Single-Entity Electrochemistry in the Agarose Hydrogel: Observation of Enhanced Signal Uniformity and Signal-to-Noise Ratio
Abstract
1. Introduction
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Reagents
4.2. Characterization of Pt NPs
4.3. Preparation of Agarose Hydrogel
4.4. Preparation of UMEs
4.5. Instruments
4.6. Electrochemical Cell
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stark, W.J.; Stoessel, P.R.; Wohlleben, W.; Hafner, A.J.C.S.R. Industrial applications of nanoparticles. Chem. Soc. Rev. 2015, 44, 5793–5805. [Google Scholar] [CrossRef]
- Van Broekhuizen, P.; van Broekhuizen, F.; Cornelissen, R.; Reijnders, L. Use of nanomaterials in the European construction industry and some occupational health aspects thereof. J. Nanopart. Res. 2011, 13, 447–462. [Google Scholar] [CrossRef]
- Bauer, L.A.; Birenbaum, N.S.; Meyer, G.J. Biological applications of high aspect ratio nanoparticles. J. Mater. Chem. 2004, 14, 517–526. [Google Scholar] [CrossRef]
- Liebel, M.; Calderon, I.; Pazos-Perez, N.; van Hulst, N.F.; Alvarez-Puebla, R.A. Widefield SERS for High-Throughput Nanoparticle Screening. Angew. Chem. Int. Ed. 2022, 61, e202200072. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lim, B.; Lee, E.P.; Xia, Y. Shape-controlled synthesis of platinum nanocrystals for catalytic and electrocatalytic applications. Nano Today 2009, 4, 81–95. [Google Scholar] [CrossRef]
- Spendelow, J.S.; Wieckowski, A. Electrocatalysis of oxygen reduction and small alcohol oxidation in alkaline media. Phys. Chem. Chem. Phys. 2007, 9, 2654–2675. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Bard, A.J. Observing single nanoparticle collisions at an ultramicroelectrode by electrocatalytic amplification. J. Am. Chem. Soc. 2007, 129, 9610–9612. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.G.; Rees, N.V.; Compton, R.G. The electrochemical detection and characterization of silver nanoparticles in aqueous solution. Angew. Chem. Int. Ed. 2011, 50, 4219–4221. [Google Scholar] [CrossRef]
- Zhou, H.; Fan, F.R.F.; Bard, A.J. Observation of discrete au nanoparticle collisions by electrocatalytic amplification using Pt ultramicroelectrode surface modification. J. Phys. Chem. Lett. 2010, 1, 2671–2674. [Google Scholar] [CrossRef]
- Kwon, S.J.; Fan, F.R.F.; Bard, A.J. Observing iridium oxide (IrOx) single nanoparticle collisions at ultramicroelectrodes. J. Am. Chem. Soc. 2010, 132, 13165–13167. [Google Scholar] [CrossRef]
- Fernando, A.; Parajuli, F.; Alpuche-Aviles, M.A. Observation of individual semiconducting nanoparticle collisions by stochastic photoelectrochemical currents. J. Am. Chem. Soc. 2013, 135, 10894–10897. [Google Scholar] [CrossRef]
- Kim, B.K.; Kim, J.; Bard, A.J. Electrochemistry of a single attoliter emulsion droplet in collisions. J. Am. Chem. Soc. 2015, 137, 2343–2349. [Google Scholar] [CrossRef]
- Kim, B.K.; Boika, A.; Kim, J.; Dick, J.E.; Bard, A.J. Characterizing emulsions by observation of single droplet collisions—Attoliter electrochemical reactors. J. Am. Chem. Soc. 2014, 136, 4849–4852. [Google Scholar] [CrossRef] [PubMed]
- Dick, J.E.; Hilterbrand, A.T.; Boika, A.; Upton, J.W.; Bard, A.J. Electrochemical detection of a single cytomegalovirus at an ultramicroelectrode and its antibody anchoring. Proc. Natl. Acad. Sci. USA 2015, 112, 5303–5308. [Google Scholar] [CrossRef] [PubMed]
- Dick, J.E.; Renault, C.; Bard, A.J. Observation of single-protein and DNA macromolecule collisions on ultramicroelectrodes. J. Am. Chem. Soc. 2015, 137, 8376–8379. [Google Scholar] [CrossRef]
- Emory, S.R.; Haskins, W.E.; Nie, S. Direct observation of size-dependent optical enhancement in single metal nanoparticles. J. Am. Chem. Soc. 1998, 120, 8009–8010. [Google Scholar] [CrossRef]
- Balamurugan, B.; Maruyama, T. Evidence of an enhanced interband absorption in Au nanoparticles: Size-dependent electronic structure and optical properties. Appl. Phys. Lett. 2005, 87, 143105. [Google Scholar] [CrossRef]
