pH-Sensitive Hydrogel Bilayers: Investigation on Transient Swelling-Induced Bending through Analytical and FEM Approaches
Abstract
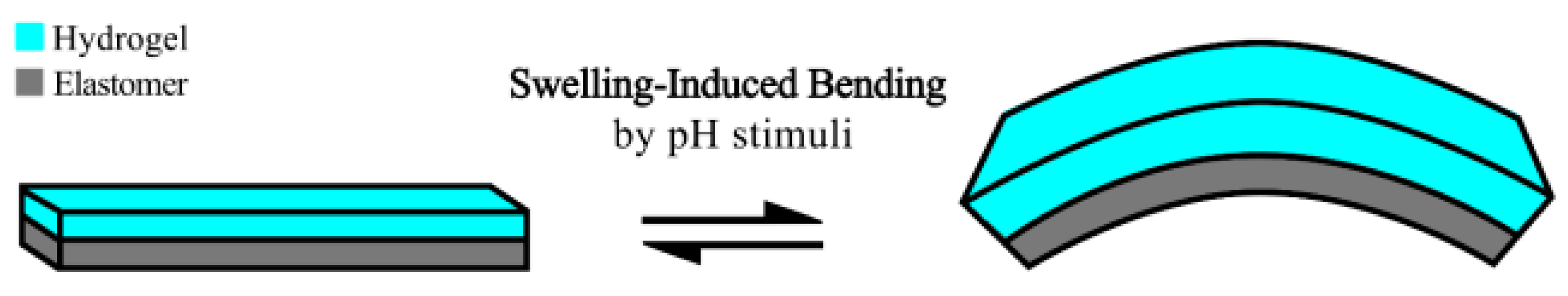
1. Introduction
- Improved drug delivery prediction: A time-dependent model can accurately capture pH-sensitive hydrogel swelling kinetics, crucial for drug release timing and rate;
- Improved mechanical stability understanding: Large-deformation theory allows insights into pH-sensitive hydrogel durability under substantial deformations. The model can assess the hydrogel’s response