Reinforcement of Acrylamide Hydrogels with Cellulose Nanocrystals Using Gamma Radiation for Antibiotic Drug Delivery
Abstract
1. Introduction
2. Results and Discussion
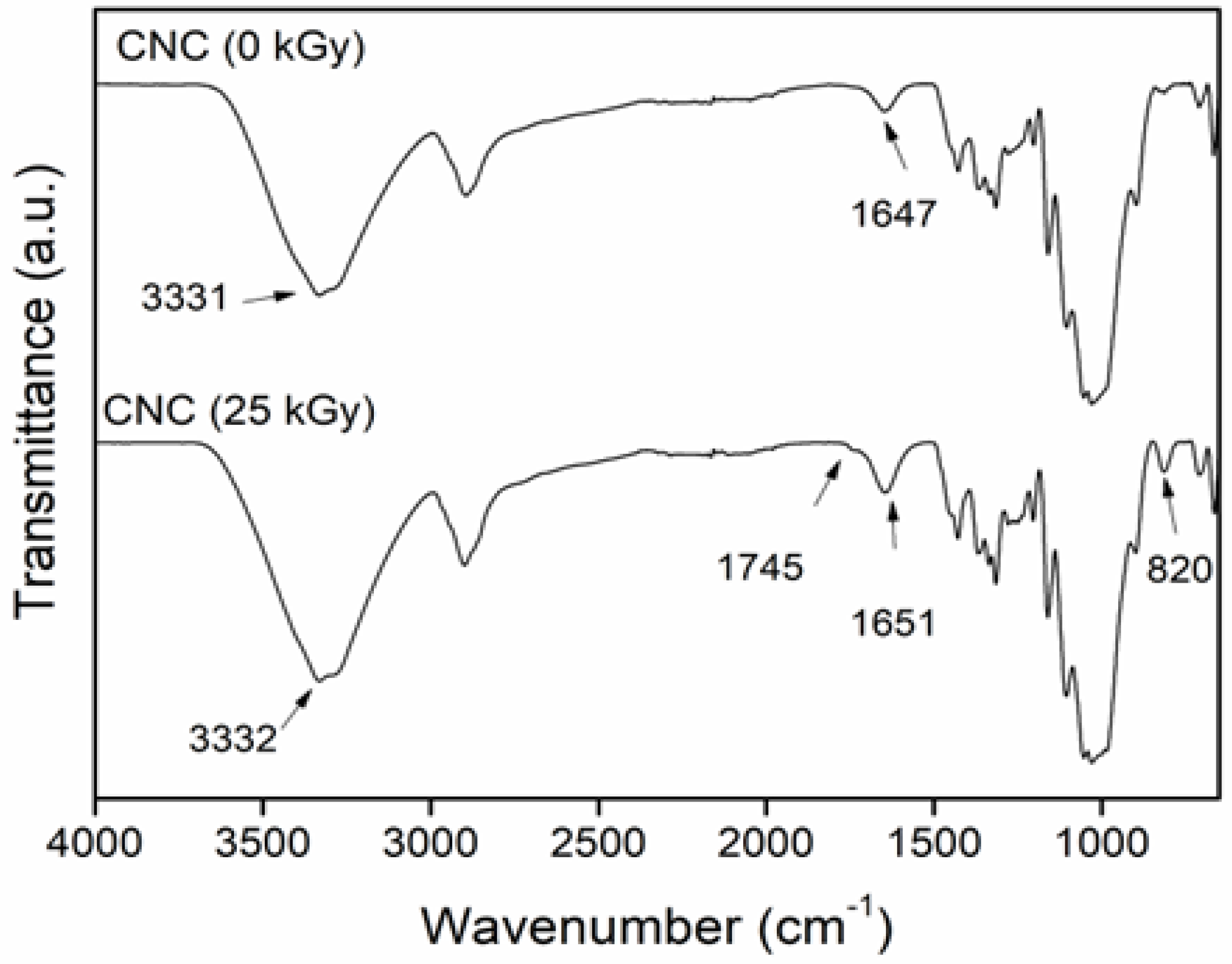
2.1. Effect of Radiation in the CNC
2.2. Hydrogels Reinforced with CNC
2.3. FTIR Determination
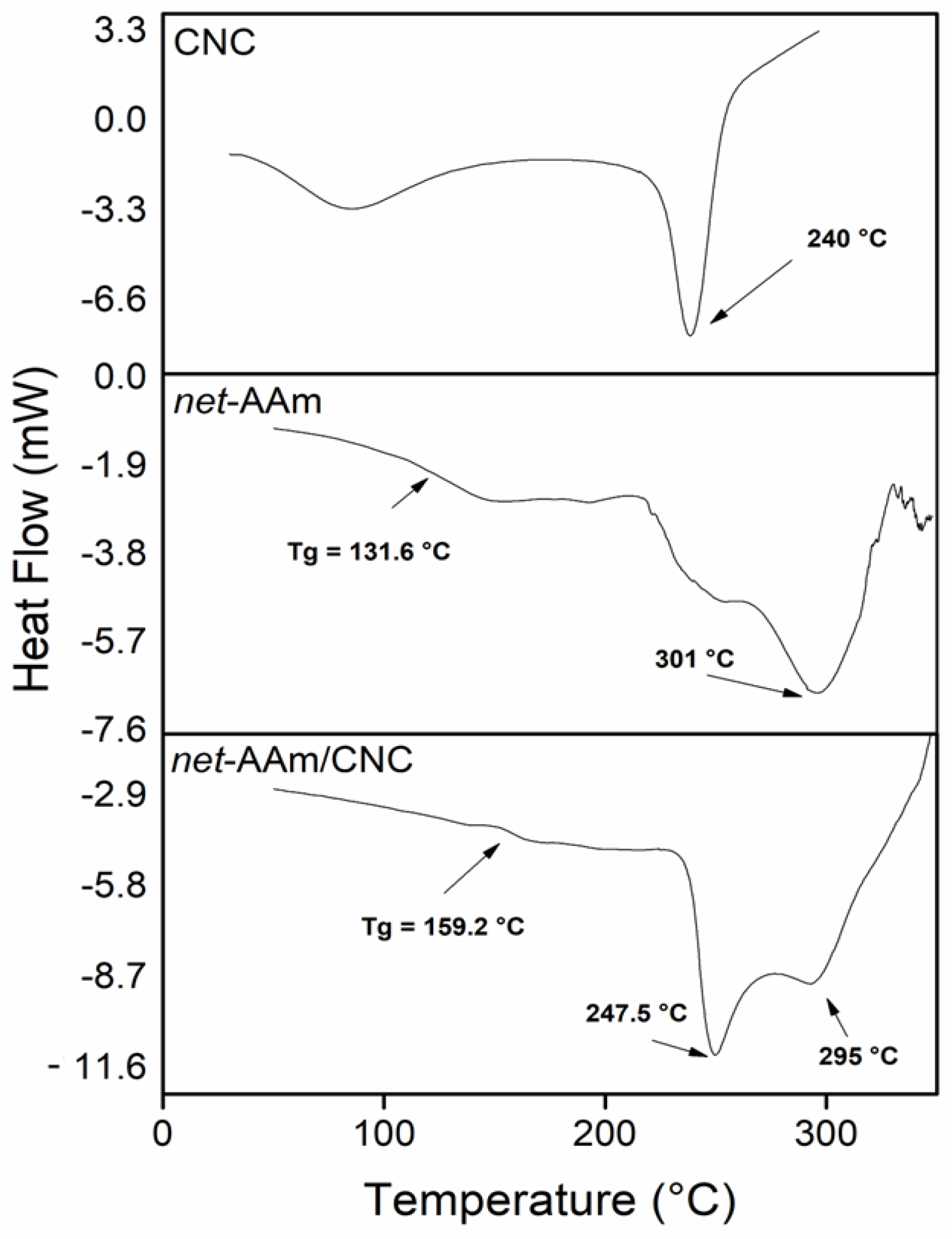
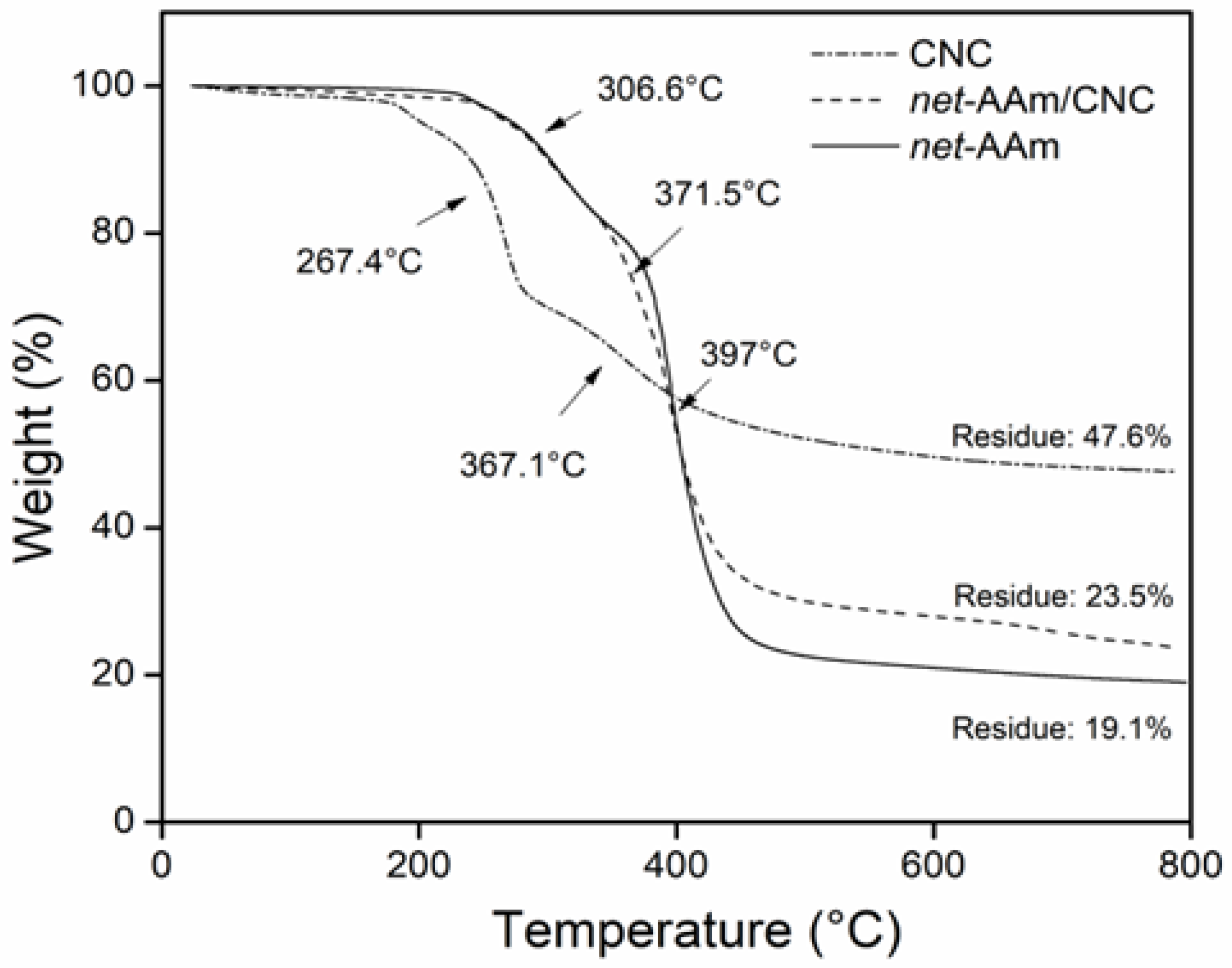
2.4. Thermal Behavior
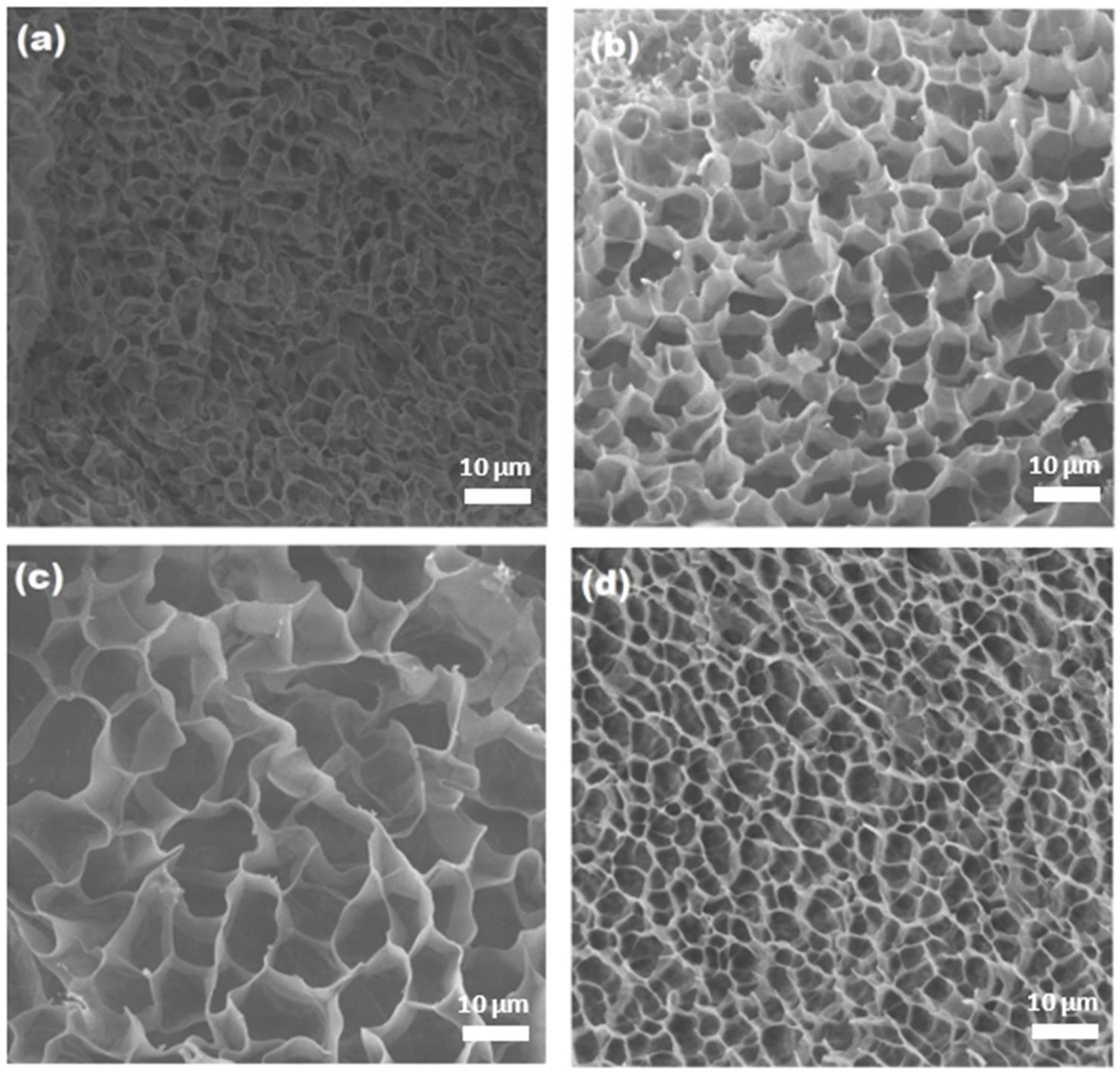
2.5. Scanning Electron Microscopy
2.6. Swelling Behavior
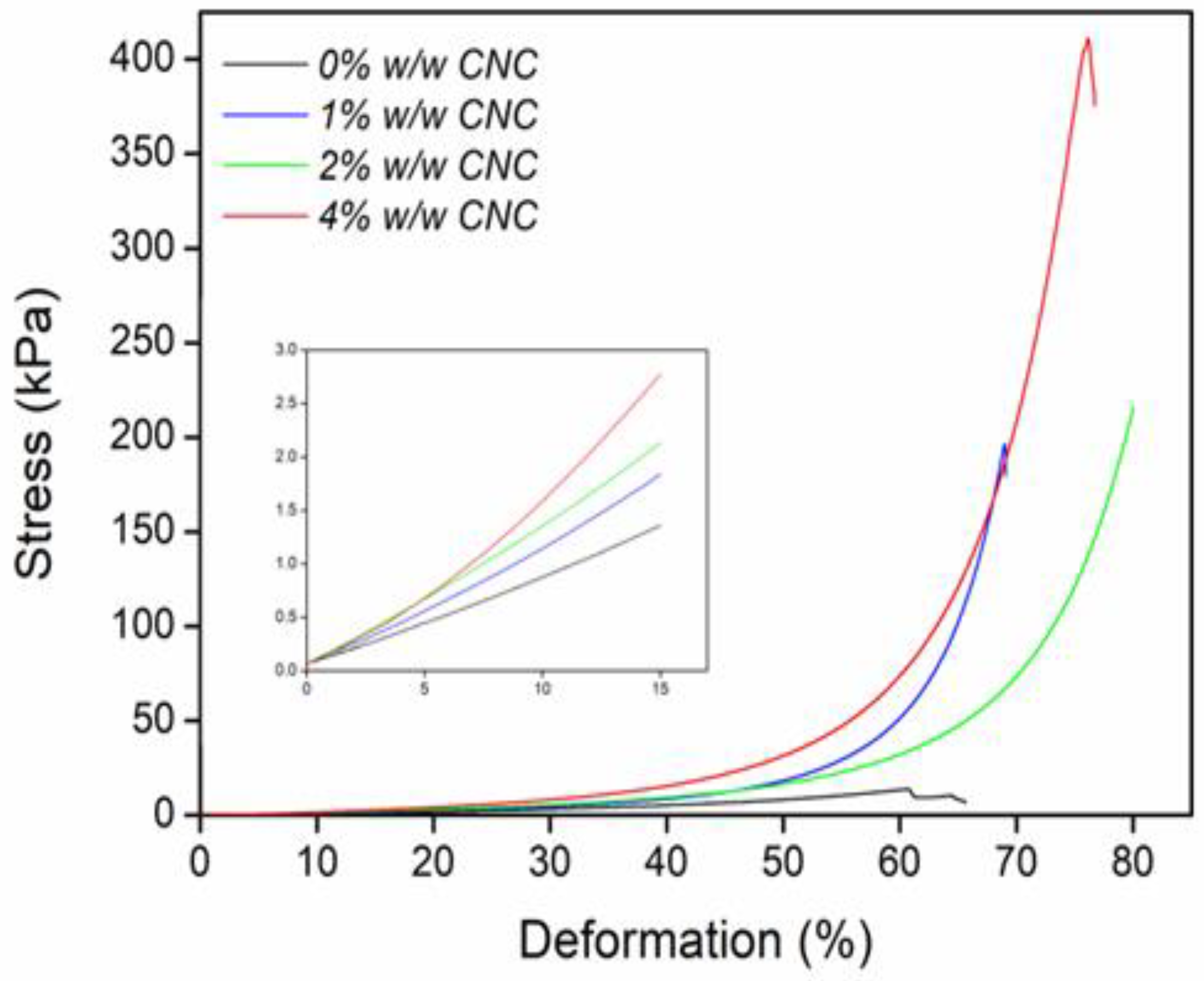
2.7. Mechanic Properties
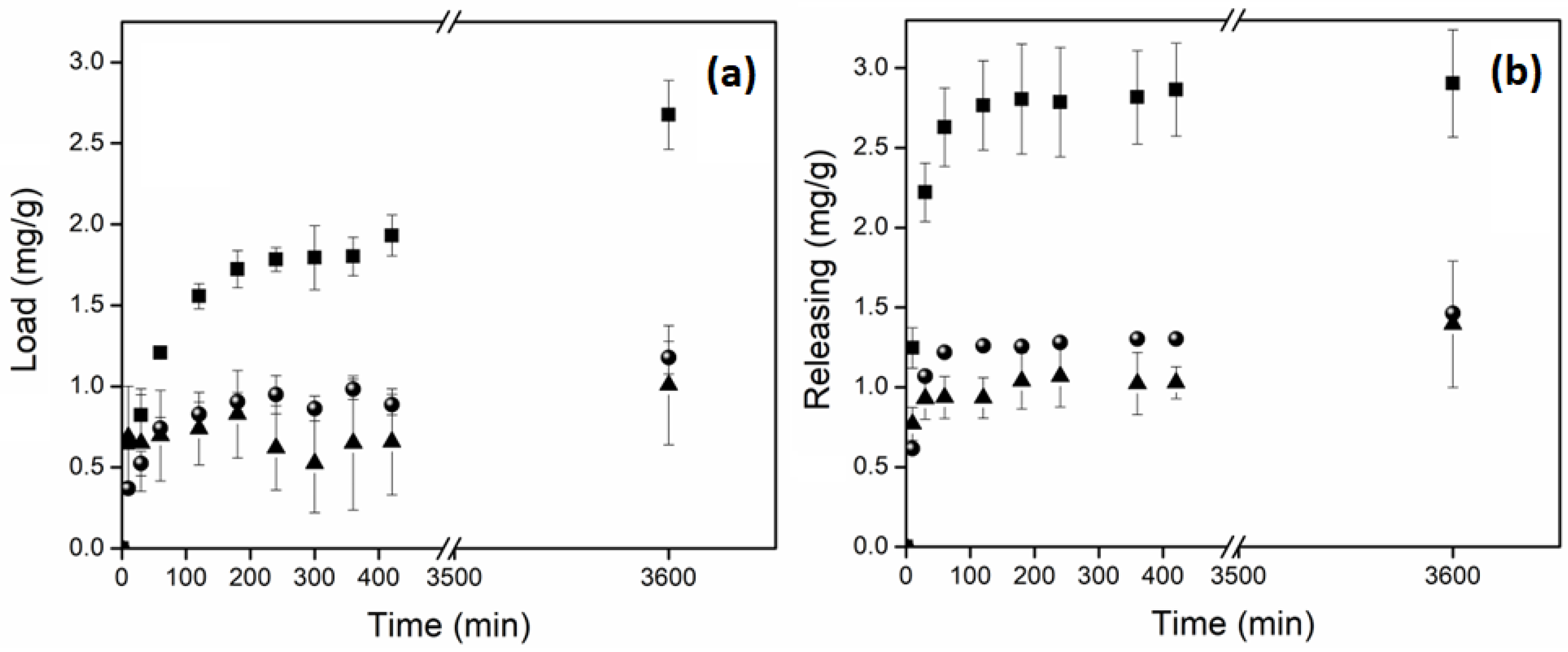
2.8. Load and Release Studies
3. Conclusions
4. Materials and Methods
4.1. CNC Synthesis
4.2. Preparation of Reinforced Hydrogels
4.3. Characterization
4.3.1. X-ray Diffraction
4.3.2. Infrared Spectroscopy
4.3.3. Thermal Properties
4.3.4. Scanning Electron Microscopy
4.3.5. Swelling Studies
4.3.6. Mechanical Tests
4.3.7. Load and Release Studies
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, A.V.; Shah, B.N. Transdermal drug delivery system: A review. Pharm. Sci. Monit. Int. J. Pharm. Sci. Res. 2018, 9, 378–390. [Google Scholar] [CrossRef][Green Version]
- Risbud, V.M.; Bhonde, M.R. Polyacrylamide-chitosan hydrogels: In vitro biocompatibility and sustained antibiotic release studies. Drug Deliv. 2000, 7, 65–79. [Google Scholar] [CrossRef]
- Hong, Y.; Lin, Z.; Yang, Y.; Jiang, T.; Shang, J.; Luo, Z. Biocompatible conductive hydrogels: Applications in the field of biomedicine. Int. J. Mol. Sci. 2022, 23, 4578. [Google Scholar] [CrossRef] [PubMed]
- Kesharwni, P.; Bisht, A.; Alexander, A.; Dave, V.; Sharma, S. Biomedical applicationsof hydrogels in drug delivery system: An update. J. Drug Deliv. Sci. Technol. 2021, 66, 102914. [Google Scholar] [CrossRef]
- Darnell, M.C.; Sun, J.Y.; Metha, C.; Johnson, C.; Arany, P.R.; Suo, Z.; Mooney, D.J. Performanceand biocompatibility of extremely tough alignate/polyacrylamide hydrogels. Biomaterials 2013, 34, 8042–8048. [Google Scholar] [CrossRef]
