Collagen-Based Hydrogels for the Eye: A Comprehensive Review
Abstract
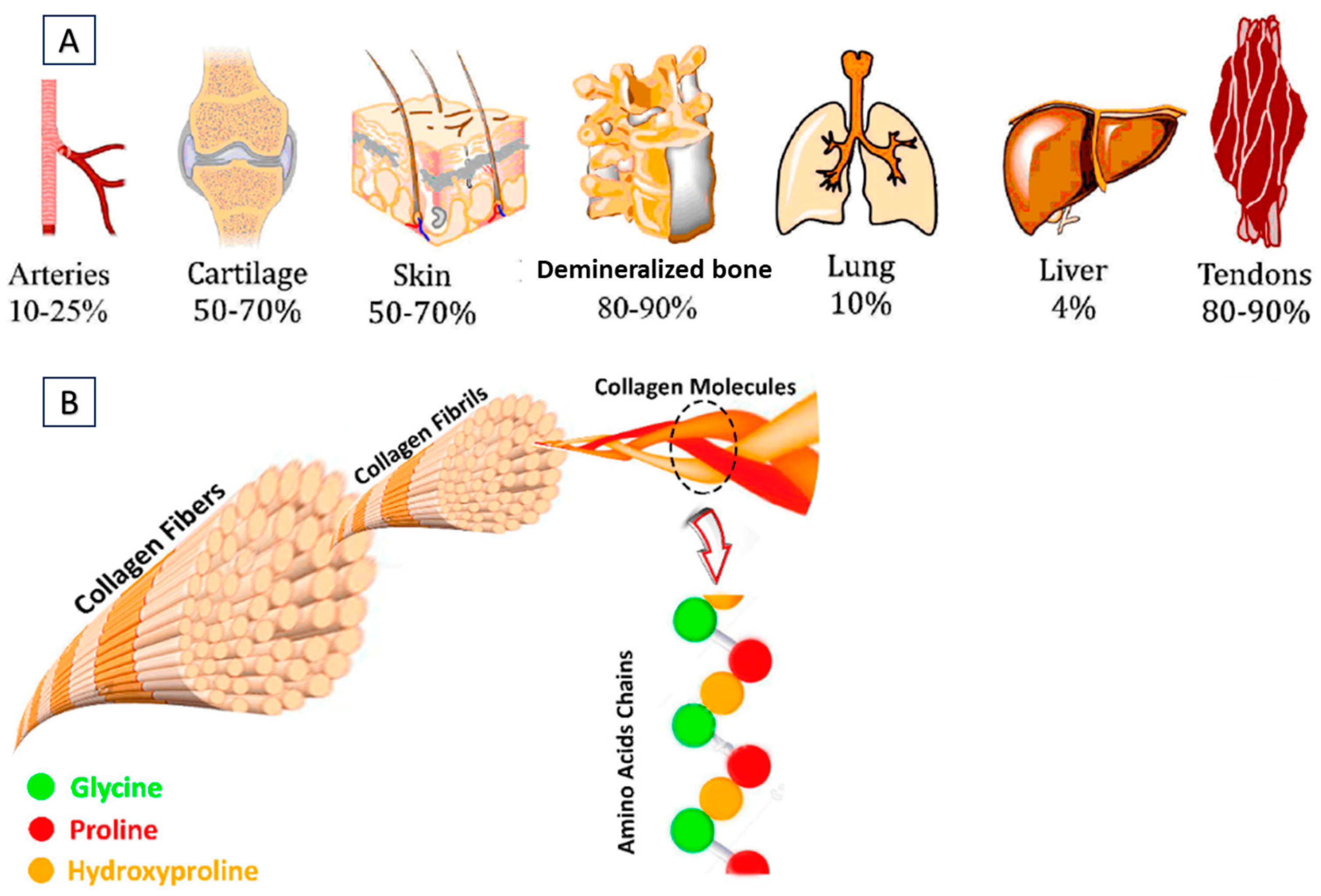
1. Introduction
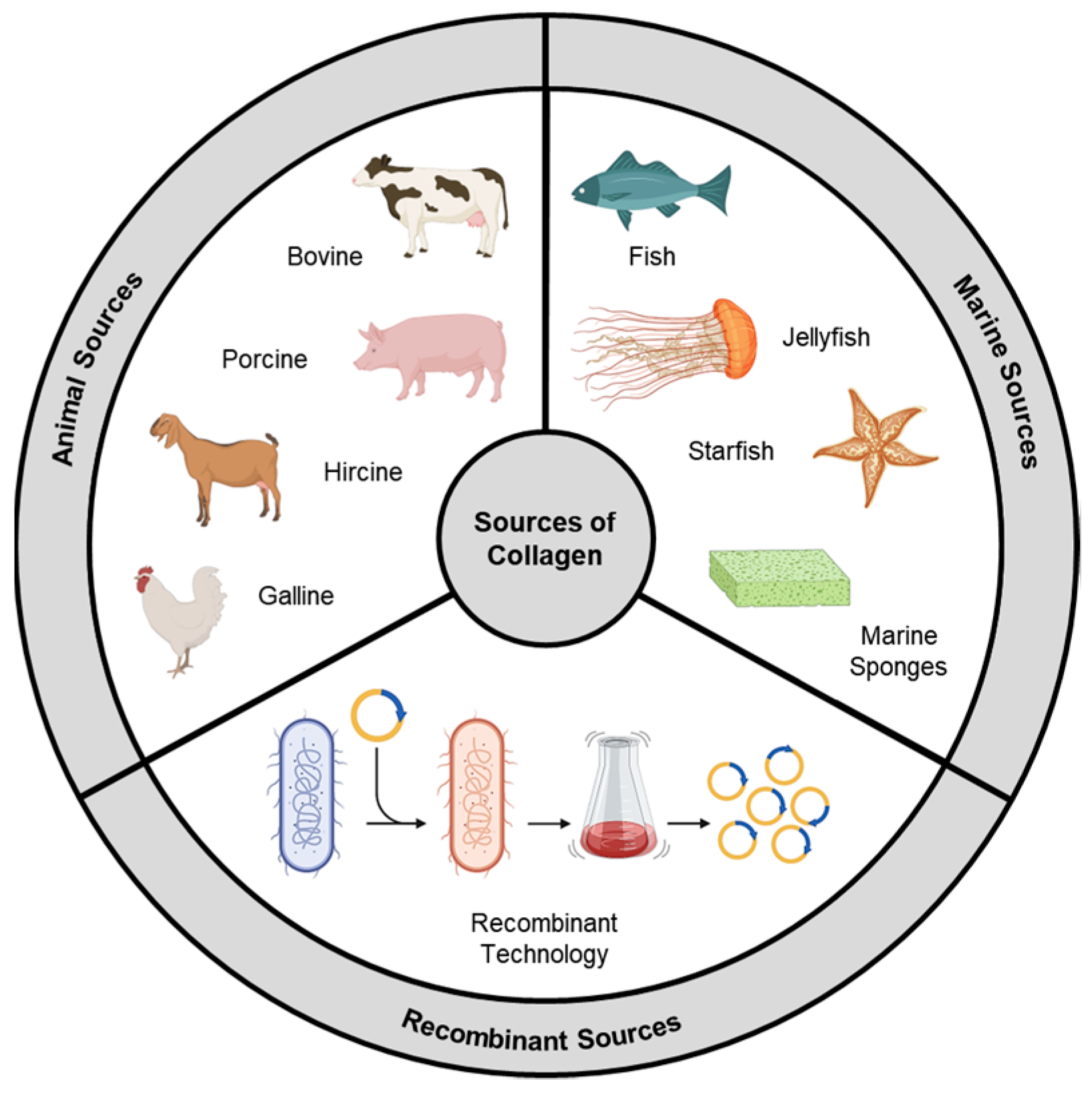
2. Types of Collagens
2.1. Animal Source
2.2. Marine Source
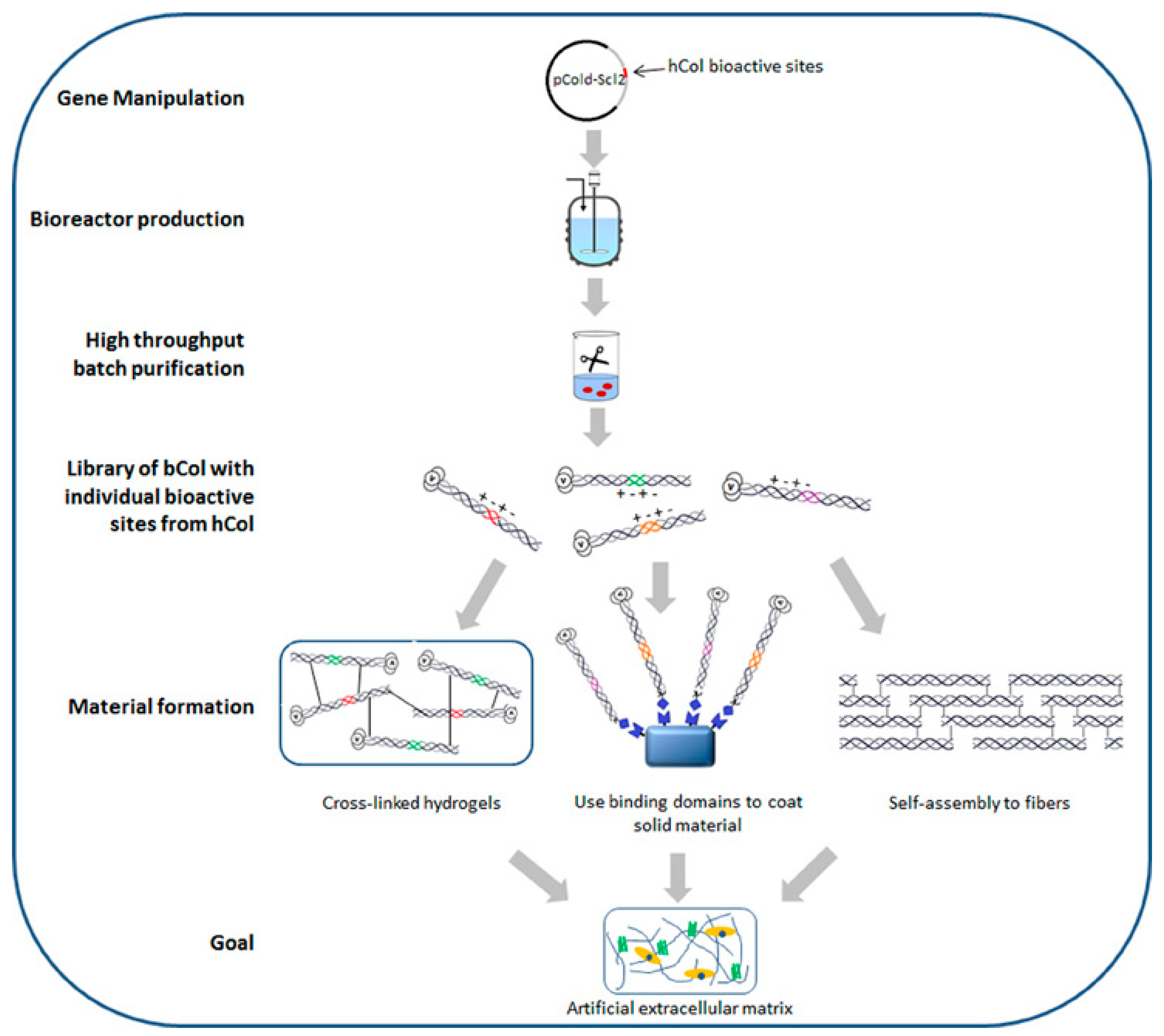
2.3. Recombinant Collagen
3. Isolation and Purification of Collagen
3.1. Pre-Treatment
3.2. Extraction
3.2.1. Acid Extraction
3.2.2. Enzymatic Digestion
3.3. Purification
3.3.1. Dialysis
3.3.2. Precipitation
3.3.3. Chromatography
4. Characterization and Quality Control
4.1. Physical Characterization
4.2. Chemical Characterization
4.3. Biological Characterization
4.4. Microbiological Quality Control
4.5. Stability Studies
4.6. Quality Control and Regulatory Compliance
5. Types of Cross-Linking Methods in Hydrogel Synthesis
5.1. Physically-Cross-Linked Hydrogel
5.1.1. Cross-Linking by Hydrophobic Interactions
5.1.2. Cross-Linking by Ionic/Electrostatic Interactions
5.1.3. Cross-Linking by UV Irradiation and De-Hydrothermal (DHT) Treatment
5.2. Chemically-Cross-Linked Hydrogel
5.2.1. Carbodiimides
5.2.2. Polyethylene Glycol
5.2.3. Genipin
6. Applications of Collagen-Based Hydrogels
6.1. Collagen-Based Hydrogels as Corneal Shields and Collasomes
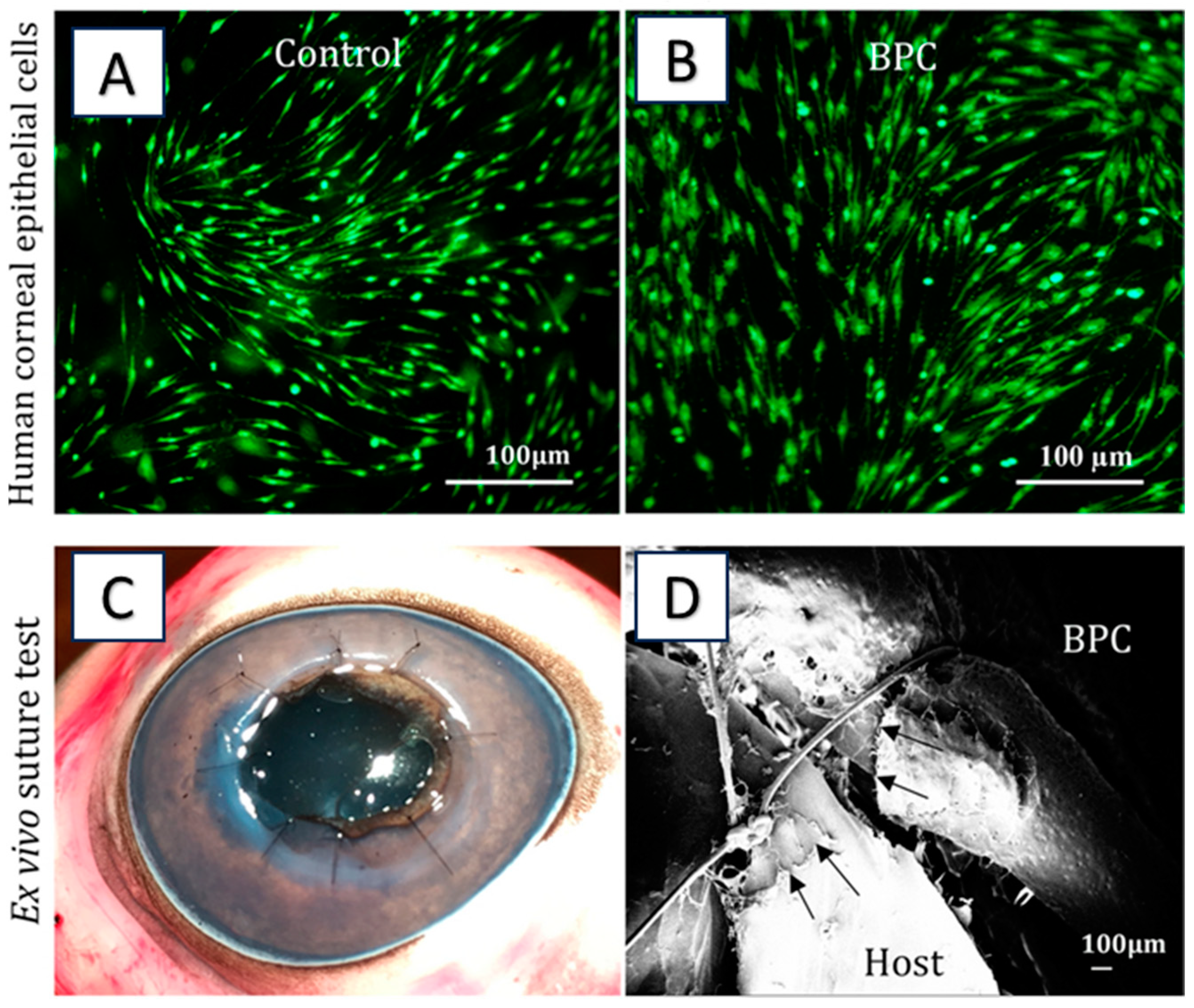
6.2. Collagen-Based Hydrogel for Corneal Regeneration
6.3. Collagen-Based Super Macroporous Cryogels
6.4. Collagen-Based Nanocarrier-Loaded Hydrogels
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-Based Biomaterials for Tissue Engineering Applications. Materials 2010, 3, 1863. [Google Scholar] [CrossRef]
- Lee, C.H.; Singla, A.; Lee, Y. Biomedical Applications of Collagen. Int. J. Pharm. 2001, 221, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Jafari, H.; Lista, A.; Siekapen, M.M.; Ghaffari-Bohlouli, P.; Nie, L.; Alimoradi, H.; Shavandi, A. Fish Collagen: Extraction, Characterization, and Applications for Biomaterials Engineering. Polymers 2020, 12, 2230. [Google Scholar] [CrossRef]
- Song, Y.; Overmass, M.; Fan, J.; Hodge, C.; Sutton, G.; Lovicu, F.J.; You, J. Application of Collagen I and IV in Bioengineering Transparent Ocular Tissues. Front. Surg. 2021, 8, 639500. [Google Scholar] [CrossRef]
- Salave, S.; Rana, D.; Sharma, A.; Bharathi, K.; Gupta, R.; Khode, S.; Benival, D.; Kommineni, N. Polysaccharide Based Implantable Drug Delivery: Development Strategies, Regulatory Requirements, and Future Perspectives. Polysaccharides 2022, 3, 625–654. [Google Scholar] [CrossRef]
- Rawat, G.; Kolhe, S.; Rana, D.; Salave, S.; Benival, D. Exploring the Therapeutic Potential of Cyclosporine for Ophthalmic Indications by Novel Carrier Systems. Crit. Rev. Ther. Drug Carr. Syst. 2023, 40, 1–45. [Google Scholar] [CrossRef] [PubMed]
- Szymczyk-Ziółkowska, P.; Łabowska, M.B.; Detyna, J.; Michalak, I.; Gruber, P. A Review of Fabrication Polymer Scaffolds for Biomedical Applications Using Additive Manufacturing Techniques. Biocybern. Biomed. Eng. 2020, 40, 624–638. [Google Scholar] [CrossRef]
- Łabowska, M.B.; Michalak, I.; Detyna, J. Methods of Extraction, Physicochemical Properties of Alginates and Their Applications in Biomedical Field—A Review. Open Chem. 2019, 17, 738–762. [Google Scholar] [CrossRef]
- Phan, C.M.; Bajgrowicz, M.; McCanna, D.J.; Subbaraman, L.N.; Jones, L. Effects of Antifungal Soaked Silicone Hydrogel Contact Lenses on Candida Albicans in an Agar Eye Model. Eye Contact Lens 2016, 42, 313–317. [Google Scholar] [CrossRef]
- Lynch, C.R.; Kondiah, P.P.D.; Choonara, Y.E.; du Toit, L.C.; Ally, N.; Pillay, V. Hydrogel Biomaterials for Application in Ocular Drug Delivery. Front. Bioeng. Biotechnol. 2020, 8, 228. [Google Scholar] [CrossRef]
- Phan, C.M.; Bajgrowicz, M.; Gao, H.; Subbaraman, L.N.; Jones, L.W. Release of Fluconazole from Contact Lenses Using a Novel In Vitro Eye Model. Optom. Vis. Sci. 2016, 93, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Rana, D.; Salave, S.; Rawat, G.; Benival, D. Recent Trends in Drug Delivery and Emerging Biomedical Applications of Gelatin for Ophthalmic Indications. Macromol. Res. 2022, 30, 687–702. [Google Scholar] [CrossRef]
