Phase Inversion-Based Doxycycline Hyclate-Incorporated Borneol In Situ Gel for Periodontitis Treatment
Abstract
1. Introduction
2. Results and Discussion
2.1. Appearance, Density, Viscosity, and Surface Tension
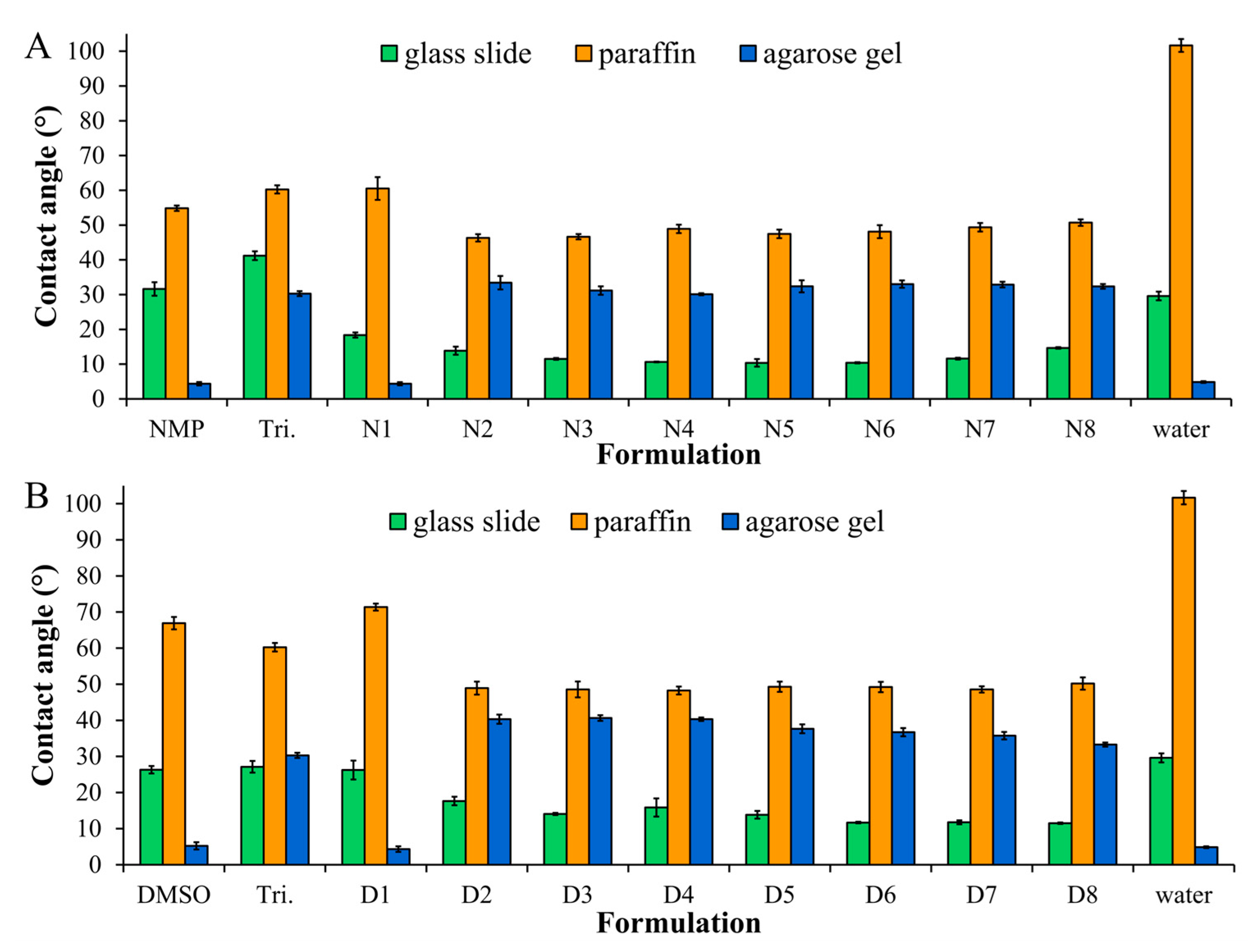
2.2. The Wettability of ISG in Different Types of Surface
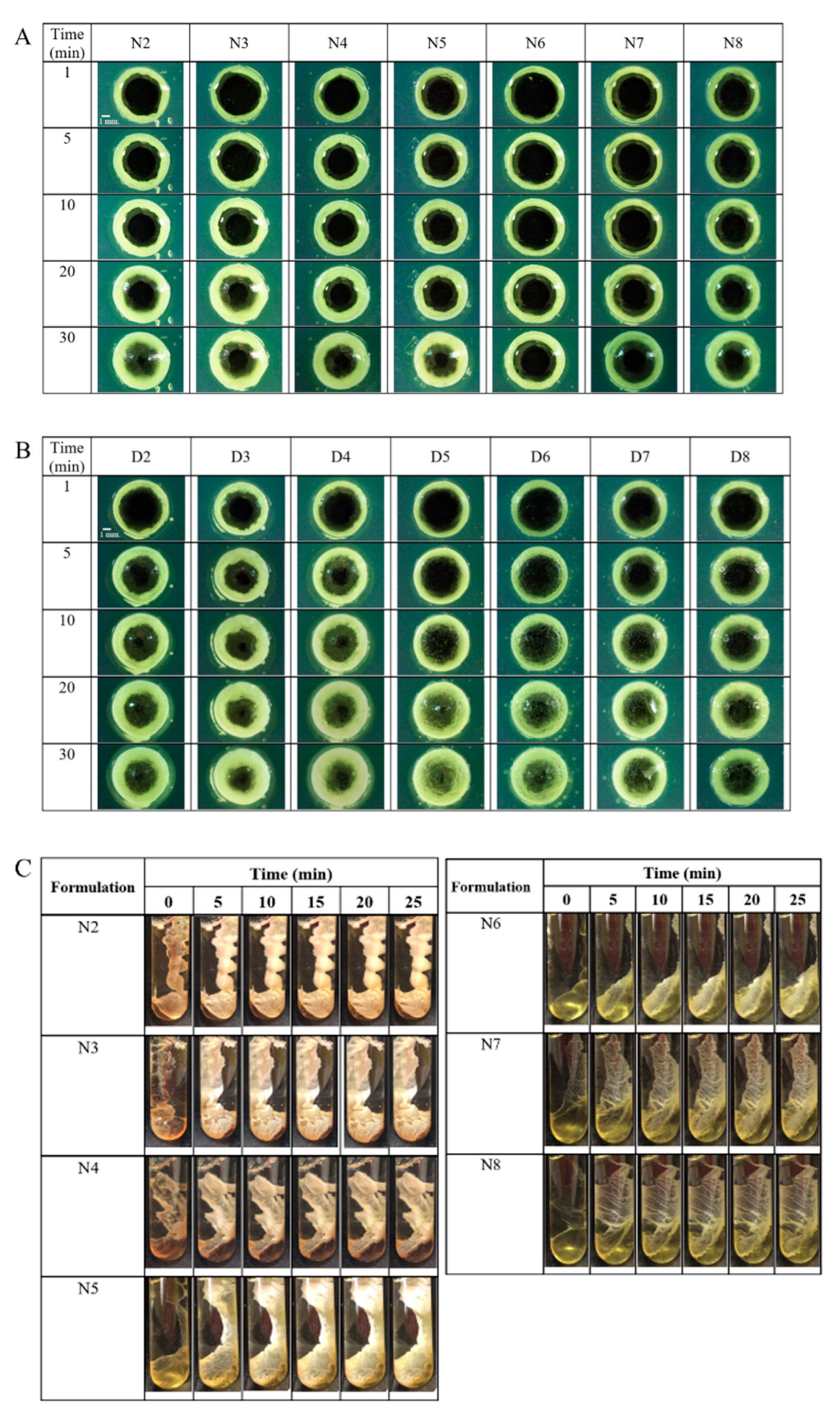
2.3. Injectability and Mechanical Properties
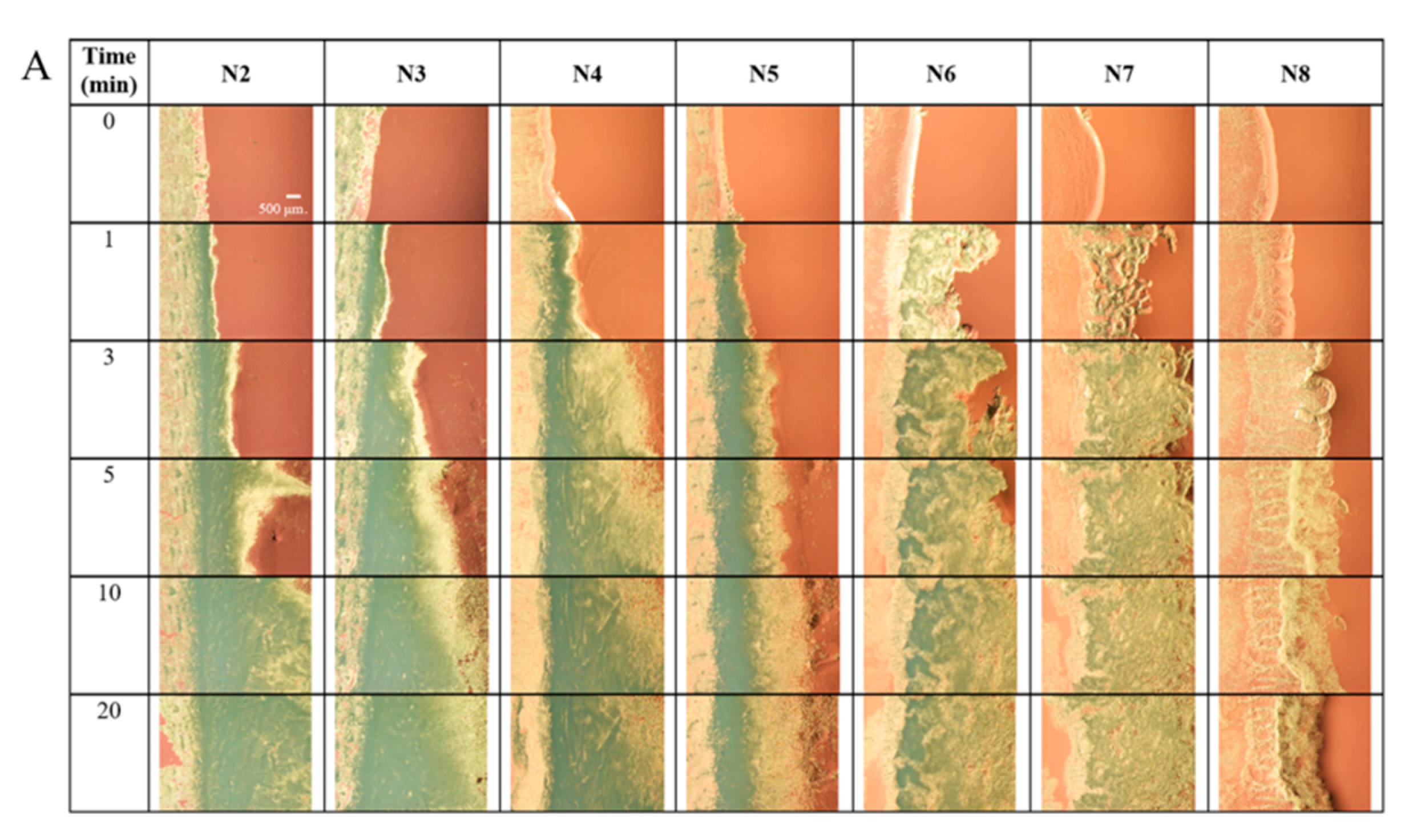
2.4. Morphology of Gel Formation
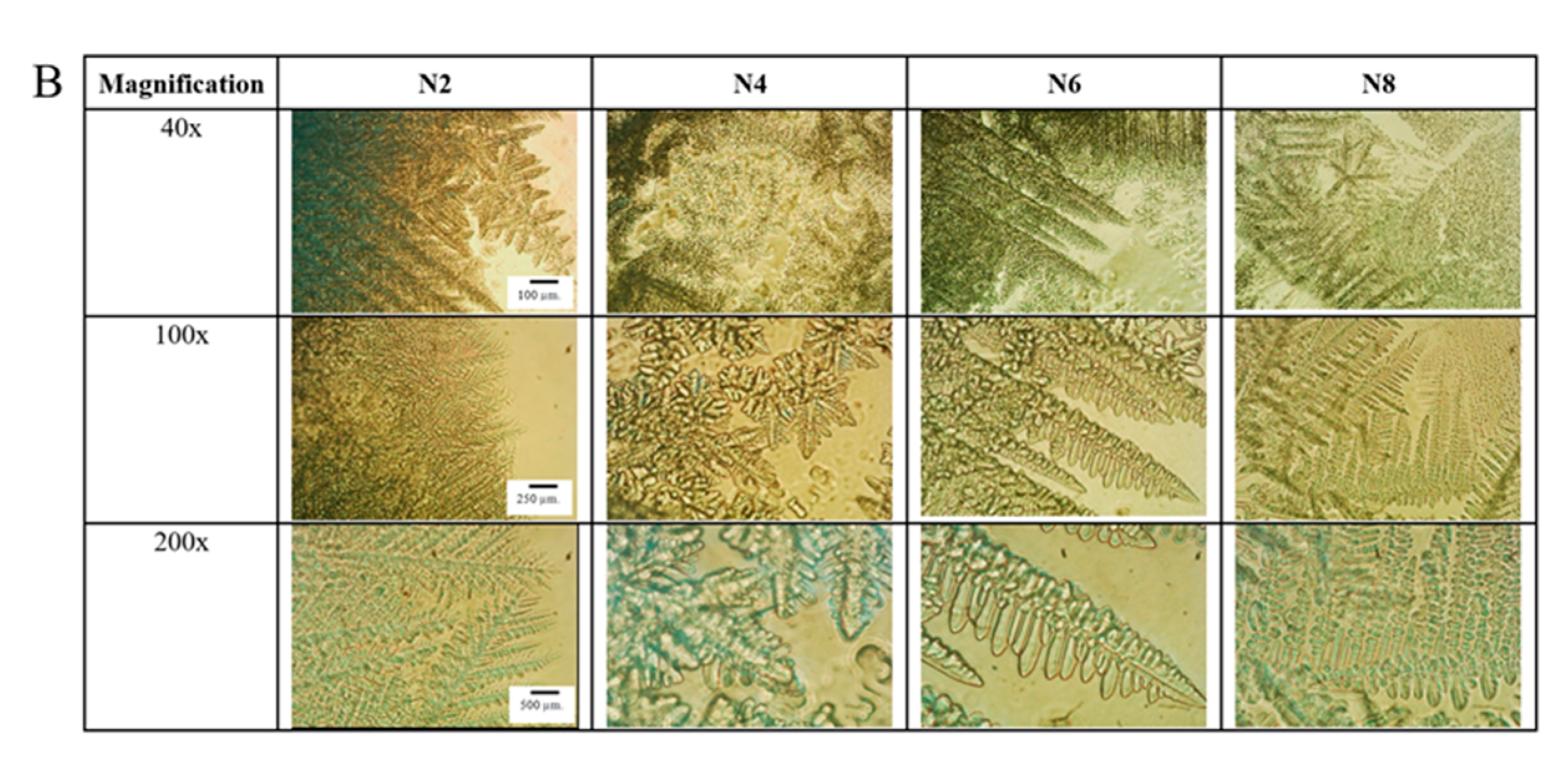
2.5. Microscopic Changes in Gel Formation
2.6. Water-Induced Gel Formation Sensitivity
2.7. In Vitro Drug Release
2.8. Drug Release Kinetics
2.9. Antimicrobial Activities
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of ISG
4.3. Physicochemical Characterization of ISG
4.3.1. Appearance and Density
4.3.2. Viscosity
4.3.3. Surface Tension, Contact Angle, and Wettability
4.3.4. Injectability and Mechanical Properties
4.4. The Evaluation of Gel Formation
4.4.1. The Morphology of Gel Formation
4.4.2. Gel Formation after Injection
4.4.3. Microscopic Changes in Gel Formation
4.4.4. Water-Induced Gel Formation Sensitivity
4.5. In Vitro Drug Release and Release Kinetics
4.6. Antimicrobial Activities
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nazir, M.A. Prevalence of periodontal disease, Its association with systemic diseases and prevention. Int. J. Health Sci. 2017, 11, 72–80. [Google Scholar]
- Kwon, T.; Lamster, I.B.; Levin, L. Current concepts in the management of periodontitis. Int. Dent. J. 2021, 71, 462–476. [Google Scholar] [CrossRef] [PubMed]
- Kinane, D.F.; Hajishengallis, G. Polymicrobial infections, biofilms, and beyond. J. Clin. Periodontol. 2009, 36, 404–405. [Google Scholar] [CrossRef] [PubMed]
- Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Zohaib, S.; Madathil, S.A.; Mali, M.; Almas, K. Efficacy of metformin in the management of periodontitis: A systematic review and meta-analysis. Saudi Pharm. J. 2018, 26, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Vieira Colombo, A.P.; Magalhaes, C.B.; Hartenbach, F.A.; Martins do Souto, R.; Maciel da Silva-Boghossian, C. Periodontal-disease-associated biofilm: A reservoir for pathogens of medical importance. Microb. Pathog. 2016, 94, 27–34. [Google Scholar] [CrossRef]
