Cellulose-Based Metallogels—Part 2: Physico-Chemical Properties and Biological Stability
Abstract
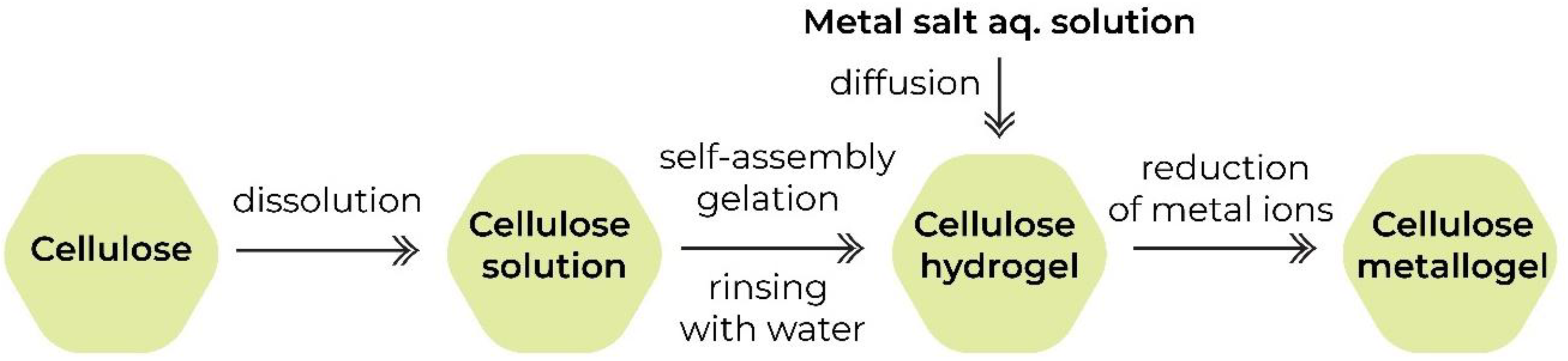
1. Introduction
- Wood pulp is a common and abundant source of cellulose. It can be obtained from different types of trees and processed to extract cellulose fibers, which can then be used to produce cellulose [23].
- Plant-based fibers come from various plants, such as cotton, bamboo, jute, hemp, and flax, and contain up to 99% of cellulose in their cell walls [24]. Fibers from these plants can be processed to extract cellulose.
- Agricultural waste contains large quantities of annually renewed cellulose that can be isolated and extracted from rice husks, sugarcane bagasse, wheat straw [25], corn stalks [26], rapeseed stalks [27], and others. These waste materials can be processed for various applications, contributing to the sustainable utilization of agricultural by-products.
- Bacteria and microorganisms: cellulose-producing bacteria and microorganisms, for instance, Acetobacter xylinum, can be used to produce cellulose through fermentation processes [33].
- Waste paper is another significant source of cellulose [34]. However, compared to other sources, waste paper may require additional extraction steps due to its heterogeneous composition and impurities resulting from the papermaking process. The recycling of waste paper involves deinking and pulping processes to remove inks, coatings, and other contaminants before cellulose fibers can be obtained.
2. Physical Properties
2.1. Appearance
2.2. Swelling Ability
2.3. Porosity
2.4. Mechanical Properties
2.5. Thermal Stability
3. Chemical Properties
3.1. Chemical Composition
3.2. Supramolecular Structure
4. Biological Properties
4.1. Biodegradation
4.2. Cytocompatibility
4.3. Biocidal Properties
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stan, L.; Malutan, T.; Volf, I.; Popa, M.; Tincu, C.E.; Stan, C.S. Photoluminescent Polymer Aerogels with R, G and B Emission. Int. J. Mol. Sci. 2022, 23, 16004. [Google Scholar] [CrossRef]
- Tofanica, B.-M.; Belosinschi, D.; Volf, I. Gels, Aerogels and Hydrogels: A Challenge for the Cellulose-Based Product Industries. Gels 2022, 8, 497. [Google Scholar] [CrossRef]
- Wu, H.; Zheng, J.; Kjøniksen, A.-L.; Wang, W.; Zhang, Y.; Ma, J. Metallogels: Availability, Applicability, and Advanceability. Adv. Mater. 2019, 31, 1806204. [Google Scholar] [CrossRef] [PubMed]
- Esposito, C.L.; Kirilov, P.; Roullin, V.G. Organogels, Promising Drug Delivery Systems: An Update of State-of-the-Art and Recent Applications. J. Control. Release 2018, 271, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Long, L.-Y.; Weng, Y.-X.; Wang, Y.-Z. Cellulose Aerogels: Synthesis, Applications, and Prospects. Polymers 2018, 10, 623. [Google Scholar] [CrossRef] [PubMed]
- De France, K.J.; Hoare, T.; Cranston, E.D. Review of Hydrogels and Aerogels Containing Nanocellulose. Chem. Mater. 2017, 29, 4609–4631. [Google Scholar] [CrossRef]
- Dastidar, P.; Ganguly, S.; Sarkar, K. Metallogels from Coordination Complexes, Organometallic, and Coordination Polymers. Chem.–Asian J. 2016, 11, 2484–2498. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Yang, X.; Cranston, E.D.; Zhu, S. Flexible and Porous Nanocellulose Aerogels with High Loadings of Metal–Organic-Framework Particles for Separations Applications. Adv. Mater. 2016, 28, 7652–7657. [Google Scholar] [CrossRef] [PubMed]
- Ungureanu, E.; Trofin, A.; Trincă, L.C.; Ariton, A.M.; Ungureanu, O.C.; Fortună, M.E.; Jităreanu, C.D.; Popa, V.I. Studies on Kinetics and Adsorption Equilibrium of Lead and Zinc Ions from Aqueous Solutions on Sarkanda Grass Lignin. Cell. Chem. Technol. 2021, 55, 939–948. [Google Scholar] [CrossRef]
- Ungureanu, E.; Jităreanu, C.D.; Trofin, A.; Fortună, M.E.; Ungureanu, O.C.; Ariton, A.M.; Trincă, L.C.; Brezuleanu, S.; Popa, V.I. Use of Sarkanda Grass Lignin as a Possible Adsorbent for As (III) from Aqueous Solutions—Kinetic and Equilibrium Studies. Cell. Chem. Technol 2022, 56, 681–689. [Google Scholar] [CrossRef]
