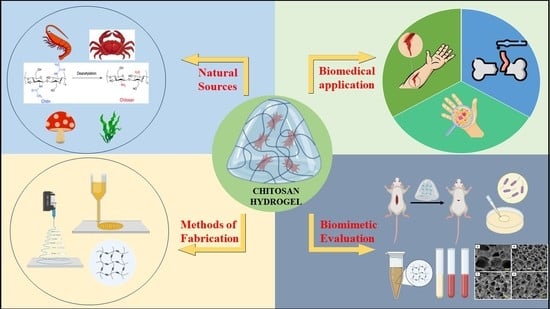
Chitosan-Based Hydrogel in the Management of Dermal Infections: A Review
Abstract
1. Introduction
2. Skin: The First Line of Defense

3. Types of Skin Infections (Bacterial, Fungal, and Parasitic)
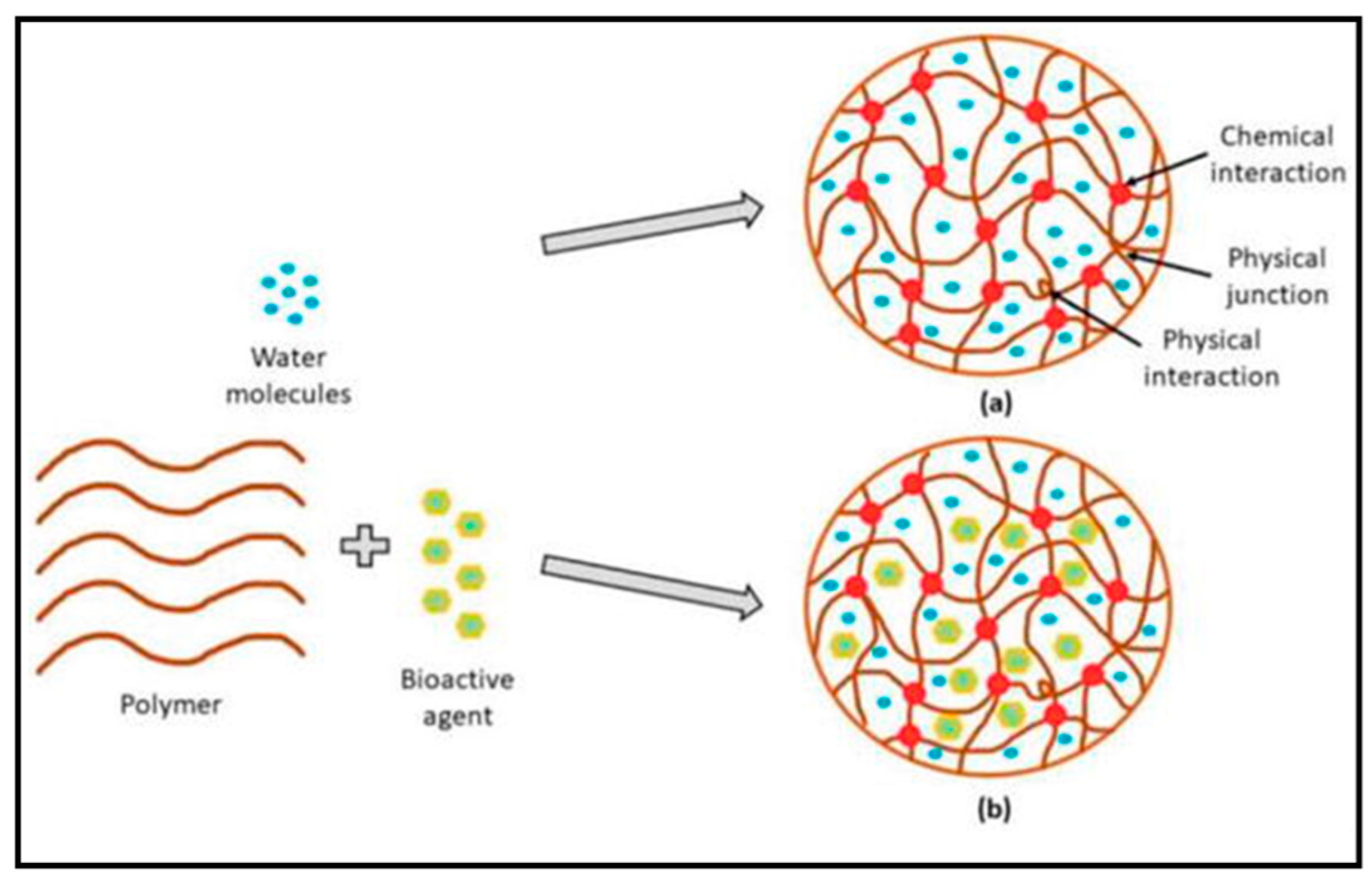
3.1. Molecular Structure of Hydrogels
3.2. Classification of Hydrogel Products
3.2.1. Based on Sources
Synthetic Source
Natural Source
3.2.2. Based on Crosslinking
Chemically Crosslinked Hydrogels
Physically Crosslinked Hydrogels
3.2.3. Classification Based on Response to Stimuli
4. Technologies Adopted in Hydrogel Preparation
4.1. Three-Dimensional Printing
4.2. Electro-Spraying
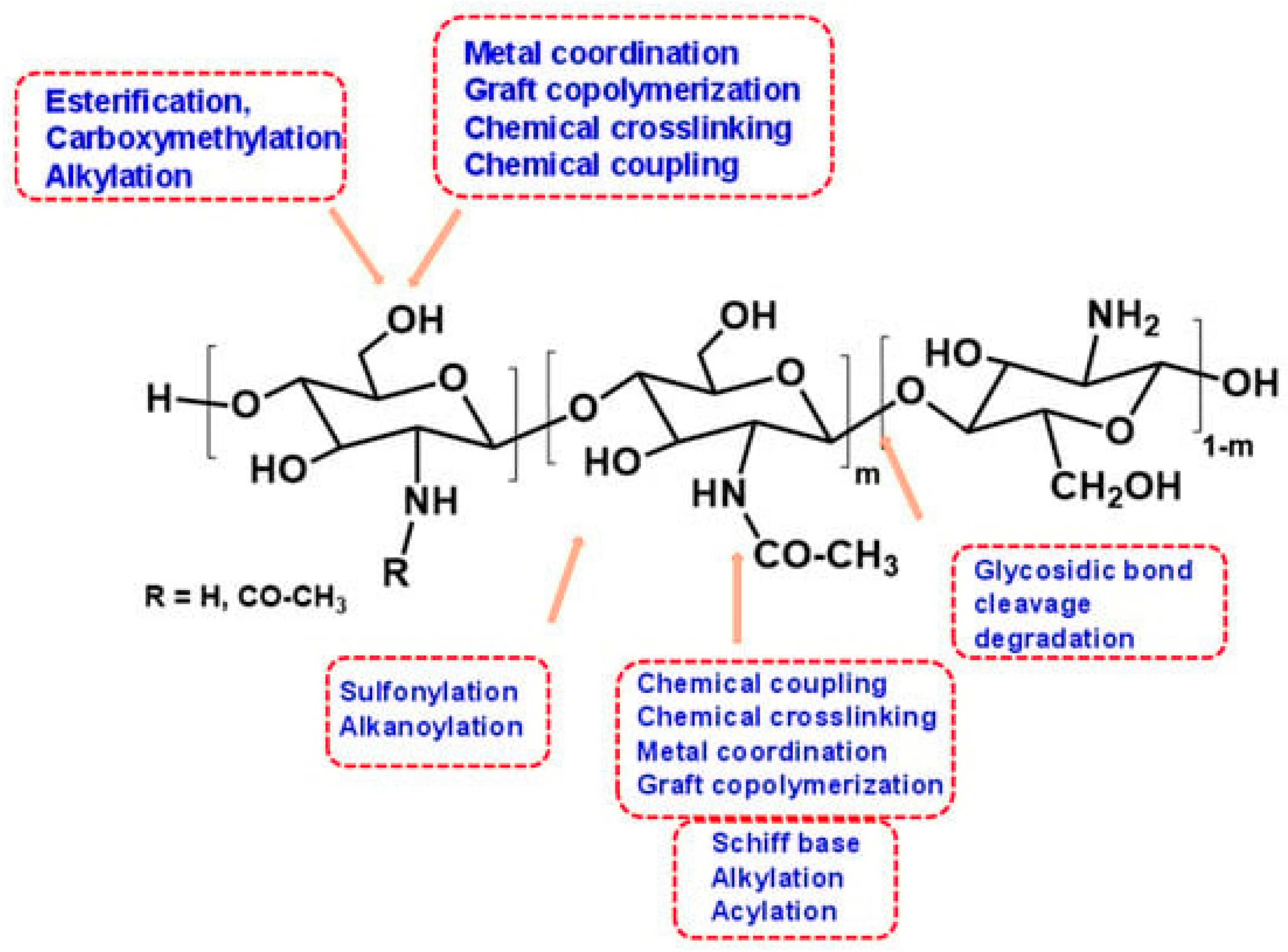
5. Chitosan as a Natural Bioactive Polymer
5.1. Effects and Mechanisms of Chitosan on Skin Wounds
5.2. Role of Chitosan in Stages of Skin Wound Healing
5.3. Antibacterial Activity of Chitosan in Skin Wound Healing
6. Preparation, Properties, and Process Optimization of Hydrogel-Based Grafted Chitosan
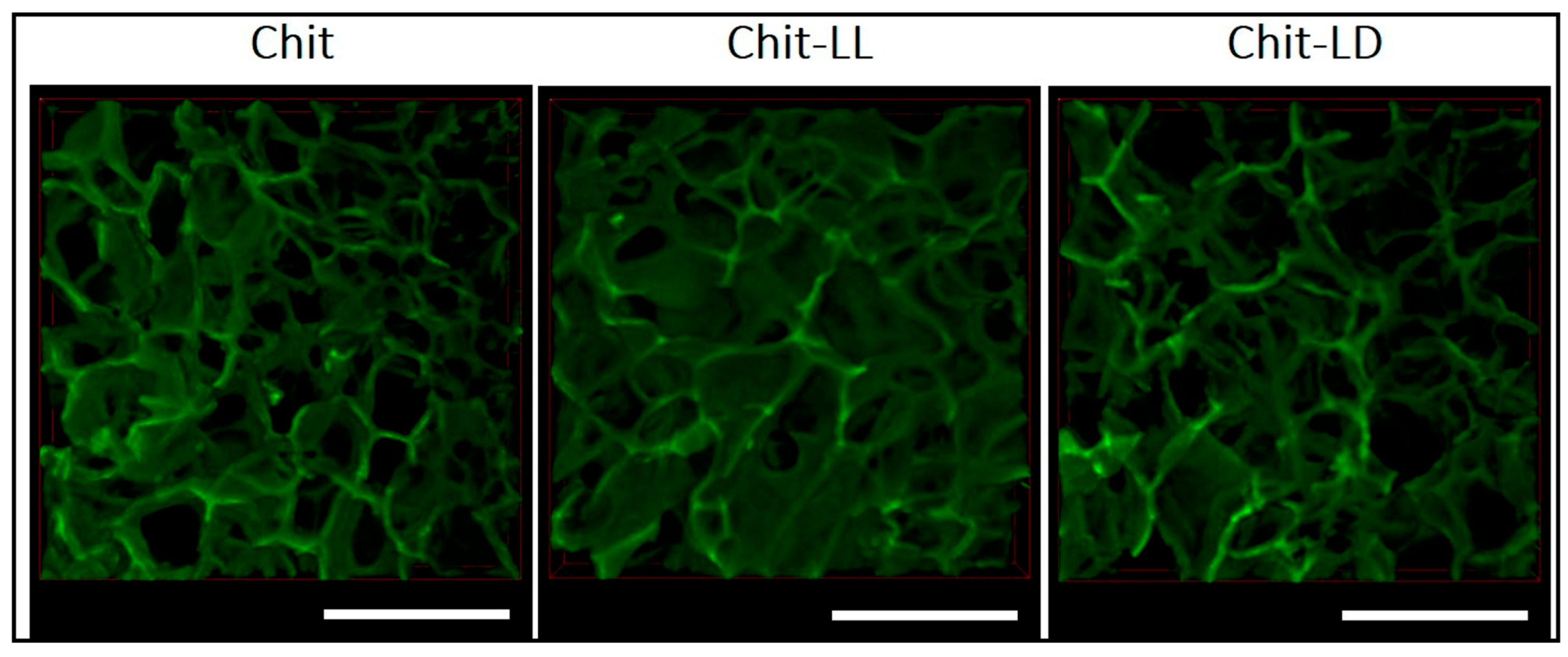
6.1. Preparation of Chitosan Crosslinking
6.2. Properties
6.3. Process Optimization
6.4. Self-Healing Chitosan Hydrogels
7. Chitosan-Based Nanoparticle-Incorporated Hydrogels
7.1. Chitosan/Drug Hydrogels
7.2. Chitosan/Bioactive Substance Hydrogels
7.3. Chitosan-Reduced/Capped Metallic Particle Gels
7.4. Other Chitosan Composite Hydrogels
7.5. Evaluations of Chitosan-Based Hydrogels
8. Chitosan Hydrogels Are Currently in Clinical Trials, and Patents
9. Challenges with Chitosan Hydrogels
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Rohr, J.R.; Barrett, C.B.; Civitello, D.J.; Craft, M.E.; Delius, B.; DeLeo, G.A.; Hudson, P.J.; Jouanard, N.; Nguyen, K.H.; Ostfeld, R.S. Emerging human infectious diseases and the links to global food production. Nat. Sustain. 2019, 2, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Tizek, L.; Schielein, M.C.; Seifert, F.; Biedermann, T.; Böhner, A.; Zink, A. Skin diseases are more common than we think: Screening results of an unreferred population at the Munich Oktoberfest. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Seth, D.; Cheldize, K.; Brown, D.; Freeman, E.F. Global Burden of Skin Disease: Inequities and Innovations. Curr. Dermatol. Rep. 2017, 6, 204–210. [Google Scholar] [CrossRef]
- Kwiecien, K.; Zegar, A.; Jung, J.; Brzoza, P.; Kwitniewski, M.; Godlewska, U.; Grygier, B.; Kwiecinska, P.; Morytko, A.; Cichy, J. Architecture of antimicrobial skin defense. Cytokine Growth Factor Rev. 2019, 49, 70–84. [Google Scholar] [CrossRef]
- Ho, T.C.; Chang, C.C.; Chan, H.P.; Chung, T.W.; Shu, C.W.; Chuang, K.P.; Duh, T.H.; Yang, M.H.; Tyan, Y.C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Khan, S.; Ullah, A.; Ullah, K.; Rehman, N.-U. Insight into hydrogels. Des. Monomers Polym. 2016, 19, 456–478. [Google Scholar] [CrossRef]
- Ahmad, Z.; Salman, S.; Khan, S.A.; Amin, A.; Rahman, Z.U.; Al-Ghamdi, Y.O.; Akhtar, K.; Bakhsh, E.M.; Khan, S.B. Versatility of Hydrogels: From Synthetic Strategies, Classification, and Properties to Biomedical Applications. Gels 2022, 8, 167. [Google Scholar] [CrossRef]
- Gasperini, L.; Mano, J.F.; Reis, R.L. Natural polymers for the microencapsulation of cells. J. R. Soc. Interface 2014, 11, 20140817. [Google Scholar] [CrossRef]
- Almajed, A.; Lemboye, K.; Moghal, A.A. A Critical Review on the Feasibility of Synthetic Polymers Inclusion in Enhancing the Geotechnical Behavior of Soils. Polymers 2022, 14, 5004. [Google Scholar] [CrossRef]
- Ertl, P.; Altmann, E.; McKenna, J.M. The Most Common Functional Groups in Bioactive Molecules and How Their Popularity Has Evolved over Time. J. Med. Chem. 2020, 63, 8408–8418. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Peters, J.T.; Wechsler, M.E.; Peppas, N.A. Advanced biomedical hydrogels: Molecular architecture and its impact on medical applications. Regen. Biomater. 2021, 8, rbab060. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Nair, A.B.; Shah, J.; Sreeharsha, N.; Gupta, S.; Shinu, P. Emerging Role of Hydrogels in Drug Delivery Systems, Tissue Engineering and Wound Management. Pharmaceutics 2021, 13, 357. [Google Scholar] [CrossRef]
- Teixeira, M.A.; Paiva, M.C.; Amorim, M.T.P.; Felgueiras, H.P. Electrospun nanocomposites containing cellulose and its derivatives modified with specialized biomolecules for an enhanced wound healing. Nanomaterials 2020, 10, 557. [Google Scholar] [CrossRef]
- Miranda, C.S.; Ribeiro, A.R.M.; Homem, N.C.; Felgueiras, H.P. Spun biotextiles in tissue engineering and biomolecules delivery systems. Antibiotics 2020, 9, 174. [Google Scholar] [CrossRef]
- Agarwal, S.; Krishnamurthy, K. Histology, Skin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar] [PubMed]
- Nawrath, C. Unraveling the complex network of cuticular structure and function. Curr. Opin. Plant Biol. 2006, 9, 281–287. [Google Scholar] [CrossRef]
