3D Printing, Histological, and Radiological Analysis of Nanosilicate-Polysaccharide Composite Hydrogel as a Tissue-Equivalent Material for Complex Biological Bone Phantom
Abstract
1. Introduction
- Each type of phantom requires specific materials that closely simulate the desired properties of bone tissue. Spatial and biomechanical properties are represented by high-fidelity anatomical models [1] or benchmark devices for biomechanical testing [2,3] that require materials with minimal thermal deformation (thermo- or photopolymers), and can be 3D printed with FDM (fused deposition modeling), SLS (selective laser sintering), or SLA (stereolithography) at optimal spatial resolutions.
- Biological and optical properties are represented by bioscaffolds [4] or organ-on-chip devices [5] made of biomaterials such as hydrogel biopolymers with optimal cell culturing characteristics and staining properties that do not impede histological examination and analysis; these can be 3D printed with extrusion-based 3D printing.
- Radiological properties are represented by imaging phantoms [6], which require materials with an atomic mass and X-ray attenuation coefficient similar to that of natural bone (for instance, thermopolymers or polymer-inorganic clay composites); these can be 3D printed with FDM.
1.1. Composite Polysaccharide–Nanosilicate Hydrogels
1.2. 3D Printing of Bioscaffolds
- Biocompatibility: the scaffold must provide the necessary base for adequate cellular adhesion, proliferation, and differentiation [38]. If the scaffold is implantable, it should not cause any inflammatory or immune reaction, which disrupts tissue regeneration and may cause rejection by the recipient.
- Bioresorption: the materials of the scaffold must be bioresorbable and eventually replaced by a newly generated extracellular matrix [39]. The byproducts of biodegradation should be nontoxic and easy to eliminate from the organism without interference with other organs and systems.
- Mechanical properties: the scaffold should possess mechanical properties corresponding to those of the tissue in which it will be implanted [40] and must preserve its integrity from the moment of implantation to the completion of the remodeling process. This condition is especially important for bone and cartilage engineering.
- Scaffold architecture: the scaffold should possess a porous structure specific to the engineered tissue, with interconnected spaces occupying a sufficient part of the total volume [41]. High porosity ensures adequate cell migration, diffusion of nutrients, and elimination of waste products. Adequate vascularization of the scaffold prevents necrosis, inflammation, and rejection of the implant [42]. Another key concern is cell adhesion, as cells bind to chemical groups (ligands) that are naturally present only in extracellular fibrillar glycoproteins. In non-natural materials, active adhesion sites can be engineered by adding binding sequences (such as Arg-Gly-Asp, RGD) or by other means to facilitate cell adhesion [43].
- Radiological properties: as an implantable structure, the bioscaffold should be controlled using imaging methods. This requires tissue-equivalent radiological properties that ensure proper control over scaffold implantation [44].
- Histological properties: staining qualities must ensure that the engineered matrix does not interfere with histological and cytological analysis during scaffold development and testing [45].
- Manufacturing technology: bioscaffold production with 3D printing or other spatially controlled technology requires high reproducibility as well as proper quality control and certification [46].
1.3. 3D Printing of Imaging Phantoms
1.4. Development of Complex Multipurpose Biological–Radiological Phantoms
2. Results and Discussion
2.1. Preparation of the Hydrogel
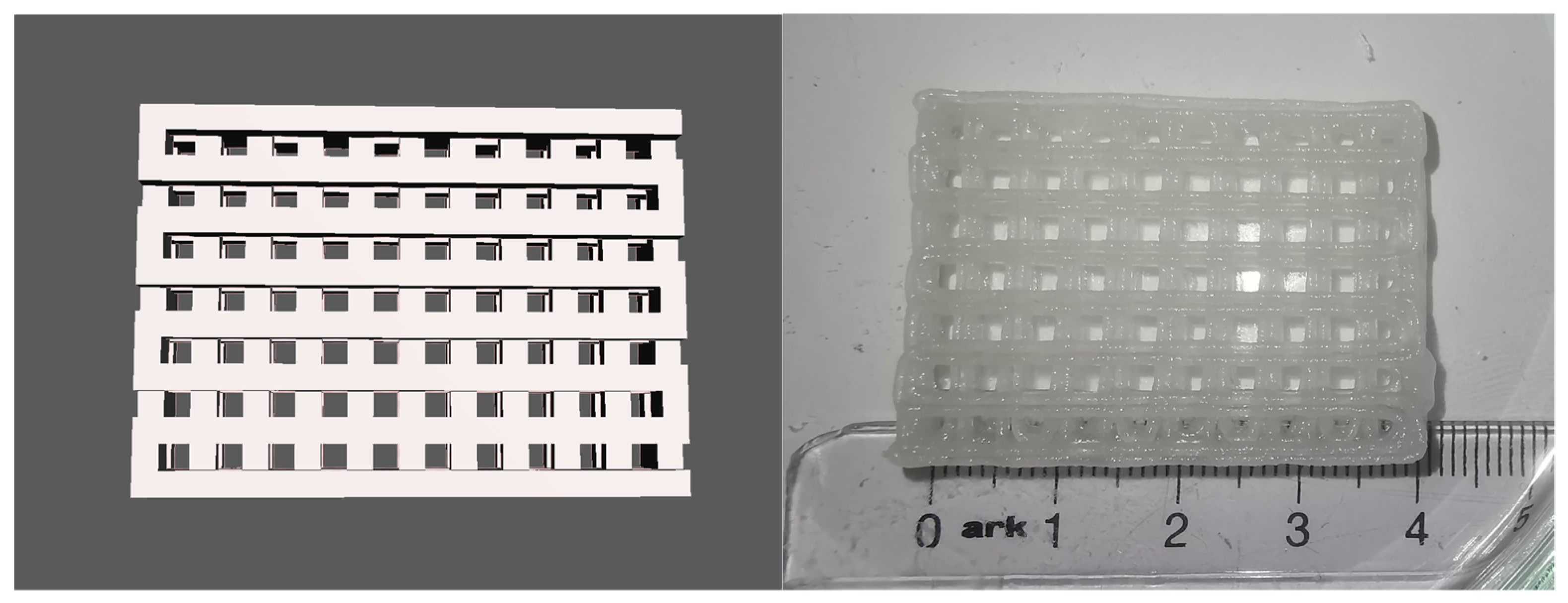
2.2. 3D Printing of Test Models and Complex Scaffolds
- Square prisms of 40 × 40 × 10 mm, 30 × 30 × 10 mm, and 20 × 20 × 10 mm;
- A cube with a size of 20 × 20 × 20 mm3;
- A cylinder with a diameter of 20 mm and height of 20 mm.