- Shou, W.; Yang, S.-T.; Wang, Y.-L.; Guo, L.-H. Preparation of Noble Metal Nanoparticles and Hydrogel Composite Materials and Their Application in Analytical Chemistry. Chin. J. Anal. Chem. 2021, 49, 676–685. [Google Scholar] [CrossRef]
- Ungureanu, E.; Ungureanu, O.; Iacob, V.; Ulea, E.; Popa, V.I. On the Biocide Properties of Some Products Based on Natural Aromatic Compounds. Cell. Chem. Technol. 2008, 42, 381–386. [Google Scholar]
- Ungureanu, E.; Căpraru, A.-M.; Ungureanu, O.; Jităreanu, C.D.; Iacob, V.; Ulea, E.; Popa, V.I. Ecologycal Biocide Systems Based on Unmodified and Epoxydation Lignins, Furan Resin and Copper. Cell. Chem. Technol. 2012, 46, 599–603. [Google Scholar]
- Ariton, A.-M.; Greanga, S.; Trinca, L.M.; Bors, S.I.; Ungureanu, E.; Malutan, T.; Popa, V. Valorization of Lignin Modified by Hydroxymethylation to Ensure Birch Veneer Bioprotection. Cellul. Chem. Technol. 2015, 49, 765–774. [Google Scholar]
- Ungureanu, E.; Trofin, A.-E.; Ariton, A.-M.; Jitareanu, D.C.; Ungureanu, O.; Gilca, V.; Bors, S.-I.; Popa, V.I. Applications of Epoxidated Lignins for Bioprotection of Lignocellulosic Materials. Methods 2016, 15, 17. [Google Scholar]
- Iftime, M.M.; Ailiesei, G.L.; Ungureanu, E.; Marin, L. Designing Chitosan Based Eco-Friendly Multifunctional Soil Conditioner Systems with Urea Controlled Release and Water Retention. Carbohydr. Polym. 2019, 223, 115040. [Google Scholar] [CrossRef]
- Fortună, M.E.; Ungureanu, E.; Jitareanu, C.D. Calcium Carbonate–Carboxymethyl Chitosan Hybrid Materials. Materials 2021, 14, 3336. [Google Scholar] [CrossRef] [PubMed]
- Zanchetta, E.; Damergi, E.; Patel, B.; Borgmeyer, T.; Pick, H.; Pulgarin, A.; Ludwig, C. Algal Cellulose, Production and Potential Use in Plastics: Challenges and Opportunities. Algal Res. 2021, 56, 102288. [Google Scholar] [CrossRef]
- Ungureanu, E.; Fortună, M.E.; Țopa, D.C.; Lobiuc, A.; Ungureanu, O.C.; Jităreanu, D.C. Design of Functional Polymer Systems to Optimize the Filler Retention in Obtaining Cellulosic Substrates with Improved Properties. Materials 2023, 16, 1904. [Google Scholar] [CrossRef]
- Magalhães, M.I.; Almeida, A.P.C. Nature-Inspired Cellulose-Based Active Materials: From 2D to 4D. Appl. Biosci. 2023, 2, 94–114. [Google Scholar] [CrossRef]
- Popa, V.I.; Volf, I. Biomass as Renewable Raw Material to Obtain Bioproducts of High-Tech Value; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Simionescu, C.I.; Rusan, V.; Popa, V.I. Options Concerning Phytomass Valorification. Cellul. Chem. Technol. 1987, 21, 3–16. [Google Scholar]
- Fengel, D.; Wegener, G. Wood: Chemistry, Ultrastructure, Reactions; De Gruyter: Berlin, Germany; New York, NY, USA, 1983; ISBN 978-3-11-083965-4. [Google Scholar]
- Gavrilescu, D.; Tofănică, B.M.; Puițel, A.C.; Petrea, P. Sustainable Use of Vegetal Fibers in Composite Materials. Sources Veg. Fibers. Environ. Eng. Manag. J. 2009, 8, 429–438. [Google Scholar] [CrossRef]
- Puițel, A.C.; Moisei, N.; Tofănică, B.M.; Gavrilescu, D. Turning Wheat Straw in a Sustainable Raw Material for Paper Industry. Environ. Eng. Manag. J. 2017, 16, 1027–1032. [Google Scholar] [CrossRef]
- Chesca, A.M.; Nicu, R.; Tofănică, B.M.; Puiţel, A.C.; Vlase, R.; Gavrilescu, D. Pulping of Corn Stalks—Assessment in Bio-Based Packaging Materials. Cellul. Chem. Technol. 2018, 52, 645–653. [Google Scholar]
- Tofanica, B.M.; Puitel, A.C.; Gavrilescu, D. Environmental Friendly Pulping and Bleaching of Rapeseed Stalk Fibers. Environ. Eng. Manag. J. 2012, 11, 681–686. [Google Scholar] [CrossRef]
- Ungureanu, G.; Filote, C.; Santos, S.C.R.; Boaventura, R.A.R.; Volf, I.; Botelho, C.M.S. Antimony Oxyanions Uptake by Green Marine Macroalgae. J. Environ. Chem. Eng. 2016, 4, 3441–3450. [Google Scholar] [CrossRef]
- Filote, C.; Santos, S.C.R.; Popa, V.I.; Botelho, C.M.S.; Volf, I. Biorefinery of Marine Macroalgae into High-Tech Bioproducts: A Review. Environ. Chem Lett 2021, 19, 969–1000. [Google Scholar] [CrossRef]