- Amel, Y.; Bouziane, D.; Leila, M.; Ahmed, B. Microbiological study of periodontitis in the west of Algeria. World J. Med. Sci. 2010, 5, 7–12. [Google Scholar]
- Fritschi, B.Z.; Albert-Kiszely, A.; Persson, G.R. Staphylococcus aureus and other bacteria in untreated periodontitis. J. Dent. Res. 2008, 87, 589–593. [Google Scholar] [CrossRef]
- Genco, R.J. Antibiotics in the treatment of human periodontal diseases. J. Periodontol. 1981, 52, 545–558. [Google Scholar] [CrossRef]
- Vyas, S.P.; Sihorkar, V.; Mishra, V. Controlled and targeted drug delivery strategies towards intraperiodontal pocket diseases. J. Clin. Pharm. Ther. 2000, 25, 21–42. [Google Scholar] [CrossRef]
- Schwach-Abdellaoui, K.; Vivien-Castioni, N.; Gurny, R. Local delivery of antimicrobial agents for the treatment of periodontal diseases. Eur. J. Pharm. Biopharm. 2000, 50, 83–99. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D. The bacterial etiology of destructive periodontal disease: Current concepts. J. Periodontol. 1992, 63 (Suppl. 4), 322–331. [Google Scholar] [CrossRef]
- Phaechamud, T.; Thurein, S.M.; Chantadee, T. Role of clove oil in solvent exchange-induced doxycycline hyclate-loaded Eudragit RS in situ forming gel. Asian J. Pharm. Sci. 2018, 13, 131–142. [Google Scholar] [CrossRef]
- Prakasam, A.; Elavarasu, S.S.; Natarajan, R.K. Antibiotics in the management of aggressive periodontitis. J. Pharm. Bioallied Sci. 2012, 4 (Suppl. 2), S252–S255. [Google Scholar] [CrossRef] [PubMed]
- Garrett, S.; Johnson, L.; Drisko, C.H.; Adams, D.F.; Bandt, C.; Beiswanger, B.; Bogle, G.; Donly, K.; Hallmon, W.W.; Hancock, E.B.; et al. Two multi-center studies evaluating locally delivered doxycycline hyclate, placebo control, oral hygiene, and scaling and root planing in the treatment of periodontitis. J. Periodontol. 1999, 70, 490–503. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.C.; Anoop, K.R. Intraperiodontal pocket: An ideal route for local antimicrobial drug delivery. J. Adv. Pharm. Technol. Res. 2012, 3, 9–15. [Google Scholar] [PubMed]