- Driskell, R.R.; Jahoda, C.A.B.; Chuong, C.M.; Watt, F.M.; Horsley, V. Defining dermal adipose tissue. Exp. Dermatol. 2014, 23, 629–631. [Google Scholar] [CrossRef]
- Baroni, A.; Buommino, E.; De Gregorio, V.; Ruocco, E.; Ruocco, V.; Wolf, R. Structure and function of the epidermis related to barrier properties. Clin. Dermatol. 2012, 30, 257–262. [Google Scholar] [CrossRef]
- Nguyen, A.V.; Soulika, A.M. The dynamics of the skin’s immune system. Int. J. Mol. Sci. 2019, 20, 1811. [Google Scholar] [CrossRef] [PubMed]
- Kolarsick, P.A.J.; Kolarsick, M.A.; Goodwin, C. Anatomy and physiology of the skin. J. Dermatol. Nurses Assoc. 2011, 3, 203–213. [Google Scholar] [CrossRef]
- Godlewska, U.; Brzoza, P.; Kwiecień, K.; Kwitniewski, M.; Cichy, J. Metagenomic Studies in Inflammatory Skin Diseases. Curr. Microbiol. 2020, 77, 3201–3212. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv. 2018, 8, 7533–7549. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for biomedical applications: Their characteristics and the mechanisms behind them. Gels 2017, 3, 6. [Google Scholar] [CrossRef]
- Buwalda, S.J.; Boere, K.W.M.; Dijkstra, P.J.; Feijen, J.; Vermonden, T.; Hennink, W.E. Hydrogels in a historical perspective: From simple networks to smart materials. J. Control. Release 2014, 190, 254–273. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Nadolna, K.; Owczarek, A. The physical and chemical properties of hydrogels based on natural polymers. In Hydrogels Based on Natural Polymers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 151–172. [Google Scholar]
- Bhatia, S.; Bhatia, S. Natural polymers vs synthetic polymer. In Natural Polymer Drug Delivery Systems: Nanoparticles, Plants; Springer: Berlin/Heidelberg, Germany, 2016; pp. 95–118. [Google Scholar]
- Parhi, R. Cross-Linked Hydrogel for Pharmaceutical Applications: A Review. Adv. Pharm. Bull. 2017, 7, 515–530. [Google Scholar] [CrossRef]
- Vildanova, R.R.; Petrova, S.F.; Kolesov, S.V.; Khutoryanskiy, V.V. Biodegradable Hydrogels Based on Chitosan and Pectin for Cisplatin Delivery. Gels 2023, 9, 342. [Google Scholar] [CrossRef]
- Rebers, L.; Reichsöllner, R.; Regett, S.; Tovar, G.E.M.; Borchers, K.; Baudis, S.; Southan, A. Differentiation of physical and chemical cross-linking in gelatin methacryloyl hydrogels. Sci. Rep. 2021, 11, 3256. [Google Scholar] [CrossRef]
- Lu, L.; Yuan, S.; Wang, J.; Shen, Y.; Deng, S.; Xie, L.; Yang, Q. The Formation Mechanism of Hydrogels. Curr. Stem. Cell Res. Ther. 2018, 13, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Violeta, G.M.; Elena, D.-P.C.; Lăcrămioara, P.; Elena-Emilia, T.; Diana-Georgiana, I.; Claudia-Maria, B. Promising Hydrogels-Based Dressings for Optimal Treatment of Cutaneous Lesions. In Hydrogels; Lăcrămioara, P., Violeta, G.M., Cristina-Elena, D.-P., Eds.; IntechOpen: Rijeka, Croatia, 2022; Chapter 2. [Google Scholar] [CrossRef]
- Gyles, D.A.; Castro, L.D.; Silva, J.O.C., Jr.; Ribeiro-Costa, R.M. A review of the designs and prominent biomedical advances of natural and synthetic hydrogel formulations. Eur. Polym. J. 2017, 88, 373–392. [Google Scholar] [CrossRef]
- Crocini, C.; Walker, C.J.; Anseth, K.S.; Leinwand, L.A. Three-dimensional encapsulation of adult mouse cardiomyocytes in hydrogels with tunable stiffness. Prog. Biophys. Mol. Biol. 2020, 154, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Cheng, G.; Xing, X.; Yin, C.; Cheng, Y.; Zhou, X.; Jiang, S.; Tao, F.; Deng, H.; Li, Z. Controlled release of adenosine from core-shell nanofibers to promote bone regeneration through STAT3 signaling pathway. J. Control. Release 2020, 319, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Bhatnagar, I.; Manivasagan, P.; Kang, K.-H.; Kim, S.-K. Alginate composites for bone tissue engineering: A review. Int. J. Biol. Macromol. 2015, 72, 269–281. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y. Rational design of smart hydrogels for biomedical applications. Front. Chem. 2021, 8, 615665. [Google Scholar] [CrossRef]
- Tao, F.; Cheng, Y.; Shi, X.; Zheng, H.; Du, Y.; Xiang, W.; Deng, H. Applications of chitin and chitosan nanofibers in bone regenerative engineering. Carbohydr. Polym. 2020, 230, 115658. [Google Scholar] [CrossRef]
- Sheng, L.; Zhang, Z.; Zhang, Y.; Wang, E.; Ma, B.; Xu, Q.; Ma, L.; Zhang, M.; Pei, G.; Chang, J. A novel “hot spring”-mimetic hydrogel with excellent angiogenic properties for chronic wound healing. Biomaterials 2021, 264, 120414. [Google Scholar] [CrossRef]
- Karoyo, A.H.; Wilson, L.D. A review on the design and hydration properties of natural polymer-based hydrogels. Materials 2021, 14, 1095. [Google Scholar] [CrossRef]
- Bao, Z.; Xian, C.; Yuan, Q.; Liu, G.; Wu, J. Natural polymer-based hydrogels with enhanced mechanical performances: Preparation, structure, and property. Adv. Healthc. Mater. 2019, 8, 1900670. [Google Scholar] [CrossRef]