- Popper, Z.A.; Michel, G.; Hervé, C.; Domozych, D.S.; Willats, W.G.T.; Tuohy, M.G.; Kloareg, B.; Stengel, D.B. Evolution and Diversity of Plant Cell Walls: From Algae to Flowering Plants. Annu. Rev. Plant Biol. 2011, 62, 567–590. [Google Scholar] [CrossRef]
- Domozych, D.; Ciancia, M.; Fangel, J.; Mikkelsen, M.; Ulvskov, P.; Willats, W. The Cell Walls of Green Algae: A Journey through Evolution and Diversity. Front. Plant Sci. 2012, 3, 82. [Google Scholar] [CrossRef]
- Borowitzka, M.A.; Beardall, J.; Raven, J.A. (Eds.) The Physiology of Microalgae; Developments in Applied Phycology; Springer International Publishing: Cham, Switzerland, 2016; Volume 6, ISBN 978-3-319-24943-8. [Google Scholar]
- Blanco, A.; Monte, M.C.; Campano, C.; Balea, A.; Merayo, N.; Negro, C. Chapter 5—Nanocellulose for Industrial Use: Cellulose Nanofibers (CNF), Cellulose Nanocrystals (CNC), and Bacterial Cellulose (BC). In Handbook of Nanomaterials for Industrial Applications; Mustansar Hussain, C., Ed.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2018; pp. 74–126. ISBN 978-0-12-813351-4. [Google Scholar]
- Mikhailidi, A.M.; Kotel’nikova, N.Y. Functional Materials from Paper Wastes: II–Cellulose Hydrogels with High Water Retention Capacity Obtained from Solutions of Waste Paper in DMAc/LiCl. Russ. J. Bioorganic Chem. 2022, 48, 1486–1497. [Google Scholar] [CrossRef]
- Nasir, M.; Aziz, M.A.; Zubair, M.; Manzar, M.S.; Ashraf, N.; Mu’azu, N.D.; Al-Harthi, M.A. Recent Review on Synthesis, Evaluation, and SWOT Analysis of Nanostructured Cellulose in Construction Applications. J. Build. Eng. 2022, 46, 103747. [Google Scholar] [CrossRef]
- Huang, K.; Wang, Y. Recent Applications of Regenerated Cellulose Films and Hydrogels in Food Packaging. Curr. Opin. Food Sci. 2022, 43, 7–17. [Google Scholar] [CrossRef]
- Felgueiras, C.; Azoia, N.G.; Gonçalves, C.; Gama, M.; Dourado, F. Trends on the Cellulose-Based Textiles: Raw Materials and Technologies. Front. Bioeng. Biotechnol. 2021, 9, 608826. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.; Yang, X.; Liu, H. Temperature-Responsive Hydrogel Prepared from Carboxymethyl Cellulose-Stabilized N-Vinylcaprolactam with Potential for Fertilizer Delivery. Carbohydr. Polym. 2023, 313, 120875. [Google Scholar] [CrossRef]
- Mu, R.; Hong, X.; Ni, Y.; Li, Y.; Pang, J.; Wang, Q.; Xiao, J.; Zheng, Y. Recent Trends and Applications of Cellulose Nanocrystals in Food Industry. Trends Food Sci. Technol. 2019, 93, 136–144. [Google Scholar] [CrossRef]
- Patidar, A.K.; Sharma, A.; Joshi, D. Formulation of Cellulose Using Groundnut Husk as an Environment-Friendly Fluid Loss Retarder Additive and Rheological Modifier Comparable to PAC for WBM. J. Pet. Explor. Prod. Technol. 2020, 10, 3449–3466. [Google Scholar] [CrossRef]
- Peng, B.; Yao, Z.; Wang, X.; Crombeen, M.; Sweeney, D.G.; Tam, K.C. Cellulose-Based Materials in Wastewater Treatment of Petroleum Industry. Green Energy Environ. 2020, 5, 37–49. [Google Scholar] [CrossRef]
- Ciolacu, D.E.; Suflet, D.M. Cellulose-Based Hydrogels for Medical/Pharmaceutical Applications. In Biomass as Renewable Raw Material to Obtain Bioproducts of High-Tech Value; Popa, V., Volf, I., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 401–439. ISBN 978-0-444-63774-1. [Google Scholar]
- Meftahi, A.; Samyn, P.; Geravand, S.A.; Khajavi, R.; Alibkhshi, S.; Bechelany, M.; Barhoum, A. Nanocelluloses as Skin Biocompatible Materials for Skincare, Cosmetics, and Healthcare: Formulations, Regulations, and Emerging Applications. Carbohydr. Polym. 2022, 278, 118956. [Google Scholar] [CrossRef] [PubMed]
- Bashari, A.; Rouhani Shirvan, A.; Shakeri, M. Cellulose-Based Hydrogels for Personal Care Products. Polym. Adv. Technol. 2018, 29, 2853–2867. [Google Scholar] [CrossRef]
- Belosinschi, D.; Tofanica, B.-M. A New Bio-Material with 3D Lightweight Network for Energy and Advanced Applications. Cellulose 2018, 25, 897–902. [Google Scholar] [CrossRef]
