Upcycling Wool Waste into Keratin Gel-Based Nanofibers Using Deep Eutectic Solvents
Abstract
:1. Introduction
2. Results and Discussion
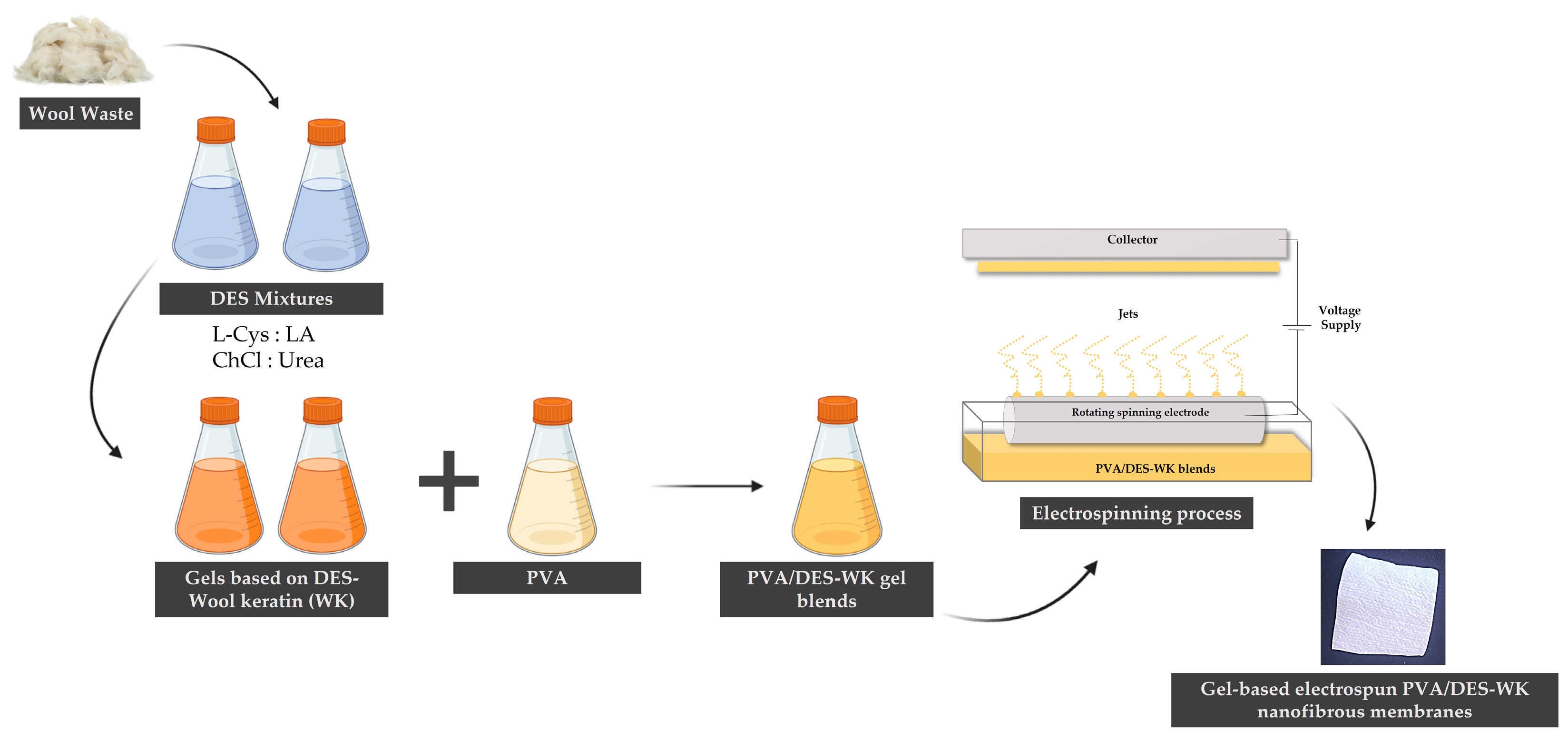
2.1. Dissolution of WK into DES Mixtures
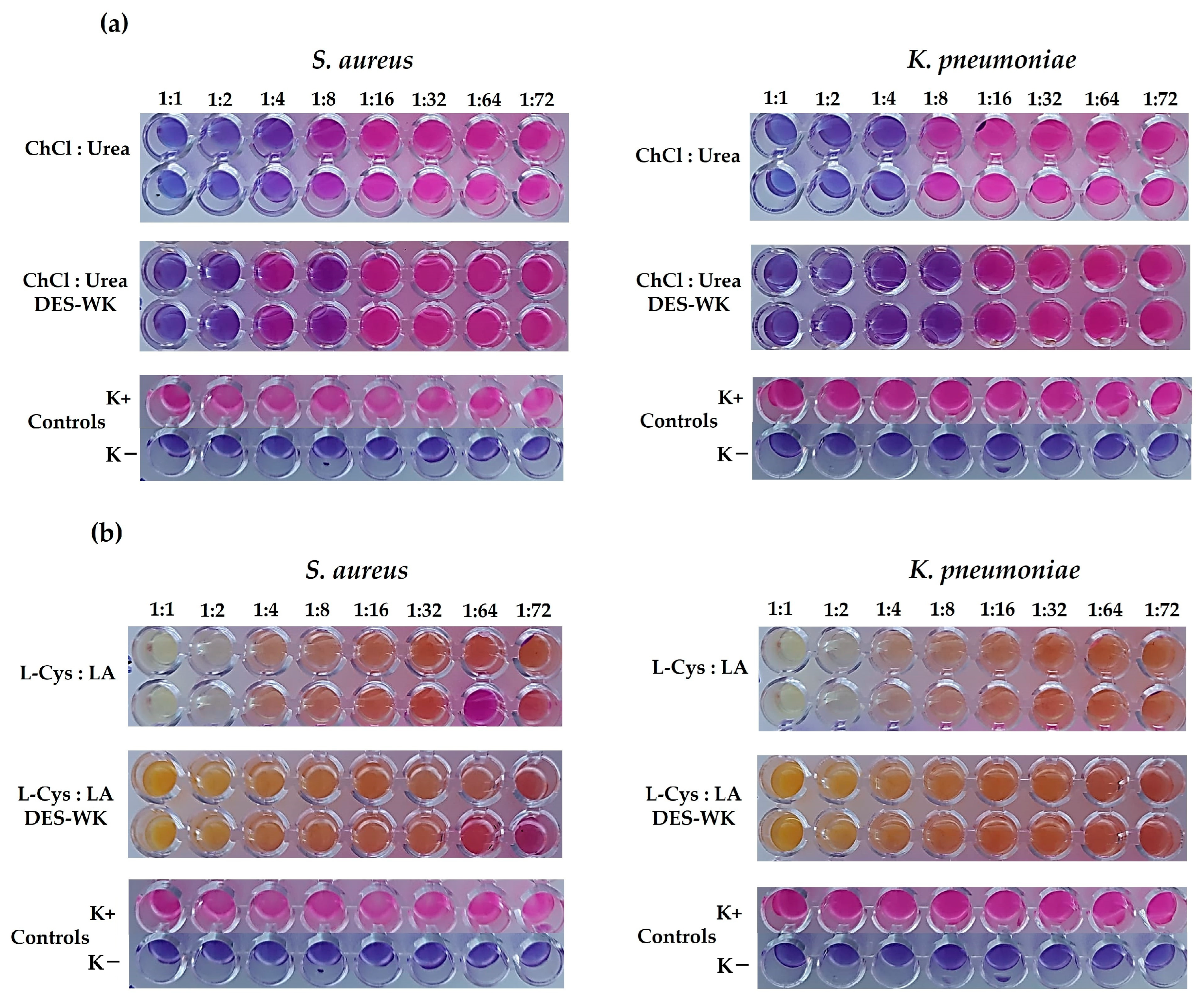
2.2. Determination of the Antibacterial Activity of the DES Mixtures and the Gels Based on DES-WK
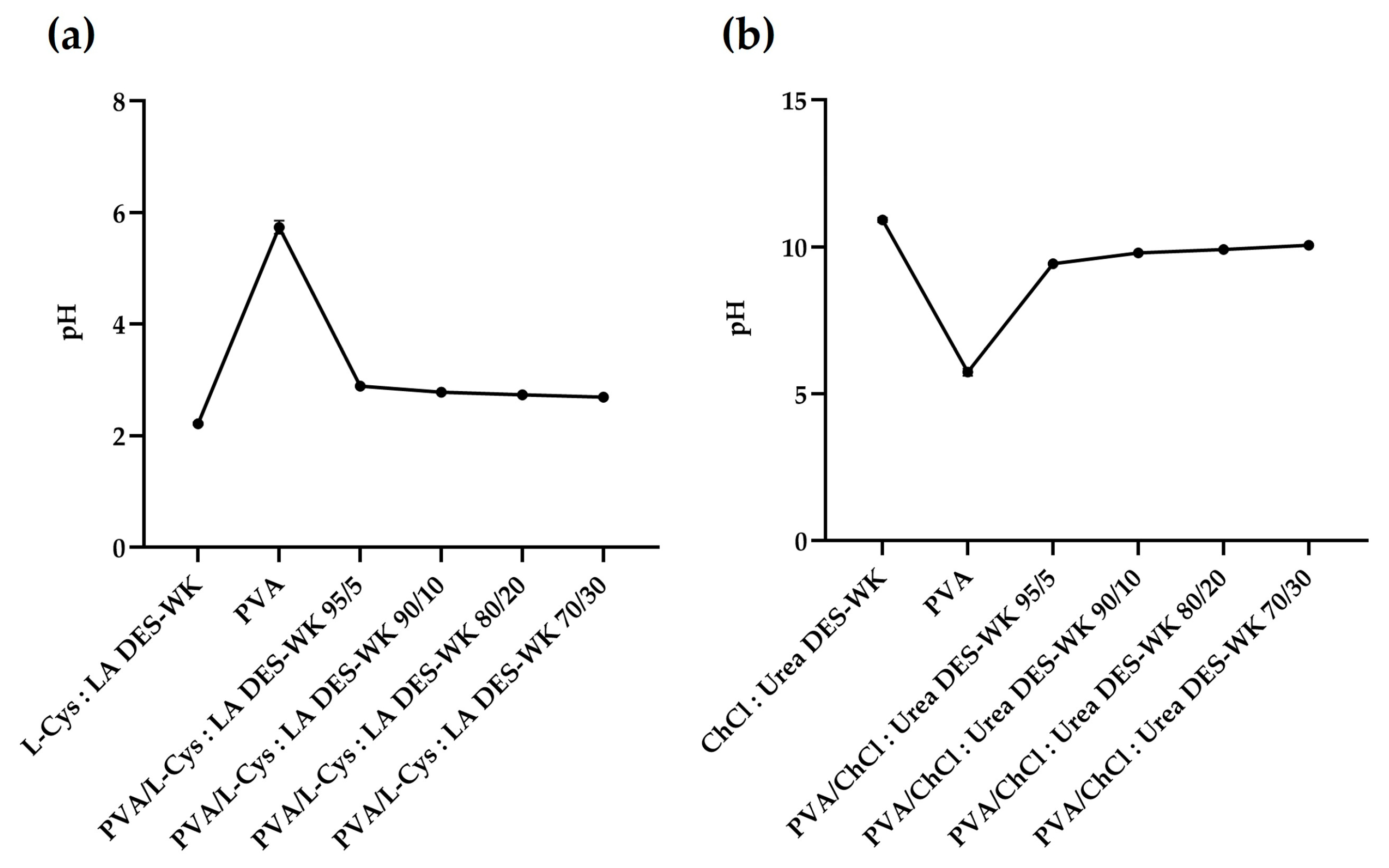
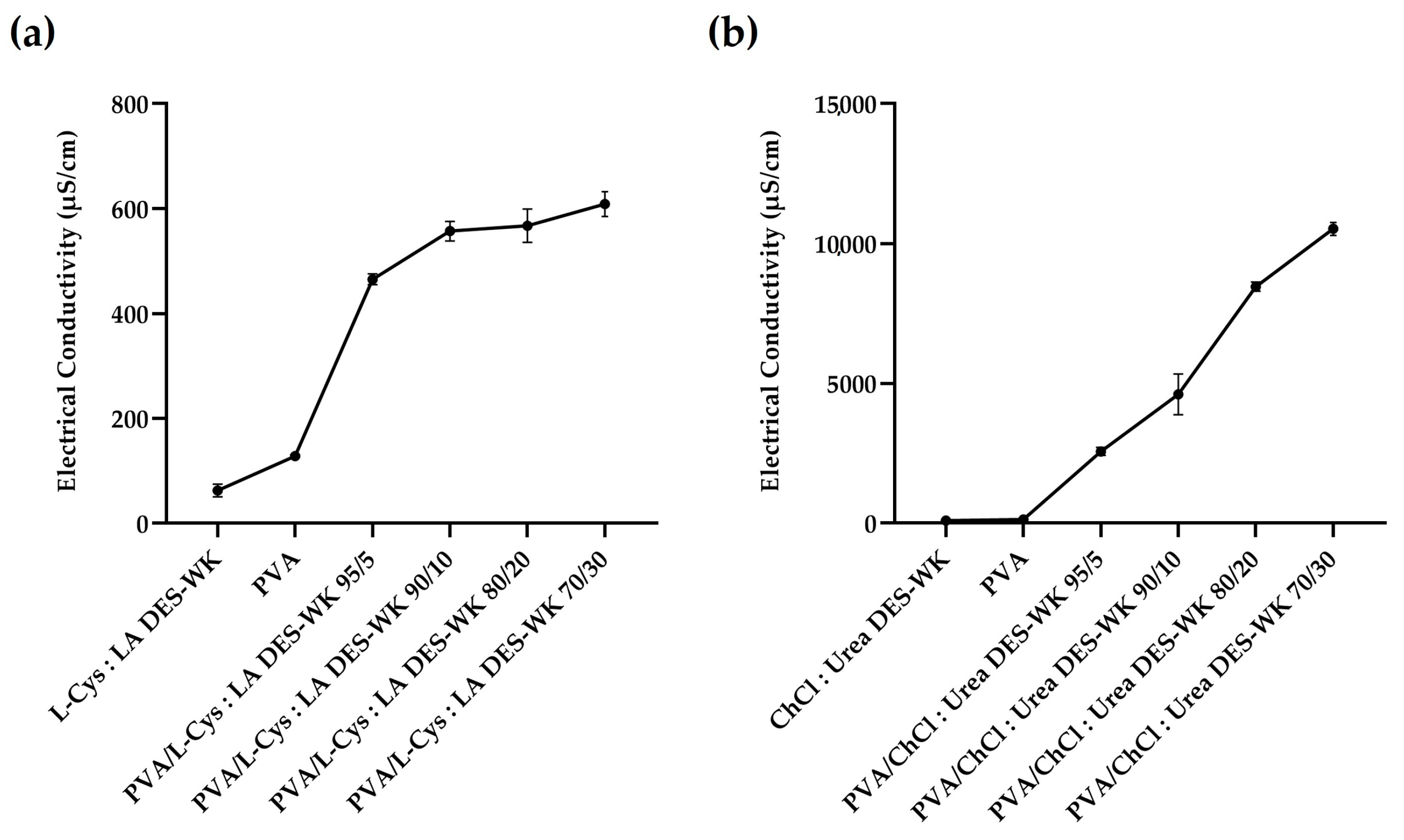
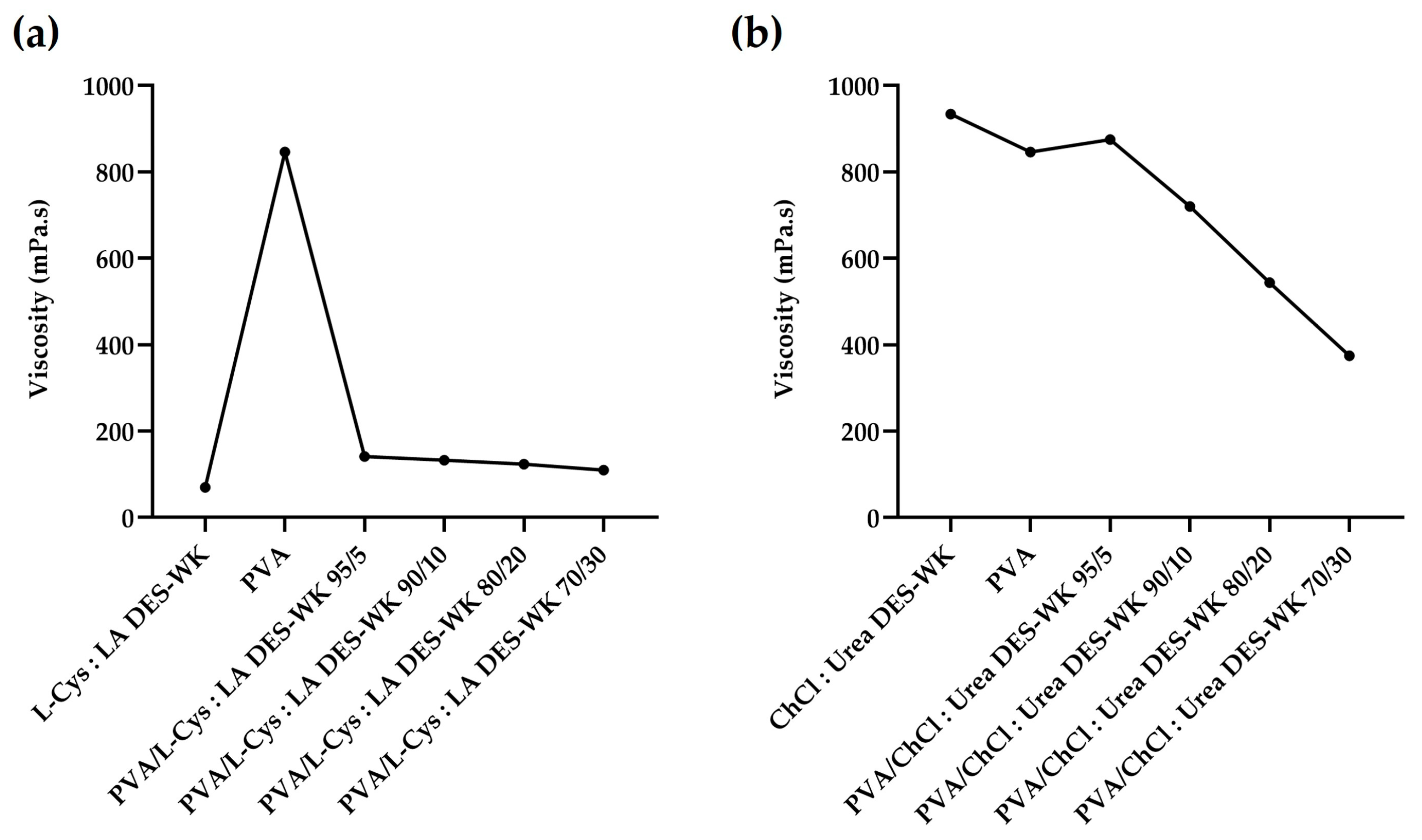
2.3. Evaluation of the pH and the Electrospinning Solution Properties
2.3.1. pH
2.3.2. Electrical Conductivity
2.3.3. Viscosity
2.4. Characterization of the Gel-Based Electrospun PVA/DES-WK Nanofibrous Membranes
2.4.1. Characterization of the Gel-Based Nanofibers’ Surface Morphology through Scanning Electron Microscopy (SEM) Analysis
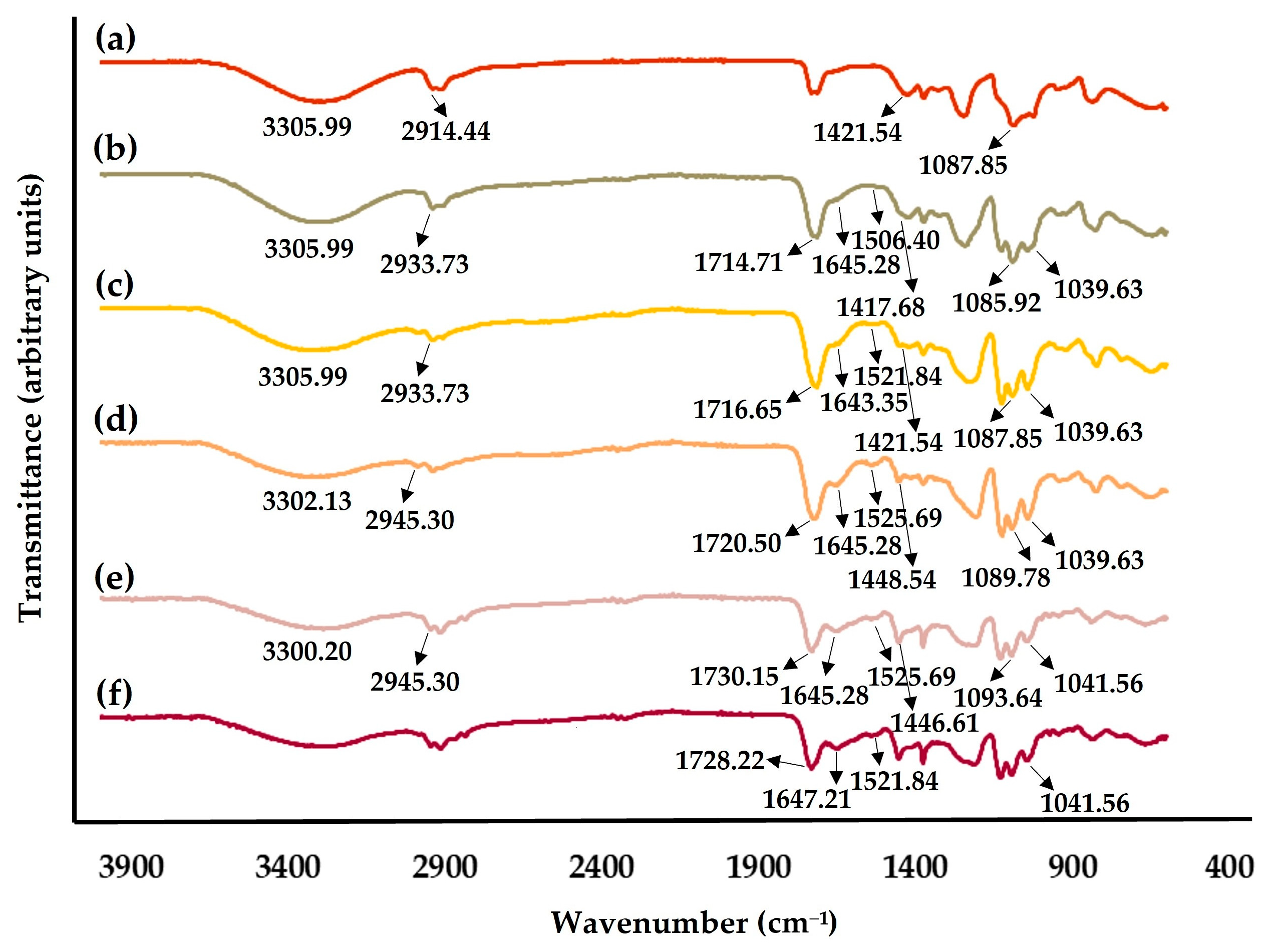
2.4.2. Fourier-Transform Infrared Spectroscopic (FTIR) Analysis
2.4.3. Characterization of the Gel-Based Nanofibers’ Mechanical Properties
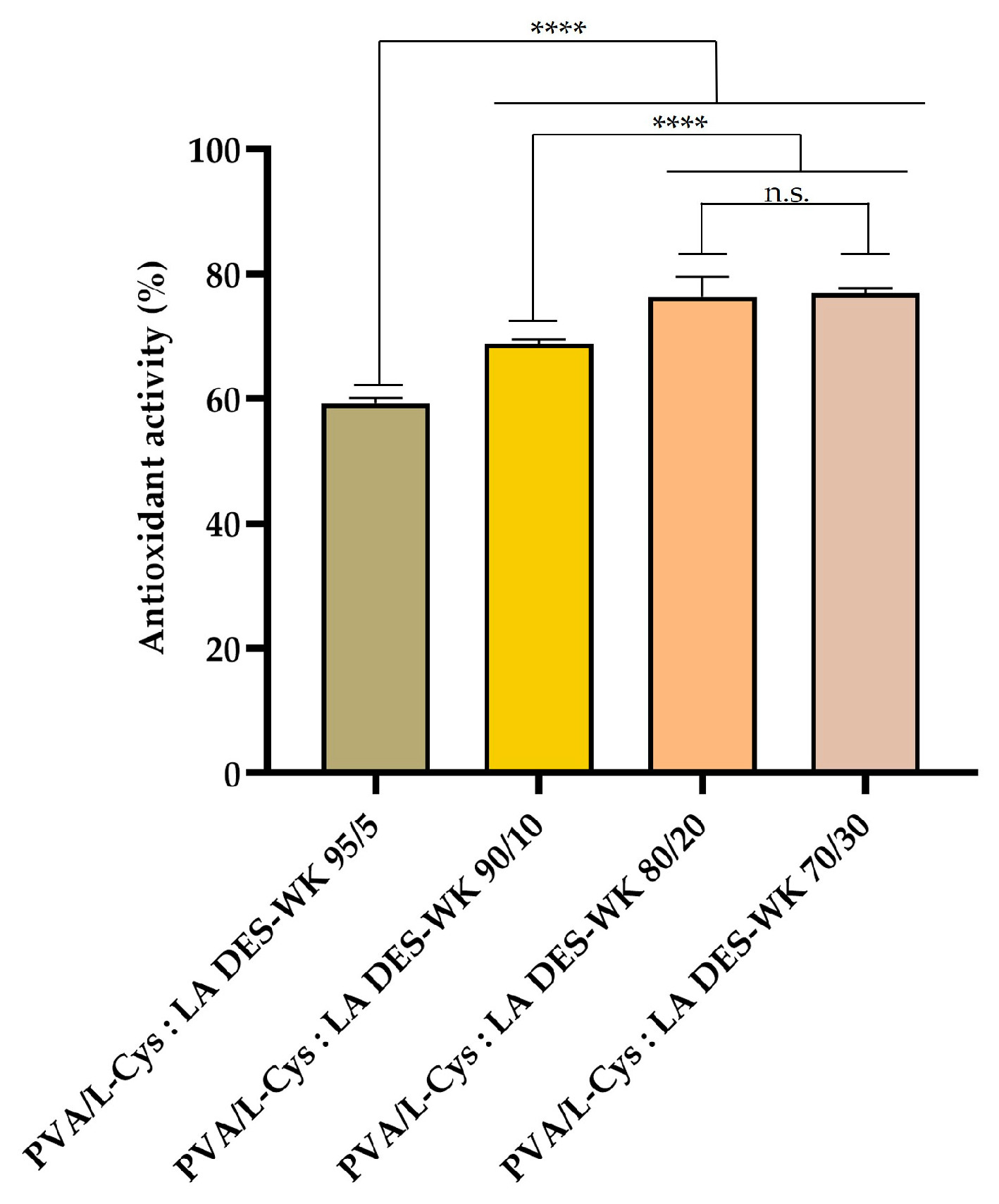
2.4.4. Evaluation of the Gel-Based Nanofibers’ Antioxidant Activity
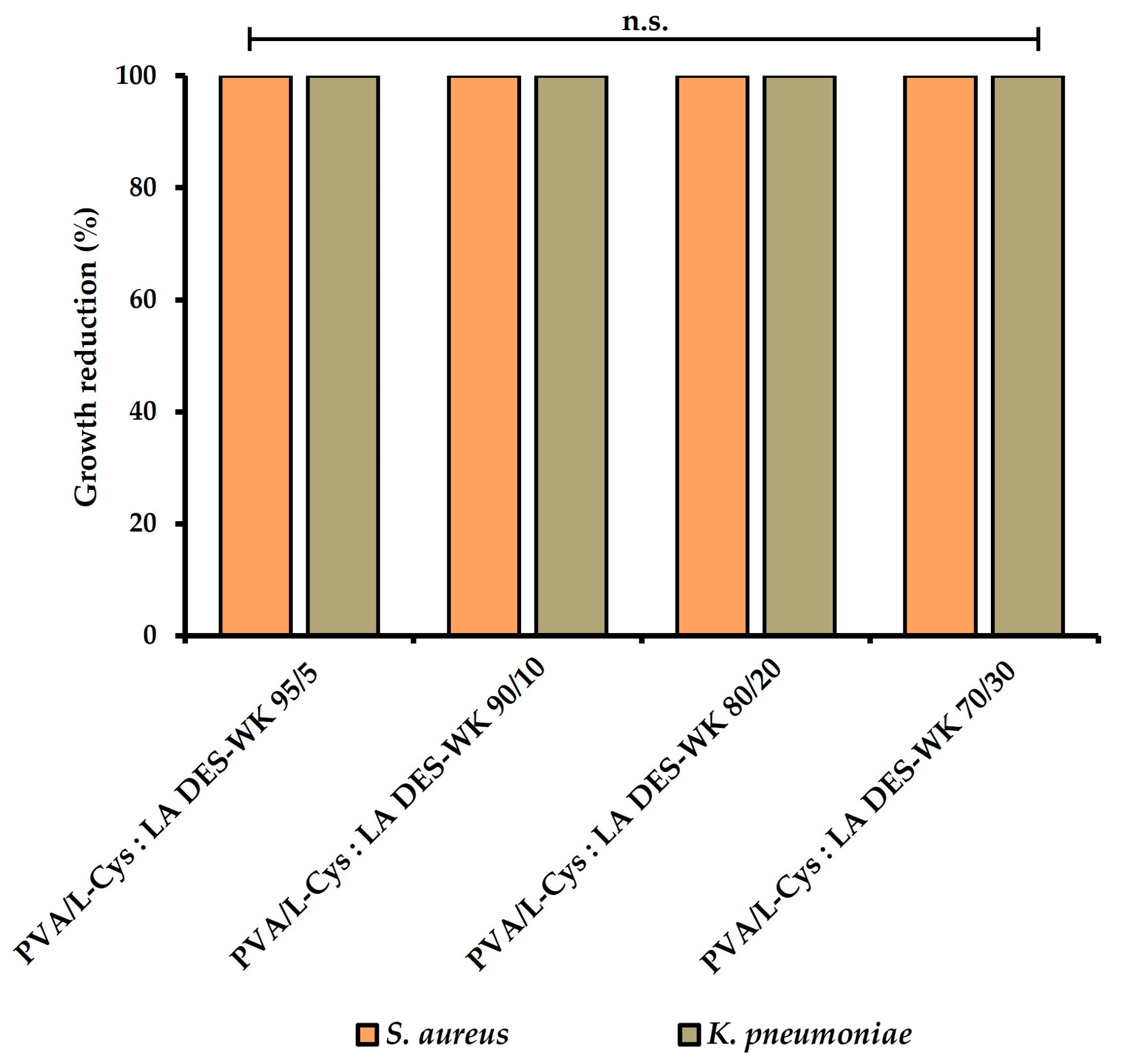
2.4.5. Evaluation of the Gel-Based Nanofibers’ Antimicrobial Properties
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of DES Mixtures
4.3. Dissolution of WK into DES Mixtures
4.4. Determination of the Antibacterial Activity of the DES Mixtures and the Gels Based on DES-WK
4.5. Production of the Gel-Based Electrospun PVA/DES-WK Nanofibrous Membranes
4.5.1. Preparation of the Electrospinning Solutions
4.5.2. Measurement of pH of the Electrospinning Gel Solutions
4.5.3. Measurement of the Electrical Conductivity of the Electrospinning Gel Solutions
4.5.4. Measurement of the Viscosity of the Electrospinning Gel Solutions