to real-world mechanical forces, aiding in designing hydrogels for specific applications, such as tissue engineering or responsive coatings;
- The bidirectional coupling of chemo-mechanical fields: Considering the influence of transient chemical kinetics on mechanical deformation, they offer great potential in advancing our understanding of mechanical phenomena such as creep and viscoelasticity, particularly in tough hydrogels.
2. Results
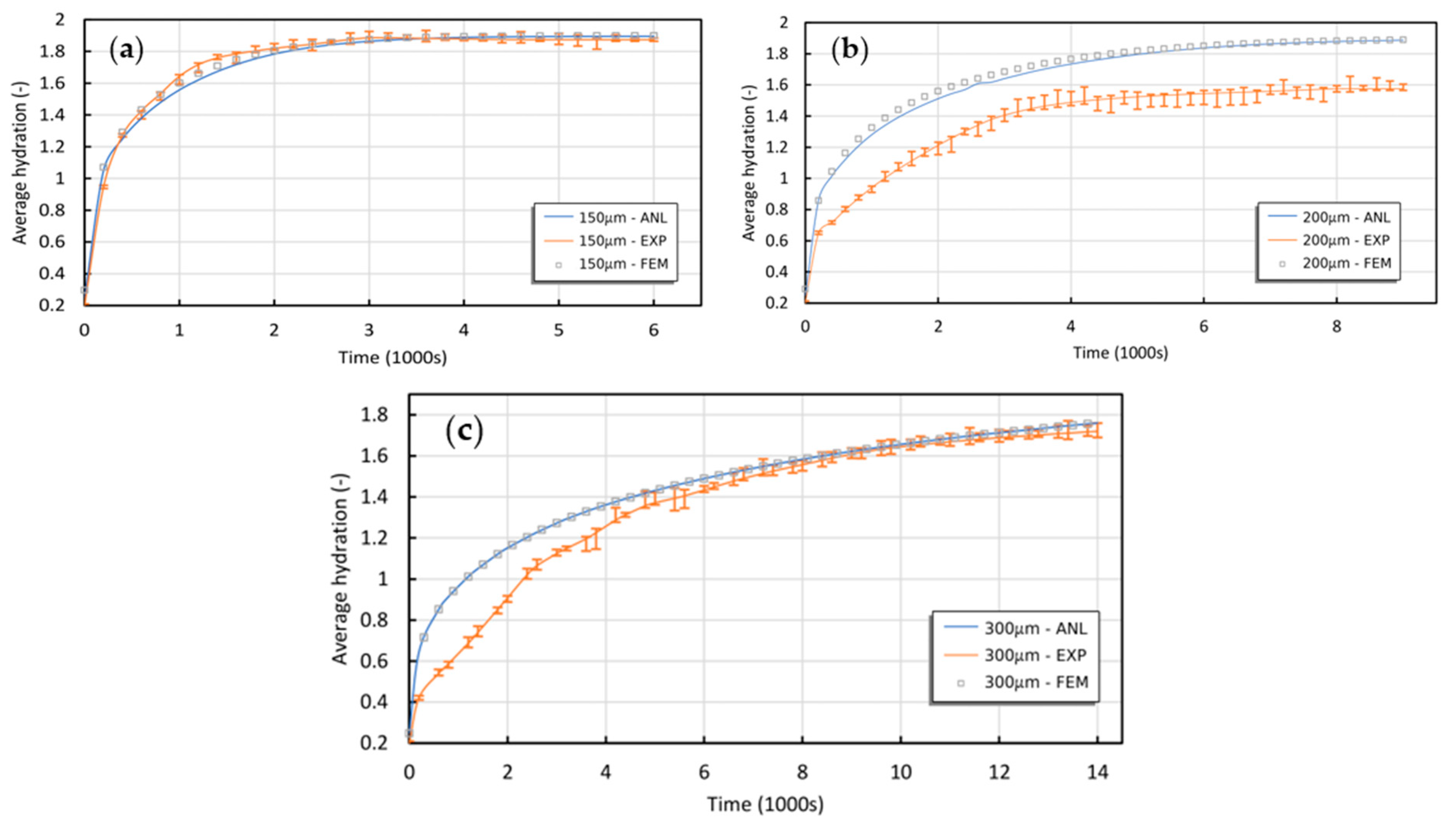
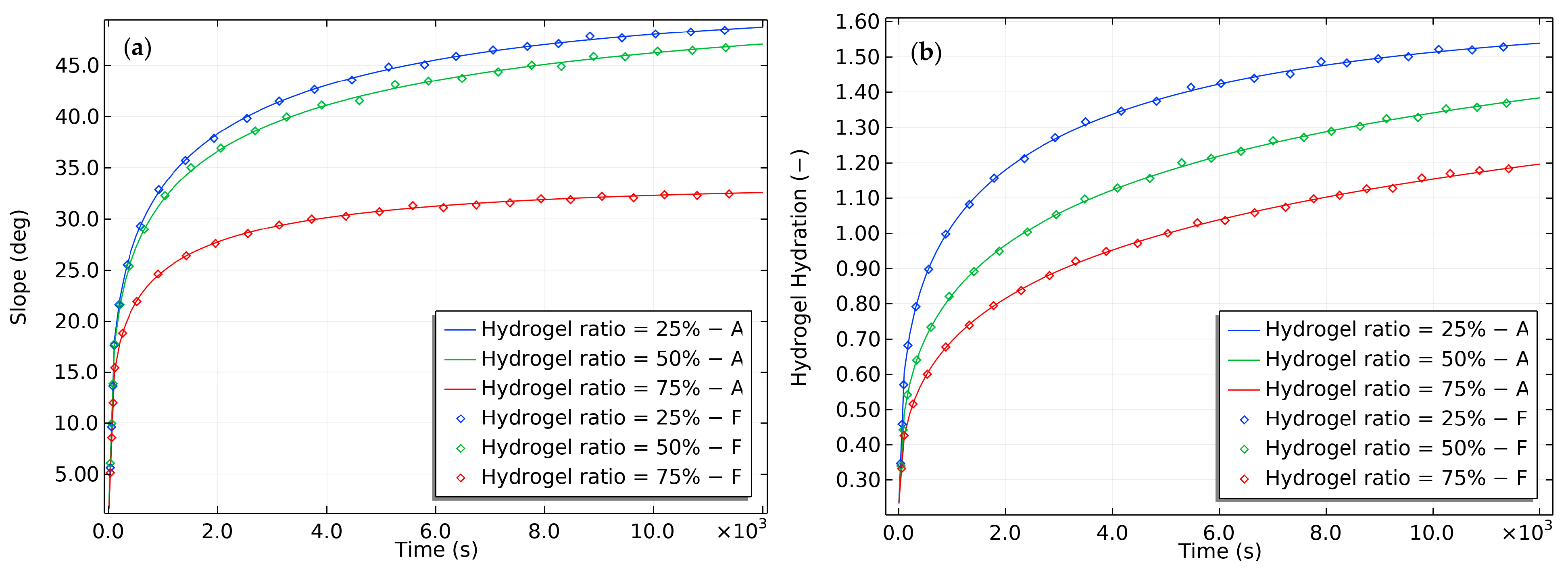
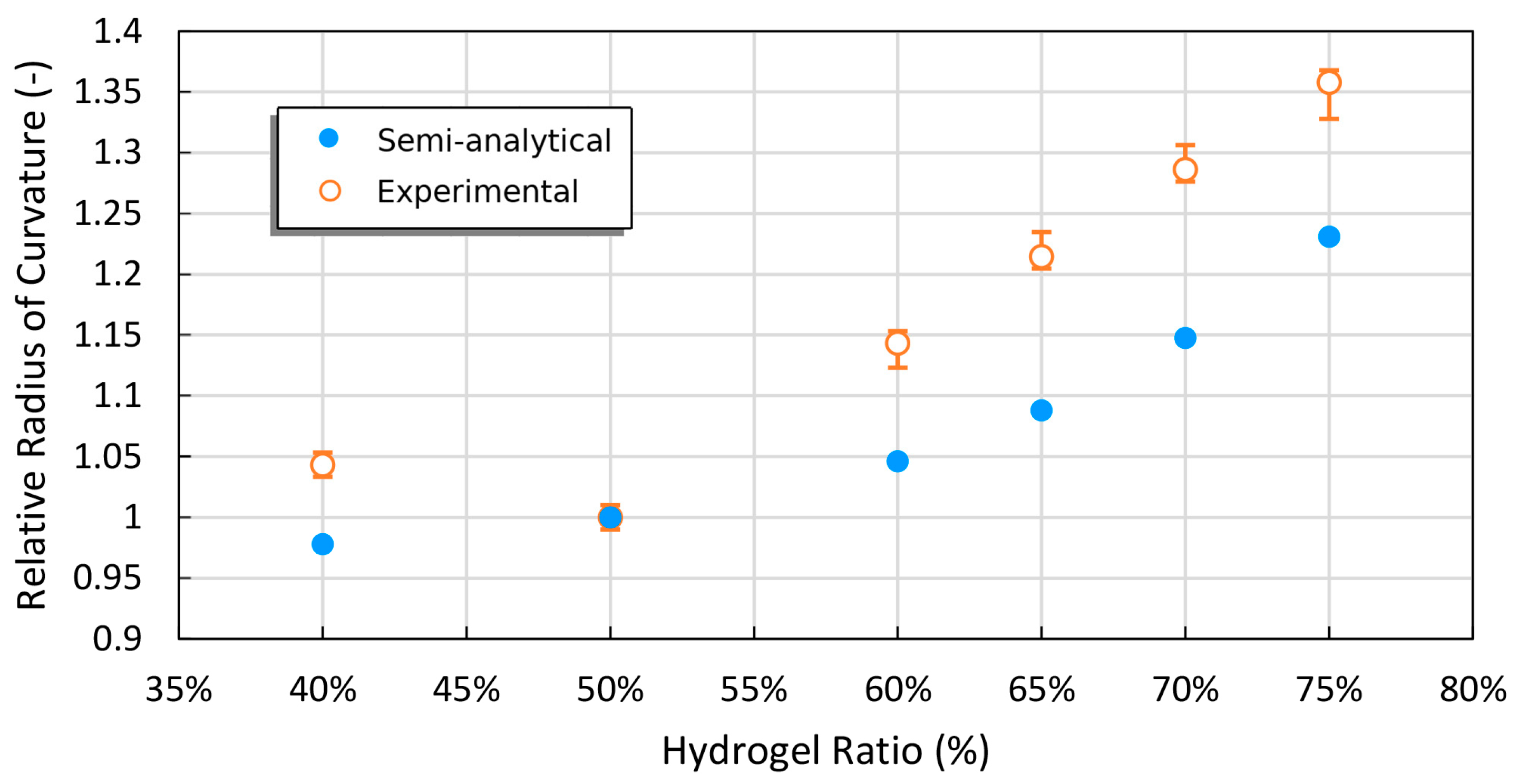