- Martin, C.; Merino, S.; González-Domínguez, J.M.; Rauti, R.; Ballerini, L.; Prato, M.; Vázquez, E. Graphene improves the biocompatibility of polyacrylamide hydrogels: 3Dpolymeric scaffolds for neuronal growth. Sci. Rep. 2017, 8, 10942. [Google Scholar] [CrossRef]
- Chan, D.; Maikawa, C.L.; D’aquino, A.; Raghavan, S.; Troxell, M.L.; Appel, E.A. Polyacrylamide-based hydrogel coatings improve biocompatibility of implanted pump devices. J. Biomed. Mater. Res. A 2023, 111, 910–920. [Google Scholar] [CrossRef]
- Elsayed, M.M. Hydrogel Preparation Technologies: Relevance Kinetics, Thermodynamics and Scaling up Aspects. J. Polym. Environ. 2019, 27, 871–891. [Google Scholar] [CrossRef]
- Li, R.; Lin, J.; Fang, Y.; Yu, C.; Zhang, J.; Xue, Y.; Liu, Z.; Zhang, J.; Tang, C.; Huang, Y. Porous boron nitride nanofibers/PVA hydrogels with improved mechanical property and thermal stability. Ceram. Int. 2018, 44, 22439–22444. [Google Scholar] [CrossRef]
- Hamzah, Y.B.; Hashim, S.; Rahman, W.A. Synthesis of polymeric nano/microgels: A review. J. Polym. Res. 2017, 24, 134. [Google Scholar] [CrossRef]
- Prusty, K.; Swain, S. Cellulose-based nanohydrogels for tissue engineering applications. In Nanocellulose and Nanohydrogel Matrices: Biotechnological and Biomedical Applications; Jawaid, M., Mohammad, F., Eds.; Wiley-VCH: Weinheim, Germany, 2017; pp. 67–90. [Google Scholar] [CrossRef]
- George, J.; Sabapathi, S.N. Cellulose Nanocrystals: Synthesis, Functional Properties, and Applications. Nanotechnol. Sci. Appl. 2015, 8, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Rueda, L.; Saralegui, A.; Fernandez d´Arlas, B.; Zhou, Q.; Berglund, L.A.; Corcuera, M.A.; Mondragon, I.; Eceiza, A. Cellulose nanocrystals/polyurethane nanocomposites. Study from the viewpoint of microphase separated structure. Carbohydr. Polym. 2013, 92, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, J.; Kumar, S.; Weder, C.; Foster, E.J. Influence of Processing Conditions on Properties of Poly (Vinyl acetate)/Cellulose Nanocrystal Nanocomposites. Macromol. Mater. Eng. 2015, 300, 562–571. [Google Scholar] [CrossRef]