4.5.5. Electrospinning of the PVA/DES-WK Blend Gel Solutions
4.6. Characterization of the Gel-Based Electrospun PVA/DES-WK Nanofibrous Membranes
4.6.1. Characterization of the Gel-Based Nanofibers’ Surface Morphology through Scanning Electron Microscopy (SEM) Analysis
4.6.2. Fourier-Transform Infrared Spectroscopic (FTIR) Analysis
4.6.3. Characterization of the Gel-Based Nanofibers’ Mechanical Properties
4.6.4. Evaluation of the Gel-Based Nanofibers’ Antioxidant Activity
4.6.5. Evaluation of the Gel-Based Nanofibers’ Antimicrobial Properties
4.6.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ben Seghir, B.; Hemmami, H.; Soumeia, Z.; Laouini, S.E.; Rebiai, A.; Amor, B.; Souici, I.; Beki, A. Preparation Methods Keratin and Nanoparticles Keratin from Wool: A Review. Alger. J. Chem. Eng. 2020, 1, 5–11. [Google Scholar] [CrossRef]
- Okoro, O.V.; Jafari, H.; Hobbi, P.; Nie, L.; Alimoradi, H.; Shavandi, A. Enhanced Keratin Extraction from Wool Waste Using a Deep Eutectic Solvent. Chem. Pap. 2022, 76, 2637–2648. [Google Scholar] [CrossRef]
- Jiang, Z.; Yuan, J.; Wang, P.; Fan, X.; Xu, J.; Wang, Q.; Zhang, L. Dissolution and Regeneration of Wool Keratin in the Deep Eutectic Solvent of Choline Chloride-Urea. Int. J. Biol. Macromol. 2018, 119, 423–430. [Google Scholar] [CrossRef]
- Wang, D.; Tang, R.C. Dissolution of Wool in the Choline Chloride/Oxalic Acid Deep Eutectic Solvent. Mater. Lett. 2018, 231, 217–220. [Google Scholar] [CrossRef]
- Jablonský, M.; Škulcová, A.; Malvis, A.; Šima, J. Extraction of Value-Added Components from Food Industry Based and Agro-Forest Biowastes by Deep Eutectic Solvents. J. Biotechnol. 2018, 282, 46–66. [Google Scholar] [CrossRef] [PubMed]