- Desai, N.; Rana, D.; Salave, S.; Gupta, R.; Patel, P.; Karunakaran, B.; Sharma, A.; Giri, J.; Benival, D.; Kommineni, N. Chitosan: A Potential Biopolymer in Drug Delivery and Biomedical Applications. Pharmaceutics 2023, 15, 1313. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.; Zhao, Y.; Qian, T.; Wang, H.; Li, Z. Functionalized Hydrogels in Ophthalmic Applications: Ocular Inflammation, Corneal Injuries, Vitreous Substitutes and Intravitreal Injection. Mater. Des. 2022, 224, 111277. [Google Scholar] [CrossRef]
- Bajgrowicz-Cieslak, M.; Alqurashi, Y.; Elshereif, M.I.; Yetisen, A.K.; Hassan, M.U.; Butt, H. Optical Glucose Sensors Based on Hexagonally-Packed 2.5-Dimensional Photonic Concavities Imprinted in Phenylboronic Acid Functionalized Hydrogel Films. RSC Adv. 2017, 7, 53916–53924. [Google Scholar] [CrossRef]
- Chen, D.X.B.; Zhu, N.; Ning, L.; Li, M.; Sklenářová, R.; Akla, N.; Latorre, M.J.; Ulrichová, J.; Franková, J. Collagen as a Biomaterial for Skin and Corneal Wound Healing. J. Funct. Biomater. 2022, 13, 249. [Google Scholar] [CrossRef]
- Xeroudaki, M.; Thangavelu, M.; Lennikov, A.; Ratnayake, A.; Bisevac, J.; Petrovski, G.; Fagerholm, P.; Rafat, M.; Lagali, N. A Porous Collagen-Based Hydrogel and Implantation Method for Corneal Stromal Regeneration and Sustained Local Drug Delivery. Sci. Rep. 2020, 10, 16936. [Google Scholar] [CrossRef]
- El-Feky, G.S.; Zayed, G.M.; Elshaier, Y.A.M.M.; Alsharif, F.M. Chitosan-Gelatin Hydrogel Crosslinked With Oxidized Sucrose for the Ocular Delivery of Timolol Maleate. J. Pharm. Sci. 2018, 107, 3098–3104. [Google Scholar] [CrossRef]
- Liu, W.; Griffith, M.; Li, F. Alginate Microsphere-Collagen Composite Hydrogel for Ocular Drug Delivery and Implantation. J. Mater. Sci. Mater. Med. 2008, 19, 3365–3371. [Google Scholar] [CrossRef]
- Noorzai, S.; Verbeek, C.J.R.; Noorzai, S.; Verbeek, C.J.R. Collagen: From Waste to Gold. In Biotechnological Applications of Biomass; InTech Open: London, UK, 2020. [Google Scholar] [CrossRef]
- Wang, H. A Review of the Effects of Collagen Treatment in Clinical Studies. Polymers 2021, 13, 3868. [Google Scholar] [CrossRef] [PubMed]
- Sorushanova, A.; Skoufos, I.; Tzora, A.; Mullen, A.M.; Zeugolis, D.I. The Influence of Animal Species, Gender and Tissue on the Structural, Biophysical, Biochemical and Biological Properties of Collagen Sponges. J. Mater. Sci. Mater. Med. 2021, 32, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.M.; Patrawalla, N.Y.; Kajave, N.S.; Brown, A.B.; Kishore, V. Species-Based Differences in Mechanical Properties, Cytocompatibility, and Printability of Methacrylated Collagen Hydrogels. Biomacromolecules 2022, 23, 5137–5147. [Google Scholar] [CrossRef]
- Zeltz, C.; Gullberg, D. The Integrin-Collagen Connection—A Glue for Tissue Repair? J. Cell Sci. 2016, 129, 653–664. [Google Scholar] [CrossRef]
- Lutfee, T.; Alwan, N.F.; Alsaffar, M.A.; Ghany, M.A.R.A.; Mageed, A.K.; AbdulRazak, A.A. An Overview of the Prospects of Extracting Collagens from Waste Sources and Its Applications. Chem. Pap. 2021, 75, 6025–6033. [Google Scholar] [CrossRef]
- Thorpe, C.T.; Birch, H.L.; Clegg, P.D.; Screen, H.R.C. The Role of the Non-Collagenous Matrix in Tendon Function. Int. J. Exp. Pathol. 2013, 94, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Mokrejs, P.; Langmaier, F.; Mladek, M.; Janacova, D.; Kolomaznik, K.; Vasek, V. Extraction of Collagen and Gelatine from Meat Industry By-Products for Food and Non Food Uses. Waste Manag. Res. 2009, 27, 31–37. [Google Scholar] [CrossRef]
- Ferraro, V.; Gaillard-Martinie, B.; Sayd, T.; Chambon, C.; Anton, M.; Santé-Lhoutellier, V. Collagen Type I from Bovine Bone. Effect of Animal Age, Bone Anatomy and Drying Methodology on Extraction Yield, Self-Assembly, Thermal Behaviour and Electrokinetic Potential. Int. J. Biol. Macromol. 2017, 97, 55–66. [Google Scholar] [CrossRef]