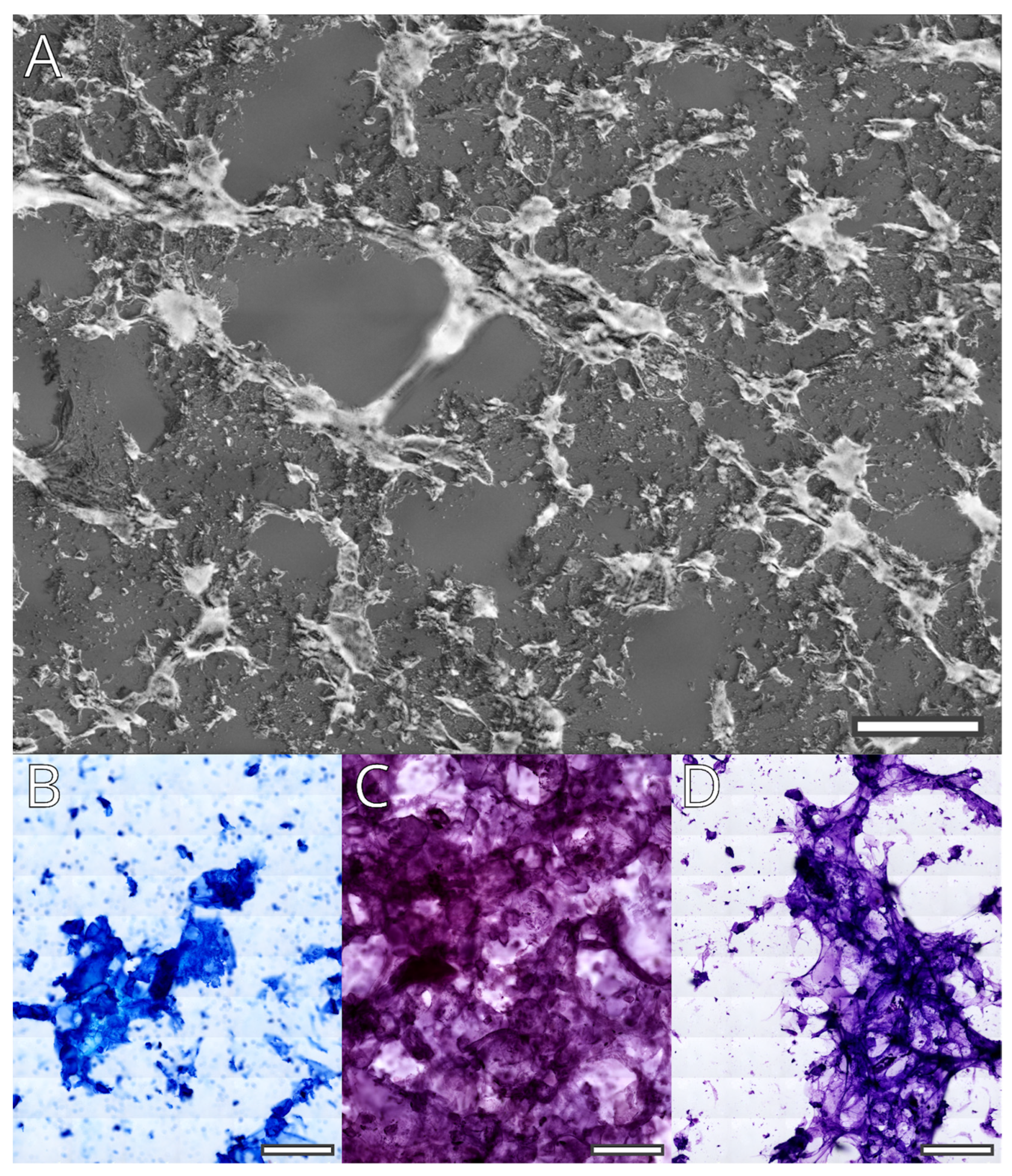
2.3. Staining and Histological Properties of the Hydrogel
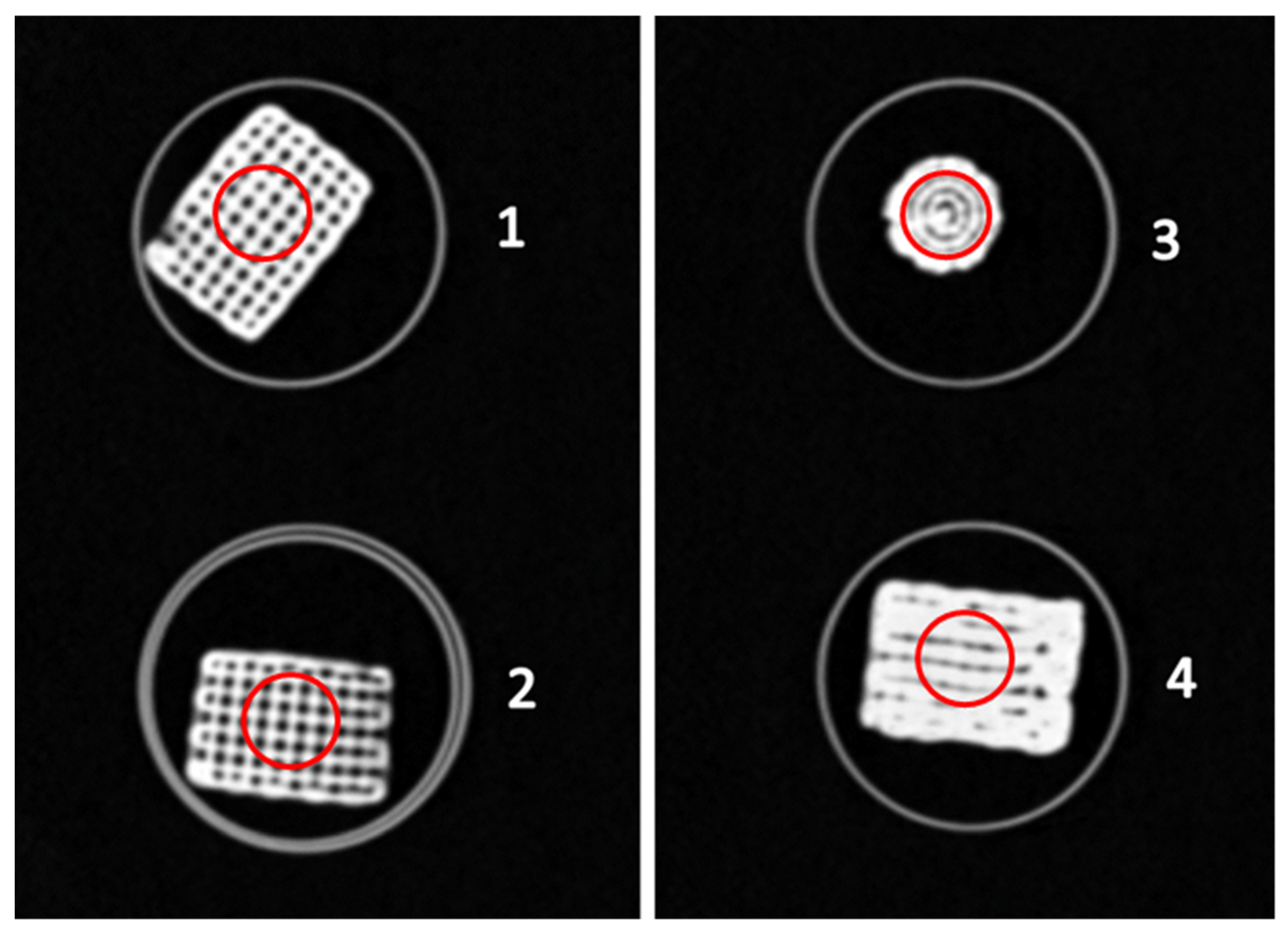