- Senarat, S.; Pichayakorn, W.; Phaechamud, T.; Tuntarawongsa, S. Antisolvent Eudragit® polymers based in situ forming IS for periodontal controlled drug delivery. J. Drug Deliv. Sci. Technol. 2023, 82, 104361. [Google Scholar] [CrossRef]
- Puyathorn, N.; Senarat, S.; Lertsuphotvanit, N.; Phaechamud, T. Physicochemical and bioactivity characteristics of doxycycline hyclate-loaded solvent removal-induced ibuprofen-based in situ forming gel. Gels 2023, 9, 128. [Google Scholar] [CrossRef]
- Lertsuphotvanit, N.; Santimaleeworagun, W.; Narakornwit, W.; Chuenbarn, T.; Mahadlek, J.; Chantadee, T.; Phaechamud, T. Borneol-based antisolvent-induced in situ forming matrix for crevicular pocket delivery of vancomycin hydrochloride. Int. J. Pharm. 2022, 617, 121603. [Google Scholar] [CrossRef]
- Phaechamud, T.; Setthajindalert, O. Antimicrobial in-situ forming gels based on bleached shellac and different solvents. J. Drug Deliv. Sci. Technol. 2018, 46, 285–293. [Google Scholar] [CrossRef]
- Javali, M.A.; Vandana, K.L. A comparative evaluation of Atrigel delivery system (10% doxycycline hyclate) Atridox with scaling and root planing and combination therapy in treatment of periodontitis: A clinical study. J. Indian Soc. Periodontol. 2012, 16, 43–48. [Google Scholar] [CrossRef]
- Api, A.M.; Belsito, D.; Botelho, D.; Bruze, M.; Burton, G.A.; Buschmann, J.; Cancellieri, M.A.; Dagli, M.L.; Date, M.; Decant, W.; et al. Update to RIFM fragrance ingredient safety assessment, borneol, CAS Registry Number 507-70-0. Food Chem. Toxicol. 2022, 163, 113025. [Google Scholar] [CrossRef]
- Lertsuphotvanit, N.; Tuntarawongsa, S.; Sirirak, J.; Phaechamud, T. Morphological and physicochemical behaviors of borneol precipitates. Mater. Today Proc. 2022, 65, 2315–2321. [Google Scholar] [CrossRef]