2.1. Experimental Verification of the Theory’s Predictions
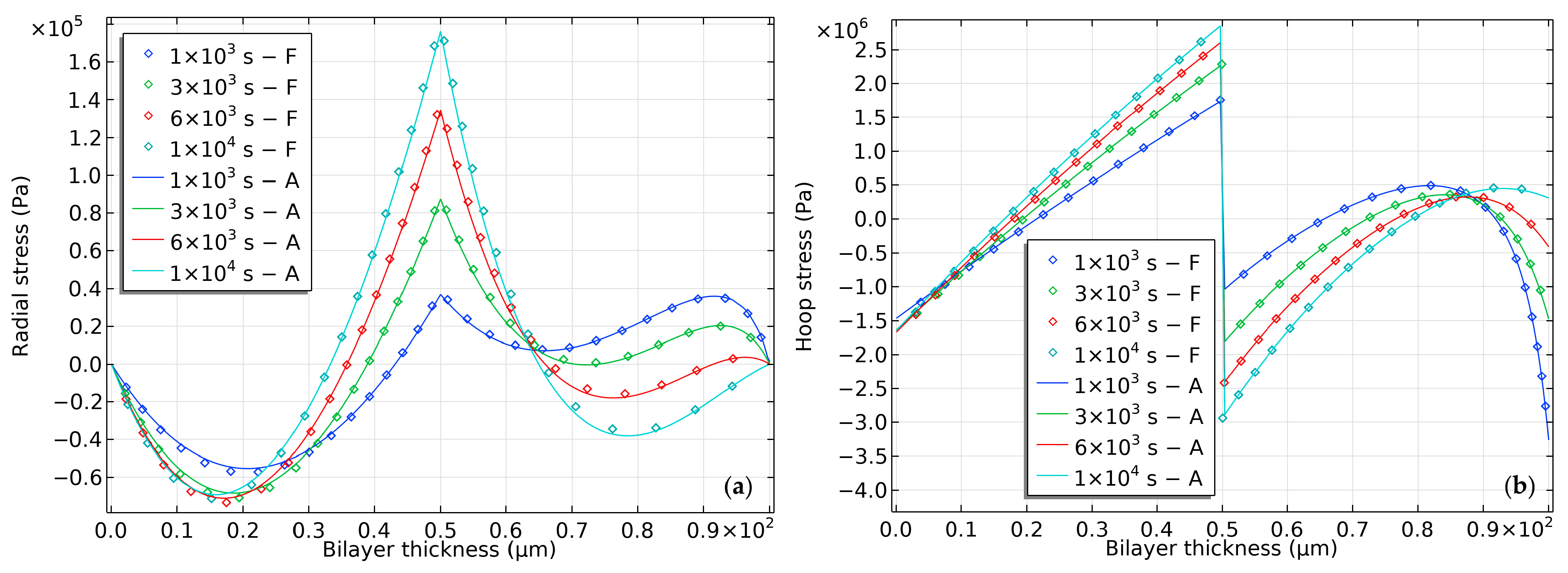
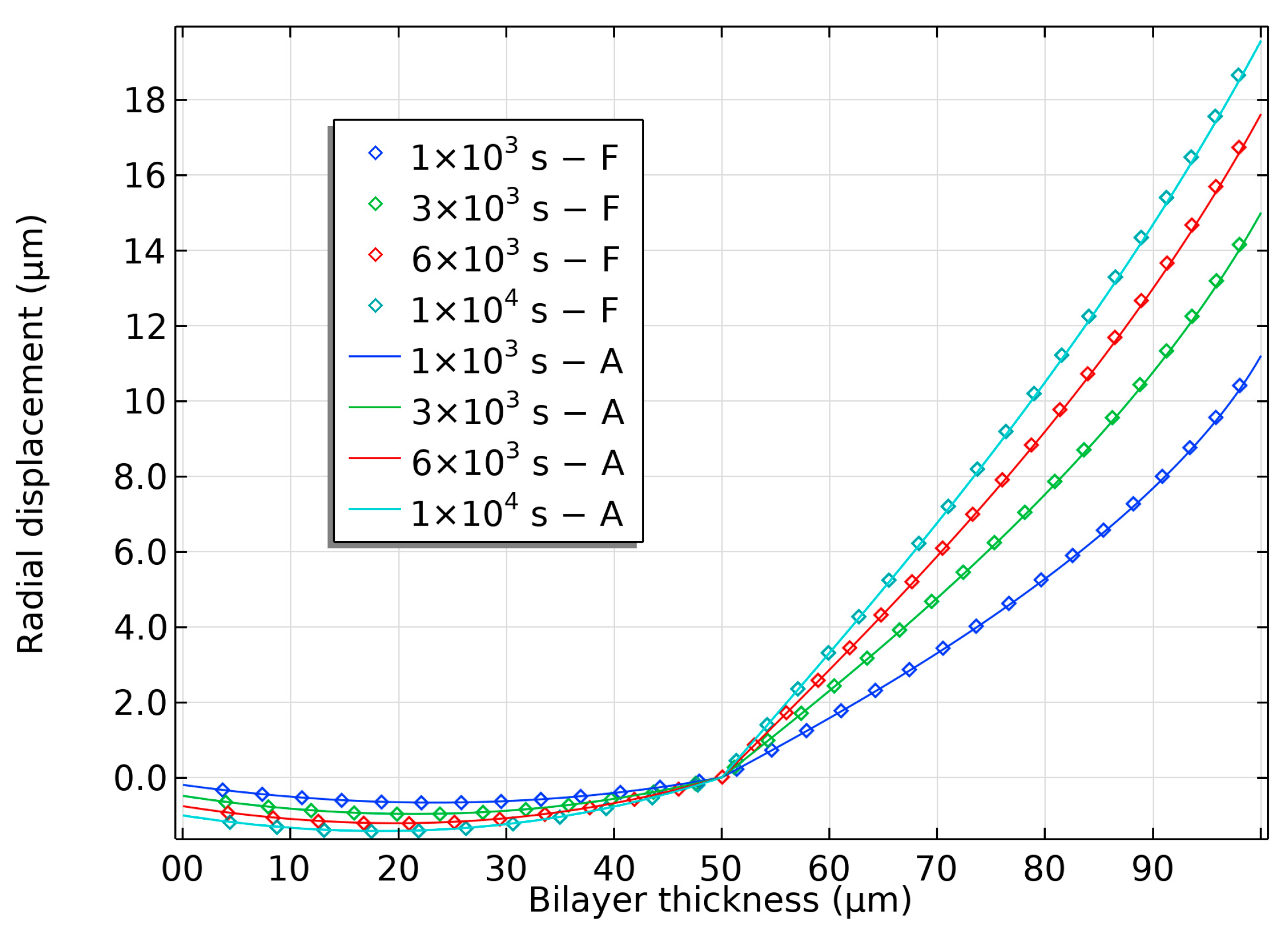
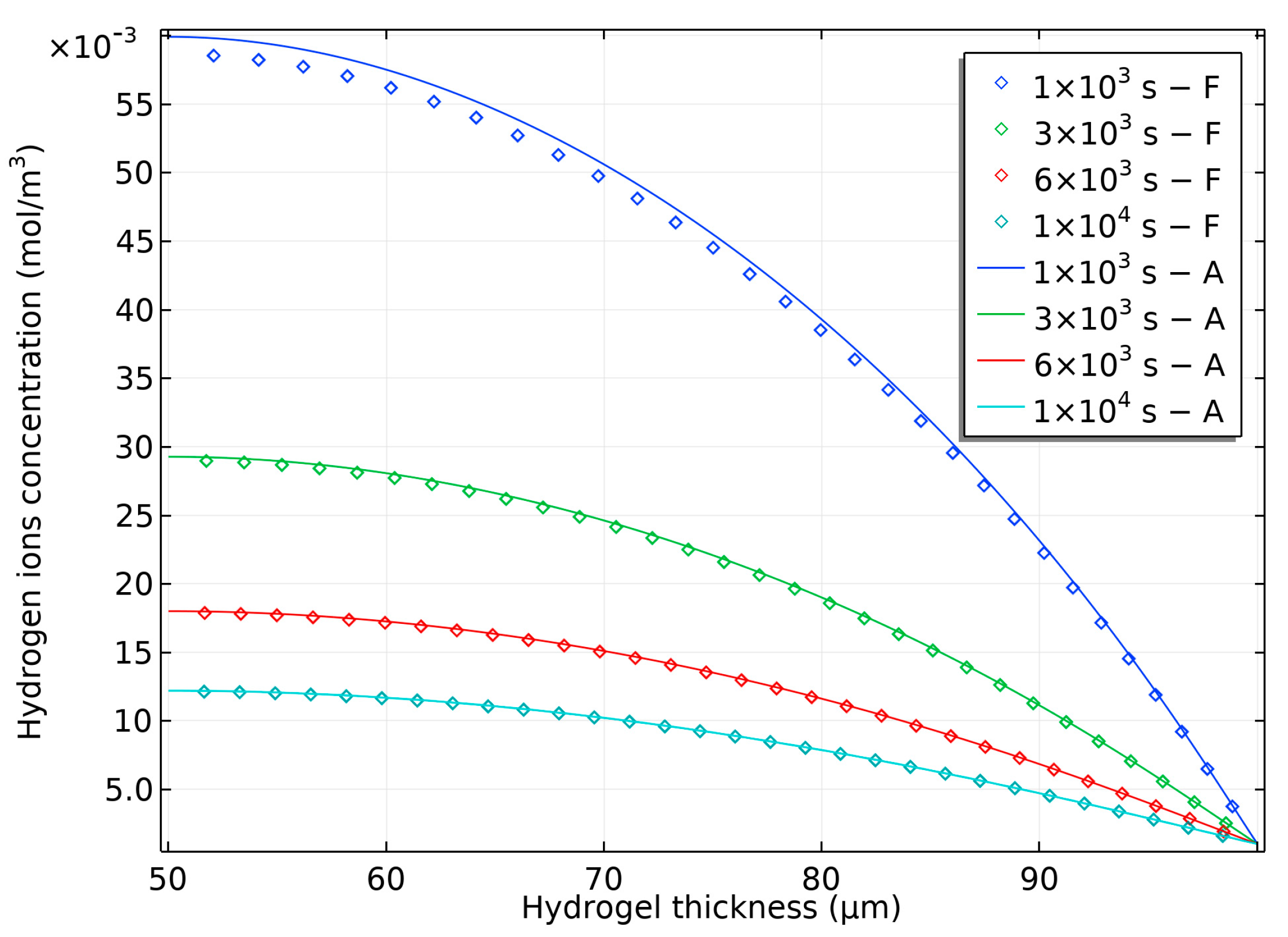
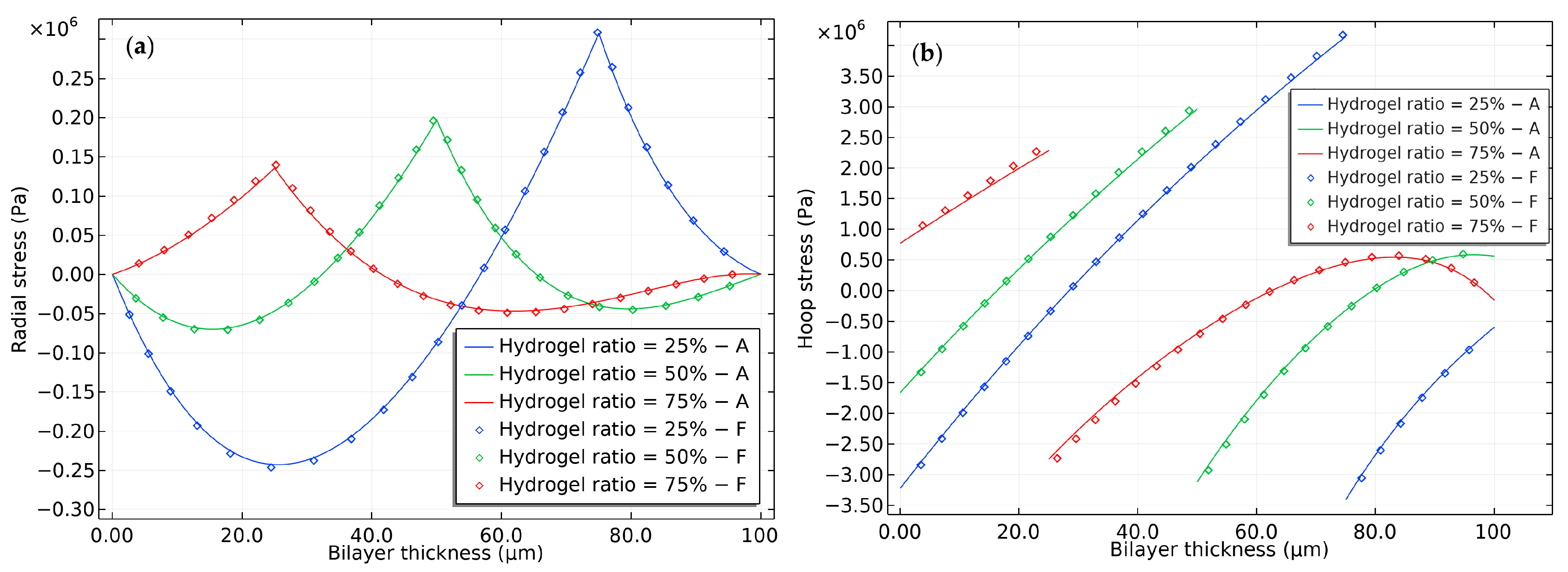
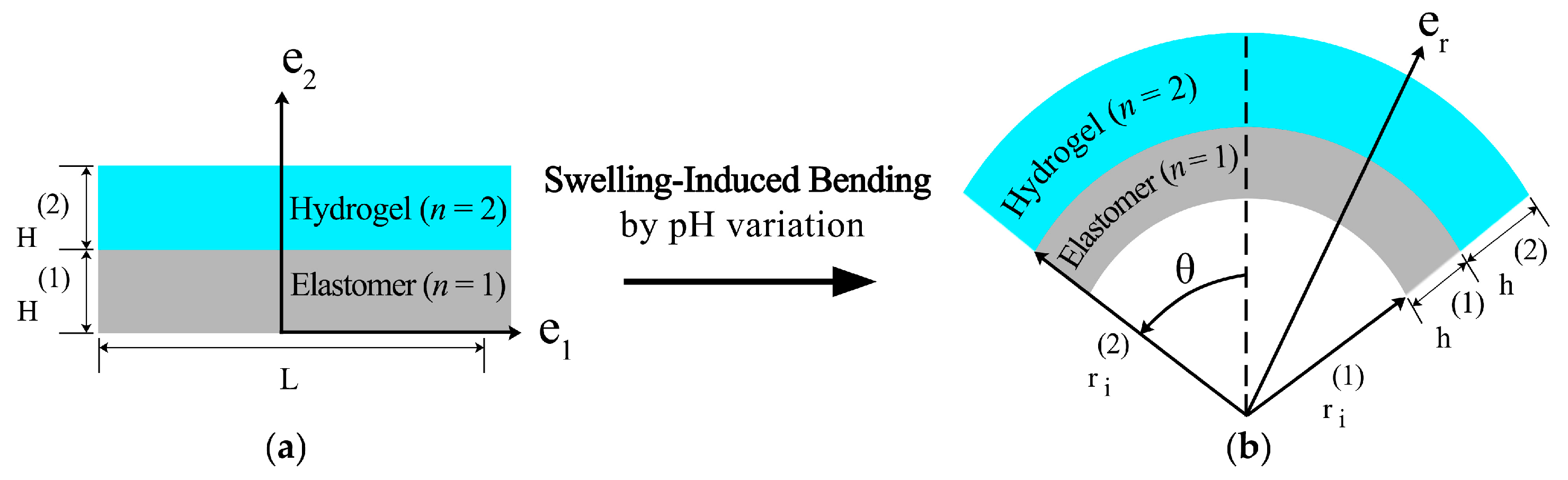
2.2. Modeling of the Hydrogel-Based Bilayer Bending
3. Conclusions
4. Theoretical Background
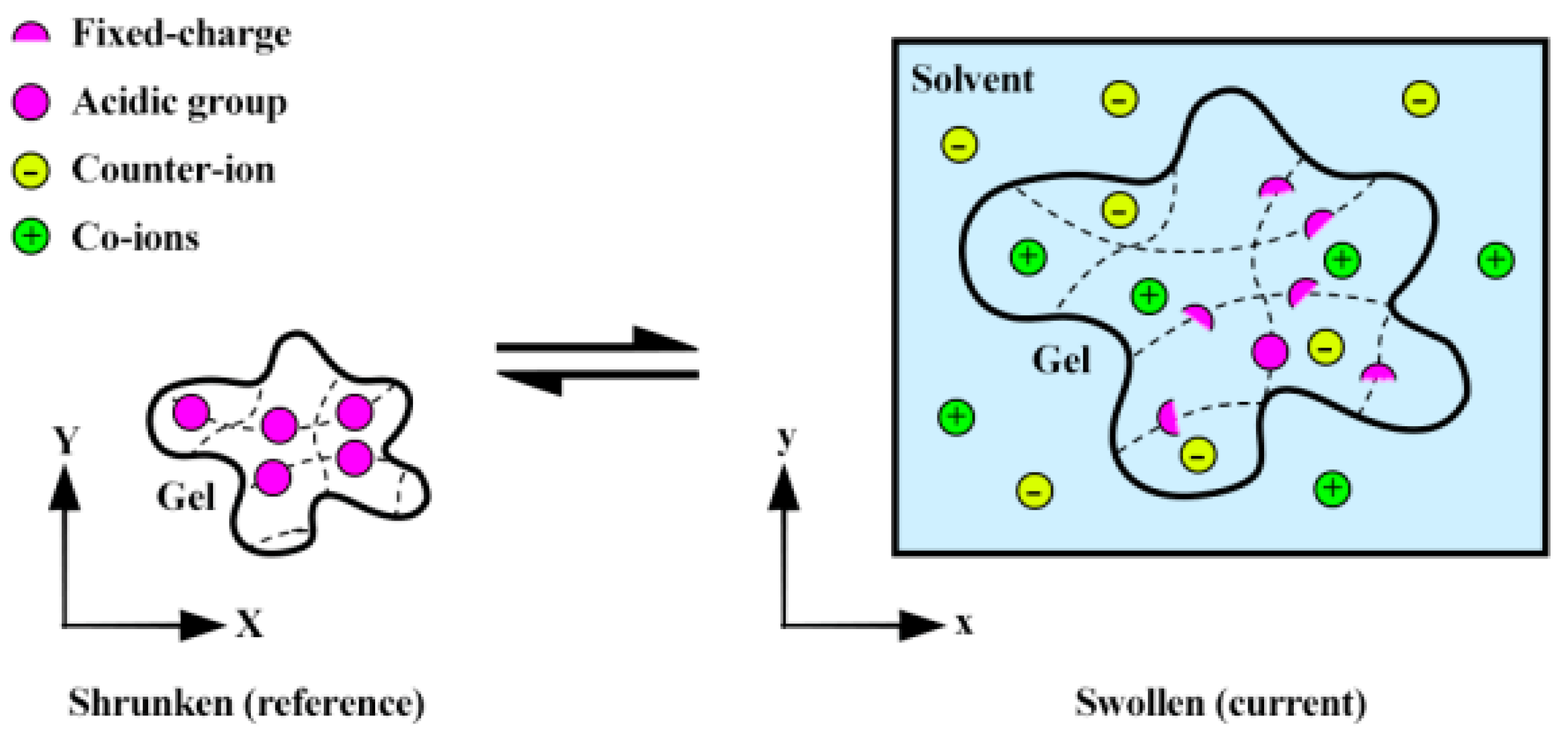
4.1. Electrochemical Field
4.2. Finite Bending of Bilayer Kinematics
4.3. Governing Equations
4.4. Boundary-Value Problem
4.5. Limiting Assumptions of the Solution