- Rajabinejad, H.; Zoccola, M.; Patrucco, A.; Montarsolo, A.; Rovero, G.; Tonin, C. Physicochemical Properties of Keratin Extracted from Wool by Various Methods. Text. Res. J. 2018, 88, 2415–2424. [Google Scholar] [CrossRef]
- Moore, K.E.; Mangos, D.N.; Slattery, A.D.; Raston, C.L.; Boulos, R.A. Wool Deconstruction Using a Benign Eutectic Melt. RSC Adv. 2016, 6, 20095–20101. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298. [Google Scholar] [CrossRef]
- Nadaf, A.; Gupta, A.; Hasan, N.; Fauziya, N.; Ahmad, S.; Kesharwani, P.; Ahmad, F.J. Recent Update on Electrospinning and Electrospun Nanofibers: Current Trends and Their Applications. RSC Adv. 2022, 12, 23808–23828. [Google Scholar] [CrossRef]
- Zulkifli, M.Z.A.; Nordin, D.; Shaari, N.; Kamarudin, S.K. Overview of Electrospinning for Tissue Engineering Applications. Polymers 2023, 15, 2418. [Google Scholar] [CrossRef]
- Avossa, J.; Herwig, G.; Toncelli, C.; Itel, F.; Rossi, R.M. Electrospinning Based on Benign Solvents: Current Definitions, Implications and Strategies. Green Chem. 2022, 24, 2347–2375. [Google Scholar] [CrossRef]
- Bongiovanni Abel, S.; Liverani, L.; Boccaccini, A.R.; Abraham, G.A. Effect of Benign Solvents Composition on Poly(ε-Caprolactone) Electrospun Fiber Properties. Mater. Lett. 2019, 245, 86–89. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kang, I.K. A Comprehensive Review Summarizing the Effect of Electrospinning Parameters and Potential Applications of Nanofibers in Biomedical and Biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Rong, K.; Wei, J.; Wang, Y.; Liu, J.; Qiao, Z.A.; Fang, Y.; Dong, S. Deep Eutectic Solvent Assisted Zero-Waste Electrospinning of Lignin Fiber Aerogels. Green Chem. 2021, 23, 6065–6075. [Google Scholar] [CrossRef]