- Santana, D.V.S.; Trindade, I.A.S.; Carvalho, Y.M.B.G.; Carvalho-Neto, A.G.; Silva, E.C.D.; Silva-Júnior, E.F.; Leite, R.F.S.; Quintans-Junior, L.J.; Aquino, T.M.; Sarafini, M.R.; et al. Analytical techniques to recognize inclusion complexes formation involving monoterpenes and cyclodextrins: A study case with (-) borneol, a food ingredient. Food Chem. 2021, 339, 127791. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, D.; Wu, J.; Ou, Y.; Mu, C.; Han, B.; Zhang, Q. Improved blood-brain barrier distribution: Effect of borneol on the brain pharmacokinetics of kaempferol in rats by in vivo microdialysis sampling. J. Ethnopharmacol. 2015, 162, 270–277. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Fu, B.M.; Zhang, Z.J. Borneol, a novel agent that improves central nervous system drug delivery by enhancing blood–brain barrier permeability. Drug Deliv. 2017, 24, 1037–1044. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.-H.; Seok, S.H.; Park, E.-S. Enhanced permeability and oral absorption of Panax notoginseng saponins by borneol. J. Drug Deliv. Sci. Technol. 2021, 66, 102819. [Google Scholar] [CrossRef]
- Dai, J.P.; Chen, J.; Bei, Y.F.; Han, B.X.; Wang, S. Influence of borneol on primary mice oral fibroblasts: A penetration enhancer may be used in oral submucous fibrosis. J. Oral Pathol. Med. 2009, 38, 276–281. [Google Scholar] [CrossRef]
- Bhatia, S.P.; Letizia, C.S.; Api, A.M. Fragrance material review on borneol. Food Chem. Toxicol. 2008, 46 (Suppl. 11), S77–S80. [Google Scholar] [CrossRef]
- Brodbeck, K.J.; DesNoyer, J.R.; McHugh, A.J. Phase inversion dynamics of PLGA solutions related to drug delivery. Part II. The role of solution thermodynamics and bath-side mass transfer. J. Control. Release 1999, 62, 333–344. [Google Scholar] [CrossRef]
- Liu, H.; Venkatraman, S.S. Cosolvent effects on the drug release and depot swelling in injectable in situ depot-forming systems. J. Pharm. Sci. 2012, 101, 1783–1793. [Google Scholar] [CrossRef]
- PubChem Compound Summary for CID 5541, Triacetin: National Center for Biotechnology Information. 2023. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Triacetin (accessed on 3 May 2023).