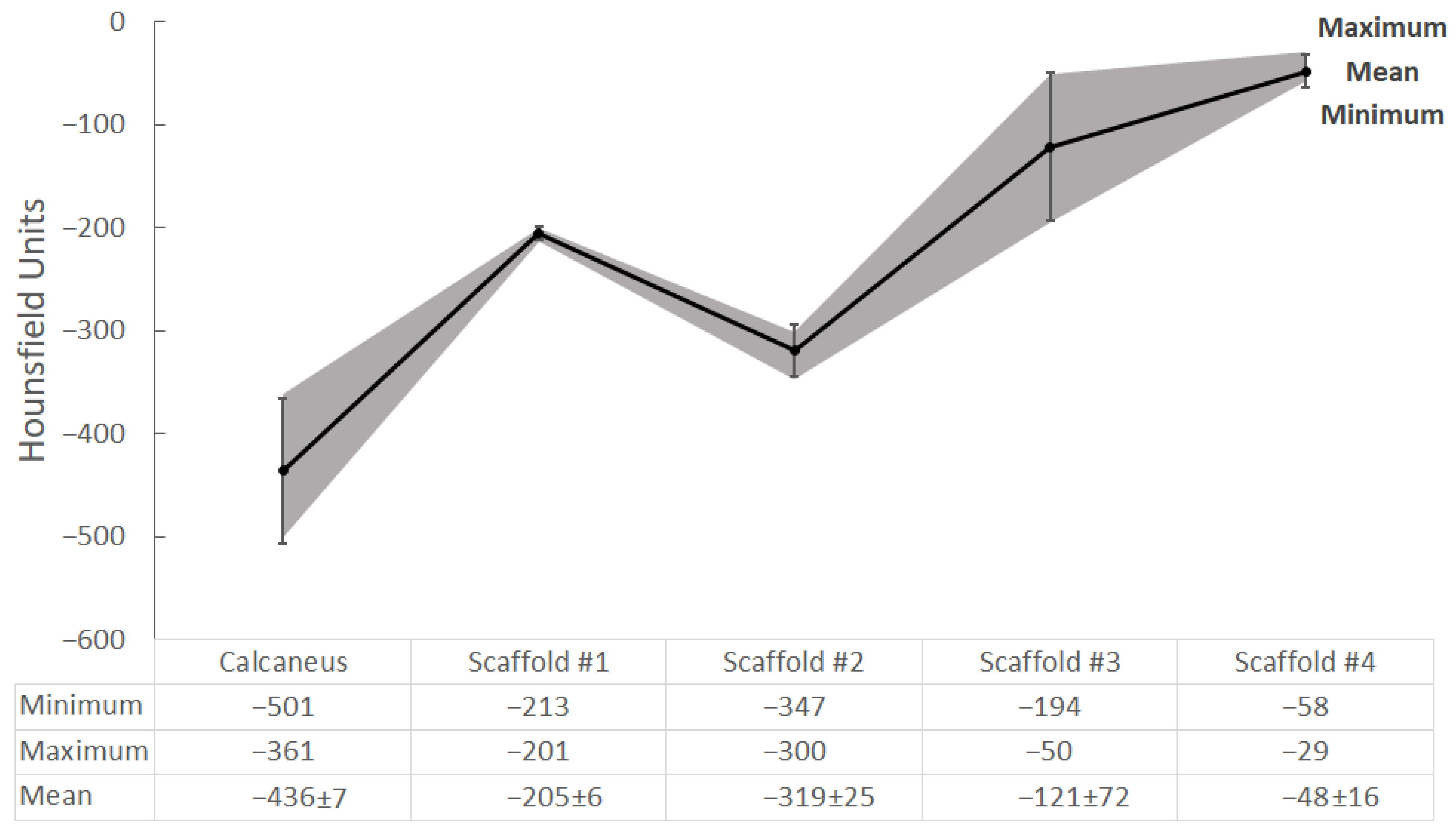
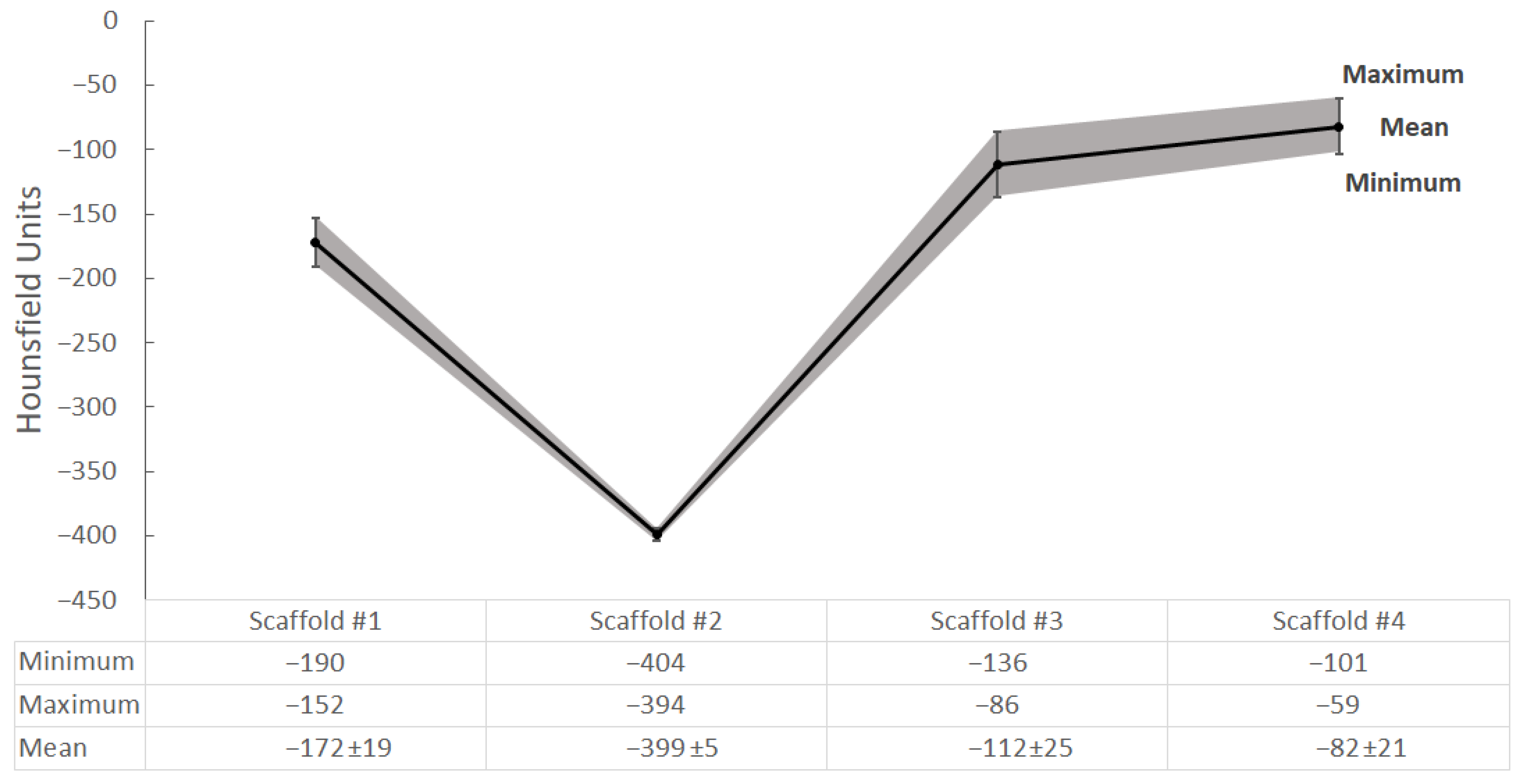
2.4. CT Scanning and Radiological Analysis of 3D-Printed Hydrogel Scaffolds
3. Conclusions
4. Materials and Methods
4.1. Preparation of the Hydrogel
- 0.3 g. Alginic acid sodium salt from brown algae, middle viscosity, Sigma-Aldrich (Burlington, MA, USA).