- Salawi, A.; Khan, A.; Zaman, M.; Riaz, T.; Ihsan, H.; Butt, M.H.; Aman, W.; Khan, R.; Majeed, I.; Almoshari, Y.; et al. Development of Statistically Optimized Chemically Cross-Linked Hydrogel for the Sustained-Release Delivery of Favipiravir. Polymers 2022, 14, 2369. [Google Scholar] [CrossRef]
- Karoyo, A.H.; Wilson, L.D. Preparation and Characterization of a polymer-based “Molecular Accordion”. Langmuir 2016, 32, 3066–3078. [Google Scholar] [CrossRef]
- Xue, X.; Hu, Y.; Wang, S.; Chen, X.; Jiang, Y.; Su, J. Fabrication of physical and chemical crosslinked hydrogels for bone tissue engineering. Bioact. Mater. 2022, 12, 327–339. [Google Scholar] [CrossRef]
- Fletes-Vargas, G.; Espinosa-Andrews, H.; Cervantes-Uc, J.M.; Limón-Rocha, I.; Luna-Bárcenas, G.; Vázquez-Lepe, M.; Morales-Hernández, N.; Jiménez-Ávalos, J.A.; Mejía-Torres, D.G.; Ramos-Martínez, P. Porous Chitosan Hydrogels Produced by Physical Crosslinking: Physicochemical, Structural, and Cytotoxic Properties. Polymers 2023, 15, 2203. [Google Scholar] [CrossRef]
- Bustamante-Torres, M.; Romero-Fierro, D.; Arcentales-Vera, B.; Palomino, K.; Magaña, H.; Bucio, E. Hydrogels Classification According to the Physical or Chemical Interactions and as Stimuli-Sensitive Materials. Gels 2021, 7, 182. [Google Scholar] [CrossRef]
- Mondal, S.; Haldar, D. A transient non-covalent hydrogel by a supramolecular gelator with dynamic covalent bonds. New J. Chem. 2021, 45, 4773–4779. [Google Scholar] [CrossRef]
- Patel, V.M.; Prajapati, B.G.; Patel, M.M. Design and characterization of chitosan-containing mucoadhesive buccal patches of propranolol hydrochloride. Acta Pharm. 2007, 57, 61–72. [Google Scholar] [CrossRef]
- Acharya, G.; Shin, C.S.; McDermott, M.; Mishra, H.; Park, H.; Kwon, I.C.; Park, K. The hydrogel template method for fabrication of homogeneous nano/microparticles. J. Control. Release 2010, 141, 314–319. [Google Scholar] [CrossRef]
- Hinton, T.J.; Jallerat, Q.; Palchesko, R.N.; Park, J.H.; Grodzicki, M.S.; Shue, H.J.; Ramadan, M.H.; Hudson, A.R.; Feinberg, A.W. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv. 2015, 1, e1500758. [Google Scholar] [CrossRef]
- Lee, U.N.; Day, J.H.; Haack, A.J.; Bretherton, R.C.; Lu, W.; DeForest, C.A.; Theberge, A.B.; Berthier, E. Layer-by-layer fabrication of 3D hydrogel structures using open microfluidics. Lab Chip 2020, 20, 525–536. [Google Scholar] [CrossRef]
- Wu, Q.; Maire, M.; Lerouge, S.; Therriault, D.; Heuzey, M.C. 3D printing of microstructured and stretchable chitosan hydrogel for guided cell growth. Adv. Biosyst. 2017, 1, 1700058. [Google Scholar] [CrossRef]
- Huang, J.; Xiong, J.; Wang, D.; Zhang, J.; Yang, L.; Sun, S.; Liang, Y. 3D Bioprinting of Hydrogels for Cartilage Tissue Engineering. Gels 2021, 7, 144. [Google Scholar] [CrossRef] [PubMed]
- Bishop, E.S.; Mostafa, S.; Pakvasa, M.; Luu, H.H.; Lee, M.J.; Wolf, J.M.; Ameer, G.A.; He, T.C.; Reid, R.R. 3-D bioprinting technologies in tissue engineering and regenerative medicine: Current and future trends. Genes Dis. 2017, 4, 185–195. [Google Scholar] [CrossRef]
- He, Y.; Yang, F.; Zhao, H.; Gao, Q.; Xia, B.; Fu, J. Research on the printability of hydrogels in 3D bioprinting. Sci. Rep. 2016, 6, 29977. [Google Scholar] [CrossRef] [PubMed]
- Roehm, K.D.; Madihally, S.V. Bioprinted chitosan-gelatin thermosensitive hydrogels using an inexpensive 3D printer. Biofabrication 2017, 10, 015002. [Google Scholar] [CrossRef]
- Demirtaş, T.T.; Irmak, G.; Gümüşderelioğlu, M. A bioprintable form of chitosan hydrogel for bone tissue engineering. Biofabrication 2017, 9, 035003. [Google Scholar] [CrossRef]
- Zhang, X.N.; Zheng, Q.; Wu, Z.L. Recent advances in 3D printing of tough hydrogels: A review. Compos. Part B Eng. 2022, 238, 109895. [Google Scholar] [CrossRef]
- Li, J.; Wu, C.; Chu, P.K.; Gelinsky, M. 3D printing of hydrogels: Rational design strategies and emerging biomedical applications. Mater. Sci. Eng. R Rep. 2020, 140, 100543. [Google Scholar] [CrossRef]
- Wang, X.; Qi, J.; Zhang, W.; Pu, Y.; Yang, R.; Wang, P.; Liu, S.; Tan, X.; Chi, B. 3D-printed antioxidant antibacterial carboxymethyl cellulose/ε-polylysine hydrogel promoted skin wound repair. Int. J. Biol. Macromol. 2021, 187, 91–104. [Google Scholar] [CrossRef]
- Miguel, S.P.; Cabral, C.S.D.; Moreira, A.F.; Correia, I.J. Production and characterization of a novel asymmetric 3D printed construct aimed for skin tissue regeneration. Colloids Surf. B Biointerfaces 2019, 181, 994–1003. [Google Scholar] [CrossRef]
- Linh, N.V.V.; Thinh, N.T.; Kien, P.T.; Quyen, T.N.; Phu, H.D. Injectable Nanocomposite Hydrogels and Electrosprayed Nano(Micro)Particles for Biomedical Applications. Adv. Exp. Med. Biol. 2018, 1077, 225–249. [Google Scholar] [CrossRef]
- Zhao, S.; Agarwal, P.; Rao, W.; Huang, H.; Zhang, R.; Liu, Z.; Yu, J.; Weisleder, N.; Zhang, W.; He, X. Coaxial electrospray of liquid core–hydrogel shell microcapsules for encapsulation and miniaturized 3D culture of pluripotent stem cells. Integr. Biol. 2014, 6, 874–884. [Google Scholar] [CrossRef]