- Majstorović, D.M.; Petrović, P.I.; Lj. Kijevčanin, M.; Živković, E.M. Thermodynamic study of triacetin or ethyl levulinate and alcohol binary mixtures. J. Chem. Thermodyn. 2023, 180, 107004. [Google Scholar] [CrossRef]
- Gomaa, E.; Eissa, N.G.; Ibrahim, T.M.; El-Bassossy, H.M.; El-Nahas, H.M.; Ayoub, M.M. Development of depot PLGA-based in-situ implant of Linagliptin: Sustained release and glycemic control. Saudi Pharm. J. 2023, 31, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Brodbeck, K.J.; Gaynor-Duarte, A.T.; Shen, T.T. InventorsGel Composition and Methods. Canada Patent CA2591581A1, 2 July 1998. [Google Scholar]
- Rey, C.; Joel, Z. InventorsLiquid Polymeric Compositions for Controlled Release of Bioactive Substances. USA Patent US20040146557A1, 29 July 2004. [Google Scholar]
- Chantadee, T.; Santimaleeworagun, W.; Phorom, Y.; Chuenbarn, T.; Phaechamud, T. Vancomycin HCl-loaded lauric acid in situ-forming gel with phase inversion for periodontitis treatment. J. Drug Deliv. Sci. Technol. 2020, 57, 101615. [Google Scholar] [CrossRef]
- Lertsuphotvanit, N.; Tuntarawongsa, S.; Mahadlek, J.; Phaechamud, T. Surface tension/contact angle characters of aprotic binary borneol-dimethyl sulphoxide mixture. Key Eng. Mater. 2020, 859, 74–80. [Google Scholar] [CrossRef]
- Donaldson, E.C.; Alam, W. Wettability. In Wettability; Donaldson, E.C., Alam, W., Eds.; Gulf Publishing Company: Houston, TX, USA, 2008; pp. 1–55. [Google Scholar]
- Ershad, A.L.; Rajabi-Siahboomi, A.; Missaghi, S.; Kirby, D.; Mohammed, A.R. Multi-analytical framework to assess the in vitro swallowability of solid oral dosage forms targeting patient acceptability and adherence. Pharmaceutics 2021, 13, 411. [Google Scholar] [CrossRef]
- Phaechamud, T.; Mahadlek, J.; Chuenbarn, T. In situ forming gel comprising bleached shellac loaded with antimicrobial drugs for periodontitis treatment. Mater. Des. 2016, 89, 294–303. [Google Scholar] [CrossRef]
- Srichan, T.; Phaechamud, T. Designing solvent exchange-induced in situ forming gel from aqueous insoluble polymers as matrix base for periodontitis treatment. AAPS PharmSciTech 2017, 18, 194–201. [Google Scholar] [CrossRef]
- Rungseevijitprapa, W.; Bodmeier, R. Injectability of biodegradable in situ forming microparticle systems (ISM). Eur. J. Pharm. Sci. 2009, 36, 524–531. [Google Scholar] [CrossRef]
- Do, M.P.; Neut, C.; Delcourt, E.; Seixas Certo, T.; Siepmann, J.; Siepmann, F. In situ forming implants for periodontitis treatment with improved adhesive properties. Eur. J. Pharm. Biopharm. 2014, 88, 342–350. [Google Scholar] [CrossRef]
- Khaing, E.M.; Intaraphairot, T.; Chuenbarn, T.; Chantadee, T.; Phaechamud, T. Natural resin-based solvent exchange induced in-situ forming gel for vancomycin HCl delivery to periodontal pocket. Mater. Today Proc. 2021, 47, 3585–3593. [Google Scholar] [CrossRef]
- Chang, D.P.; Garripelli, V.K.; Rea, J.; Kelley, R.; Rajagopal, K. Investigation of fragment antibody stability and its release mechanism from poly(lactide-co-glycolide)-triacetin depots for sustained-release applications. J. Pharm. Sci. 2015, 104, 3404–3417. [Google Scholar] [CrossRef]
- Persson, G.R.; Salvi, G.E.; Heitz-Mayfield, L.J.; Lang, N.P. Antimicrobial therapy using a local drug delivery system (Arestin) in the treatment of peri-implantitis. I: Microbiological outcomes. Clin. Oral Implants Res. 2006, 17, 386–393. [Google Scholar] [CrossRef]
- Wei, Y.; Deng, Y.; Ma, S.; Ran, M.; Jia, Y.; Meng, J.; Han, F.; Guo, J.; Yin, T.; He, H.; et al. Local drug delivery systems as therapeutic strategies against periodontitis: A systematic review. J. Control. Release 2021, 333, 269–282. [Google Scholar] [CrossRef]
- Yao, W.; Xu, P.; Pang, Z.; Zhao, J.; Chai, Z.; Li, X.; Li, H.; Jiang, M.; Cheng, H.; Zhang, B.; et al. Local delivery of minocycline-loaded PEG-PLA nanoparticles for the enhanced treatment of periodontitis in dogs. Int. J. Nanomed. 2014, 9, 3963–3970. [Google Scholar] [CrossRef]
- Agrawal, A.G.; Kumar, A.; Gide, P.S. Toxicity study of a self-nanoemulsifying drug delivery system containing N-methyl pyrrolidone. Drug. Res. (Stuttg) 2015, 65, 446–448. [Google Scholar] [CrossRef]
- Jouyban, A.; Fakhree, M.A.; Shayanfar, A. Review of pharmaceutical applications of N-methyl-2-pyrrolidone. J. Pharm. Sci. 2010, 13, 524–535. [Google Scholar] [CrossRef]