- 0.3 g. Methyl Cellulose, viscosity 4000 cP, 2% in H2O (20 C) (lit.); Sigma-Aldrich (Burlington, MA, USA).
- 0.3 g Laponite RD; BYK.
- 10 mL deionized water CHROMASOLV™ Plus, for HPLC; Honeywell (Charlotte, NC, USA).
- Calcium chloride, anhydrous, granular; 96%, Sigma-Aldrich (Burlington, MA, USA).
4.2. 3D Modelling and 3D Printing
- Layer height: 0.5 mm for Tests 1, 2, and 3; 0.4 mm for Tests 4 and 5.
- Shell thickness: 0.8 mm.
- Nozzle diameter: 0.838 mm (for a nozzle, we used commercially available hypodermic needles at 18 G caliber, which were shortened and had the tip ground flat).
- Speed: 10 mm/s for Tests 1 and 2; 5 mm/s for Tests 3, 4, and 5; Perimeters: 4.
- For the generation of g-code, we used Slic3r (Version 1.9) for all tests.
4.3. Staining
- Diff Quik is a commercial variant of Wright’s stain. In our implementation, we skipped the fixation step with methanol, rehydrated in distilled water for 10 min, and stained sequentially with a buffered solution of Methylene blue and Azure A (nuclear stain) followed by buffered Eosin Y (contrast stain), dehydration, clearing in xylol, and covering.
- Standard Hemalaun–Eosin protocol: rehydration in distilled water for 10 min, staining in Hemalaun for 5 min, fixation and bluing of the He stain in tap water, staining with Eosin, dehydration, clearing in xylol, and covering.
- Staining with Cresyl violet: rehydration in distilled water for 10 min, staining for 1 to 5 min in 0.5% solution of Cresyl violet, differentiation in 1.5% acetic acid in 90% ethanol, dehydration clearing, and covering.
4.4. CT Scanning
4.5. Radiological Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bücking, T.M.; Hill, E.R.; Robertson, J.L.; Maneas, E.; Plumb, A.A.; Nikitichev, D.I. From medical imaging data to 3D printed anatomical models. PLoS ONE 2017, 12, e0178540. [Google Scholar] [CrossRef] [PubMed]
- Verstraete, M.A.; Willemot, L.; Van Onsem, S.; Stevens, C.; Arnout, N.; Victor, J. 3D printed guides for controlled alignment in biomechanics tests. J. Biomech. 2016, 49, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, S.; Stefanov, A.; Zderic, I.; Rodemund, C.; Schepers, T.; Gehweiler, D.; Dauwe, J.; Pastor, T.; Makelov, B.; Raykov, D.; et al. Percutaneous fixation of intraarticular joint-depression calcaneal fractures with different screw configurations—A biomechanical human cadaveric analysis. Eur. J. Trauma Emerg. Surg. 2022, 48, 3305–3315. [Google Scholar] [CrossRef] [PubMed]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.-G.; Lee, H.; Cho, D.-W. 3D Printing of Organs-On-Chips. Bioengineering 2017, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Bliznakova, K.; Buliev, I.; Bliznakov, Z. Design and composition of anthropomorphic phantoms. In Anthropomorphic Phantoms in Image Quality and Patient Dose Optimization; IOP Publishing: Bristol, UK, 2018; pp. 3-1–3-19. [Google Scholar] [CrossRef]
- Gelinsky, M. Biopolymer hydrogel bioinks. In 3D Bioprinting for Reconstructive Surgery: Techniques and Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 125–136. [Google Scholar] [CrossRef]
- Xavier, J.R.; Thakur, T.; Desai, P.; Jaiswal, M.K.; Sears, N.; Cosgriff-Hernandez, E.; Kaunas, R.; Gaharwar, A.K. Bioactive Nanoengineered Hydrogels for Bone Tissue Engineering: A Growth-Factor-Free Approach. ACS Nano 2015, 9, 3109–3118. [Google Scholar] [CrossRef]
- GhavamiNejad, A.; Ashammakhi, N.; Wu, X.Y.; Khademhosseini, A. Crosslinking Strategies for 3D Bioprinting of Polymeric Hydrogels. Small 2020, 16, 2002931. [Google Scholar] [CrossRef]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef]
- Zhai, X.; Ma, Y.; Hou, C.; Gao, F.; Zhang, Y.; Ruan, C.; Pan, H.; Lu, W.W.; Liu, W. 3D-Printed High Strength Bioactive Supramolecular Polymer/Clay Nanocomposite Hydrogel Scaffold for Bone Regeneration. ACS Biomater. Sci. Eng. 2017, 3, 1109–1118. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Janarthanan, G.; Noh, I. Nano-biomaterials for designing functional bioinks towards complex tissue and organ regeneration in 3D bioprinting. Addit. Manuf. 2021, 37, 101639. [Google Scholar] [CrossRef]
- Koons, G.L.; Mikos, A.G. Progress in three-dimensional printing with growth factors. J. Control. Release 2019, 295, 50–59. [Google Scholar] [CrossRef]