- Li, S.; Yang, X.H. Fabrication and Characterization of Electrospun Wool Keratin/Poly(Vinyl Alcohol) Blend Nanofibers. Adv. Mater. Sci. Eng. 2014, 2014, 163678. [Google Scholar] [CrossRef]
- Ma, H.; Shen, J.; Cao, J.; Wang, D.; Yue, B.; Mao, Z.; Wu, W.; Zhang, H. Fabrication of Wool Keratin/Polyethylene Oxide Nano-Membrane from Wool Fabric Waste. J. Clean. Prod. 2017, 161, 357–361. [Google Scholar] [CrossRef]
- Aluigi, A.; Vineis, C.; Tonin, C.; Tonetti, C.; Varesano, A.; Mazzuchetti, G. Wool Keratin-Based Nanofibres for Active Filtration of Air and Water. J. Biobased Mater. Bioenergy 2009, 3, 311–319. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Li, L.; Zhang, Y.; Ren, X. Preparation of Antibacterial Biocompatible Polycaprolactone/Keratin Nanofibrous Mats by Electrospinning. J. Appl. Polym. Sci. 2021, 138, 49862. [Google Scholar] [CrossRef]
- Fatahian, R.; Mirjalili, M.; Khajavi, R.; Rahimi, M.K.; Nasirizadeh, N. Effect of Electrospinning Parameters on Production of Polyvinyl Alcohol/Polylactic Acid Nanofiber Using a Mutual Solvent. Polym. Polym. Compos. 2021, 29, S844–S856. [Google Scholar] [CrossRef]
- Hu, X.; Wang, X.; Li, S.; Zhou, W.; Song, W. Antibacterial Electrospun Polyvinyl Alcohol Nanofibers Encapsulating Berberine-Hydroxypropyl-β-Cyclodextrin Inclusion Complex. J. Drug Deliv. Sci. Technol. 2021, 64, 102649. [Google Scholar] [CrossRef]
- Ezati, P.; Khan, A.; Rhim, J.W. Cellulose Nanofiber-Based PH Indicator Integrated with Resazurin-Modified Carbon Dots for Real-Time Monitoring of Food Freshness. Food Biosci. 2023, 53, 102679. [Google Scholar] [CrossRef]
- Labadie, M.; Randrianjatovo-Gbalou, I.; Zaidi-Ait-Salem, M.; Dossat-Létisse, V.; Fontagné-Faucher, C.; Marcato-Romain, C.E. A Dynamic Resazurin Microassay Allowing Accurate Quantification of Cells and Suitable for Acid-Forming Bacteria. J. Microbiol. Methods 2021, 183, 106172. [Google Scholar] [CrossRef]