- Kundu, R.; Mahada, P.; Chhirang, B.; Das, B. Cellulose Hydrogels: Green and Sustainable Soft Biomaterials. Curr. Res. Green Sustain. Chem. 2022, 5, 100252. [Google Scholar] [CrossRef]
- Thivya, P.; Akalya, S.; Sinija, V.R. A Comprehensive Review on Cellulose-Based Hydrogel and Its Potential Application in the Food Industry. Appl. Food Res. 2022, 2, 100161. [Google Scholar] [CrossRef]
- Zare-Akbari, Z.; Farhadnejad, H.; Furughi-Nia, B.; Abedin, S.; Yadollahi, M.; Khorsand-Ghayeni, M. PH-Sensitive Bionanocomposite Hydrogel Beads Based on Carboxymethyl Cellulose/ZnO Nanoparticle as Drug Carrier. Int. J. Biol. Macromol. 2016, 93, 1317–1327. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.S.; Kong, B.J.; Park, S.N. Physicochemical Properties of PH-Sensitive Hydrogels Based on Hydroxyethyl Cellulose-Hyaluronic Acid and for Applications as Transdermal Delivery Systems for Skin Lesions. Eur. J. Pharm. Biopharm. 2015, 92, 146–154. [Google Scholar] [CrossRef]
- Park, H.; Kim, M.H.; Yoon, Y.I.; Park, W.H. One-Pot Synthesis of Injectable Methylcellulose Hydrogel Containing Calcium Phosphate Nanoparticles. Carbohydr. Polym. 2017, 157, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Almuslem, A.S.; Alnaim, N.; Ibrahim, S.S.; Ibrahim, M.A. Green Synthesis and Characteristics of Cellulose Nanocrystal/Poly Acrylic Acid Nanocomposite Thin Film for Organic Dye Adsorption during Water Treatment. Polymers 2023, 15, 2154. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, V.; Thakur, A. Carboxymethyl Cellulose-Polyvinyl Alcohol Based Materials: A Review. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Kono, H. Characterization and Properties of Carboxymethyl Cellulose Hydrogels Crosslinked by Polyethylene Glycol. Carbohydr. Polym. 2014, 106, 84–93. [Google Scholar] [CrossRef]
- Ambreen, J.; Al-Harbi, F.F.; Sakhawat, H.; Ajmal, M.; Naeem, H.; Farooqi, Z.H.; Batool, N.; Siddiq, M. Fabrication of Poly (N-Vinylcaprolactam-Co-Acrylic Acid)-Silver Nanoparticles Composite Microgel with Substantial Potential of Hydrogen Peroxide Sensing and Catalyzing the Reduction of Water Pollutants. J. Mol. Liq. 2022, 355, 118931. [Google Scholar] [CrossRef]
- Fu, L.-H.; Qi, C.; Ma, M.-G.; Wan, P. Multifunctional Cellulose-Based Hydrogels for Biomedical Applications. J. Mater. Chem. B 2019, 7, 1541–1562. [Google Scholar] [CrossRef]
- Fu, H.; Wang, B.; Li, J.; Xu, J.; Li, J.; Zeng, J.; Gao, W.; Chen, K. A Self-Healing, Recyclable and Conductive Gelatin/Nanofibrillated Cellulose/Fe3+ Hydrogel Based on Multi-Dynamic Interactions for a Multifunctional Strain Sensor. Mater. Horiz. 2022, 9, 1412–1421. [Google Scholar] [CrossRef] [PubMed]
- Mikhailidi, A.; Volf, I.; Belosinschi, D.; Tofanica, B.-M.; Ungureanu, E. Cellulose-Based Metallogels—Part 1: Raw Materials and Preparation. Gels 2023, 9, 390. [Google Scholar] [CrossRef] [PubMed]
- Mikhailidi, A.M.; Kotel’nikova, N.E.; Shakhmin, A.L.; Andersson, S.; Saprykina, N.N.; Kudryashov, V.I.; Anan’eva, E.P.; Martakova, Y.V. Preparation, Characteristics, and Antibacterial Properties of Pulp—Silver Nanocomposites Obtained from DMA/LiCl Solutions. Fibre Chem. 2015, 47, 260–264. [Google Scholar] [CrossRef]
- Dong, H.; Snyder, J.F.; Tran, D.T.; Leadore, J.L. Hydrogel, Aerogel and Film of Cellulose Nanofibrils Functionalized with Silver Nanoparticles. Carbohydr. Polym. 2013, 95, 760–767. [Google Scholar] [CrossRef]
- Zander, N.E.; Dong, H.; Steele, J.; Grant, J.T. Metal Cation Cross-Linked Nanocellulose Hydrogels as Tissue Engineering Substrates. ACS Appl. Mater. Interfaces 2014, 6, 18502–18510. [Google Scholar] [CrossRef] [PubMed]
- Ovalle-Serrano, S.A.; Díaz-Serrano, L.A.; Hong, C.; Hinestroza, J.P.; Blanco-Tirado, C.; Combariza, M.Y. Synthesis of Cellulose Nanofiber Hydrogels from Fique Tow and Ag Nanoparticles. Cellulose 2020, 27, 9947–9961. [Google Scholar] [CrossRef]