- Zhou, X.; Hou, C.; Chang, T.-L.; Zhang, Q.; Liang, J.F. Controlled released of drug from doubled-walled PVA hydrogel/PCL microspheres prepared by single needle electrospraying method. Colloids Surf. B Biointerfaces 2020, 187, 110645. [Google Scholar] [CrossRef]
- Jain, E.; Scott, K.M.; Zustiak, S.P.; Sell, S.A. Fabrication of Polyethylene Glycol-Based Hydrogel Microspheres Through Electrospraying. Macromol. Mater. Eng. 2015, 300, 823–835. [Google Scholar] [CrossRef]
- Kumar, D.; Gihar, S.; Shrivash, M.K.; Kumar, P.; Kundu, P.P. A review on the synthesis of graft copolymers of chitosan and their potential applications. Int. J. Biol. Macromol. 2020, 163, 2097–2112. [Google Scholar] [CrossRef]
- Díaz-Montes, E.; Castro-Muñoz, R. Trends in chitosan as a primary biopolymer for functional films and coatings manufacture for food and natural products. Polymers 2021, 13, 767. [Google Scholar] [CrossRef]
- Mohite, P.; Shah, S.; Singh, S.; Rajput, T.; Munde, S.; Ade, N.; Prajapati, B.G.; Paliwal, H.; Mori, D.D.; Dudhrejiya, A.V. Chitosan and Chito-oligosaccharide: A versatile biopolymer with endless grafting possibilities for multifarious applications. Front. Bioeng. Biotechnol. 2023, 11, 718. [Google Scholar] [CrossRef]
- Eze, F.N.; Jayeoye, T.J.; Singh, S. Fabrication of intelligent pH-sensing films with antioxidant potential for monitoring shrimp freshness via the fortification of chitosan matrix with broken Riceberry phenolic extract. Food Chem. 2022, 366, 130574. [Google Scholar] [CrossRef]
- Singh, S.; Nwabor, O.F.; Syukri, D.M.; Voravuthikunchai, S.P. Chitosan-poly(vinyl alcohol) intelligent films fortified with anthocyanins isolated from Clitoria ternatea and Carissa carandas for monitoring beverage freshness. Int. J. Biol. Macromol. 2021, 182, 1015–1025. [Google Scholar] [CrossRef]
- Nwabor, O.F.; Singh, S.; Paosen, S.; Vongkamjan, K.; Voravuthikunchai, S.P. Enhancement of food shelf life with polyvinyl alcohol-chitosan nanocomposite films from bioactive Eucalyptus leaf extracts. Food Biosci. 2020, 36, 100609. [Google Scholar] [CrossRef]
- Detsi, A.; Kavetsou, E.; Kostopoulou, I.; Pitterou, I.; Pontillo, A.R.N.; Tzani, A.; Christodoulou, P.; Siliachli, A.; Zoumpoulakis, P. Nanosystems for the encapsulation of natural products: The case of chitosan biopolymer as a matrix. Pharmaceutics 2020, 12, 669. [Google Scholar] [CrossRef] [PubMed]
- Purohit, P.; Mittal, R.; Bhatt, A. Polymer Grafting and chemical reactions involved in. Front. Bioeng. Biotechnol. 2023, 10, 1044927. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Zheng, J.; Jiao, S.; Cheng, G.; Feng, C.; Du, Y.; Liu, H. A review on the preparation of chitosan oligosaccharides and application to human health, animal husbandry and agricultural production. Carbohydr. Polym. 2019, 220, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, A. The virtuous potential of chitosan oligosaccharide for promising biomedical applications. J. Mater. Res. 2020, 35, 1123–1134. [Google Scholar] [CrossRef]
- Prajapati, B. Chitosan a marine medical polymer and its lipid lowering capacity. Int. J. Health 2009, 9, 2. [Google Scholar]
- El-Hack, M.E.A.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; El-Hakim, Y.M.A.; Al-Sagheer, A.A. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: A review. Int. J. Biol. Macromol. 2020, 164, 2726–2744. [Google Scholar] [CrossRef]
- Feng, P.; Luo, Y.; Ke, C.; Qiu, H.; Wang, W.; Zhu, Y.; Hou, R.; Xu, L.; Wu, S. Chitosan-based functional materials for skin wound repair: Mechanisms and applications. Front. Bioeng. Biotechnol. 2021, 9, 650598. [Google Scholar] [CrossRef]
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Yoshikawa, K. Cutaneous wound healing: An update. J. Dermatol. 2001, 28, 521–534. [Google Scholar] [CrossRef]
- Kirfel, G.; Herzog, V. Migration of epidermal keratinocytes: Mechanisms, regulation, and biological significance. Protoplasma 2004, 223, 67–78. [Google Scholar] [CrossRef]
- Cañedo-Dorantes, L.; Cañedo-Ayala, M. Skin acute wound healing: A comprehensive review. Int. J. Inflamm. 2019, 2019, 3706315. [Google Scholar] [CrossRef]
- Velazquez, O.C. Angiogenesis and vasculogenesis: Inducing the growth of new blood vessels and wound healing by stimulation of bone marrow–derived progenitor cell mobilization and homing. J. Vasc. Surg. 2007, 45, A39–A47. [Google Scholar] [CrossRef]
- Xue, M.; Jackson, C.J. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef]
- De Abreu, F.R.; Campana-Filho, S.P. Characteristics and properties of carboxymethylchitosan. Carbohydr. Polym. 2009, 75, 214–221. [Google Scholar] [CrossRef]
- Gaharwar, A.K. Engineered Biomaterials for In Situ Tissue Regeneration. Nat. Rev. Mater. 2022, 5, S590. [Google Scholar] [CrossRef]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin wound healing process and new emerging technologies for skin wound care and regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef]