- Dufresne, A. Nanocellulose: A new ageless bionanomaterial. Mater. Today 2013, 16, 220–227. [Google Scholar] [CrossRef]
- Foster, E.J.; Moon, R.J.; Agarwal, U.P.; Bortner, M.J.; Bras, J.; Camarero-Espinosa, S.; Chan, K.J.; Clift, M.J.D.; Cranston, E.D.; Eichhorn, S.J.; et al. Current characterization methods for cellulose nanomaterials. Chem. Soc. Rev. 2018, 47, 2609. [Google Scholar] [CrossRef] [PubMed]
- Wadood Hamad, Y. Cellulose Nanocrystals Properties, Production, and Applications, 1st ed.; John Wiley and Sons: Chichester, UK, 2017. [Google Scholar]
- Alexandre, M.; Dubois, P. Polymer-layered silicate nanocomposites: Preparation, properties and uses of a new class of materials. Mater. Sci. Eng. R Rep. 2000, 28, 1–63. [Google Scholar] [CrossRef]
- Ambreen, J.; Haleem, A.; Shah, A.A.; Mushtaq, F.; Siddiq, M.; Bukhari, S.N.U.S.; Chandio, A.D.; Mahdi, W.; Alshehri, S. Facile synthesis and fabrication of NIPAAM-based cryogels for environmental remediation. Gels 2023, 9, 64. [Google Scholar] [CrossRef]
- Ghorpade, V.S. Preparation of hydrogels based on natural polymers via chemical reaction and cross-linking. In Hydrogels Based on Natural Polymers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 91–118. [Google Scholar]
- Darwis, D.; Abbas, A.B.; Nurlidar, F.; Putra, P.D. Radiation Processing of Polymers for Medical and Pharmaceutical Applications. Macromol. Symp. 2015, 353, 15–23. [Google Scholar] [CrossRef]
- Moghaddam, R.H.; Dadfarnia, S.; Shabani, A.M.H.; Moghaddam, Z.H.; Tavakol, M. Electron beam irradiation synthesis of porous and non-porous pectin based hydrogels for a tetracycline drug delivery system. Mater. Sci. Eng. C 2019, 102, 391–404. [Google Scholar] [CrossRef]