- Al-Akayleh, F.; Khalid, R.M.; Hawash, D.; Al-Kaissi, E.; Al-Adham, I.S.I.; Al-Muhtaseb, N.; Jaber, N.; Al-Remawi, M.; Collier, P.J. Antimicrobial Potential of Natural Deep Eutectic Solvents. Lett. Appl. Microbiol. 2022, 75, 607–615. [Google Scholar] [CrossRef]
- Akbar, N.; Khan, N.A.; Ibrahim, T.; Khamis, M.; Khan, A.S.; Alharbi, A.M.; Alfahemi, H.; Siddiqui, R. Antimicrobial Activity of Novel Deep Eutectic Solvents. Sci. Pharm. 2023, 91, 9. [Google Scholar] [CrossRef]
- Nogueira, F.; Granadeiro, L.; Mouro, C.; Gouveia, I.C. Antimicrobial and Antioxidant Surface Modification toward a New Silk-Fibroin (SF)-l-Cysteine Material for Skin Disease Management. Appl. Surf. Sci. 2016, 364, 552–559. [Google Scholar] [CrossRef]
- Giteru, S.G.; Ramsey, D.H.; Hou, Y.; Cong, L.; Mohan, A.; Bekhit, A.E.D.A. Wool Keratin as a Novel Alternative Protein: A Comprehensive Review of Extraction, Purification, Nutrition, Safety, and Food Applications. Compr. Rev. Food Sci. Food Saf. 2023, 22, 643–687. [Google Scholar] [CrossRef] [PubMed]
- Vieira Sanches, M.; Freitas, R.; Oliva, M.; Mero, A.; De Marchi, L.; Cuccaro, A.; Fumagalli, G.; Mezzetta, A.; Colombo Dugoni, G.; Ferro, M.; et al. Are Natural Deep Eutectic Solvents Always a Sustainable Option? A Bioassay-Based Study. Env. Environ. Sci. Pollut. Res. 2023, 30, 17268–17279. [Google Scholar] [CrossRef]
- Shekaari, H.; Zafarani-Moattar, M.T.; Mokhtarpour, M.; Faraji, S. Solubility of Hesperidin Drug in Aqueous Biodegradable Acidic Choline Chloride-Based Deep Eutectic Solvents. Sci. Rep. 2023, 13, 11276. [Google Scholar] [CrossRef]