- Gushiken, L.F.S.; Beserra, F.P.; Bastos, J.K.; Jackson, C.J.; Pellizzon, C.H. Cutaneous wound healing: An update from physiopathology to current therapies. Life 2021, 11, 665. [Google Scholar] [CrossRef]
- Shi, Q.; Qian, Z.; Liu, D.; Sun, J.; Wang, X.; Liu, H.; Xu, J.; Guo, X. GMSC-Derived Exosomes Combined with a Chitosan/Silk Hydrogel Sponge Accelerates Wound Healing in a Diabetic Rat Skin Defect Model. Front. Physiol. 2017, 8, 904. [Google Scholar] [CrossRef]
- Wei, L.; Tan, J.; Li, L.; Wang, H.; Liu, S.; Chen, J.; Weng, Y.; Liu, T. Chitosan/Alginate Hydrogel Dressing Loaded FGF/VE-Cadherin to Accelerate Full-Thickness Skin Regeneration and More Normal Skin Repairs. Int. J. Mol. Sci. 2022, 23, 1249. [Google Scholar] [CrossRef]
- Nunes, C.; Coimbra, M.A.; Ferreira, P. Tailoring Functional Chitosan-Based Composites for Food Applications. Chem. Rec. 2018, 18, 1138. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bai, H.; Yang, Y.; Yoon, J.; Wang, S.; Zhang, X. Supramolecular antibacterial materials for combatting antibiotic resistance. Adv. Mater. 2019, 31, 1805092. [Google Scholar] [CrossRef] [PubMed]
- Abid, S.; Hussain, T.; Nazir, A.; Zahir, A.; Ramakrishna, S.; Hameed, M.; Khenoussi, N. Enhanced antibacterial activity of PEO-chitosan nanofibers with potential application in burn infection management. Int. J. Biol. Macromol. 2019, 135, 1222–1236. [Google Scholar] [CrossRef] [PubMed]
- Augustine, R.; Rehman, S.R.U.; Ahmed, R.; Zahid, A.A.; Sharifi, M.; Falahati, M.; Hasan, A. Electrospun chitosan membranes containing bioactive and therapeutic agents for enhanced wound healing. Int. J. Biol. Macromol. 2020, 156, 153–170. [Google Scholar] [CrossRef]
- Atay, H.Y. Antibacterial Activity of Chitosan-Based Systems. In Functional Chitosan; Jana, S., Jana, S., Eds.; Springer: Singapore, 2019; pp. 457–489. [Google Scholar] [CrossRef]
- Sharma, P.K.; Halder, M.; Srivastava, U.; Singh, Y. Antibacterial PEG-Chitosan Hydrogels for Controlled Antibiotic/Protein Delivery. ACS Appl. Bio Mater. 2019, 2, 5313–5322. [Google Scholar] [CrossRef]
- Manal, A.A.; Jaydee, D.C.; Heather, J.L.B.; Stephen, C.M.; Lyall, R.H. Antimicrobial Properties of a Chitosan Dextran-Based Hydrogel for Surgical Use. Antimicrob. Agents Chemother. 2012, 56, 280–287. [Google Scholar] [CrossRef]
- Suflet, D.M.; Popescu, I.; Pelin, I.M.; Ichim, D.L.; Daraba, O.M.; Constantin, M.; Fundueanu, G. Dual Cross-Linked Chitosan/PVA Hydrogels Containing Silver Nanoparticles with Antimicrobial Properties. Pharmaceutics 2021, 13, 1461. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Qing, X.; He, G.; Liu, Z.; Yin, Y.; Cai, W.; Fan, L.; Fardim, P. Preparation and properties of polyvinyl alcohol/N–succinyl chitosan/lincomycin composite antibacterial hydrogels for wound dressing. Carbohydr. Polym. 2021, 261, 117875. [Google Scholar] [CrossRef]
- Baghaie, S.; Khorasani, M.T.; Zarrabi, A.; Moshtaghian, J. Wound healing properties of PVA/starch/chitosan hydrogel membranes with nano Zinc oxide as antibacterial wound dressing material. J. Biomater. Sci. Polym. Ed. 2017, 28, 2220–2241. [Google Scholar] [CrossRef]
- Chopra, H.; Bibi, S.; Kumar, S.; Khan, M.S.; Kumar, P.; Singh, I. Preparation and Evaluation of Chitosan/PVA Based Hydrogel Films Loaded with Honey for Wound Healing Application. Gels 2022, 8, 111. [Google Scholar] [CrossRef]
- Ahmadi, F.; Oveisi, Z.; Samani, S.M.; Amoozgar, Z. Chitosan based hydrogels: Characteristics and pharmaceutical applications. Res. Pharm. Sci. 2015, 10, 1–16. [Google Scholar]
- Summonte, S.; Racaniello, G.F.; Lopedota, A.; Denora, N.; Bernkop-Schnürch, A. Thiolated polymeric hydrogels for biomedical application: Cross-linking mechanisms. J. Control. Release 2021, 330, 470–482. [Google Scholar] [CrossRef]
- Thirupathi, K.; Raorane, C.J.; Ramkumar, V.; Ulagesan, S.; Santhamoorthy, M.; Raj, V.; Krishnakumar, G.S.; Phan, T.T.; Kim, S.-C. Update on Chitosan-Based Hydrogels: Preparation, Characterization, and Its Antimicrobial and Antibiofilm Applications. Gels 2023, 9, 35. [Google Scholar] [CrossRef]
- Khorasani, M.T.; Joorabloo, A.; Adeli, H.; Mansoori-Moghadam, Z.; Moghaddam, A. Design and optimization of process parameters of polyvinyl (alcohol)/chitosan/nano zinc oxide hydrogels as wound healing materials. Carbohydr. Polym. 2019, 207, 542–554. [Google Scholar] [CrossRef]
- Negi, P.; Singh, B.; Sharma, G.; Beg, S.; Raza, K.; Katare, O.P. Phospholipid microemulsion-based hydrogel for enhanced topical delivery of lidocaine and prilocaine: QbD-based development and evaluation. Drug Deliv. 2016, 23, 941–957. [Google Scholar] [CrossRef]
- Dalwadi, C.; Patel, G. Implementation of “quality by design (QbD)” approach for the development of 5-fluorouracil loaded thermosensitive hydrogel. Curr. Drug Deliv. 2016, 13, 512–527. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Joshi, N.; Thorat, K.; Kaur, S.; Chandan, R.; Banerjee, R. A tumor responsive self healing prodrug hydrogel enables synergistic action of doxorubicin and miltefosine for focal combination chemotherapy. J. Mater. Chem. B 2019, 7, 2920–2925. [Google Scholar] [CrossRef]