- Sheehy, E.J.; Cunniffe, G.M.; O’Brien, F.J. Collagen-Based Biomaterials for Tissue Regeneration and Repair. In Peptides and Proteins as Biomaterials for Tissue Regeneration and Repair; Elsevier: Amsterdam, The Netherlands, 2018; pp. 127–150. [Google Scholar] [CrossRef]
- Lin, Z.; Nica, C.; Sculean, A.; Asparuhova, M.B. Enhanced Wound Healing Potential of Primary Human Oral Fibroblasts and Periodontal Ligament Cells Cultured on Four Different Porcine-Derived Collagen Matrices. Materials 2020, 13, 3819. [Google Scholar] [CrossRef]
- Rezvani Ghomi, E.; Nourbakhsh, N.; Akbari Kenari, M.; Zare, M.; Ramakrishna, S. Collagen-Based Biomaterials for Biomedical Applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 1986–1999. [Google Scholar] [CrossRef]
- Rittié, L. Type I Collagen Purification from Rat Tail Tendons. Methods Mol. Biol. 2017, 1627, 287–308. [Google Scholar] [CrossRef]
- Eser, B.E.; Gozde, K.I. Marine Collagen. Stud. Nat. Prod. Chem. 2021, 71, 121–139. [Google Scholar] [CrossRef]
- Coppola, D.; Oliviero, M.; Vitale, G.A.; Lauritano, C.; D’Ambra, I.; Iannace, S.; de Pascale, D. Marine Collagen from Alternative and Sustainable Sources: Extraction, Processing and Applications. Mar. Drugs 2020, 18, 214. [Google Scholar] [CrossRef] [PubMed]
- Pal, G.K.; Suresh, P.V. Sustainable Valorisation of Seafood By-Products: Recovery of Collagen and Development of Collagen-Based Novel Functional Food Ingredients. Innov. Food Sci. Emerg. Technol. 2016, 37, 201–215. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, H.; Wang, H.; Li, Y.; Wang, M.; Zhou, J. Biochemical Properties of Skin Collagens Isolated from Black Carp (Mylopharyngodon Piceus). Food Sci. Biotechnol. 2012, 21, 1585–1592. [Google Scholar] [CrossRef]
- Pal, G.K.; Nidheesh, T.; Suresh, P.V. Comparative Study on Characteristics and in Vitro Fibril Formation Ability of Acid and Pepsin Soluble Collagen from the Skin of Catla (Catla Catla) and Rohu (Labeo Rohita). Food Res. Int. 2015, 76, 804–812. [Google Scholar] [CrossRef]
- Krishnan, S.; Sekar, S.; Katheem, M.F.; Krishnakumar, S.; Sastry, T.P. Fish Scale Collagen—A Novel Material for Corneal Tissue Engineering. Artif. Organs 2012, 36, 829–835. [Google Scholar] [CrossRef]
- Salvatore, L.; Gallo, N.; Natali, M.L.; Campa, L.; Lunetti, P.; Madaghiele, M.; Blasi, F.S.; Corallo, A.; Capobianco, L.; Sannino, A. Marine Collagen and Its Derivatives: Versatile and Sustainable Bio-Resources for Healthcare. Mater. Sci. Eng. C 2020, 113, 110963. [Google Scholar] [CrossRef]
- Bao, Z.; Sun, Y.; Rai, K.; Peng, X.; Wang, S.; Nian, R.; Xian, M. The Promising Indicators of the Thermal and Mechanical Properties of Collagen from Bass and Tilapia: Synergistic Effects of Hydroxyproline and Cysteine. Biomater. Sci. 2018, 6, 3042–3052. [Google Scholar] [CrossRef]
- Liu, S.; Lau, C.S.; Liang, K.; Wen, F.; Teoh, S.H. Marine Collagen Scaffolds in Tissue Engineering. Curr. Opin. Biotechnol. 2022, 74, 92–103. [Google Scholar] [CrossRef]
- Wang, T.; Lew, J.; Premkumar, J.; Poh, C.L.; Naing, M.W. Production of Recombinant Collagen: State of the Art and Challenges. Eng. Biol. 2017, 1, 18–23. [Google Scholar] [CrossRef]
- Ramshaw, J.A.M.; Werkmeister, J.A.; Glattauer, V. Recent Progress with Recombinant Collagens Produced in Escherichia Coli. Curr. Opin. Biomed. Eng. 2019, 10, 149–155. [Google Scholar] [CrossRef]
- An, B.; Kaplan, D.L.; Brodsky, B. Engineered Recombinant Bacterial Collagen as an Alternative Collagen-Based Biomaterial for Tissue Engineering. Front. Chem. 2014, 2, 91698. [Google Scholar] [CrossRef] [PubMed]
- Fertala, A. Three Decades of Research on Recombinant Collagens: Reinventing the Wheel or Developing New Biomedical Products? Bioengineering 2020, 7, 155. [Google Scholar] [CrossRef]