- Tam, N.; Oguz, S.; Aydogdu, A.; Sumnu, G.; Sahin, S. Influence of Solution Properties and PH on the Fabrication of Electrospun Lentil Flour/HPMC Blend Nanofibers. Food Res. Int. 2017, 102, 616–624. [Google Scholar] [CrossRef]
- Saxena, S.K. POLYVINYL ALCOHOL (PVA) Chemical and Technical Assessment (CTA) First Draft Prepared By. In Proceedings of the Joint FAO/WHO Expert Committee on Food Additives, 61st Meeting, Rome, Italy, 10–19 June 2003; pp. 1–3. [Google Scholar]
- Keirouz, A.; Wang, Z.; Reddy, V.S.; Nagy, Z.K.; Vass, P.; Buzgo, M.; Ramakrishna, S.; Radacsi, N. The History of Electrospinning: Past, Present, and Future Developments. Adv. Mater. Technol. 2023, 8, 2201723. [Google Scholar] [CrossRef]
- Sharma, G.K.; James, N.R.; Sharma, G.K.; James, N.R. Electrospinning: The Technique and Applications. Recent. Dev. Nanofibers Res. 2022. [Google Scholar] [CrossRef]
- Yalcinkaya, F.; Yalcinkaya, B.; Jirsak, O.; Yalcinkaya, F.; Yalcinkaya, B.; Jirsak, O. Dependent and Independent Parameters of Needleless Electrospinning. Vlak. A Text. 2016, 2015, 75–79. [Google Scholar] [CrossRef]
- Supaphol, P.; Chuangchote, S. On the Electrospinning of Poly(Vinyl Alcohol) Nanofiber Mats: A Revisit. J. Appl. Polym. Sci. 2008, 108, 969–978. [Google Scholar] [CrossRef]
- Gazioglu Ruzgar, D.; Altun Kurtoglu, S.; Bhullar, S.K. A Study on Extraction and Characterization of Keratin Films and Nanofibers from Waste Wool Fiber. J. Nat. Fibers 2020, 17, 427–436. [Google Scholar] [CrossRef]
- Teixeira, M.A.; Amorim, M.T.P.; Felgueiras, H.P. Poly(Vinyl Alcohol)-Based Nanofibrous Electrospun Scaffolds for Tissue Engineering Applications. Polymer 2020, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Riyadh, S.M.; Khalil, K.D.; Bashal, A.H. Structural Properties and Catalytic Activity of Binary Poly (Vinyl Alcohol)/Al2O3 Nanocomposite Film for Synthesis of Thiazoles. Catalysts 2020, 10, 100. [Google Scholar] [CrossRef]