- Aldrich, A.; Kuss, M.A.; Duan, B.; Kielian, T. 3D Bioprinted Scaffolds Containing Viable Macrophages and Antibiotics Promote Clearance of Staphylococcus aureus Craniotomy-Associated Biofilm Infection. ACS Appl. Mater. Interfaces 2019, 11, 12298–12307. [Google Scholar] [CrossRef] [PubMed]
- Valchanov, P.; Pavlov, S.; Chervenkov, T. Composite hydrogels and their application for 3D Bioprinting in the Regenerative medicine. In Proceedings of the 2020 International Conference on Biomedical Innovations and Applications (BIA), Varna, Bulgaria, 24–27 September 2020; IEEE: Washington, DC, USA, 2020; pp. 25–28. [Google Scholar] [CrossRef]
- Chimutengwende-Gordon, M. Advances in the Use of Stem Cells and Tissue Engineering Applications in Bone Repair. Curr. Stem Cell Res. Ther. 2012, 7, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Vega, S.; Kwon, M.; Burdick, J. Recent advances in hydrogels for cartilage tissue engineering. Eur. Cells Mater. 2017, 33, 59–75. [Google Scholar] [CrossRef]
- Bakirci, E.; Guenat, O.; Ahmad, S.; Gantenbein, B. Tissue engineering approaches for the repair and regeneration of the anterior cruciate ligament: Towards 3D bioprinted ACL-on-chip. Eur. Cells Mater. 2022, 44, 21–42. [Google Scholar] [CrossRef]
- Bentzinger, C.F.; Wang, Y.X.; von Maltzahn, J.; Rudnicki, M.A. The emerging biology of muscle stem cells: Implications for cell-based therapies. Bioessays 2013, 35, 231–241. [Google Scholar] [CrossRef] [PubMed]
- O’connor, C.; Brady, E.; Zheng, Y.; Moore, E.; Stevens, K.R. Engineering the multiscale complexity of vascular networks. Nat. Rev. Mater. 2022, 7, 702–716. [Google Scholar] [CrossRef] [PubMed]
- Tomás, H.; Alves, C.S.; Rodrigues, J. Laponite®: A key nanoplatform for biomedical applications? Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2407–2420. [Google Scholar] [CrossRef]
- Becher, T.B.; Braga, C.B.; Bertuzzi, D.L.; Ramos, M.D.; Hassan, A.; Crespilho, F.N.; Ornelas, C. The structure–property relationship in LAPONITE® materials: From Wigner glasses to strong self-healing hydrogels formed by non-covalent interactions. Soft Matter 2019, 15, 1278–1289. [Google Scholar] [CrossRef]
- Miao, S.; Zhou, J.; Liu, B.; Lei, X.; Wang, T.; Hao, X.; Cheng, P.; Wu, H.; Song, Y.; Pei, G.; et al. A 3D bioprinted nano-laponite hydrogel construct promotes osteogenesis by activating PI3K/AKT signaling pathway. Mater. Today Bio. 2022, 16, 100342. [Google Scholar] [CrossRef]
- Axpe, E.; Oyen, M.L. Applications of alginate-based bioinks in 3D bioprinting. Int. J. Mol. Sci. 2016, 17, 1976. [Google Scholar] [CrossRef] [PubMed]
- Murizan, N.I.S.; Mustafa, N.S.; Ngadiman, N.H.A.; Yusof, N.M.; Idris, A. Review on Nanocrystalline Cellulose in Bone Tissue Engineering Applications. Polymers 2020, 12, 2818. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, D.; Chen, J.; Zhang, X.; Li, X.; Zhao, W.; Xu, T. Biomaterials based on marine resources for 3D bioprinting applications. Mar. Drugs 2019, 17, 555. [Google Scholar] [CrossRef] [PubMed]
- Petta, D.; D’Amora, U.; Ambrosio, L.; Grijpma, D.; Eglin, D.; D’Este, M. Hyaluronic acid as a bioink for extrusion-based 3D printing. Biofabrication 2020, 12, 032001. [Google Scholar] [CrossRef]
- Osidak, E.O.; Kozhukhov, V.I.; Osidak, M.S.; Domogatskiy, S.P. Collagen as Bioink for Bioprinting: A Comprehensive Review. Int. J. Bioprinting 2020, 6, 270. [Google Scholar] [CrossRef]
- Shpichka, A.; Osipova, D.; Efremov, Y.; Bikmulina, P.; Kosheleva, N.; Lipina, M.; Bezrukov, E.A.; Sukhanov, R.B.; Solovieva, A.B.; Vosough, M.; et al. Fibrin-based bioinks: New tricks from an old dog. Int. J. Bioprinting 2020, 6, 269. [Google Scholar] [CrossRef]
- Bae, S.-W.; Lee, K.-W.; Park, J.-H.; Lee, J.; Jung, C.-R.; Yu, J.; Kim, H.-Y.; Kim, D.-H. 3D bioprinted artificial trachea with epithelial cells and chondrogenic-differentiated bone marrow-derived mesenchymal stem cells. Int. J. Mol. Sci. 2018, 19, 1624. [Google Scholar] [CrossRef]
- Augello, A.; Kurth, T.B.; De Bari, C. Mesenchymal stem cells: A perspective from in vitro cultures to in vivo migration and niches. Eur. Cells Mater. 2010, 20, 121–133. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Caddeo, S.; Boffito, M.; Sartori, S. Tissue Engineering Approaches in the Design of Healthy and Pathological in vitro Tissue Models. Front. Bioeng. Biotechnol. 2017, 5, 40. [Google Scholar] [CrossRef]