- Davison-Kotler, E.; Marshall, W.S.; García-Gareta, E. Sources of Collagen for Biomaterials in Skin Wound Healing. Bioengineering 2019, 6, 56. [Google Scholar] [CrossRef] [PubMed]
- Ilamaran, M.; Janeena, A.; Valappil, S.; Ramudu, K.N.; Shanmugam, G.; Niraikulam, A. A Self-Assembly and Higher Order Structure Forming Triple Helical Protein as a Novel Biomaterial for Cell Proliferation. Biomater. Sci. 2019, 7, 2191–2199. [Google Scholar] [CrossRef]
- Beygmoradi, A.; Homaei, A.; Hemmati, R.; Fernandes, P. Recombinant Protein Expression: Challenges in Production and Folding Related Matters. Int. J. Biol. Macromol. 2023, 233, 123407. [Google Scholar] [CrossRef]
- Gibney, R.; Patterson, J.; Ferraris, E. High-Resolution Bioprinting of Recombinant Human Collagen Type Iii. Polymers 2021, 13, 2973. [Google Scholar] [CrossRef]
- Liu, W.; Lin, H.; Zhao, P.; Xing, L.; Li, J.; Wang, Z.; Ju, S.; Shi, X.L.; Liu, Y.; Deng, G.; et al. A Regulatory Perspective on Recombinant Collagen-Based Medical Devices. Bioact. Mater. 2022, 12, 198–202. [Google Scholar] [CrossRef]
- Amirrah, I.N.; Lokanathan, Y.; Zulkiflee, I.; Wee, M.F.M.R.; Motta, A.; Fauzi, M.B. A Comprehensive Review on Collagen Type I Development of Biomaterials for Tissue Engineering: From Biosynthesis to Bioscaffold. Biomedicines 2022, 10, 2307. [Google Scholar] [CrossRef]
- Pacak, C.A.; MacKay, A.A.; Cowan, D.B. An Improved Method for the Preparation of Type I Collagen from Skin. J. Vis. Exp. 2014, e51011. [Google Scholar] [CrossRef]
- Nurubhasha, R.; Sampath Kumar, N.S.; Thirumalasetti, S.K.; Simhachalam, G.; Dirisala, V.R. Extraction and Characterization of Collagen from the Skin of Pterygoplichthys Pardalis and Its Potential Application in Food Industries. Food Sci. Biotechnol. 2019, 28, 1811–1817. [Google Scholar] [CrossRef]
- Nilsuwan, K.; Chantakun, K.; Chotphruethipong, L.; Benjakul, S. Development of Hydrolysis and Defatting Processes for Production of Lowered Fishy Odor Hydrolyzed Collagen from Fatty Skin of Sockeye Salmon (Oncorhynchus nerka). Foods 2021, 10, 2257. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Taddei, P.; Pondrelli, A.; Zamparini, F.; Prati, C.; Spagnuolo, G. Demineralization, Collagen Modification and Remineralization Degree of Human Dentin after EDTA and Citric Acid Treatments. Materials 2018, 12, 25. [Google Scholar] [CrossRef]
- Tao, M.; Ao, T.; Mao, X.; Yan, X.; Javed, R.; Hou, W.; Wang, Y.; Sun, C.; Lin, S.; Yu, T.; et al. Sterilization and Disinfection Methods for Decellularized Matrix Materials: Review, Consideration and Proposal. Bioact. Mater. 2021, 6, 2927–2945. [Google Scholar] [CrossRef] [PubMed]
- Bhuimbar, M.V.; Bhagwat, P.K.; Dandge, P.B. Extraction and Characterization of Acid Soluble Collagen from Fish Waste: Development of Collagen-Chitosan Blend as Food Packaging Film. J. Environ. Chem. Eng. 2019, 7, 102983. [Google Scholar] [CrossRef]
- Vidal, A.R.; Duarte, L.P.; Schmidt, M.M.; Cansian, R.L.; Fernandes, I.A.; de Oliveira Mello, R.; Demiate, I.M.; Dornelles, R.C.P. Extraction and Characterization of Collagen from Sheep Slaughter By-Products. Waste Manag. 2020, 102, 838–846. [Google Scholar] [CrossRef] [PubMed]
- León-López, A.; Morales-Peñaloza, A.; Martínez-Juárez, V.M.; Vargas-Torres, A.; Zeugolis, D.I.; Aguirre-Álvarez, G. Hydrolyzed Collagen—Sources and Applications. Molecules 2019, 24, 4031. [Google Scholar] [CrossRef]
- Sousa, R.O.; Alves, A.L.; Carvalho, D.N.; Martins, E.; Oliveira, C.; Silva, T.H.; Reis, R.L. Acid and Enzymatic Extraction of Collagen from Atlantic Cod (Gadus Morhua) Swim Bladders Envisaging Health-Related Applications. J. Biomater. Sci. Polym. Ed. 2020, 31, 20–37. [Google Scholar] [CrossRef]
- Vate, N.K.; Undeland, I.; Abdollahi, M. Resource Efficient Collagen Extraction from Common Starfish with the Aid of High Shear Mechanical Homogenization and Ultrasound. Food Chem. 2022, 393, 133426. [Google Scholar] [CrossRef]