- Gonzalez-Gomez, R.; Ortega, A.; Lazo, L.M.; Burillo, G. Retention of heavy metal ion on comb-type hydrogels based on acrylic acid and 4-vinylpyridine, synthesized by gamma radiation. Radiat. Phys. Chem. 2014, 102, 117–123. [Google Scholar] [CrossRef]
- Charlesby, A. Atomic Radiation and Polymers; Pergamon Press: Oxford, UK, 1960; Chapter 9; pp. 134–158. [Google Scholar]
- Rosiak, J.M.; Ulansky, P.; Pajewski, L.A.; Yoshii, F.; Makuuchi, K. Radiation formation of hydrogels for biomedical purposes. Some remarks and comments. Radiat. Phys. Chem. 1995, 46, 161–168. [Google Scholar] [CrossRef]
- Yu, J.; Wang, C.; Wang, J.; Chu, F. In situ development of self-reinforced cellulose nanocrystals based thermoplastic elastomers by atom transfer radical polymerization. Carbohydr. Polym. 2016, 141, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Tissot, C.; Gordanovska, S.; Barkatt, B.; Silverman, J.; Al-Sheikhly, M. On the mechanism of the radiation-induced degradation of cellulosic substances. Radiat. Phys. Chem. 2013, 84, 185–190. [Google Scholar] [CrossRef]
- Criado, P.; Fraschini, C.; Jamshidian, M.; Salmieri, S.; Safrany, A.; Lacroix, M. Gamma-irradiation of cellulose nanocrystals (CNCs): Investigation of physicochemical and antioxidant properties. Cellulose 2001, 24, 2111–2124. [Google Scholar] [CrossRef]
- Sharmin, N.; Khan, R.A.; Salmien, S.; Dussault, D.; Bouchard, J.; Lacroix, M. Modification and characterization of biodegradable methylcellulose films with trimethylpropane trimethacrylate (TMPTMA) by radiation: Effect of nanocrystalline cellulose. J. Agric. Food Chem. 2012, 60, 623–629. [Google Scholar] [CrossRef]
- Madrid, J.F.; Abad, L.V. Modification of microcrystalline cellulose by gamma Radiation-induced grafting. Radiat. Phys. Chem. 2015, 115, 143–147. [Google Scholar] [CrossRef]