- Negrini, N.C.; Ricci, C.; Bongiorni, F.; Trombi, L.; D’alessandro, D.; Danti, S.; Farè, S. An Osteosarcoma Model by 3D Printed Polyurethane Scaffold and In Vitro Generated Bone Extracellular Matrix. Cancers 2022, 14, 2003. [Google Scholar] [CrossRef]
- Palo, M.; Holländer, J.; Suominen, J.; Yliruusi, J.; Sandler, N. 3D printed drug delivery devices: Perspectives and technical challenges. Expert Rev. Med. Devices 2017, 14, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Valchanov, P.S. 3D Printing in medicine—Principles, applications and challenges. Scr. Sci. Vox Stud. 2017, 1, 18–22. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Naahidi, S.; Jafari, M.; Logan, M.; Wang, Y.; Yuan, Y.; Bae, H.; Dixon, B.; Chen, P. Biocompatibility of hydrogel-based scaffolds for tissue engineering applications. Biotechnol. Adv. 2017, 35, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Freed, L.E.; Vunjak-Novakovic, G.; Biron, R.J.; Eagles, D.B.; Lesnoy, D.C.; Barlow, S.K.; Langer, R. Biodegradable Polymer Scaffolds for Tissue Engineering. Nat. Biotechnol. 1994, 12, 689–693. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Kishore, V.; Rivera, C.; Bullock, W.; Wu, C.-J.; Akkus, O.; Schmidt, G. Physically Crosslinked Nanocomposites from Silicate-Crosslinked PEO: Mechanical Properties and Osteogenic Differentiation of Human Mesenchymal Stem Cells. Macromol. Biosci. 2012, 12, 779–793. [Google Scholar] [CrossRef]
- Ma, T.; Li, Y.; Yang, S.-T.; Kniss, D.A. Effects of pore size in 3-D fibrous matrix on human trophoblast tissue development. Biotechnol. Bioeng. 2000, 70, 606–618. [Google Scholar] [CrossRef]
- Mori, N.; Akagi, Y.; Imai, Y.; Takayama, Y.; Kida, Y.S. Fabrication of Perfusable Vascular Channels and Capillaries in 3D Liver-like Tissue. Sci. Rep. 2020, 10, 5646. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, C.-Y.; Hollister, S.J. The interaction between bone marrow stromal cells and RGD-modified three-dimensional porous polycaprolactone scaffolds. Biomaterials 2009, 30, 4063–4069. [Google Scholar] [CrossRef]
- Petri, M.; Namazian, A.; Wilke, F.; Ettinger, M.; Stübig, T.; Brand, S.; Bengel, F.; Krettek, C.; Berding, G.; Jagodzinski, M. Repair of segmental long-bone defects by stem cell concentrate augmented scaffolds: A clinical and positron emission tomography-computed tomography analysis. Int. Orthop. 2013, 37, 2231–2237. [Google Scholar] [CrossRef] [PubMed]
- Valentin, J.E.; Badylak, J.S.; McCabe, G.P.; Badylak, S. Extracellular Matrix Bioscaffolds for Orthopaedic Applications. J. Bone Jt. Surg. 2006, 88, 2673–2686. [Google Scholar] [CrossRef] [PubMed]
- Kanters, D.; de Vries, A.; Boon, H.; Urbach, J.; Becht, A.; Kooistra, H.-A. Quality Assurance in Medical 3D-Printing. In Proceedings of the World Congress on Medical Physics and Biomedical Engineering 2018, Prague, Czech Republic, 3–8 June 2018; pp. 669–674. [Google Scholar] [CrossRef]
- Okkalidis, N. 3D printing methods for radiological anthropomorphic phantoms. Phys. Med. Biol. 2022, 67, 15TR04. [Google Scholar] [CrossRef]
- Sarno, A.; Valero, C.; Tucciariello, R.M.; Dukov, N.; Costa, P.R.; Tomal, A. Physical and digital phantoms for 2D and 3D x-ray breast imaging: Review on the state-of-the-art and future prospects. Radiat. Phys. Chem. 2023, 204, 110715. [Google Scholar] [CrossRef]
- Aimar, A.; Palermo, A.; Innocenti, B. The Role of 3D Printing in Medical Applications: A State of the Art. J. Health Eng. 2019, 2019, 5340616. [Google Scholar] [CrossRef]
- Dahal, E.; Badal, A.; Zidan, A.; Alayoubi, A.; Hagio, T.; Glick, S.J.; Badano, A.; Ghammraoui, B. Stable gelatin-based phantom materials with tunable X-ray attenuation properties and 3D printability for x-ray imaging. Phys. Med. Biol. 2018, 63, 09NT01. [Google Scholar] [CrossRef] [PubMed]
- Ahlfeld, T.; Cidonio, G.; Kilian, D.; Duin, S.; Akkineni, A.R.; Dawson, J.I.; Yang, S.; Lode, A.; Oreffo, R.O.C.; Gelinsky, M. Development of a clay based bioink for 3D cell printing for skeletal application. Biofabrication 2017, 9, 034103. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, C.; Chai, W.; Compaan, A.; Huang, Y. Self-Supporting Nanoclay as Internal Scaffold Material for Direct Printing of Soft Hydrogel Composite Structures in Air. ACS Appl. Mater. Interfaces 2017, 9, 17456–17465. [Google Scholar] [CrossRef] [PubMed]
- Autodesk Meshmixer. Available online: http://www.meshmixer.com/ (accessed on 17 April 2018).