- The main focus of this study is on macroscopic hydrogels, which are assumed to satisfy electro-neutrality conditions, given Debye’s length in the nanometer range. The hypotheses, assumptions, and theories are chosen and refined with care to ensure that the conclusions drawn are specific and targeted;
- In this study, chemical reactions are assumed to be reversible and infinitely fast, resulting in the instantaneous attainment of equilibrium in the time dimension. Moreover, the activity coefficient of mobile ions in the hydrogel is considered equivalent to that of the equilibrium solution;
- Rather than using a multi-phase approach, we treat the hydrogel and solvent as a single-phase domain. While this simplified model may not fully capture all the complexities of hydrogel behavior, it enables more efficient and practical analysis of the system under investigation. Even with this simplified approach, valuable insights into the behavior of hydrogels and their potential applications can still be gained;
- The effect of fluid dynamics in the hydrogel domain is overlooked in this study due to its negligible impact [31]. Instead, diffusion is identified as the hydrogel’s primary cause of ion migration. Therefore, it is appropriate to assume a quasi-static condition to study the mechanical response;
- According to Kurnia, Birgersson, and Mujumdar [38], osmotic pressure primarily influences the swelling behavior of pH-responsive polymers when no external electric field is present. As a result, we neglect the contribution of electrostatic forces and the tendency of the polymer network to mix with the surrounding solution in this analysis.