- Khan, A.S.; Farooq, I.; Ali, S.; Khan, S.Q.; Hakeem, A.S. Comparative Evaluation of Two Chelating Agents in Collagen Fiber Network Modification over Dentinal Tubules: An in Vitro Analysis. Saudi Pharm. J. 2020, 28, 657–661. [Google Scholar] [CrossRef]
- Mohammadi, R.; Mohammadifar, M.A.; Mortazavian, A.M.; Rouhi, M.; Ghasemi, J.B.; Delshadian, Z. Extraction Optimization of Pepsin-Soluble Collagen from Eggshell Membrane by Response Surface Methodology (RSM). Food Chem. 2016, 190, 186–193. [Google Scholar] [CrossRef]
- Meyer, M. Processing of Collagen Based Biomaterials and the Resulting Materials Properties. Biomed. Eng. 2019, 18, 1–74. [Google Scholar] [CrossRef]
- Andrew, S.M.; Titus, J.A.; Zumstein, L. Dialysis and Concentration of Protein Solutions. Curr. Protoc. Toxicol. 2001, 10, A.3H.1–A.3H.5. [Google Scholar] [CrossRef]
- Wood, G.C. The Precipitation of Collagen Fibers from Solution. Int. Rev. Connect. Tissue Res. 1964, 2, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Kumar Vate, N.; Pawel Strachowski, P.; Undeland, I.; Abdollahi, M. Structural and Functional Properties of Collagen Isolated from Lumpfish and Starfish Using Isoelectric Precipitation vs Salting Out. Food Chem. X 2023, 18, 100646. [Google Scholar] [CrossRef] [PubMed]
- Meredith, S.C.; Kézdy, F.J. The Chromatographic Purification of Native Types I, II, and III Collagens. Biochim. Biophys. Acta Protein Struct. 1981, 668, 357–369. [Google Scholar] [CrossRef]
- Ke, X.; Hu, X.; Li, L.; Yang, X.; Chen, S.; Wu, Y.; Xue, C. A Novel Zinc-Binding Peptide Identified from Tilapia (Oreochromis niloticus) Skin Collagen and Transport Pathway across Caco-2 Monolayers. Food Biosci. 2021, 42, 101127. [Google Scholar] [CrossRef]
- Saallah, S.; Roslan, J.; Julius, F.S.; Saallah, S.; Mohamad Razali, U.H.; Pindi, W.; Sulaiman, M.R.; Pa’ee, K.F.; Mustapa Kamal, S.M. Comparative Study of The Yield and Physicochemical Properties of Collagen from Sea Cucumber (Holothuria scabra), Obtained through Dialysis and the Ultrafiltration Membrane. Molecules 2021, 26, 2564. [Google Scholar] [CrossRef]
- Cleland, T.P.; Voegele, K.; Schweitzer, M.H. Empirical Evaluation of Bone Extraction Protocols. PLoS ONE 2012, 7, e31443. [Google Scholar] [CrossRef]
- Valenzuela-Rojo, R.D.; López-Cervantes, J.; Sánchez-Machado, D.I.; Escárcega-Galaz, A.A.; del Rosario Martínez-Macias, M. Antibacterial, Mechanical and Physical Properties of Collagen-Chitosan Sponges from Aquatic Source. Sustain. Chem. Pharm. 2020, 15, 100218. [Google Scholar] [CrossRef]
- De Melo Oliveira, V.; Assis, C.R.D.; Costa, B.D.A.M.; de Araújo Neri, R.C.; Monte, F.T.D.; da Costa Vasconcelos, H.M.S.; França, R.C.P.; Santos, J.; de Souza Bezerra, R.; Porto, A.L.F. Physical, Biochemical, Densitometric and Spectroscopic Techniques for Characterization Collagen from Alternative Sources: A Review Based on the Sustainable Valorization of Aquatic by-Products. J. Mol. Struct. 2021, 1224, 129023. [Google Scholar] [CrossRef]
- Zeng, S.; Yin, J.; Yang, S.; Zhang, C.; Yang, P.; Wu, W. Structure and Characteristics of Acid and Pepsin-Solubilized Collagens from the Skin of Cobia (Rachycentron canadum). Food Chem. 2012, 135, 1975–1984. [Google Scholar] [CrossRef] [PubMed]
- De Melo Oliveira, V.; de Araújo Neri, R.C.; do Monte, F.T.D.; Roberto, N.A.; Costa, H.M.S.; Assis, C.R.D.; Santos, J.F.; Bezerra, R.S.; Porto, A.L.F. Crosslink-Free Collagen from Cichla Ocellaris: Structural Characterization by FT-IR Spectroscopy and Densitometric Evaluation. J. Mol. Struct. 2019, 1176, 751–758. [Google Scholar] [CrossRef]
- Jeevithan, E.; Bao, B.; Bu, Y.; Zhou, Y.; Zhao, Q.; Wu, W. Type II Collagen and Gelatin from Silvertip Shark (Carcharhinus albimarginatus) Cartilage: Isolation, Purification, Physicochemical and Antioxidant Properties. Mar. Drugs 2014, 12, 3852–3873. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Li, R.; Bai, H.; Zhu, Z.; Zhu, L.; Zhu, C.; Che, Z.; Liu, H.; Wang, J.; et al. Collagen-Based Biomaterials for Bone Tissue Engineering. Mater. Des. 2021, 210, 110049. [Google Scholar] [CrossRef]
