar] [CrossRef]
- Dong, K.; Xu, K.; Wei, N.; Fang, Y.; Qin, Z. Three-dimensional porous sodium alginate/gellan gum environmentally friendly aerogel: Preparation, characterization, adsorption, and kinetics studies. Chem. Eng. Res. Des. 2022, 179, 227–236. [Google Scholar] [CrossRef]
- Dhua, S.; Gupta, A.K.; Mishra, P. Aerogel: Functional Emerging Material for Potential Application in Food: A Review. Food Bioprocess Technol. 2022, 15, 2396–2421. [Google Scholar] [CrossRef]
- Sun, X.; Fu, H.; Bao, M.; Zhang, F.; Liu, W.; Li, Y.; Li, Y.; Lu, J. Preparation of slow-release microencapsulated fertilizer—Biostimulation remediation of marine oil spill pollution. J. Environ. Chem. Eng. 2023, 11, 109283. [Google Scholar] [CrossRef]
- Del Gaudio, P.; Auriemma, G.; Mencherini, T.; Della Porta, G.; Reverchon, E.; Aquino, R.P. Design of alginate-based aerogel for nonsteroidal anti-inflammatory drugs controlled delivery systems using prilling and supercritical-assisted drying. J. Pharm. Sci. 2013, 102, 185–194. [Google Scholar] [CrossRef]
- Guastaferro, M.; Reverchon, E.; Baldino, L. Agarose, alginate and chitosan nanostructured aerogels for pharmaceutical applications: A short review. Front. Bioeng. Biotechnol. 2021, 9, 688477. [Google Scholar] [CrossRef]
- Wu, C.; Dan, Y.; Tian, D.; Zheng, Y.; Wei, S.; Xiang, D. Facile fabrication of MOF(Fe)@alginate aerogel and its application for a high-performance slow-release N-fertilizer. Int. J. Biol. Macromol. 2020, 145, 1073–1079. [Google Scholar] [CrossRef]
- Fan, X.; Li, Y.; Li, X.; Wu, Y.; Tang, K.; Liu, J.; Zheng, X.; Wan, G. Injectable antibacterial cellulose nanofiber/chitosan aerogel with rapid shape recovery for noncompressible hemorrhage. Int. J. Biol. Macromol. 2020, 154, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Jiang, S.; Han, F.; Li, M.; Wang, N.; Liu, L. Anisotropic cellulose nanofiber/chitosan aerogel with thermal management and oil absorption properties. Carbohydr. Polym. 2021, 264, 118033. [Google Scholar] [CrossRef] [PubMed]
- Gorshkova, N.; Brovko, O.; Palamarchuk, I.; Bogolitsyn, K.; Ivakhnov, A. Preparation of bioactive aerogel material based on sodium alginate and chitosan for controlled release of levomycetin. Polym. Adv. Technol. 2021, 32, 3474–3482. [Google Scholar] [CrossRef]
- Mutlaq, M.S.; Jabrail, F.H. Controlled Delivery System for NPK Agrochemical Release from Chitosan Copolymer Hydrogels. Am. J. Appl. Sci. 2022, 19, 84–92. [Google Scholar]
- Takeshita, S.; Zhao, S.; Malfait, W.J.; Koebel, M.M. Chemistry of chitosan aerogels: Three-dimensional pore control for tailored applications. Angew. Chem. Int. Ed. 2021, 60, 9828–9851. [Google Scholar] [CrossRef]
- Druel, L.; Bardl, R.; Vorwerg, W.; Budtova, T. Starch aerogels: A member of the family of thermal superinsulating materials. Biomacromolecules 2017, 18, 4232–4239. [Google Scholar] [CrossRef]
- da Silva, F.T.; de Oliveira, J.P.; Fonseca, L.M.; Bruni, G.P.; da Rosa Zavareze, E.; Dias, A.R.G. Physically cross-linked aerogels based on germinated and non-germinated wheat starch and PEO for application as water absorbers for food packaging. Int. J. Biol. Macromol. 2020, 155, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Esquivel-Castro, T.A.; Ibarra-Alonso, M.; Oliva, J.; Martínez-Luévanos, A. Porous aerogel and core/shell nanoparticles for controlled drug delivery: A review. Mater. Sci. Eng. C 2019, 96, 915–940. [Google Scholar] [CrossRef] [PubMed]