5. Computational Framework
6. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, C.; Peng, S.; Chen, L.; Cao, X.; Sun, Y.; Chen, L.; Yang, L.; Ma, C.; Liu, Q.; Liu, Z.; et al. Anisotropic Bi-Layer Hydrogel Actuator with pH-Responsive Color-Changing and Photothermal-Responsive Shape-Changing Bi-Functional Synergy. Gels 2023, 9, 438. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiyari, A.; Baghani, M.; Sohrabpour, S. An investigation on multilayer shape memory polymers under finite bending through nonlinear thermo-visco-hyperelasticity. Appl. Math. Mech. 2023, 44, 73–88. [Google Scholar] [CrossRef]
- Ullah, G.; Nawaz, A.; Latif, M.S.; Shah, K.U.; Ahmad, S.; Javed, F.; Alfatama, M.; Abd Ghafar, S.A.; Lim, V. Clarithromycin and Pantoprazole Gastro-Retentive Floating Bilayer Tablet for the Treatment of Helicobacter Pylori: Formulation and Characterization. Gels 2023, 9, 43. [Google Scholar] [CrossRef]
- Peng, S.; Cao, X.; Sun, Y.; Chen, L.; Ma, C.; Yang, L.; Zhao, H.; Liu, Q.; Liu, Z.; Ma, C. Polyurethane Shape Memory Polymer/pH-Responsive Hydrogel Hybrid for Bi-Function Synergistic Actuations. Gels 2023, 9, 428. [Google Scholar] [CrossRef] [PubMed]
- Luneva, O.; Olekhnovich, R.; Uspenskaya, M. Bilayer Hydrogels for Wound Dressing and Tissue Engineering. Polymers 2022, 14, 3135. [Google Scholar] [CrossRef]
- Amiri, A.; Baniassadi, M.; Bayat, M.R.; Baghani, M. Transient swelling of cylindrical hydrogels under coupled extension-torsion: Analytical and 3D FEM solutions. J. Intell. Mater. Syst. Struct. 2023, 34, 415–424. [Google Scholar] [CrossRef]
- Rao, K.-M.; Rao, K.S.V.K.; Palem, R.-R.; Uthappa, U.-T.; Ha, C.-S.; Han, S.-S. pH Sensitive Drug Delivery Behavior of Palmyra Palm Kernel Hydrogel of Chemotherapeutic Agent. Gels 2023, 9, 38. [Google Scholar] [CrossRef]
- Liu, Q.; Ye, X.; Wu, H.; Zhang, X. A multiphysics model of magnetic hydrogel under a moving magnet for targeted drug delivery. Int. J. Mech. Sci. 2022, 215, 106963. [Google Scholar] [CrossRef]
- Dehghany, M.; Zhang, H.; Naghdabadi, R.; Hu, Y. A thermodynamically-consistent large deformation theory coupling photochemical reaction and electrochemistry for light-responsive gels. J. Mech. Phys. Solids 2018, 116, 239–266. [Google Scholar] [CrossRef]
- Ge, P.; Chang, S.; Wang, T.; Zhao, Q.; Wang, G.; He, B. An antioxidant and antibacterial polydopamine-modified thermo-sensitive hydrogel dressing for Staphylococcus aureus-infected wound healing. Nanoscale 2023, 15, 644–656. [Google Scholar] [CrossRef]
- Loyez, M.; Adolphson, M.; Liao, J.; Thakur, S.; Yang, L. pH-sensitive optical micro-resonator based on PAA/PVA gel swelling. In Optics and Biophotonics in Low-Resource Settings IX; SPIE: Bellingham, WA, USA, 2023; p. 1236907. [Google Scholar]
- Khodabandehloo, A.; Mazaheri, H. Analytic and finite element studies on deformation of bilayers with a functionally graded pH-responsive hydrogel layer. Int. J. Appl. Mech. 2022, 14, 2250053. [Google Scholar] [CrossRef]
- Amjadi, A.; Sirousazar, M.; Kokabi, M. Dual stimuli responsive neutral/cationic polymers/clay nanocomposite hydrogels. J. Appl. Polym. Sci. 2020, 137, 48797. [Google Scholar] [CrossRef]
- Shojaeifard, M.; Niroumandi, S.; Baghani, M. Programming shape-shifting of flat bilayers composed of tough hydrogels under transient swelling. Acta Mech. 2022, 233, 213–232. [Google Scholar] [CrossRef]
- Huang, R.; Xue, Y.; Li, Z.; Liu, Z. Programmable spiral and helical deformation behaviors of hydrogel-based bi-material beam structures. Int. J. Struct. Stab. Dyn. 2020, 20, 2041010. [Google Scholar] [CrossRef]
- Li, C.; Lau, G.C.; Yuan, H.; Aggarwal, A.; Dominguez, V.L.; Liu, S.; Sai, H.; Palmer, L.C.; Sather, N.A.; Pearson, T.J.; et al. Fast and programmable locomotion of hydrogel-metal hybrids under light and magnetic fields. Sci. Robot. 2020, 5, eabb9822. [Google Scholar] [CrossRef]
- Askari-Sedeh, M.; Baghani, M. Coupled chemo-mechanical swelling behavior of pH-sensitive hollow cylinder hydrogels under extension-torsion and internal pressure: Analytical and 3D FEM Solutions. Int. J. Appl. Mech. 2023, 15, 2350030. [Google Scholar] [CrossRef]
- Topol, H.; Al-Chlaihawi, M.J.; Demirkoparan, H.; Merodio, J. Bifurcation of Fiber-Reinforced Cylindrical Membranes under Extension, Inflation, and Swelling. J. Appl. Comput. Mech. 2023, 9, 113–128. [Google Scholar] [CrossRef]
- Liu, H.; Sun, Y.; Yu, T.; Zhang, J.; Zhang, X.; Zhang, H.; Zhao, K.; Wei, J. Plant-mediated biosynthesis of iron nanoparticles-calcium alginate hydrogel membrane and its eminent performance in removal of Cr(VI). Chem. Eng. J. 2019, 378, 122120. [Google Scholar] [CrossRef]
- Feyzioğlu-Demir, E.; Üzüm, Ö.B.; Akgöl, S. Swelling and diffusion behaviour of spherical morphological polymeric hydrogel membranes (SMPHMs) containing epoxy groups and their application as drug release systems. Polym. Bull. 2023, 80, 6567–6590. [Google Scholar] [CrossRef]
- Hanzly, L.E.; Chauhan, N.; Barone, J.R. Mechanically cycling gelatin bilayers. Smart Mater. Struct. 2022, 31, 085005. [Google Scholar] [CrossRef]
- Shiblee, M.N.I.; Ahmed, K.; Kawakami, M.; Furukawa, H. 4D printing of shape-memory hydrogels for soft-robotic functions. Adv. Mater. Technol. 2019, 4, 1900071. [Google Scholar] [CrossRef]