- Agossa, K.; Delepierre, A.; Lizambard, M.; Delcourt-Debruyne, E.; Siepmann, J.; Siepmann, F.; Neut, C. In-situ forming implants for dual controlled release of chlorhexidine and ibuprofen for periodontitis treatment: Microbiological and mechanical key properties. J. Drug Deliv. Sci. Technol. 2020, 60, 101956. [Google Scholar] [CrossRef]
- Ovrutsky, A.M.; Prokhoda, A.S.; Rasshchupkyna, M.S. Basic concepts of theory of phase transformations. In Computational Materials Science; Ovrutsky, A.M., Prokhoda, A.S., Rasshchupkyna, M.S., Eds.; Elsevier: Oxford, UK, 2014; pp. 35–69. [Google Scholar]
- Chantadee, T.; Santimaleeworagun, W.; Phorom, Y.; Phaechamud, T. Saturated fatty acid-based in situ forming matrices for localized antimicrobial delivery. Pharmaceutics 2020, 12, 808. [Google Scholar] [CrossRef]
- Chantadee, T.; Sirirak, J.; Hoshino, T.; Phaechamud, T. Augmentative molecular aspect for phase inversion of vancomycin hydrochloride-loaded fatty acid in situ forming matrices. Mater. Des. 2021, 199, 109429. [Google Scholar] [CrossRef]
- Cubillas, P.; Anderson, M.W. Synthesis mechanism: Crystal growth and nucleation. In Zeolites and Catalysis: Synthesis, Reactions and Applications; Jiři, Č., Avelino, C., Stacey, Z., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, MA, USA, 2010; pp. 1–55. [Google Scholar]
- Parent, M.; Nouvel, C.; Koerber, M.; Sapin, A.; Maincent, P.; Boudier, A. PLGA in situ implants formed by phase inversion: Critical physicochemical parameters to modulate drug release. J. Control. Release 2013, 172, 292–304. [Google Scholar] [CrossRef]
- Vekilov, P.G. The two-step mechanism of nucleation of crystals in solution. Nanoscale 2010, 2, 2346–2357. [Google Scholar] [CrossRef] [PubMed]
- Karthika, S.; Radhakrishnan, T.K.; Kalaichelvi, P. A review of classical and nonclassical nucleation theories. Cryst. Growth Des. 2016, 16, 6663–6681. [Google Scholar] [CrossRef]
- Thurein, S.M.; Lertsuphotvanit, N.; Phaechamud, T. Physicochemical properties of beta-cyclodextrin solutions and precipitates prepared from injectable vehicles. Asian J. Pharm. Sci. 2018, 13, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Kojima, M.; Mori, Y.; Nakamura, J.; Shibasaki, J. Enhancing effect of pyrrolidone derivatives on transdermal drug delivery. I. Int. J. Pharm. 1988, 44, 15–24. [Google Scholar] [CrossRef]
- Phaechamud, T.; Mahadlek, J.; Tuntarawongsa, S. Peppermint oil/doxycycline hyclate-loaded Eudragit RS in situ forming gel for periodontitis treatment. J. Pharm. Investig. 2018, 48, 451–464. [Google Scholar] [CrossRef]
- Chuenbarn, T.; Tuntarawongsa, S.; Janmahasatian, S.; Phaechamud, T. Bleached shellac in situ forming micro-particle fabricated with different oils as antibacterial delivery system for periodontitis treatment. Mater. Today Proc. 2021, 47, 3546–3553. [Google Scholar] [CrossRef]
- Phaechamud, T.; Senarat, S.; Puyathorn, N.; Praphanwittaya, P. Solvent exchange and drug release characteristics of doxycycline hyclate-loaded bleached shellac in situ-forming gel and microparticle. Int. J. Biol. Macromol. 2019, 135, 1261–1272. [Google Scholar] [CrossRef]
- Reza, M.S.; Quadir, M.A.; Haider, S.S. Comparative evaluation of plastic, hydrophobic and hydrophilic polymers as matrices for controlled-release drug delivery. J. Pharm. Sci. 2003, 6, 282–291. [Google Scholar]
- Thakur, R.R.; McMillan, H.L.; Jones, D.S. Solvent induced phase inversion-based in situ forming controlled release drug delivery implants. J. Control. Release 2014, 176, 8–23. [Google Scholar] [CrossRef]
- Ibrahim, T.M.; El-Megrab, N.A.; El-Nahas, H.M. Optimization of injectable PLGA in-situ forming implants of anti-psychotic risperidone via Box-Behnken Design. J. Drug Deliv. Sci. Technol. 2020, 58, 101803. [Google Scholar] [CrossRef]
- Bruschi, M.L. Mathematical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Bruschi, M.L., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 63–86. [Google Scholar]
- Popova, C.; Dosseva-Panova, V.; Panov, V. Microbiology of periodontal diseases: A Review. Biotechnol. Biotechnol. Equip. 2013, 27, 3754–3759. [Google Scholar] [CrossRef]
- Mergoni, G.; Percudani, D.; Lodi, G.; Bertani, P.; Manfredi, M. Prevalence of Candida species in endodontic infections: Systematic review and meta-analysis. J. Endod. 2018, 44, 1616–1625. [Google Scholar] [CrossRef]
- Seymour, R.A.; Heasman, P.A. Pharmacological control of periodontal disease. II. Antimicrobial agents. J. Dent. 1995, 23, 5–14. [Google Scholar] [CrossRef]