- Gupta, A.; Briffa, S.M.; Swingler, S.; Gibson, H.; Kannappan, V.; Adamus, G.; Kowalczuk, M.; Martin, C.; Radecka, I. Synthesis of Silver Nanoparticles Using Curcumin-Cyclodextrins Loaded into Bacterial Cellulose-Based Hydrogels for Wound Dressing Applications. Biomacromolecules 2020, 21, 1802–1811. [Google Scholar] [CrossRef]
- Sood, A.; Granick, M.S.; Tomaselli, N.L. Wound Dressings and Comparative Effectiveness Data. Adv. Wound Care 2014, 3, 511–529. [Google Scholar] [CrossRef]
- Mikhailidi, A.; Saurov, S.K.; Andersson, S.; Kotelnikova, N. Lignocellulose Fibers Elaborating Super-Swollen Three-Dimensional Cellulose Hydrogels from Solution in N, N-Dimethylacetamide/Lithium Chloride. TAPPI J. 2018, 17, 81–88. [Google Scholar] [CrossRef]
- Tang, L.; Tang, F.; Li, M.; Li, L. Facile Synthesis of Ag@AgCl-Contained Cellulose Hydrogels and Their Application. Colloids Surf. A Physicochem. Eng. Asp. 2018, 553, 618–623. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Z.; Li, Q.; Yang, L.; Liu, H.; Yan, R.; Xiao, L.; Liu, H.; Wang, J.; Yang, B.; et al. Transparent Conductive Supramolecular Hydrogels with Stimuli-Responsive Properties for On-Demand Dissolvable Diabetic Foot Wound Dressings. Macromol. Rapid Commun. 2020, 41, 2000441. [Google Scholar] [CrossRef]
- Cai, J.; Kimura, S.; Wada, M.; Kuga, S. Nanoporous Cellulose as Metal Nanoparticles Support. Biomacromolecules 2009, 10, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Mikhailidi, A.; Kotelnikova, N. Antibacterial Cellulose Hydrogels with Noble Metals Nanoparticles: Synthesis, Mechanism, and Properties. In Proceedings of the International Conference “Progress in Organic and Macromolecular Compounds”, Iasi, Romania, 7 October 2021; pp. 129–130. [Google Scholar]
- Huang, C.; Ye, Q.; Dong, J.; Li, L.; Wang, M.; Zhang, Y.; Zhang, Y.; Wang, X.; Wang, P.; Jiang, Q. Biofabrication of Natural Au/Bacterial Cellulose Hydrogel for Bone Tissue Regeneration via in-Situ Fermentation. Smart Mater. Med. 2023, 4, 1–14. [Google Scholar] [CrossRef]
- Lin, X.; Han, X.; Wang, J. In Situ Synthesis of Easily Separable Au Nanoparticles Catalysts Based on Cellulose Hydrogels. Polym. J. 2018, 50, 495–501. [Google Scholar] [CrossRef]
- Mikhailidi, A.; Saprykina, N.; Mokeev, M.; Zinchenko, A.; Kotelnikova, N. Highly Porous Hybrid Composite Hydrogels Based on Cellulose and 1,10-Phenanthrocyanine of Zn (II): Synthesis and Characterization with WAXS, FTIR, 13C CP/MAS NMR and SEM. Cellulose Chem. Technol. 2020, 54, 875–888. [Google Scholar] [CrossRef]
- Mikhailidi, A.M.; Ivanova, M.V.; Shakhmin, A.L.; Saprikina, N.N.; Kotelnikova, N.E. Antimicrobial Cellulose Hydrogels for Medical Applications. In Proceedings of the XIX International Conference “New Polymer Composite Materials. Mikitaev Readings”, Elbrus, Russia, 3 July 2023; p. 272. (In Russian). [Google Scholar]
- Jampi, A.L.W.; Chin, S.-F.; Wasli, M.E.; Chia, C.-H. Preparation of Cellulose Hydrogel from Sago Pith Waste as a Medium for Seed Germination. J. Phys. Sci. 2021, 32, 13–26. [Google Scholar] [CrossRef]
- Tovar-Carrillo, K.L.; Tagaya, M.; Kobayashi, T. Bamboo Fibers Elaborating Cellulose Hydrogel Films for Medical Applications. J. Mater. Sci. Chem. Eng. 2013, 1, 7–12. [Google Scholar] [CrossRef][Green Version]
- Song, S.; Liu, Z.; Abubaker, M.A.; Ding, L.; Zhang, J.; Yang, S.; Fan, Z. Antibacterial Polyvinyl Alcohol/Bacterial Cellulose/Nano-Silver Hydrogels That Effectively Promote Wound Healing. Mater. Sci. Eng. C 2021, 126, 112171. [Google Scholar] [CrossRef] [PubMed]
- Fortună, M.E.; Ungureanu, E.; Jităreanu, D.C.; Țopa, D.C.; Harabagiu, V. Effects of Hybrid Polymeric Material Based on Polycaprolactone on the Environment. Materials 2022, 15, 4868. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Mathew, A.P. Cellulose–Metal Organic Frameworks (CelloMOFs) Hybrid Materials and Their Multifaceted Applications: A Review. Coord. Chem. Rev. 2022, 451, 214263. [Google Scholar] [CrossRef]
- Ungureanu, E. Aspects of Composites Biodegradation Based on Lignocelluloses Materials. Ph.D. Thesis, “Gh. Asachi” Polytechnic University of Iasi, Iasi, Romania, 2008. [Google Scholar]