- Devi, V.K.A.; Shyam, R.; Palaniappan, A.; Jaiswal, A.K.; Oh, T.-H.; Nathanael, A.J. Self-Healing Hydrogels: Preparation, Mechanism and Advancement in Biomedical Applications. Polymers 2021, 13, 3782. [Google Scholar] [CrossRef]
- Craciun, A.M.; Morariu, S.; Marin, L. Self-Healing Chitosan Hydrogels: Preparation and Rheological Characterization. Polymers 2022, 14, 2570. [Google Scholar] [CrossRef]
- Guo, S.; Ren, Y.; Chang, R.; He, Y.; Zhang, D.; Guan, F.; Yao, M. Injectable Self-Healing Adhesive Chitosan Hydrogel with Antioxidative, Antibacterial, and Hemostatic Activities for Rapid Hemostasis and Skin Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 34455–34469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tan, B.; Wu, Y.; Zhang, M.; Xie, X.; Liao, J. An injectable, self-healing carboxymethylated chitosan hydrogel with mild photothermal stimulation for wound healing. Carbohydr. Polym. 2022, 293, 119722. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Chen, B.; Wang, L.; Sun, Y.; Jin, Z.; Liu, X.; Ouyang, L.; Liao, Y. Chitosan@ Puerarin hydrogel for accelerated wound healing in diabetic subjects by miR-29ab1 mediated inflammatory axis suppression. Bioact. Mater. 2023, 19, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Tian, M.; Feng, R.; Dang, Y.; Zhang, M. Novel Self-Healing Hydrogel with Injectable, pH-Responsive, Strain-Sensitive, Promoting Wound-Healing, and Hemostatic Properties Based on Collagen and Chitosan. ACS Biomater. Sci. Eng. 2020, 6, 3855–3867. [Google Scholar] [CrossRef]
- Jiang, Y.; Krishnan, N.; Heo, J.; Fang, R.H.; Zhang, L. Nanoparticle–hydrogel superstructures for biomedical applications. J. Control. Release 2020, 324, 505–521. [Google Scholar] [CrossRef]
- Dannert, C.; Stokke, B.T.; Dias, R.S. Nanoparticle-Hydrogel Composites: From Molecular Interactions to Macroscopic Behavior. Polymers 2019, 11, 275. [Google Scholar] [CrossRef]
- Shafique, M.; Sohail, M.; Minhas, M.U.; Khaliq, T.; Kousar, M.; Khan, S.; Hussain, Z.; Mahmood, A.; Abbasi, M.; Aziz, H.C. Bio-functional hydrogel membranes loaded with chitosan nanoparticles for accelerated wound healing. Int. J. Biol. Macromol. 2021, 170, 207–221. [Google Scholar] [CrossRef]
- Mohamed, R.R.; Sabaa, M.W. Synthesis and characterization of antimicrobial crosslinked carboxymethyl chitosan nanoparticles loaded with silver. Int. J. Biol. Macromol. 2014, 69, 95–99. [Google Scholar] [CrossRef]
- Zhang, M.; Qiao, X.; Han, W.; Jiang, T.; Liu, F.; Zhao, X. Alginate-chitosan oligosaccharide-ZnO composite hydrogel for accelerating wound healing. Carbohydr. Polym. 2021, 266, 118100. [Google Scholar] [CrossRef]
- George, D.; Maheswari, P.U.; Begum, K.M.M.S. Synergic formulation of onion peel quercetin loaded chitosan-cellulose hydrogel with green zinc oxide nanoparticles towards controlled release, biocompatibility, antimicrobial and anticancer activity. Int. J. Biol. Macromol. 2019, 132, 784–794. [Google Scholar] [CrossRef]
- Fu, J.; Yang, F.; Guo, Z. The chitosan hydrogels: From structure to function. New J. Chem. 2018, 42, 17162–17180. [Google Scholar] [CrossRef]
- Peers, S.; Montembault, A.; Ladaviere, C. Chitosan hydrogels incorporating colloids for sustained drug delivery. Carbohydr. Polym. 2022, 275, 118689. [Google Scholar] [CrossRef]
- Hoang, H.T.; Vu, T.T.; Karthika, V.; Jo, S.-H.; Jo, Y.-J.; Seo, J.-W.; Oh, C.-W.; Park, S.-H.; Lim, K.T. Dual cross-linked chitosan/alginate hydrogels prepared by Nb-Tz ‘click’reaction for pH responsive drug delivery. Carbohydr. Polym. 2022, 288, 119389. [Google Scholar] [CrossRef]
- Shah, S.A.; Sohail, M.; Karperien, M.; Johnbosco, C.; Mahmood, A.; Kousar, M. Chitosan and carboxymethyl cellulose-based 3D multifunctional bioactive hydrogels loaded with nano-curcumin for synergistic diabetic wound repair. Int. J. Biol. Macromol. 2023, 227, 1203–1220. [Google Scholar] [CrossRef]
- Tolstova, T.; Drozdova, M.; Popyrina, T.; Matveeva, D.; Demina, T.; Akopova, T.; Andreeva, E.; Markvicheva, E. Preparation and In Vitro Evaluation of Chitosan-g-Oligolactide Based Films and Macroporous Hydrogels for Tissue Engineering. Polymers 2023, 15, 907. [Google Scholar] [CrossRef]
- Islam, M.M.; Shahruzzaman, M.; Biswas, S.; Sakib, M.N.; Rashid, T.U. Chitosan based bioactive materials in tissue engineering applications-A review. Bioact. Mater. 2020, 5, 164–183. [Google Scholar] [CrossRef]
- Magli, S.; Rossi, G.B.; Risi, G.; Bertini, S.; Cosentino, C.; Crippa, L.; Ballarini, E.; Cavaletti, G.; Piazza, L.; Masseroni, E. Design and Synthesis of Chitosan—Gelatin Hybrid Hydrogels for 3D Printable in vitro Models. Front. Chem. 2020, 8, 524. [Google Scholar] [CrossRef]
- Saberian, M.; Seyedjafari, E.; Zargar, S.J.; Mahdavi, F.S.; Sanaei-rad, P. Fabrication and characterization of alginate/chitosan hydrogel combined with honey and aloe vera for wound dressing applications. J. Appl. Polym. Sci. 2021, 138, 51398. [Google Scholar] [CrossRef]