- Łojewska, J.; Lubańska, A.; Miśkowiec, P. FTIR in situ transmission studies on the kinetics of paper degradation via hydrolytic and oxidative reaction paths. Appl. Phys. A 2006, 83, 597–603. [Google Scholar] [CrossRef]
- Ashfaq, A.; Clochard, M.C.; Coqueret, X.; Dispenza, C.; Driscoll, M.S.; Ulański, P.; Al-Sheikhly, M. Polymerization Reactions and Modifications of Polymers by Ionizing Radiation. Polymers 2020, 12, 2877. [Google Scholar] [CrossRef]
- Lugo-Lugo, V.; Ureña-Nuñez, F.; Barrera-Díaz, C. Gamma irradiated orange peel for Cr (VI) bioreduction. Sep. Sci. Technol. 2017, 52, 2443–2455. [Google Scholar] [CrossRef]
- Sánchez-Orozco, R.; Balderas-Hernández, P.; Flore-Ramírez, N.; Roa-Morales, G.; Luna, J.; Montoya-Castro, A.J. Gamma Irradiation Induced Degradation of Orange Peels. Energies 2012, 5, 3051–3063. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Salleh, W.N.W.; Jaafar, J.; Asri, S.E.A.M.; Ismail, A.F. Physicochemical properties of green nanocrystalline cellulose isolated from recycled newspaper. RSC Adv. 2015, 5, 29842–29849. [Google Scholar] [CrossRef]
- Voronova, M.I.; Surov, O.V.; Afineevskii, A.V.; Zakharov, A.G. Properties of polyacrylamide composites reinforced by cellulose nanocrystals. Heliyon 2020, 6, e05529. [Google Scholar] [CrossRef]
- Murugan, R.; Mohan, S.; Bigotto, S.A. FTIR and polarized Raman spectra of acrylamide and polyacrylamide. J. Korean Phys. Soc. 1998, 32, 505–512. [Google Scholar]
- Zhou, C.; Wu, Q.; Yue, Y.; Zhang, Q. Application of rod-shaped cellulose nanocrystals in polyacrylamide hydrogels. J. Colloid Interface Sci. 2011, 325, 116–123. [Google Scholar] [CrossRef]
- Schwanninger, M.; Rodriguez, J.C.; Pereira, H.; Hinterstoisser, B. Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose. Vib. Spectrosc. 2004, 36, 23–40. [Google Scholar] [CrossRef]