- Williams, D.F. Biocompatibility Pathways and Mechanisms for Bioactive Materials: The Bioactivity Zone. Bioact. Mater. 2022, 10, 306–322. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, C.; Fan, D.; Ma, X.; Mi, Y.; Xue, W. A two-step protocol to remove endotoxins from human-like collagen. Sep. Sci. Technol. 2015, 50, 993–1001. [Google Scholar] [CrossRef]
- Ma, R.; Fan, D.D.; Xue, W.J.; Xing, J.Y.; Zhu, C.H.; Ma, X.X. Endotoxin Removal during the Purification Process of Human-like Collagen. Sep. Sci. Technol. 2010, 45, 2400–2405. [Google Scholar] [CrossRef]
- Kirkness, M.W.; Lehmann, K.; Forde, N.R. Mechanics and Structural Stability of the Collagen Triple Helix. Curr. Opin. Chem. Biol. 2019, 53, 98–105. [Google Scholar] [CrossRef]
- Badea, E.; Della Gatta, G.; Usacheva, T. Effects of Temperature and Relative Humidity on Fibrillar Collagen in Parchment: A Micro Differential Scanning Calorimetry (Micro DSC) Study. Polym. Degrad. Stab. 2012, 97, 346–353. [Google Scholar] [CrossRef]
- Ramshaw, J.A.M.; Glattauer, V. Evaluation of the Quality of Collagen Preparations. In Biophysical and Chemical Properties of Collagen Biophysical Applications; IOP Publishing Ltd.: Bristol, UK, 2019. [Google Scholar] [CrossRef]
- O’Grady, J.E.; Bordon, D.M. Global Regulatory Registration Requirements for Collagen-Based Combination Products: Points to Consider. Adv. Drug Deliv. Rev. 2003, 55, 1699–1721. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wang, Z.; Xiao, Y.; Zhang, S.; Wang, J. Advances in Crosslinking Strategies of Biomedical Hydrogels. Biomater. Sci. 2019, 7, 843–855. [Google Scholar] [CrossRef] [PubMed]
- Parhi, R. Cross-Linked Hydrogel for Pharmaceutical Applications: A Review. Adv. Pharm. Bull. 2017, 7, 515–530. [Google Scholar] [CrossRef] [PubMed]
- Klouda, L.; Mikos, A.G. Thermoresponsive Hydrogels in Biomedical Applications. Eur. J. Pharm. Biopharm. 2008, 68, 34–45. [Google Scholar] [CrossRef]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in Pharmaceutical Formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Sarrigiannidis, S.O.; Rey, J.M.; Dobre, O.; González-García, C.; Dalby, M.J.; Salmeron-Sanchez, M. A Tough Act to Follow: Collagen Hydrogel Modifications to Improve Mechanical and Growth Factor Loading Capabilities. Mater. Today Bio 2021, 10, 100098. [Google Scholar] [CrossRef]
- Jiang, Y.-H.; Lou, Y.-Y.; Li, T.-H.; Liu, B.-Z.; Chen, K.; Zhang, D.; Li, T. Cross-Linking Methods of Type I Collagen-Based Scaffolds for Cartilage Tissue Engineering. Am. J. Transl. Res. 2022, 14, 1146–1159. [Google Scholar]
- Weadock, K.; Olson, R.M.; Silver, F.H. Evaluation of Collagen Crosslinking Techniques. Biomater. Med. Devices Artif. Organs 1983, 11, 293–318. [Google Scholar] [CrossRef]
- Hapach, L.A.; Vanderburgh, J.A.; Miller, J.P.; Reinhart-King, C.A. Manipulation of in Vitro Collagen Matrix Architecture for Scaffolds of Improved Physiological Relevance. Phys. Biol. 2015, 12, 61002. [Google Scholar] [CrossRef]
- Merrill, E.W. Poly(Ethylene Oxide) Star Molecules: Synthesis, Characterization, and Applications in Medicine and Biology. J. Biomater. Sci. Polym. Ed. 1994, 5, 1–11. [Google Scholar] [CrossRef]
- Sargeant, T.D.; Desai, A.P.; Banerjee, S.; Agawu, A.; Stopek, J.B. An in Situ Forming Collagen-PEG Hydrogel for Tissue Regeneration. Acta Biomater. 2012, 8, 124–132. [Google Scholar] [CrossRef] [PubMed]
- MacAya, D.; Ng, K.K.; Spector, M. Injectable Collagen-Genipin Gel for the Treatment of Spinal Cord Injury: In Vitro Studies. Adv. Funct. Mater. 2011, 21, 4788–4797. [Google Scholar] [CrossRef]
- Gorantla, S.; Rapalli, V.K.; Waghule, T.; Singh, P.P.; Dubey, S.K.; Saha, R.N.; Singhvi, G. Nanocarriers for Ocular Drug Delivery: Current Status and Translational Opportunity. RSC Adv. 2020, 10, 27835–27855. [Google Scholar] [CrossRef] [PubMed]