- Fridkin, S.K. Increasing prevalence of antimicrobial resistance in intensive care units. Crit. Care Med. 2001, 29, N64–N68. [Google Scholar] [CrossRef]
- Senarat, S.; Rojviriya, C.; Puyathorn, N.; Lertsuphotvanit, N.; Phaechamud, T. Levofloxacin HCl-incorporated zein-based solvent removal phase inversion in situ forming gel for periodontitis treatment. Pharmaceutics 2023, 15, 1199. [Google Scholar] [CrossRef]
- Larsen, T. Susceptibility of Porphyromonas gingivalis in biofilms to amoxicillin, doxycycline and metronidazole. Oral Microbiol. Immunol. 2002, 17, 267–271. [Google Scholar] [CrossRef]
- Oettinger-Barak, O.; Dashper, S.G.; Catmull, D.V.; Adams, G.G.; Sela, M.N.; Machtei, E.E.; Reynolds, E.C. Antibiotic susceptibility of Aggregatibacter actinomycetemcomitans JP2 in a biofilm. J. Oral Microbiol. 2013, 5, 20320. [Google Scholar] [CrossRef]
- Tabanca, N.; Kirimer, N.; Demirci, B.; Demirci, F.; Baser, K.H. Composition and antimicrobial activity of the essential oils of Micromeria cristata subsp. phrygia and the enantiomeric distribution of borneol. J. Agric. Food Chem. 2001, 49, 4300–4303. [Google Scholar] [CrossRef]
- Phaechamud, T.; Mahadlek, J.; Charoenteeraboon, J.; Choopun, S. Characterization and antimicrobial activity of N-methyl-2-pyrrolidone-loaded ethylene oxide-propylene oxide block copolymer thermosensitive gel. Indian J. Pharm. Sci. 2012, 74, 498–504. [Google Scholar] [CrossRef]
- Hammerschmidt, F.J.; Clark, A.M.; Soliman, F.M.; el-Kashoury, E.S.; Abd el-Kawy, M.M.; el-Fishawy, A.M. Chemical composition and antimicrobial activity of essential oils of Jasonia candicans and J. montana. Planta Med. 1993, 59, 68–70. [Google Scholar] [CrossRef]
- Johnson, S.A.M.; Tuura, J.L. Glyceryl triacetate (triacetin) as a fungicide. Arch. Dermatol. 1956, 74, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Cunha, B.A.; Domenico, P.; Cunha, C.B. Pharmacodynamics of doxycycline. Clin. Microbiol. Infect. 2000, 6, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, B.T.; Rybak, M.J.; Cheung, C.M.; Amjad, M.; Kaatz, G.W. Community- and health care-associated methicillin-resistant Staphylococcus aureus: A comparison of molecular epidemiology and antimicrobial activities of various agents. Diagn. Microbiol. Infect. Dis. 2007, 58, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, L.; Biasi, M.D.; Lorenzon, M.G.; Frattini, C.; Angerame, D. Volumetric analysis of gingival crevicular fluid and peri-implant sulcus fluid in healthy and diseased sites: A cross-sectional split-mouth pilot study. Open Dent. J. 2016, 10, 131–138. [Google Scholar] [CrossRef]



| Formulation | Density (g/cm3) | Viscosity (cPs) | Surface Tension (mN/m) |
|---|---|---|---|
| Triacetin | 1.1472 ± 0.0002 | 25.53 ± 0.52 | 38.87 ± 0.55 |
| NMP | 1.0283 ± 0.0004 | 1.88 ± 0.03 | 39.62 ± 0.02 |
| N1 | 1.0425 ± 0.0009 | 2.59 ± 0.16 | 40.33 ± 0.42 |
| N2 | 1.0204 ± 0.0014 a,b | 5.38 ± 0.09 e,f | 35.18 ± 0.16 |
| N3 | 1.0228 ± 0.0011 | 5.55 ± 0.12 | 34.65 ± 0.23 |
| N4 | 1.0268 ± 0.0017 a | 6.16 ± 0.07 e | 34.61 ± 0.22 |
| N5 | 1.0290 ± 0.0009 | 6.66 ± 0.13 | 34.08 ± 0.17 |
| N6 | 1.0340 ± 0.0014 | 7.06 ± 0.06 | 33.43 ± 0.07 |
| N7 | 1.0386 ± 0.0017 | 8.84 ± 0.09 | 33.29 ± 0.08 |
| N8 | 1.0435 ± 0.0013 b | 9.67 ± 0.04 f | 33.14 ± 0.09 |
| DMSO | 1.0924 ± 0.0003 | 2.25 ± 0.04 | 42.61 ± 0.46 |
| D1 | 1.1090 ± 0.0003 | 3.07 ± 0.08 | 44.59 ± 0.36 |
| D2 | 1.0524 ± 0.0009 c,d | 5.49 ± 0.12 g,h | 34.28 ± 0.95 |
| D3 | 1.0538 ± 0.0008 | 5.92 ± 0.33 | 35.49 ± 0.15 |
| D4 | 1.0557 ± 0.0013 c | 6.30 ± 0.19 g | 34.94 ± 0.38 |
| D5 | 1.0579 ± 0.0009 | 7.19 ± 0.09 | 34.23 ± 0.38 |
| D6 | 1.0601 ± 0.0004 | 8.19 ± 0.33 | 34.69 ± 0.15 |
| D7 | 1.0626 ± 0.0011 | 8.76 ± 0.11 | 35.06 ± 0.25 |
| D8 | 1.0656 ± 0.0006 d | 10.43 ± 0.62 h | 34.86 ± 0.31 |
| Formulation | Modeling | Criteria for Model Selection | Kinetic Parameters | ||||
|---|---|---|---|---|---|---|---|
| R2 | AIC | MSC | |||||
| N1 | Zero order | 0.9480 | 32.8196 | 2.3843 | k0 = 0.058 | ||
| First order | 0.9831 | 24.9566 | 3.5076 | k1 = 0.001 | |||
| Higuchi | 0.9799 | 26.1750 | 3.3335 | kH = 2.578 | |||
| Korsmeyer–Peppas | 0.9956 | 17.5847 | 4.5607 | kKP = 9.011 | n = 0.318 | ||
| Hixson–Crowell | 0.9833 | 33.9840 | 3.5898 | kHC = 0.0000 | |||
| Hopfenberg | 0.9921 | 30.0182 | 4.0855 | kHB = 0.0000 | n = 185.258 | ||
| Peppas–Sahlin | 0.9979 | 21.2822 | 5.1775 | k1 = 8.630 | k2 = −0.048 | m = 0.332 | |