- Tang, W.; Wang, B.; Li, J.; Li, Y.; Zhang, Y.; Quan, H.; Huang, Z. Facile Pyrolysis Synthesis of Ionic Liquid Capped Carbon Dots and Subsequent Application as the Water-Based Lubricant Additives. J. Mater. Sci. 2019, 54, 1171–1183. [Google Scholar] [CrossRef]
- Amorim, L.F.A.; Fangueiro, R.; Gouveia, I.C. Characterization of Bioactive Colored Materials Produced from Bacterial Cellulose and Bacterial Pigments. Materials 2022, 15, 2069. [Google Scholar] [CrossRef]
- Martins, R.; Mouro, C.; Pontes, R.; Nunes, J.; Gouveia, I. Natural Deep Eutectic Solvent Extraction of Bioactive Pigments from Spirulina Platensis and Electrospinning Ability Assessment. Polymer 2023, 15, 1574. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, Y.; Jia, X.; Liu, D.; Huang, X.; Wang, C.; Zhu, Y.; Yue, C.; Deng, S.; Lyu, Y. Lactic Acid Bacteria with a Strong Antioxidant Function Isolated from “Jiangshui”, Pickles, and Feces. Front. Microbiol. 2023, 14, 1163662. [Google Scholar] [CrossRef]

| DES Mixture | Time (h) | T (°C) | Solubility (%) |
|---|---|---|---|
| ChCl:Urea molar ratio 1:2 | 3 | 130 | 42.88 ± 0.83 |
| 1.6 g L-Cys in 20 mL LA | 3 | 130 | 68.83 ± 5.10 |
| Sample | Degree of Confidence | Corresponding Polymer/Solvent |
|---|---|---|
| PVA | 855—Medium | PVA |
| PVA/L-Cys LA DES-WK 95/5 | 825—Medium | PVA |
| PVA/L-Cys LA DES-WK 90/10 | 786—Medium | Ethyl Lactate |
| PVA/L-Cys LA DES-WK 80/20 | 805—Medium | Ethyl Lactate |
| PVA/L-Cys LA DES-WK 70/30 | 752—Medium | Ethyl Lactate |
| L-Cys LA DES-WK | 758—Medium | Ethyl Lactate |
| Tensile Strength (MPa) | Young’s Modulus (MPa) | Elongation at Break (%) | Thickness (mm) | |
|---|---|---|---|---|