- Gorantla, S.; Waghule, T.; Rapalli, V.K.; Singh, P.P.; Dubey, S.K.; Saha, R.N.; Singhvi, G. Advanced Hydrogels Based Drug Delivery Systems for Ophthalmic Delivery. Recent Pat. Drug Deliv. Formul. 2020, 13, 291–300. [Google Scholar] [CrossRef]
- Collagen Corneal Shields. Available online: https://chicago.medicine.uic.edu/departments/academic-departments/ophthalmology-visual-sciences/our-department/resources-center/eye-facts/collagen-corneal-shields/ (accessed on 31 July 2023).
- Tannebaum, S. Svyatoslav Fyodorov, M.D.: Innovative eye surgeon. J. Am. Optom. Assoc. 1995, 66, 652–654. [Google Scholar]
- Bio-Cor Collagen Corneal Shield. Available online: https://fda.report/PMN/K944872 (accessed on 31 July 2023).
- Robin, J.D.; Keys, C.L.; Kaminski, L.A.; Viana, M.A.G. The Effect of Collagen Shields on Rabbit Corneal Reepithelialization after Chemical Debridement. Investig. Ophthalmol. Vis. Sci. 1990, 31, 1294–1300. [Google Scholar]
- Angella, G.J.; Sherwood, M.B.; Balasubramanian, L.; Doyle, J.W.; Smith, M.F.; van Setten, G.; Goldstein, M.; Schultz, G.S. Enhanced short-term plasmid transfection of filtration surgery tissues. Investig. Ophthalmol. Vis. Sci. 2000, 41, 4158–4162. [Google Scholar]
- Kaufman, H.E.; Steinemann, T.L.; Lehman, E.; Thompson, H.W.; Varnell, E.D.; Jacob-Labarre, J.T.; Gebhardt, B.M. Collagen-Based Drug Delivery and Artificial Tears. J. Ocul. Pharmacol. Ther. 2009, 10, 17–27. [Google Scholar] [CrossRef]
- Mishima, S. Clinical pharmacokinetics of the eye. Proctor lecture. Investig. Ophthalmol. Vis. Sci. 1981, 21, 504–541. [Google Scholar]
- Meek, K.M. Corneal Collagen—Its Role in Maintaining Corneal Shape and Transparency. Biophys. Rev. 2009, 1, 83. [Google Scholar] [CrossRef]
- Osidak, E.O.; Andreev, A.Y.; Avetisov, S.E.; Voronin, G.V.; Surnina, Z.V.; Zhuravleva, A.V.; Grigoriev, T.E.; Krasheninnikov, S.V.; Sukhinich, K.K.; Zayratyants, O.V.; et al. Corneal Stroma Regeneration with Collagen-Based Hydrogel as an Artificial Stroma Equivalent: A Comprehensive In Vivo Study. Polymers 2022, 14, 4017. [Google Scholar] [CrossRef] [PubMed]
- Yellepeddi, V.K.; Sheshala, R.; McMillan, H.; Gujral, C.; Jones, D.; Raghu Raj Singh, T. Punctal Plug: A Medical Device to Treat Dry Eye Syndrome and for Sustained Drug Delivery to the Eye. Drug Discov. Today 2015, 20, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Saraswathy, K.; Agarwal, G.; Srivastava, A. Hyaluronic Acid Microneedles-Laden Collagen Cryogel Plugs for Ocular Drug Delivery. J. Appl. Polym. Sci. 2020, 137, 49285. [Google Scholar] [CrossRef]
- Lengyel, M.; Kállai-Szabó, N.; Antal, V.; Laki, A.J.; Antal, I. Microparticles, Microspheres, and Microcapsules for Advanced Drug Delivery. Sci. Pharm. 2019, 87, 20. [Google Scholar] [CrossRef]
- Varela-Fernández, R.; Díaz-Tomé, V.; Luaces-Rodríguez, A.; Conde-Penedo, A.; García-Otero, X.; Luzardo-álvarez, A.; Fernández-Ferreiro, A.; Otero-Espinar, F.J. Drug Delivery to the Posterior Segment of the Eye: Biopharmaceutic and Pharmacokinetic Considerations. Pharmaceutics 2020, 12, 269. [Google Scholar] [CrossRef]
- Jiang, J.; Pi, J.; Cai, J. The Advancing of Zinc Oxide Nanoparticles for Biomedical Applications. Bioinorg. Chem. Appl. 2018, 2018, 1062562. [Google Scholar] [CrossRef]
- Agban, Y.; Mugisho, O.O.; Thakur, S.S.; Rupenthal, I.D. Characterization of Zinc Oxide Nanoparticle Cross-Linked Collagen Hydrogels. Gels 2020, 6, 37. [Google Scholar] [CrossRef]
- Gupta, R.; Salave, S.; Rana, D.; Karunakaran, B.; Butreddy, A.; Benival, D.; Kommineni, N.; Mousavifar, L.; Gupta, R.; Salave, S.; et al. Versatility of Liposomes for Antisense Oligonucleotide Delivery: A Special Focus on Various Therapeutic Areas. Pharmaceutics 2023, 15, 1435. [Google Scholar] [CrossRef]
- Rana, D.; Salave, S.; Jain, S.; Shah, R.; Benival, D. Systematic Development and Optimization of Teriparatide-Loaded Nanoliposomes Employing Quality by Design Approach for Osteoporosis. J. Pharm. Innov. 2022, 1–15. [Google Scholar] [CrossRef]