- Timmis, H. Modeling with Fusion 360. In Practical Arduino Engineering; Apress: Berkeley, CA, USA, 2021; pp. 57–127. [Google Scholar] [CrossRef]
- Shiwarski, D.J.; Hudson, A.R.; Tashman, J.W.; Feinberg, A.W. Emergence of FRESH 3D printing as a platform for advanced tissue biofabrication. APL Bioeng. 2021, 5, 010904. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]


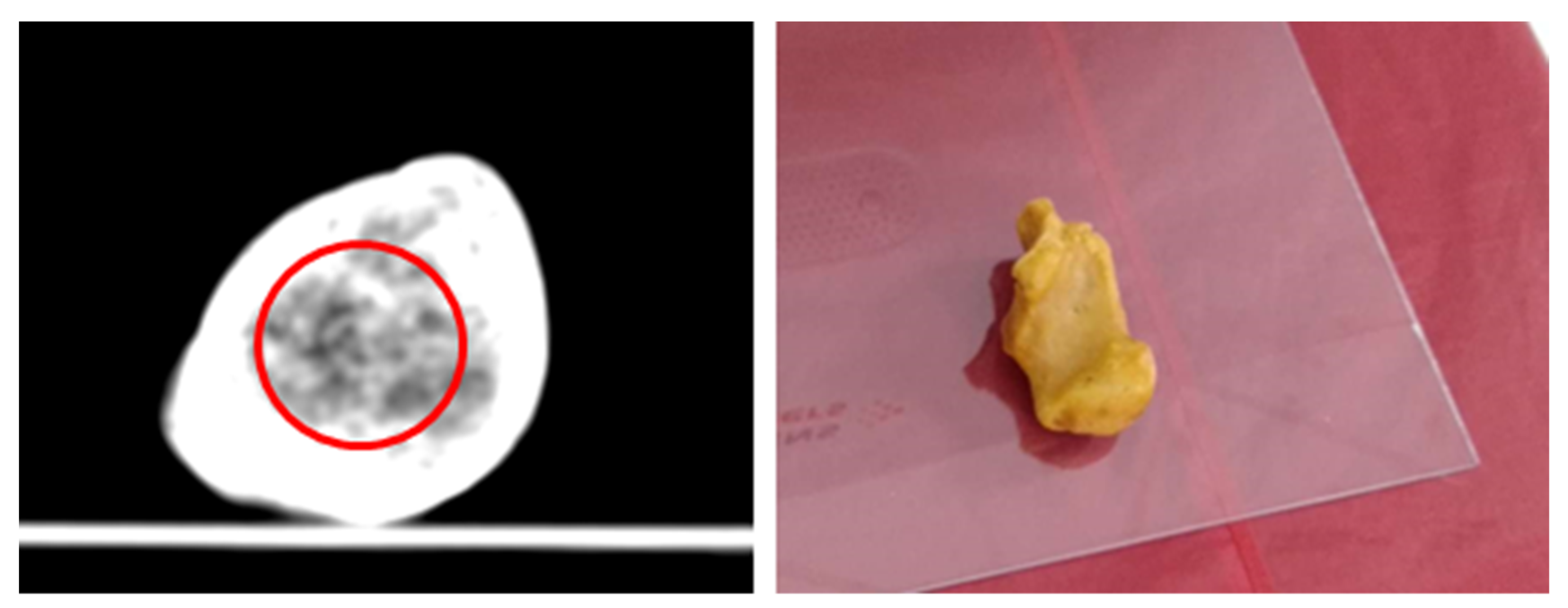
| Alginate w/v% | Methylcellulose w/v% | Laponite w/v% | |
|---|---|---|---|
| Modification 1 | 3 | 3 | 3 |
| Modification 2 | 5 | 5 | 6 |
| w/v% | Viscosity | Stability | Crosslinking | 3D Printing | |
|---|---|---|---|---|---|
| Modification 1 | 3/3/3 | low | low | low | low |
| Modification 2 | 5/5/6 | medium | high | excellent | excellent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valchanov, P.; Dukov, N.; Pavlov, S.; Kontny, A.; Dikova, T. 3D Printing, Histological, and Radiological Analysis of Nanosilicate-Polysaccharide Composite Hydrogel as a Tissue-Equivalent Material for Complex Biological Bone Phantom. Gels 2023, 9, 547. https://doi.org/10.3390/gels9070547
Valchanov P, Dukov N, Pavlov S, Kontny A, Dikova T. 3D Printing, Histological, and Radiological Analysis of Nanosilicate-Polysaccharide Composite Hydrogel as a Tissue-Equivalent Material for Complex Biological Bone Phantom. Gels. 2023; 9(7):547. https://doi.org/10.3390/gels9070547
Chicago/Turabian StyleValchanov, Petar, Nikolay Dukov, Stoyan Pavlov, Andreas Kontny, and Tsanka Dikova. 2023. "3D Printing, Histological, and Radiological Analysis of Nanosilicate-Polysaccharide Composite Hydrogel as a Tissue-Equivalent Material for Complex Biological Bone Phantom" Gels 9, no. 7: 547. https://doi.org/10.3390/gels9070547
APA StyleValchanov, P., Dukov, N., Pavlov, S., Kontny, A., & Dikova, T. (2023). 3D Printing, Histological, and Radiological Analysis of Nanosilicate-Polysaccharide Composite Hydrogel as a Tissue-Equivalent Material for Complex Biological Bone Phantom. Gels, 9(7), 547. https://doi.org/10.3390/gels9070547