| N2 | Zero order | 0.9111 | 90.8974 | 2.1756 | k0 = 0.023 | ||
| First order | 0.9889 | 63.8739 | 4.2543 | k1 = 0.000 | |||
| Higuchi | 0.9762 | 73.7490 | 3.4947 | kH = 1.253 | |||
| Korsmeyer–Peppas | 0.9984 | 40.7684 | 6.0316 | kKP = 0.461 | n = 0.629 | ||
| Hixson–Crowell | 0.9812 | 70.7012 | 3.7291 | kHC = 0.000 | |||
| Hopfenberg | 0.9889 | 65.8890 | 4.0993 | kHB = 0.000 | n = 653.884 | ||
| Peppas–Sahlin | 0.9984 | 42.7074 | 5.8825 | k1 = −0.157 | k2 = 0.518 | m = 0.309 | |
| N4 | Zero order | 0.8408 | 121.9651 | 1.6132 | k0 = 0.011 | ||
| First order | 0.9471 | 104.3304 | 2.7154 | k1 = 0.000 | |||
| Higuchi | 0.9969 | 58.8696 | 5.5567 | kH = 0.889 | |||
| Korsmeyer–Peppas | 0.9974 | 57.9719 | 5.6128 | kKP = 0.761 | n = 0.518 | ||
| Hixson–Crowell | 0.9288 | 109.0962 | 2.4175 | kHC = 0.000 | |||
| Hopfenberg | 0.9471 | 106.3399 | 2.5898 | kHB = 0.000 | n = 1295.786 | ||
| Peppas–Sahlin | 0.9987 | 48.5514 | 6.2015 | k1 = 1.291 | k2 = 0.026 | m = 0.399 | |
| N8 | Zero order | 0.9310 | 69.2509 | 2.3960 | k0 = 0.036 | ||
| First order | 0.9943 | 41.8565 | 4.8864 | k1 = 0.001 | |||
| Higuchi | 0.9411 | 67.5029 | 2.5550 | kH = 1.417 | |||
| Korsmeyer–Peppas | 0.9985 | 10.5336 | 5.7222 | kKP = 0.399 | n = 0.679 | ||
| Hixson–Crowell | 0.9901 | 47.8600 | 4.3407 | kHC = 0.000 | |||
| Hopfenberg | 0.9943 | 43.7404 | 4.7152 | kHB = 0.000 | n = 26.098 | ||
| Peppas–Sahlin | 0.9986 | 12.3015 | 5.3686 | k1 = 0.652 | k2 = 0.191 | m = 0.376 | |
| Microbes | Inhibition Zone Diameter (Mean ± S.D.) (mm) | |||||
|---|---|---|---|---|---|---|
| NMP | Triacetin | N1 | N2 | N4 | N8 | |
| P. gingivalis ATCC 33277 | 15.00 ± 1.73 a,b | NC | 40.33 ± 1.53 a | 35.33 ± 0.58 | 34.33 ± 1.15 b | 32.67 ± 0.58 |
| A. actinomycetemcomitans ATCC 29522 | 46.33 ± 0.58 c | 21.33 ± 0.58 | 50.00 ± 1.00 c | 37.33 ± 1.15 | 36.67 ± 0.58 | 30.67 ± 0.58 |
| S. aureus (MRSA) ATCC 43300 | 17.00 ± 1.00 d,e | 12.33 ± 2.52 | 42.67 ± 1.15 d | 39.33 ± 1.15 | 38.00 ± 0.58 e | 36.67 ± 1.53 |
| S. aureus ATCC 25923 | 18.67 ± 0.58 f,g | NC | 38.67 ± 0.58 f | 37.00 ± 1.00 | 35.33 ± 1.15 g | 33.33 ± 0.58 |
| E. coli ATCC 8739 | 22.33 ± 0.58 h,i | NC | 29.33 ± 0.58 h | 28.67 ± 0.58 | 27.33 ± 0.00 i | 25.67 ± 0.58 |
| C. albicans ATCC 17100 | 26.00 ± 1.00 | 12.00 ± 1.00 | 26.33 ± 0.58 | 22.33 ± 1.00 | 20.00 ± 0.00 | 17.33 ± 1.15 |
| C. krusei TISTR 5259 | 31.30 ± 1.20 | NC | 32.33 ± 0.58 | 21.00 ± 1.00 | 18.33 ± 1.53 | NC |
| C. lusitaniae TISTR 5156 | 38.33 ± 1.15 | 12.33 ± 0.58 | 38.33 ± 0.58 | 35.33 ± 1.15 | 34.33 ± 1.53 | 26.00 ± 1.00 |
| C. tropicalis TISTR 5306 | 32.67 ± 0.58 | NC | 31.67 ± 0.58 | 29.67 ± 2.08 | 25.67 ± 1.53 | 18.00 ± 2.00 |
| Formulation | Amount (% w/w) | ||||
|---|---|---|---|---|---|
| Dox | Borneol | Triacetin | Solvent | ||
| NMP | DMSO | ||||
| NMP | - | - | - | 100 | - |
| N1 | 5 | - | 95 | - | |
| N2 | 5 | 40 | - | 55 | - |
| N3 | 5 | 40 | 2.5 | 52.5 | - |
| N4 | 5 | 40 | 5.0 | 50 | - |
| N5 | 5 | 40 | 10.0 | 45 | - |
| N6 | 5 | 40 | 15.0 | 40 | - |
| N7 | 5 | 40 | 20.0 | 35 | - |
| N8 | 5 | 40 | 25.0 | 30 | - |
| DMSO | - | - | - | - | 100 |
| D1 | 5 | - | - | - | 95 |
| D2 | 5 | 40 | - | - | 55 |
| D3 | 5 | 40 | 2.5 | - | 52.5 |
| D4 | 5 | 40 | 5.0 | - | 50 |
| D5 | 5 | 40 | 10.0 | - | 45 |
| D6 | 5 | 40 | 15.0 | - | 40 |
| D7 | 5 | 40 | 20.0 | - | 35 |
| D8 | 5 | 40 | 25.0 | - | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lertsuphotvanit, N.; Tuntarawongsa, S.; Chantadee, T.; Phaechamud, T. Phase Inversion-Based Doxycycline Hyclate-Incorporated Borneol In Situ Gel for Periodontitis Treatment. Gels 2023, 9, 557. https://doi.org/10.3390/gels9070557
Lertsuphotvanit N, Tuntarawongsa S, Chantadee T, Phaechamud T. Phase Inversion-Based Doxycycline Hyclate-Incorporated Borneol In Situ Gel for Periodontitis Treatment. Gels. 2023; 9(7):557. https://doi.org/10.3390/gels9070557
Chicago/Turabian StyleLertsuphotvanit, Nutdanai, Sarun Tuntarawongsa, Takron Chantadee, and Thawatchai Phaechamud. 2023. "Phase Inversion-Based Doxycycline Hyclate-Incorporated Borneol In Situ Gel for Periodontitis Treatment" Gels 9, no. 7: 557. https://doi.org/10.3390/gels9070557
APA StyleLertsuphotvanit, N., Tuntarawongsa, S., Chantadee, T., & Phaechamud, T. (2023). Phase Inversion-Based Doxycycline Hyclate-Incorporated Borneol In Situ Gel for Periodontitis Treatment. Gels, 9(7), 557. https://doi.org/10.3390/gels9070557