- Kotelnikova, N.E.; Mikhailidi, A.M.; Martakova, Y.V. Preparation of Cellulose Hydrogels via Self-Assembly in DMAc/LiCl Solutions and Study of Their Properties. Polym. Sci. Ser. A 2017, 59, 76–87. [Google Scholar] [CrossRef]
- Yang, X.; Cranston, E.D. Chemically Cross-Linked Cellulose Nanocrystal Aerogels with Shape Recovery and Superabsorbent Properties. Chem. Mater. 2014, 26, 6016–6025. [Google Scholar] [CrossRef]
- Ashtaputrey, P.; Ashtaputrey, S. Study of Swelling Behavior and Determination of Swellin Parameters of Spherical Hydrogels in Water. J. Drug Deliv. Ther. 2018, 8, 218–222. [Google Scholar] [CrossRef]
- Qiu, Y.; Sun, X.; Lin, X.; Yi, W.; Jiang, J. An Injectable Metal Nanoparticle Containing Cellulose Derivative-Based Hydrogels: Evaluation of Antibacterial and in Vitro-Vivo Wound Healing Activity in Children with Burn Injuries. Int. Wound J. 2022, 19, 666–678. [Google Scholar] [CrossRef] [PubMed]
- Goh, C.F.; Leh, C.P.; Almashhadani, A.; Wong, L.C.; Tannimalay, H. 2—Cellulose-Based Nanocomposite Hydrogels for Wound Management. In Functional Nanocomposite Hydrogels; Kumar, A., Thakur, V.K., Eds.; Nanotechnology in Biomedicine; Elsevier: Amsterdam, The Netherlands, 2023; pp. 25–68. ISBN 978-0-323-99638-9. [Google Scholar]
- Nakasone, K.; Kobayashi, T. Cytocompatible Cellulose Hydrogels Containing Trace Lignin. Mater. Sci. Eng. C 2016, 64, 269–277. [Google Scholar] [CrossRef]
- Cui, X.; Lee, J.J.; Chen, W.N. Eco-Friendly and Biodegradable Cellulose Hydrogels Produced from Low Cost Okara: Towards Non-Toxic Flexible Electronics. Sci. Rep. 2019, 9, 18166. [Google Scholar] [CrossRef]
- Cho, C.; Aye, T.; Khaing, A.; Kobayashi, T. Comparative Study of Cellulose Hydrogel Films Prepared from Various Biomass Wastes. In Cellulose Science and Derivatives; Sand, A., Banga, S., Eds.; IntechOpen: London, UK, 2021; ISBN 978-1-83969-579-7. [Google Scholar]
- Chen, Y.; Li, L.; Li, Y.; Liu, Y.; Chen, Y.; Li, H.; Li, M.; Xu, F.; Liu, Y. Preparation of a Double-Network Hydrogel Based on Wastepaper and Its Application in the Treatment of Wastewater Containing Copper(II) and Methylene Blue. RSC Adv. 2021, 11, 18131–18143. [Google Scholar] [CrossRef]
- Abdelaziz, R.M.; El-Maghraby, A.; Sadik, W.A.-A.; El-Demerdash, A.-G.M.; Fadl, E.A. Biodegradable Cellulose Nanocrystals Hydrogels for Removal of Acid Red 8 Dye from Aqueous Solutions. Sci. Rep. 2022, 12, 6424. [Google Scholar] [CrossRef]
- Ma, J.; Li, X.; Bao, Y. Advances in Cellulose-Based Superabsorbent Hydrogels. RSC Adv. 2015, 5, 59745–59757. [Google Scholar] [CrossRef]
- Sawut, A.; Yimit, M.; Sun, W.; Nurulla, I. Photopolymerisation and Characterization of Maleylatedcellulose-g-Poly(Acrylic Acid) Superabsorbent Polymer. Carbohydr. Polym. 2014, 101, 231–239. [Google Scholar] [CrossRef]
- Wu, F.; Zhang, Y.; Liu, L.; Yao, J. Synthesis and Characterization of a Novel Cellulose-g-Poly(Acrylic Acid-Co-Acrylamide) Superabsorbent Composite Based on Flax Yarn Waste. Carbohydr. Polym. 2012, 87, 2519–2525. [Google Scholar] [CrossRef]
- Zhou, L.; Xie, F.; Li, H.; Li, W.; Li, W.J.; Li, Y.W. Preparation and Properties of Regenerated Cellulose Hydrogels. IOP Conf. Ser. Mater. Sci. Eng. 2017, 170, 012038. [Google Scholar] [CrossRef]
- Demitri, C.; Scalera, F.; Madaghiele, M.; Sannino, A.; Maffezzoli, A. Potential of Cellulose-Based Superabsorbent Hydrogels as Water Reservoir in Agriculture. Int. J. Polym. Sci. 2013, 2013, e435073. [Google Scholar] [CrossRef]
- Liu, Z.; Cao, Y.; Wang, Z.; Xia, J.; Su, C. Preparation and Characterization of Lignocellulose Gel from Soybean Stem/LiCl/DMSO Solution. Chem. Ind. For. Prod. 2016, 36, 81–88. [Google Scholar]
- Song, L.; Shu, L.; Wang, Y.; Zhang, X.-F.; Wang, Z.; Feng, Y.; Yao, J. Metal Nanoparticle-Embedded Bacterial Cellulose Aerogels via Swelling-Induced Adsorption for Nitrophenol Reduction. Int. J. Biol. Macromol. 2020, 143, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Zhang, M.; Tu, Y.; Lin, S.; Hu, J. Stimuli-Responsive Cellulose-Based Hydrogels. In Cellulose-Based Superabsorbent Hydrogels; Mondal, M.d.I.H., Ed.; Polymers and Polymeric Composites: A Reference Series; Springer International Publishing: Cham, Switzerland, 2019; pp. 269–308. ISBN 978-3-319-77830-3. [Google Scholar]