- Cheng, K.-C.; Huang, C.-F.; Wei, Y.; Hsu, S.-h. Novel chitosan–cellulose nanofiber self-healing hydrogels to correlate self-healing properties of hydrogels with neural regeneration effects. NPG Asia Mater. 2019, 11, 25. [Google Scholar] [CrossRef]
- Tenório, F.S.; do Amaral Montanheiro, T.L.; dos Santos, A.M.I.; dos Santos Silva, M.; Lemes, A.P.; Tada, D.B. Chitosan hydrogel covalently crosslinked by gold nanoparticle: Eliminating the use of toxic crosslinkers. J. Appl. Polym. Sci. 2021, 138, 49819. [Google Scholar] [CrossRef]
- Xie, Y.; Liao, X.; Zhang, J.; Yang, F.; Fan, Z. Novel chitosan hydrogels reinforced by silver nanoparticles with ultrahigh mechanical and high antibacterial properties for accelerating wound healing. Int. J. Biol. Macromol. 2018, 119, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Nwabor, O.F.; Sukri, D.M.; Wunnoo, S.; Dumjun, K.; Lethongkam, S.; Kusolphat, P.; Hemtanon, N.; Klinprathum, K.; Sunghan, J.; et al. Poly (vinyl alcohol) copolymerized with xanthan gum/hypromellose/sodium carboxymethyl cellulose dermal dressings functionalized with biogenic nanostructured materials for antibacterial and wound healing application. Int. J. Biol. Macromol. 2022, 216, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, M.; Zhang, Y.; Cao, Q.; Wang, X.; Han, Y.; Sun, G.; Li, Y.; Zhou, J. Novel lignin–chitosan–PVA composite hydrogel for wound dressing. Mater. Sci. Eng. C 2019, 104, 110002. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.C.; Liao, Y.; Karthikeyan, K.G.; Pan, X.J. Mesoporous cellulose-chitosan composite hydrogel fabricated via the co-dissolution-regeneration process as biosorbent of heavy metals. Environ. Pollut. 2021, 286, 117324. [Google Scholar] [CrossRef]
- Crompton, K.E.; Prankerd, R.J.; Paganin, D.M.; Scott, T.F.; Horne, M.K.; Finkelstein, D.I.; Gross, K.A.; Forsythe, J.S. Morphology and gelation of thermosensitive chitosan hydrogels. Biophys. Chem. 2005, 117, 47–53. [Google Scholar] [CrossRef]
- Park, H.; Park, K.; Kim, D. Preparation and swelling behavior of chitosan-based superporous hydrogels for gastric retention application. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2006, 76, 144–150. [Google Scholar] [CrossRef]
- Rohindra, D.R.; Nand, A.V.; Khurma, J.R. Swelling properties of chitosan hydrogels. South Pac. J. Nat. Appl. Sci. 2004, 22, 32–35. [Google Scholar] [CrossRef]
- Ahn, J.; Ryu, J.; Song, G.; Whang, M.; Kim, J. Network structure and enzymatic degradation of chitosan hydrogels determined by crosslinking methods. Carbohydr. Polym. 2019, 217, 160–167. [Google Scholar] [CrossRef]
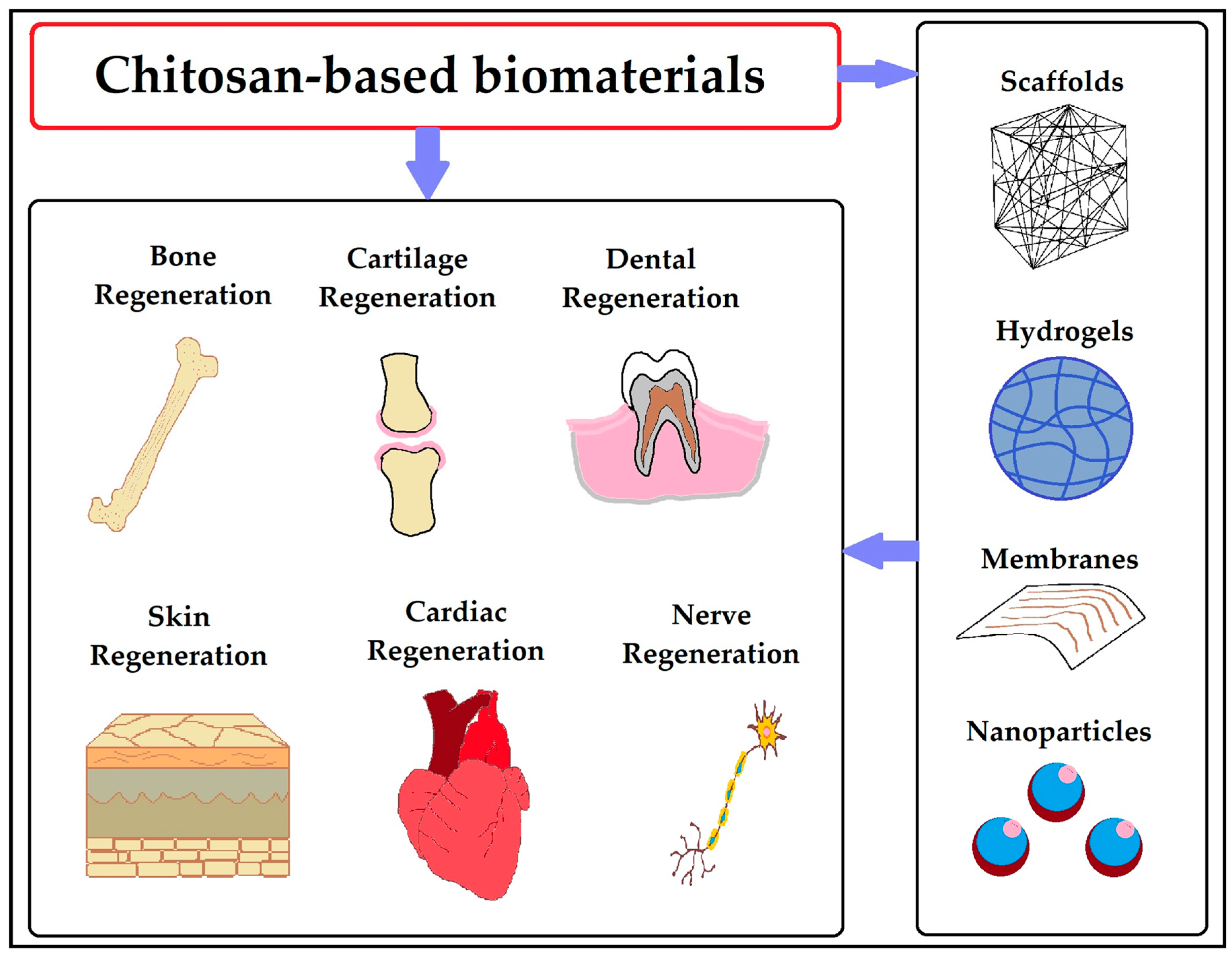
- Kim, Y.; Zharkinbekov, Z.; Raziyeva, K.; Tabyldiyeva, L.; Berikova, K.; Zhumagul, D.; Temirkhanova, K.; Saparov, A. Chitosan-Based Biomaterials for Tissue Regeneration. Pharmaceutics 2023, 15, 807. [Google Scholar] [CrossRef]
- Yan, J.; Yang, L.; Wang, G.; Xiao, Y.; Zhang, B.; Qi, N. Biocompatibility evaluation of chitosan-based injectable hydrogels for the culturing mice mesenchymal stem cells in vitro. J. Biomater. Appl. 2010, 24, 625–637. [Google Scholar] [CrossRef]
- Akakuru, O.U.; Isiuku, B.O. Chitosan hydrogels and their glutaraldehyde-crosslinked counterparts as potential drug release and tissue engineering systems-synthesis, characterization, swelling kinetics and mechanism. J. Phys. Chem. Biophys. 2017, 7, 3. [Google Scholar]
- Dutta, P.K. Chitin and Chitosan for Regenerative Medicine; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Giri, T.K.; Thakur, A.; Alexander, A.; Badwaik, H.; Tripathi, D.K. Modified chitosan hydrogels as drug delivery and tissue engineering systems: Present status and applications. Acta Pharm. Sin. B 2012, 2, 439–449. [Google Scholar] [CrossRef]
- Yu, Y.; Cheng, Y.; Tong, J.; Zhang, L.; Wei, Y.; Tian, M. Recent advances in thermo-sensitive hydrogels for drug delivery. J. Mater. Chem. B 2021, 9, 2979–2992. [Google Scholar] [CrossRef]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Kibungu, C.; Kondiah, P.P.D.; Kumar, P.; Choonara, Y.E. This Review Recent Advances in Chitosan and Alginate-Based Hydrogels for Wound Healing Application. Front. Mater. 2021, 8, 681960. [Google Scholar] [CrossRef]
- Dextran-Chitosan-Based In-Situ Gelling Hydrogels for Biomedical Applications. Available online: https://patents.google.com/patent/US20110076332A1 (accessed on 9 May 2023).
- Skin Repair Composition Containing Chitosan Hydrogel. Available online: https://patents.google.com/patent/JP2005517043A (accessed on 9 May 2023).
- Composite Chitosan Hydrogel Dressing, as Well as Preparation Method and Applications Thereof. Available online: https://patents.google.com/patent/CN104707164B (accessed on 9 May 2023).
- Chitosan-Containing hydrogel and Method of Cosmetic Skin Care with Its Use. Available online: https://patents.google.com/patent/RU2667130C1 (accessed on 9 May 2023).