- Salave, S.; Rana, D.; Pardhe, R.; Bule, P.; Benival, D. Unravelling Micro and Nano Vesicular System in Intranasal Drug Delivery for Epilepsy. Pharm. Nanotechnol. 2022, 10, 182–193. [Google Scholar] [CrossRef]
- Salave, S.; Rana, D.; Benival, D. Encapsulation of Anabolic Peptide in Lipid Nano Vesicles for Osteoporosis. Curr. Protein Pept. Sci. 2022, 23, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Salave, S.; Jain, S.; Shah, R.; Benival, D. Quantification of Anti-Osteoporotic Anabolic Peptide in Stealth Lipid Nanovesicles Through Validated RP-HPLC Method. J. AOAC Int. 2022, 106, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Salave, S.; Rana, D.; Kumar, H.; Kommineni, N.; Benival, D. Anabolic Peptide-Enriched Stealth Nanoliposomes for Effective Anti-Osteoporotic Therapy. Pharmaceutics 2022, 14, 2417. [Google Scholar] [CrossRef] [PubMed]
- Salave, S.; Rana, D.; Benival, D. Dual Targeting Anti-Osteoporotic Therapy Through Potential Nanotherapeutic Approaches. Pharm. Nanotechnol. 2022, 10, 384–392. [Google Scholar] [CrossRef]
- Karunakaran, B.; Gupta, R.; Patel, P.; Salave, S.; Sharma, A.; Desai, D.; Benival, D.; Kommineni, N. Emerging Trends in Lipid-Based Vaccine Delivery: A Special Focus on Developmental Strategies, Fabrication Methods, and Applications. Vaccines 2023, 11, 661. [Google Scholar] [CrossRef]
- Salave, S.; Shinde, S.D.; Rana, D.; Sahu, B.; Kumar, H.; Patel, R.; Benival, D.; Kommineni, N. Peptide Engraftment on PEGylated Nanoliposomes for Bone Specific Delivery of PTH (1-34) in Osteoporosis. Pharmaceutics 2023, 15, 608. [Google Scholar] [CrossRef]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef]
- Desai, N.; Rana, D.; Pande, S.; Salave, S.; Giri, J.; Benival, D.; Kommineni, N. “Bioinspired” Membrane-Coated Nanosystems in Cancer Theranostics: A Comprehensive Review. Pharmaceutics 2023, 15, 1677. [Google Scholar] [CrossRef]
- Chang, M.C.; Kuo, Y.J.; Hung, K.H.; Peng, C.L.; Chen, K.Y.; Yeh, L.K. Liposomal Dexamethasone–Moxifloxacin Nanoparticle Combinations with Collagen/Gelatin/Alginate Hydrogel for Corneal Infection Treatment and Wound Healing. Biomed. Mater. 2020, 15, 055022. [Google Scholar] [CrossRef]


| Expression System | Transduced Gene | Expressed Collagen | |
|---|---|---|---|
| Prokaryote | Escherichia coli | COL1A1 | Type I |
| Escherichia coli | COL3A1 | Type III | |
| Yeast | Pichia pastoris | COL3A1, PH4A/B | Type III |
| Saccharomyces cerevisiae | COL3A1, PH4A/B | Type III | |
| Human cell lines | HT1080 fibrosarcoma cells | COL1A1 | Type I |
| HEK 293 kidney epithelial cells | COL5A1 | Type V | |
| Mammal | Mus Musculus (Mammary gland) | COL1A1 | Type I |
| Insect | Spodoptera frugiperda (Sf9 cells) | COL3A1 | Type III |
| Bombyx mori | COL1A1 | Gly-X-Y collage-like homodimer | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rana, D.; Desai, N.; Salave, S.; Karunakaran, B.; Giri, J.; Benival, D.; Gorantla, S.; Kommineni, N. Collagen-Based Hydrogels for the Eye: A Comprehensive Review. Gels 2023, 9, 643. https://doi.org/10.3390/gels9080643
Rana D, Desai N, Salave S, Karunakaran B, Giri J, Benival D, Gorantla S, Kommineni N. Collagen-Based Hydrogels for the Eye: A Comprehensive Review. Gels. 2023; 9(8):643. https://doi.org/10.3390/gels9080643
Chicago/Turabian StyleRana, Dhwani, Nimeet Desai, Sagar Salave, Bharathi Karunakaran, Jyotsnendu Giri, Derajram Benival, Srividya Gorantla, and Nagavendra Kommineni. 2023. "Collagen-Based Hydrogels for the Eye: A Comprehensive Review" Gels 9, no. 8: 643. https://doi.org/10.3390/gels9080643
APA StyleRana, D., Desai, N., Salave, S., Karunakaran, B., Giri, J., Benival, D., Gorantla, S., & Kommineni, N. (2023). Collagen-Based Hydrogels for the Eye: A Comprehensive Review. Gels, 9(8), 643. https://doi.org/10.3390/gels9080643