- Behera, S.K.; Kumar, D.; Sarangi, S. Modeling of electro–viscoelastic dielectric elastomer: A continuum mechanics approach. Eur. J. Mech.-A/Solids 2021, 90, 104369. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, X.; Zhao, Y.; Li, H.; Xiao, S.; Chen, Z.; Huang, G.; Sun, Y.; Liu, Z.; He, Z. An Anisotropic Hydrogel by Programmable Ionic Crosslinking for Sequential Two-Stage Actuation under Single Stimulus. Gels 2023, 9, 279. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qiu, Y.; Zhang, H.; Zheng, Y.; Ye, H. A solid-shell finite element method for the anisotropic swelling of hydrogels with reinforced fibers. Eur. J. Mech. A/Solids 2021, 86, 104197. [Google Scholar] [CrossRef]
- Joshi, A.; Choudhury, S.; Baghel, V.S.; Ghosh, S.; Gupta, S.; Lahiri, D.; Ananthasuresh, G.; Chatterjee, K. 4D Printed Programmable Shape-morphing Hydrogels as Intraoperative Self-folding Nerve Conduits for Sutureless Neurorrhaphy. Adv. Healthc. Mater. 2023, 2300701. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, T.; Ashida, F. Temperature-responsive bending of a bilayer gel. Int. J. Solids Struct. 2015, 56, 20–28. [Google Scholar] [CrossRef]
- Abdolahi, J.; Baghani, M.; Arbabi, N.; Mazaheri, H. Analytical and numerical analysis of swelling-induced large bending of thermally-activated hydrogel bilayers. Int. J. Solids Struct. 2016, 99, 1–11. [Google Scholar] [CrossRef]
- Arbabi, N.; Baghani, M.; Abdolahi, J.; Mazaheri, H.; Mashhadi, M.M. Finite bending of bilayer pH-responsive hydrogels: A novel analytic method and finite element analysis. Compos. Part B Eng. 2017, 110, 116–123. [Google Scholar] [CrossRef]
- Wu, Y.; Hao, X.; Xiao, R.; Lin, J.; Wu, Z.L.; Yin, J.; Qian, J. Controllable Bending of Bi-hydrogel Strips with Differential Swelling. Acta Mech. Solida Sin. 2019, 32, 652–662. [Google Scholar] [CrossRef]
- De, S.K.; Aluru, N.R.; Johnson, B.; Crone, W.C.; Beebe, D.J.; Moore, J. Equilibrium swelling and kinetics of pH-responsive hydrogels: Models, experiments, and simulations. J. Microelectromech. Syst. 2002, 11, 544–555. [Google Scholar] [CrossRef]
- Azeem, M.K.; Islam, A.; Rizwan, M.; Rasool, A.; Gul, N.; Khan, R.U.; Khan, S.M.; Rasheed, T. Sustainable and environment Friendlier carrageenan-based pH-responsive hydrogels: Swelling behavior and controlled release of fertilizers. Colloid Polym. Sci. 2023, 301, 209–219. [Google Scholar] [CrossRef]
- Kim, J.; Kim, C.; Song, Y.; Jeong, S.-G.; Kim, T.-S.; Lee, C.-S. Reversible self-bending soft hydrogel microstructures with mechanically optimized designs. Chem. Eng. J. 2017, 321, 384–393. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, M.; Li, H.; Lam, K.Y. Multiphysics modeling of responsive deformation of dual magnetic-pH-sensitive hydrogel. Int. J. Solids Struct. 2020, 190, 76–92. [Google Scholar] [CrossRef]
- Shymborska, Y.; Stetsyshyn, Y.; Raczkowska, J.; Awsiuk, K.; Ohar, H.; Budkowski, A. Impact of the various buffer solutions on the temperature-responsive properties of POEGMA-grafted brush coatings. Colloid Polym. Sci. 2022, 300, 487–495. [Google Scholar] [CrossRef]
- Rajan, A.; Arockiarajan, A. Bending of hard-magnetic soft beams: A finite elasticity approach with anticlastic bending. Eur. J. Mech. A/Solids 2021, 90, 104374. [Google Scholar] [CrossRef]
- Ricker, A.; Wriggers, P. Systematic Fitting and Comparison of Hyperelastic Continuum Models for Elastomers. Arch. Comput. Methods Eng. 2023, 30, 2257–2288. [Google Scholar] [CrossRef]
- Kurnia, J.C.; Birgersson, E.; Mujumdar, A.S. Analysis of a model for pH-sensitive hydrogels. Polymer 2012, 53, 613–622. [Google Scholar] [CrossRef]
- Li, H.; Lai, F. Modeling of Ionic-Strength-Sensitive Hydrogel: Effect of Initial Distance Between the Fixed Charges. Soft Mater. 2013, 11, 13–21. [Google Scholar] [CrossRef]
- Goh, K.B.; Li, H.; Lam, K.Y. Modeling the Impact of pH- and Oxygen-Coupled Stimuli on Osmotic Pressure and Electrical Potential Responses of Hemoglobin-Loaded Polyampholyte Hydrogel. ACS Appl. Bio Mater. 2018, 1, 318–327. [Google Scholar] [CrossRef]
- Louf, J.-F.; Lu, N.B.; O’Connell, M.G.; Cho, H.J.; Datta, S.S. Under pressure: Hydrogel swelling in a granular medium. Sci. Adv. 2021, 7, eabd2711. [Google Scholar] [CrossRef]




| Scope | Boundary Conditions | Initial Conditions |
|---|---|---|
| Chemical | ||
| Mechanical | ||
| Physics | Parameter | Hydrogel | Elastomer |
|---|---|---|---|
| Mechanical | |||
| Chemical | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Askari-Sedeh, M.; Baghani, M. pH-Sensitive Hydrogel Bilayers: Investigation on Transient Swelling-Induced Bending through Analytical and FEM Approaches. Gels 2023, 9, 563. https://doi.org/10.3390/gels9070563
Askari-Sedeh M, Baghani M. pH-Sensitive Hydrogel Bilayers: Investigation on Transient Swelling-Induced Bending through Analytical and FEM Approaches. Gels. 2023; 9(7):563. https://doi.org/10.3390/gels9070563
Chicago/Turabian StyleAskari-Sedeh, Mahdi, and Mostafa Baghani. 2023. "pH-Sensitive Hydrogel Bilayers: Investigation on Transient Swelling-Induced Bending through Analytical and FEM Approaches" Gels 9, no. 7: 563. https://doi.org/10.3390/gels9070563
APA StyleAskari-Sedeh, M., & Baghani, M. (2023). pH-Sensitive Hydrogel Bilayers: Investigation on Transient Swelling-Induced Bending through Analytical and FEM Approaches. Gels, 9(7), 563. https://doi.org/10.3390/gels9070563