- Huang, S.; Zhou, L.; Li, M.C.; Wu, Q.; Zhou, D. Cellulose Nanocrystals (CNCs) from Corn Stalk: Activation Energy Analysis. Materials 2017, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Xia, W.; Sinko, R.; Keten, S. Tuning glass transition in polymer nanocomposites with functionalized cellulose nanocrystals through nanoconfinement. Nano Lett. 2015, 15, 6738–6744. [Google Scholar] [CrossRef]
- Surov, O.V.; Voronova, M.I.; Afineeskii, A.V.; Zakharov, A.V. Polyethylene oxide films reinforced by cellulose nanocrystals: Microstructure properties relationship. Carbohydr. Polym. 2018, 181, 489–498. [Google Scholar] [CrossRef]
- Kitahara, Y.; Okuyama, K.; Ozawa, K.; Suga, T.; Takahshi, S.; Fujii, T. Thermal decomposition of acrylamide from polyacrylamide. Time -resolved pyrolysis with ion-attachment mass spectroscopy. J. Therm. Anal. Calorim. 2012, 110, 423–429. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, Q. A novel polyacrylamide nanocomposite hydrogel reinforced with natural chitosan nanofibers. Colloids Surf. B Biointerfaces 2011, 84, 155–162. [Google Scholar] [CrossRef]
- Lim, L.S.; Rosli, N.A.; Ahmad, I.; Lazim, A.M.; Amin, M.C.I.M. Synthesis and swelling behavior oh pH-sensitive semi-IPN superabsorbent hydrogels based on poly(acrylic acid) reinforced with cellulose nanocrystals. Nanomaterials 2017, 7, 399. [Google Scholar] [CrossRef] [PubMed]
- Ortega, A.; Sánchez, A.; Burillo, G. Binary Graft of Poly(N-vinylcaprolactam) and Poly(acrylic acid) onto Chitosan Hydrogels Using Ionizing Radiation for the Retention and controlled Release of Therapeutic Compounds. Polymers 2021, 13, 2641. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, N.; Bensingh, R.J.; Kader, M.A.; Nayak, S.K. Synthesis and properties of hydrogels prepared by various polymerization reaction systems. In Cellulose-Based Superabsorbent Hydrogels. Polymer and Polymeric Composites: A Reference Series; Mondal, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–25. [Google Scholar] [CrossRef]