- Pseudo-Thermosetting Neutralized Chitosan Composition Forming a Hydrogel and a process for Producing the Same. Available online: https://patents.google.com/patent/US8507563B2 (accessed on 9 May 2023).
- Enhanced Targeted Drug Delivery System via Chitosan Hydrogel and Chlorotoxin. Available online: https://patents.google.com/patent/US9522114B1 (accessed on 9 May 2023).
- Chitosan Hydrogel Derivatives as a Coating Agent with a Broad Spectrum of Antimicrobial Activities. Available online: https://patents.google.com/patent/US9750847B2 (accessed on 9 May 2023).
- Cross-Linkable Chitosan Composition for Producing a Chitosan Hydrogel. Available online: https://patents.google.com/patent/NZ584996A (accessed on 9 May 2023).
- Elangwe, C.N.; Morozkina, S.N.; Olekhnovich, R.O.; Krasichkov, A.; Polyakova, V.O.; Uspenskaya, M.V. A Review on Chitosan and Cellulose Hydrogels for Wound Dressings. Polymers 2022, 14, 5163. [Google Scholar] [CrossRef]
- Wen, Y.; Li, F.; Li, C.; Yin, Y.; Li, J. High mechanical strength chitosan-based hydrogels cross-linked with poly(ethylene glycol)/polycaprolactone micelles for the controlled release of drugs/growth factors. J. Mater. Chem. B 2017, 5, 961–971. [Google Scholar] [CrossRef]
- El-Hady, M.M.A.; Saeed, S.E. Antibacterial Properties and pH Sensitive Swelling of Insitu Formed Silver-Curcumin Nanocomposite Based Chitosan Hydrogel. Polymers 2020, 12, 2451. [Google Scholar] [CrossRef]
- Raju, L.; AR, S.C.L.; Prakash, N.K.U.; Rajkumar, E. Chitosan-terephthaldehyde hydrogels—Effect of concentration of cross-linker on structural, swelling, thermal and antimicrobial properties. Materialia 2021, 16, 101082. [Google Scholar] [CrossRef]
- Sacco, P.; Furlani, F.; De Marzo, G.; Marsich, E.; Paoletti, S.; Donati, I. Concepts for Developing Physical Gels of Chitosan and of Chitosan Derivatives. Gels 2018, 4, 67. [Google Scholar] [CrossRef]
- Galante, R.; Rediguieri, C.F.; Kikuchi, I.S.; Vasquez, P.A.; Colaço, R.; Serro, A.P.; Pinto, T.J. About the Sterilization of Chitosan Hydrogel Nanoparticles. PLoS ONE 2016, 11, e0168862. [Google Scholar] [CrossRef] [PubMed]
- Bryuzgin, E.; Bryuzgina, E.; Yartseva, V.; Belina, K.; Makevnina, O.; Kolyaganova, O.; Klimov, V.; Navrotskiy, A.; Novakov, I. Biodegradation Control of Chitosan Materials by Surface Modification with Copolymers of Glycidyl Methacrylate and Alkyl Methacrylates. Fibers Polym. 2022, 23, 2502–2510. [Google Scholar] [CrossRef]
- Catoira, M.C.; González-Payo, J.; Fusaro, L.; Ramella, M.; Boccafoschi, F. Natural hydrogels R&D process: Technical and regulatory aspects for industrial implementation. J. Mater. Sci. Mater. Med. 2020, 31, 64. [Google Scholar] [CrossRef] [PubMed]
- Ben Bouali, A.; Montembault, A.; David, L.; Von Boxberg, Y.; Viallon, M.; Hamdi, B.; Nothias, F.; Fodil, R.; Féréol, S. Nanoscale mechanical properties of chitosan hydrogels as revealed by AFM. Prog. Biomater. 2020, 9, 187–201. [Google Scholar] [CrossRef]




| Bacterial Species | Hydrogel Type | Ref. |
|---|---|---|
| E. coli and S. aureus | Polyvinyl alcohol (PVA)/N–succinyl chitosan (NSCS)/lincomycin hydrogels | [104] |
| E. coli | PEG–Chitosan Hydrogel | [100] |
| E. coli and S. aureus | Chitosan/Alginate Hydrogel Dressing Loaded FGF/VE-Cadherin | [94] |
| E. coli, S. aureus and P. aeruginosa | PVA/Starch/Chitosan Hydrogel Membranes with Nano Zinc oxide | [105] |
| S. aureus | Chitosan/PVA-Based Hydrogel Films | [106] |
| Patent Title | Application | Patent No. | Year | Ref. |
|---|---|---|---|---|
| Dextran-chitosan-based in-situ gelling hydrogels for biomedical applications | Tissue engineering applications to prevent tissue ingrowth | US20110076332A1 | 2010 | [152] |
| Skin repair composition containing chitosan hydrogel | Repairing/healing chronic wounds or acute wound-type skin damage | JP2005517043A | 2002 | [153] |
| Composite chitosan hydrogel dressing, as well as preparation method and applications thereof | Extended drug delivery and wound healing | CN104707164B | 2015 | [154] |
| Chitosan-containing hydrogel and method of cosmetic skin care with its use | Cosmetic skin care | RU2667130C1 | 2017 | [155] |
| Pseudo-thermosetting neutralized chitosan composition forming a hydrogel and a process for producing the same | Drug delivery system | US8507563B2 | 2004 | [156] |
| Enhanced targeted drug delivery system via chitosan hydrogel and chlorotoxin | Drug delivery to tumour cells, such as glioma | US9522114B1 | 2015 | [157] |
| Chitosan hydrogel derivatives as a coating agent with a broad spectrum of antimicrobial activities | Antimicrobial water shield coating agent. | US9750847B2 | 2009 | [158] |
| Cross-linkable chitosan composition for producing a chitosan hydrogel | Used in pharmaceuticals, in the food industry, as a glue, as a lubricant or as a drilling servicing fluid | NZ584996A | 2008 | [159] |
| Hydrogels | Application | Ref. |
|---|---|---|
| Tegasorb ® 3M | Chitosan particles swell when they absorb exudate, generating a soft gel. Leg ulcers, sacral wounds, and chronic wounds can all benefit from this treatment. | [25] |
| Chitoseal ® Abbott | It has excellent biocompatibility and hemostatic properties. Helpful for bleeding wounds | [160] |
| Chitoflex ® HemCon | Antibacterial and biocompatible. For stuffing into a wound track to control severe bleeding | [25] |
| Chitopoly ® Fujispinning | Antimicrobial clothing made with chitosan and polynosic Junlon poly(acrylate). | [25] |
| Chitopack C ® Eisai | Repairs damaged bodily tissues and regenerated skin regularly. | [160] |
| Chitoderm ® plus | Adsorbent properties. | [160] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohite, P.; Rahayu, P.; Munde, S.; Ade, N.; Chidrawar, V.R.; Singh, S.; Jayeoye, T.J.; Prajapati, B.G.; Bhattacharya, S.; Patel, R.J. Chitosan-Based Hydrogel in the Management of Dermal Infections: A Review. Gels 2023, 9, 594. https://doi.org/10.3390/gels9070594
Mohite P, Rahayu P, Munde S, Ade N, Chidrawar VR, Singh S, Jayeoye TJ, Prajapati BG, Bhattacharya S, Patel RJ. Chitosan-Based Hydrogel in the Management of Dermal Infections: A Review. Gels. 2023; 9(7):594. https://doi.org/10.3390/gels9070594
Chicago/Turabian StyleMohite, Popat, Pudji Rahayu, Shubham Munde, Nitin Ade, Vijay R. Chidrawar, Sudarshan Singh, Titilope J. Jayeoye, Bhupendra G. Prajapati, Sankha Bhattacharya, and Ravish J. Patel. 2023. "Chitosan-Based Hydrogel in the Management of Dermal Infections: A Review" Gels 9, no. 7: 594. https://doi.org/10.3390/gels9070594
APA StyleMohite, P., Rahayu, P., Munde, S., Ade, N., Chidrawar, V. R., Singh, S., Jayeoye, T. J., Prajapati, B. G., Bhattacharya, S., & Patel, R. J. (2023). Chitosan-Based Hydrogel in the Management of Dermal Infections: A Review. Gels, 9(7), 594. https://doi.org/10.3390/gels9070594