- Deng, J.; Song, Q.; Liu, S.; Pei, W.; Wang, P.; Zheng, L.; Huang, C.; Ma, M.; Jiang, Q.; Zhang, K. Advanced Applications of Cellulose-Based Composites in Fighting Bone Diseases. Compos. Part B Eng. 2022, 245, 110221. [Google Scholar] [CrossRef]
- Rusu, D.; Ciolacu, D.; Simionescu, B. Cellulose-Based Hydrogels in Tissue Engineering Applications. Cellul. Chem. Technol. 2019, 53, 907–923. [Google Scholar] [CrossRef]
- Ciolacu, D.E.; Nicu, R.; Ciolacu, F. Cellulose-Based Hydrogels as Sustained Drug-Delivery Systems. Materials 2020, 13, 5270. [Google Scholar] [CrossRef]
- Nasution, H.; Harahap, H.; Dalimunthe, N.F.; Ginting, M.H.S.; Jaafar, M.; Tan, O.O.H.; Aruan, H.K.; Herfananda, A.L. Hydrogel and Effects of Crosslinking Agent on Cellulose-Based Hydrogels: A Review. Gels 2022, 8, 568. [Google Scholar] [CrossRef]
- Isobe, N.; Chen, X.; Kim, U.-J.; Kimura, S.; Wada, M.; Saito, T.; Isogai, A. TEMPO-Oxidized Cellulose Hydrogel as a High-Capacity and Reusable Heavy Metal Ion Adsorbent. J. Hazard. Mater. 2013, 260, 195–201. [Google Scholar] [CrossRef]
- Li, Y.; Tian, Y.; Zheng, W.; Feng, Y.; Huang, R.; Shao, J.; Tang, R.; Wang, P.; Jia, Y.; Zhang, J.; et al. Composites of Bacterial Cellulose and Small Molecule-Decorated Gold Nanoparticles for Treating Gram-Negative Bacteria-Infected Wounds. Small 2017, 13, 1700130. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, X.; Qin, Z.; Sun, X.; Zhang, H.; Yu, Q.; Yao, M.; He, S.; Dong, X.; Yao, F.; et al. Dual Physically Cross-Linked Carboxymethyl Cellulose-Based Hydrogel with High Stretchability and Toughness as Sensitive Strain Sensors. Cellulose 2020, 27, 9975–9989. [Google Scholar] [CrossRef]
- González-Ugarte, A.S.; Hafez, I.; Tajvidi, M. Characterization and Properties of Hybrid Foams from Nanocellulose and Kaolin-Microfibrillated Cellulose Composite. Sci. Rep. 2020, 10, 17459. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, K.; Gao, X.; Han, Q.; Peng, L. Improved Thermal Stability of Regenerated Cellulose Films from Corn (Zea Mays) Stalk Pith Using Facile Preparation with Low-Concentration Zinc Chloride Dissolving. Carbohydr. Polym. 2019, 217, 190–198. [Google Scholar] [CrossRef]
- Shankar, S.; Oun, A.A.; Rhim, J.-W. Preparation of Antimicrobial Hybrid Nano-Materials Using Regenerated Cellulose and Metallic Nanoparticles. Int. J. Biol. Macromol. 2018, 107, 17–27. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, N.; Zhang, X.; Xu, J. Cellulose/Silver Nanoparticles Composite Microspheres: Eco-Friendly Synthesis and Catalytic Application. Cellulose 2012, 19, 1239–1249. [Google Scholar] [CrossRef]
- Kotelnikova, N.; Demidov, V.; Wegener, G.; Windeisen, E. Mechanisms of Diffusion-Reduction Interaction of Microcrystalline Cellulose and Silver Ions. Russ. J. Gen. Chem. 2003, 73, 427–433. [Google Scholar] [CrossRef]
- Ungureanu, E.; Popa, V.I. On the Biological Stability of Wood in the Presence of Some Bioprotection Agents. Cellul. Chem. Technol. 2007, 41, 429. [Google Scholar]
- Sannino, A.; Demitri, C.; Madaghiele, M. Biodegradable Cellulose-Based Hydrogels: Design and Applications. Materials 2009, 2, 353–373. [Google Scholar] [CrossRef]
- Abou Hammad, A.B.; Abd El-Aziz, M.E.; Hasanin, M.S.; Kamel, S. A Novel Electromagnetic Biodegradable Nanocomposite Based on Cellulose, Polyaniline, and Cobalt Ferrite Nanoparticles. Carbohydr. Polym. 2019, 216, 54–62. [Google Scholar] [CrossRef]
- Singla, R.; Soni, S.; Patial, V.; Kulurkar, P.M.; Kumari, A.; Padwad, Y.S.; Yadav, S.K. Cytocompatible Anti-Microbial Dressings of Syzygium Cumini Cellulose Nanocrystals Decorated with Silver Nanoparticles Accelerate Acute and Diabetic Wound Healing. Sci. Rep. 2017, 7, 10457. [Google Scholar] [CrossRef] [PubMed]