- Burillo, G.; Ogawa, T. Effect the pressure of the radiation-induced crosslinking of some vinyl polymers. Radiat. Phys. Chem. 1985, 25, 383–388. [Google Scholar] [CrossRef]
- Francis, S.; Mitra, D.; Dhanawade, B.R.; Varshney, L.; Sabharwal, S. Gamma radiation synthesis of rapid swelling superporous polyacrylamide hydrogels. Radiat. Phys. Chem. 2009, 78, 951–953. [Google Scholar] [CrossRef]
- Wu, Y.T.; Zhou, Z.; Fan, Q.Q.; Chen, L.; Zhu, M.F. Facile in-situ fabrication of novel organic nanoparticle hydrogels with excellent mechanical properties. J. Mater. Chem. 2009, 19, 7340–7346. [Google Scholar] [CrossRef]
- Rao, K.M.; Kumar, A.; Han, S.S. Polysaccharide based bionanocomposites hydrogels reinforced with cellulose nanocrystals: Drug release and biocompatibility analyses. Int. J. Biol. Macromol. 2017, 101, 165–171. [Google Scholar]
- Daza, J.H.U.; Righetto, G.M.; Chaud, M.V.; Martinis, V.C.A.; Camargo, I.L.B.C.; Plepis, A.M.G. PVA/anionic collagen membranes as drug carriers of ciprofloxacin hydrochloride with sustained antibacterial activity and potential use in the treatment of ulcerative keratitis. J. Biomater. Appl. 2020, 35, 301–312. [Google Scholar] [CrossRef]
- Uhljar, L.E.; Kan, S.Y.; Radacsi, N.; Koutsos, V.; Szabó-Révész, P.; Ambrus, R. In vitro drug release, permeability, and structural test of ciprofloxacin-loaded nanofibers. Pharmaceutics 2021, 13, 556. [Google Scholar] [CrossRef]
- Doble, A. Ciprofloxacin. In XPharm: The Comprehensive Pharmacology Reference; Enna, S.J., Bylund, D.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 1–8. [Google Scholar] [CrossRef]
- Hachaichi, A.; Kouini, B.; Kian, L.K.; Asim, M.; Fouad, H. Nanocrystalline cellulose from microcrystalline cellulose of Date Palm fibers as a promising candidate for bio-nanocomposites: Isolation and characterization. Materials 2021, 14, 5313. [Google Scholar] [CrossRef]