| PVA | 8.18 ± 1.25 | 45.04 ± 3.58 | 18.34 ± 3.97 | 0.174 ± 0.02 |
| PVA/ L-Cys:LA DES-WK 95/5 | 4.19 ± 0.96 | 22.24 ± 3.00 | 19.28 ± 8.05 | 0.292 ± 0.01 |
| PVA/ L-Cys:LA DES-WK 90/10 | 4.43 ± 1.14 | 27.01 ± 0.18 | 16.40 ± 5.99 | 0.210 ± 0.02 |
| PVA/ L-Cys:LA DES-WK 80/20 * | - | - | - | - |
| PVA/ L-Cys:LA DES-WK 70/30 * | - | - | - | - |
| S. aureus | K. pneumoniae | |||||
|---|---|---|---|---|---|---|
| Samples | CFU/mL | Growth Reduction (%) | CFU/mL | Growth Reduction (%R) | ||
| PVA | 0 h | 7.25 × 103 | - | 0 h | 3.63 × 104 | - |
| 24 h | 3.02 × 108 | - | 24 h | 2.94 × 108 | - | |
| PVA/L-Cys:LA DES-WK 95/5 | 0.00 × 100 | 100.00% | 0.00 × 100 | 100.00% | ||
| PVA/L-Cys:LA DES-WK 90/10 | 0.00 × 100 | 100.00% | 0.00 × 100 | 100.00% | ||
| PVA/L-Cys:LA DES-WK 80/20 | 0.00 × 100 | 100.00% | 0.00 × 100 | 100.00% | ||
| PVA/L-Cys:LA DES-WK 70/30 | 0.00 × 100 | 100.00% | 0.00 × 100 | 100.00% | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mouro, C.; Martins, R.; Gomes, A.P.; Gouveia, I.C. Upcycling Wool Waste into Keratin Gel-Based Nanofibers Using Deep Eutectic Solvents. Gels 2023, 9, 661. https://doi.org/10.3390/gels9080661
Mouro C, Martins R, Gomes AP, Gouveia IC. Upcycling Wool Waste into Keratin Gel-Based Nanofibers Using Deep Eutectic Solvents. Gels. 2023; 9(8):661. https://doi.org/10.3390/gels9080661
Chicago/Turabian StyleMouro, Cláudia, Rodrigo Martins, Ana P. Gomes, and Isabel C. Gouveia. 2023. "Upcycling Wool Waste into Keratin Gel-Based Nanofibers Using Deep Eutectic Solvents" Gels 9, no. 8: 661. https://doi.org/10.3390/gels9080661
APA StyleMouro, C., Martins, R., Gomes, A. P., & Gouveia, I. C. (2023). Upcycling Wool Waste into Keratin Gel-Based Nanofibers Using Deep Eutectic Solvents. Gels, 9(8), 661. https://doi.org/10.3390/gels9080661