- Capraru, A.-M.; Ungureanu, E.; Popa, V.I. Studies on the Interaction between Birch Veneer and Compounds with Biocide Potential Action. Environ. Eng. Manag. J. 2008, 7, 525–530. [Google Scholar] [CrossRef]
- Blanchette, V.; Belosinschi, D.; Lai, T.T.; Cloutier, L.; Barnabé, S. New Antibacterial Paper Made of Silver Phosphate Cellulose Fibers: A Preliminary Study on the Elimination of Staphylococcus Aureus Involved in Diabetic Foot Ulceration. BioMed Res. Int. 2020, 2020, 1304016. [Google Scholar] [CrossRef] [PubMed]
- Thi, T.U.D.; Nguyen, T.T.; Thi, Y.D.; Thi, K.H.T.; Phan, B.T.; Pham, K.N. Green Synthesis of ZnO Nanoparticles Using Orange Fruit Peel Extract for Antibacterial Activities. RSC Adv. 2020, 10, 23899–23907. [Google Scholar] [CrossRef]
- Anagha, B.; George, D.; Maheswari, P.U.; Begum, K.M.M.S. Biomass Derived Antimicrobial Hybrid Cellulose Hydrogel with Green ZnO Nanoparticles for Curcumin Delivery and Its Kinetic Modelling. J Polym Environ. 2019, 27, 2054–2067. [Google Scholar] [CrossRef]


| Initial Material | Solvent | (Ligno)Cellulose Concentration in the Solution, % | Swelling Ability, g/g | Physico-Chemical Properties | Reference |
|---|---|---|---|---|---|
| Bamboo fibers | DMAc/LiCl (hydrogel films) | 1 | 0.31 | Tensile strength 66 N/mm2, Elongation 33.5% | [64] |
| NaOH/ water (hydrogel films) | 0.14 | Tensile strength 27 N/mm2, Elongation 13% | |||
| NaOH/urea (hydrogel films) | 0.11 | Tensile strength 21 N/mm2, Elongation 8% | |||
| Okara cellulose | LiOH/ urea/water (8:15:77), ECH | 6 | 0.88–0.94 | Tensile strength 0.024–0.245 N/mm2, Elongation 60.5–107%, Young’s modulus 0.0087–0.37 mpa | [73] |
| Flax lignocellulose | DMAc/LiCl | 1–3 | 25 | Porosity 98.9% | [52] |
| Hardwood lignocellulose | 28 | 97.4% | |||
| Cotton MCC | 20 | 86.8% | |||
| Sugarcane bagasse lignocelluloses | DMAc/LiCl (hydrogel film) | 1 | 11.53–15.25 | Tensile strength 0.43–0.80 N/mm2, Elongation 26.5–45.2% | [72] |
| Cellulose | NMMO | 3 | 3.94 | Tdecompos. 335 °C | [80] |
| 8 | 6.19 | 352 °C | |||
| Thanaka heartwood cellulose | DMSO/LiCl (hydrogel film) | 1 | 1.66 | Elongation 9% | [74] |
| Sugarcane bagasse cellulose | 1.89 | 12.45% | |||
| Rice straw cellulose | 1.69 | 35.71% | |||
| Sago pith waste cellulose | NaOH/urea aqueous (7:12), ECH | 3 | 2.4 | Tdecompos. 300–400 °C | [63] |
| 4 | 2.0 | ||||
| 5 | 19.7 | ||||
| 6 | 8.1 | ||||
| Waste paper/acrylamide | NaOH/urea aqueous (7:12), ECH | 2 | 66.75 | Porosity 98.1% Tdecompos. 407,72 °C | [75] |
| Waste office paper | NaOH/urea/ water (7:12:81), ECH | 4 | 18.0 | [76] | |
| 5 | 27.7 | ||||
| 6 | 13.4 | ||||
| Soybean stem lignocellulose with lignin content: 23.24% | DMSO/LiCl, EDA | 5 | at 25 °C 22.87 | Average pore size 20.281 nm | [82] |
| 21.33% | 11.29 | 16.301 nm | |||
| 18.91% | 7.71 | 16.709 nm | |||
| 14.24% | 13.96 | 17.813 nm | |||
| PVA/BC |
NaOH /urea/water | 4 | 13 | Tensile strength 0.051 N/mm2, elongation 160% | [65] |
| PVA/BC/AgNO3 | 16 | Tensile strength 0.2 N/mm2, elongation 180% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikhailidi, A.; Volf, I.; Belosinschi, D.; Tofanica, B.-M.; Ungureanu, E. Cellulose-Based Metallogels—Part 2: Physico-Chemical Properties and Biological Stability. Gels 2023, 9, 633. https://doi.org/10.3390/gels9080633
Mikhailidi A, Volf I, Belosinschi D, Tofanica B-M, Ungureanu E. Cellulose-Based Metallogels—Part 2: Physico-Chemical Properties and Biological Stability. Gels. 2023; 9(8):633. https://doi.org/10.3390/gels9080633
Chicago/Turabian StyleMikhailidi, Aleksandra, Irina Volf, Dan Belosinschi, Bogdan-Marian Tofanica, and Elena Ungureanu. 2023. "Cellulose-Based Metallogels—Part 2: Physico-Chemical Properties and Biological Stability" Gels 9, no. 8: 633. https://doi.org/10.3390/gels9080633
APA StyleMikhailidi, A., Volf, I., Belosinschi, D., Tofanica, B.-M., & Ungureanu, E. (2023). Cellulose-Based Metallogels—Part 2: Physico-Chemical Properties and Biological Stability. Gels, 9(8), 633. https://doi.org/10.3390/gels9080633