- Gungula, D.T.; Andrew, F.P.; Joseph, J.; Kareem, S.A.; Barminas, J.T.; Adebayo, E.F.; Saddiq, A.M.; Tame, V.T.; Dere, I.; Ahinda, W.J. Formulation and characterization of water retention and slow-release urea fertilizer based on Borassus aethiopum starch and Maesopsis eminii hydrogels. Results Mater. 2021, 12, 100223. [Google Scholar] [CrossRef]
- Wang, Y.; Shaghaleh, H.; Hamoud, Y.A.; Zhang, S.; Li, P.; Xu, X.; Liu, H. Synthesis of a pH-responsive nano-cellulose/sodium alginate/MOFs hydrogel and its application in the regulation of water and N-fertilizer. Int. J. Biol. Macromol. 2021, 187, 262–271. [Google Scholar] [CrossRef] [PubMed]
| Precursor Material | Cross-Linker | Drying Technique | Density (mg/cm3) | Porosity (%) | Surface Area (m2/g) | Application | Ref |
|---|---|---|---|---|---|---|---|
| Cellulose nanofibers | Pure CNF | Freeze-drying | 8.1 ± 0.8 | 99.1 ± 0.2 | 145.3 ± 2.7 | Biomedical | [58] |
| Chitosan | Pure chitosan | Freeze-drying | 141.4 | 90.8 | - | General applications | [59] |
| Sodium alginates | Calcium chloride | Supercritical drying | - | 98 | 402 ± 12 | Drug delivery | [60] |
| Gelatin | Glutaraldehyde | Freeze-drying | 115 | 75.37 | 9.56 | Oil–water separation | [61] |
| Starch | Corn starch | Supercritical fluid | 100.6 | 90.1 | 240 | Drug delivery of active | [62] |
| Cellulose nanocrystals | Poly (vinyl alcohol) | Freeze-drying | 22.5 | 97.7 | 38.0 | water–oil separation | [63] |
| Starch | Pure starch | Supercritical drying | 602 | - | 90.0 | Vitamins delivery | [64] |
| Carrageenan | Potassium chloride | Supercritical CO2 | 250.0 | 86.0 | 110.0 | Drug delivery | [65] |
| Biomass | Phytic acid | Freeze-drying | 52.0 | - | - | Thermal insulation | [42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdul Khalil, H.P.S.; Jha, K.; Yahya, E.B.; Panchal, S.; Patel, N.; Garai, A.; Kumari, S.; Jameel, M. Insights into the Potential of Biopolymeric Aerogels as an Advanced Soil-Fertilizer Delivery Systems. Gels 2023, 9, 666. https://doi.org/10.3390/gels9080666
Abdul Khalil HPS, Jha K, Yahya EB, Panchal S, Patel N, Garai A, Kumari S, Jameel M. Insights into the Potential of Biopolymeric Aerogels as an Advanced Soil-Fertilizer Delivery Systems. Gels. 2023; 9(8):666. https://doi.org/10.3390/gels9080666
Chicago/Turabian StyleAbdul Khalil, H. P. S., Kanchan Jha, Esam Bashir Yahya, Sandeep Panchal, Nidhi Patel, Arindam Garai, Soni Kumari, and Mohammed Jameel. 2023. "Insights into the Potential of Biopolymeric Aerogels as an Advanced Soil-Fertilizer Delivery Systems" Gels 9, no. 8: 666. https://doi.org/10.3390/gels9080666
APA StyleAbdul Khalil, H. P. S., Jha, K., Yahya, E. B., Panchal, S., Patel, N., Garai, A., Kumari, S., & Jameel, M. (2023). Insights into the Potential of Biopolymeric Aerogels as an Advanced Soil-Fertilizer Delivery Systems. Gels, 9(8), 666. https://doi.org/10.3390/gels9080666