- Kian, L.K.; Jawaid, M.; Ariffin, H.; Karim, Z. Isolation, and characterization of nanocrystalline cellulose rom roselle-derived microcrystalline cellulose. Int. J. Biol. Macromol. 2018, 114, 54–63. [Google Scholar] [CrossRef]



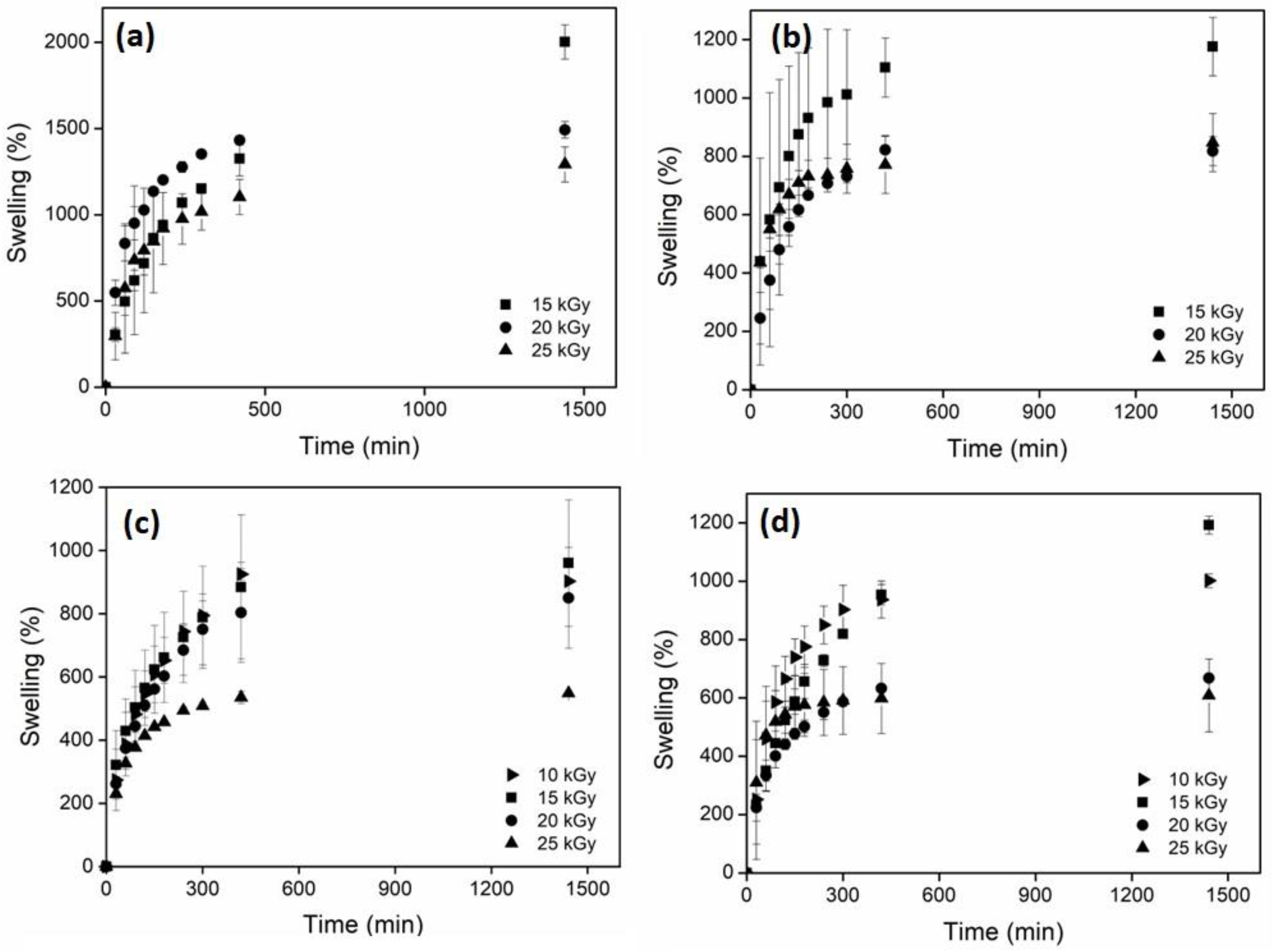

| ID | Dose (kGy) | Maximum Swelling (%) |
|---|---|---|
| net-AAm | 10 | - |
| 15 | 2214 | |
| 20 | 1472 | |
| 25 | 1290 | |
| net-AAm/CNC (1% w/w) | 10 | - |
| 15 | 1176 | |
| 20 | 832 | |
| 25 | 840 | |
| net-AAm/CNC (2% w/w) | 10 | 1001 |
| 15 | 1192 | |
| 20 | 850 | |
| 25 | 548 | |
| net-AAm/CNC (4% w/w) | 10 | 987 |
| 15 | 960 | |
| 20 | 667 | |
| 25 | 608 |
| ID | CNC (%) | E (kPa) | SD (+/−) | 6 Max (kPa) | SD (+/−) |
|---|---|---|---|---|---|
| net-AAm | 0 | 11 | 0.00058 | 15.75 | 3.1931 |
| net-AAm/CNC (1% w/w) | 1 | 14 | 0.00252 | 144.77 | 91.0468 |
| net-AAm/CNC (2% w/w) | 2 | 18 | 0.001 | 180.44 | 61.0729 |
| net-AAm/CNC (4% w/w) | 4 | 30 | 0.00289 | 433.36 | 45.5530 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega, A.; Valencia, S.; Rivera, E.; Segura, T.; Burillo, G. Reinforcement of Acrylamide Hydrogels with Cellulose Nanocrystals Using Gamma Radiation for Antibiotic Drug Delivery. Gels 2023, 9, 602. https://doi.org/10.3390/gels9080602
Ortega A, Valencia S, Rivera E, Segura T, Burillo G. Reinforcement of Acrylamide Hydrogels with Cellulose Nanocrystals Using Gamma Radiation for Antibiotic Drug Delivery. Gels. 2023; 9(8):602. https://doi.org/10.3390/gels9080602
Chicago/Turabian StyleOrtega, Alejandra, Silvia Valencia, Ernesto Rivera, Tania Segura, and Guillermina Burillo. 2023. "Reinforcement of Acrylamide Hydrogels with Cellulose Nanocrystals Using Gamma Radiation for Antibiotic Drug Delivery" Gels 9, no. 8: 602. https://doi.org/10.3390/gels9080602
APA StyleOrtega, A., Valencia, S., Rivera, E., Segura, T., & Burillo, G. (2023). Reinforcement of Acrylamide Hydrogels with Cellulose Nanocrystals Using Gamma Radiation for Antibiotic Drug Delivery. Gels, 9(8), 602. https://doi.org/10.3390/gels9080602