- Shankar, S.S.; Rai, A.; Ankamwar, B.; Singh, A.; Ahmad, A.; Sastry, M. Biological synthesis of triangular gold nanoprisms. Nat. Mater. 2004, 3, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Hao, F.; Huang, Y.; Wang, W.; Nordlander, P.; Xu, H. Polarization dependence of surface-enhanced Raman scattering in gold nanoparticle−nanowire systems. Nano Lett. 2008, 8, 2497–2502. [Google Scholar] [CrossRef]
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H.; Car, H. Nanoparticles as drug delivery systems. Pharmacol. Res. 2012, 64, 1020–1037. [Google Scholar] [CrossRef]
- Wang, C.; Yu, C. Detection of chemical pollutants in water using gold nanoparticles as sensors: A review. Rev. Anal. Chem. 2013, 32, 1–14. [Google Scholar] [CrossRef]
- Yahya, R.; Shah, A.; Kokab, T.; Ullah, N.; Hakeem, M.K.; Hayat, M.; Shah, I. Electrochemical Sensor for detection and degradation studies of ethyl violet dye. ACS Omega 2022, 7, 34154–34165. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Niu, Z.; Kim, D.; Li, M.; Yang, P. Surface and interface control in nanoparticle catalysis. Chem. Rev. 2019, 120, 1184–1249. [Google Scholar] [CrossRef] [PubMed]
- Baek, D.S.; Joo, S.H. Non-siliceous ordered mesoporous materials via nanocasting for small molecule conversion electrocatalysis. Bull. Korean Chem. Soc. 2022, 43, 1156–1168. [Google Scholar] [CrossRef]
- Luo, X.; Morrin, A.; Killard, A.J.; Smyth, M.R. Application of nanoparticles in electrochemical sensors and biosensors. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2006, 18, 319–326. [Google Scholar] [CrossRef]
- Gao, X.; Lee, J.; White, H.S. Natural convection at microelectrodes. Anal. Chem. 1995, 67, 1541–1545. [Google Scholar] [CrossRef]
- Novev, J.K.; Compton, R.G. Natural convection effects in electrochemical systems. Curr. Opin. Electrochem. 2018, 7, 118–129. [Google Scholar] [CrossRef]
- Mateescu, A.; Wang, Y.; Dostalek, J.; Jonas, U. Thin hydrogel films for optical biosensor applications. Membranes 2012, 2, 40–69. [Google Scholar] [CrossRef]
- Wang, N.; Wu, X.S. Preparation and characterization of agarose hydrogel nanoparticles for protein and peptide drug delivery. Pharm. Dev. 1997, 2, 135–142. [Google Scholar] [CrossRef]
- Fernández-Cossío, S.; León-Mateos, A.; Sampedro, F.G.; Oreja, M.T.C. Biocompatibility of agarose gel as a dermal filler: Histologic evaluation of subcutaneous implants. Plast. Reconst. Surg. 2007, 120, 1161–1169. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Manouchehri, S.; Ahmadi, Z.; Saeb, M.R.; Urbanska, A.M.; Kaplan, D.L.; Mozafari, M. Agarose-based biomaterials for tissue engineering. Carbohydr. Polym. 2018, 187, 66–84. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, A.; Kanzaki, T. Swelling of agarose gel and its related changes. Food Hydrocoll. 1987, 1, 317–325. [Google Scholar] [CrossRef]
- Rochas, C.; Hecht, A.M.; Geissler, E. Swelling properties of agarose gels. J. Chim. Phys. 1996, 93, 850–857. [Google Scholar] [CrossRef]
- Wang, S.F.; Chen, T.; Zhang, Z.L.; Shen, X.C.; Lu, Z.X.; Pang, D.W.; Wong, K.Y. Direct electrochemistry and electrocatalysis of heme proteins entrapped in agarose hydrogel films in room-temperature ionic liquids. Langmuir 2005, 21, 9260–9266. [Google Scholar] [CrossRef]
- Kim, B.K.; Park, K. Mass Transport Properties and Influence of Natural Convection for Voltammetry at the Agarose Hydrogel Interface. J. Electrochem. Sci. Technol. 2022, 13, 347–353. [Google Scholar] [CrossRef]
- Han, J.; Jang, S.; Kim, B.K.; Park, K. Electrochemical study of agarose hydrogels for natural convection on macroelectrodes and ultramicroelectrodes. J. Anal. Sci. Technol. 2023, 14, 10. [Google Scholar] [CrossRef]
- Ko, S. Multifunctional surface coating using chitosan and its chemical functionalization. Bull. Korean Chem. Soc. 2022, 43, 1207–1211. [Google Scholar] [CrossRef]
- Reddy, S.M.; Sette, G.; Phan, Q. Electrochemical probing of selective haemoglobin binding in hydrogel-based molecularly imprinted polymers. Electrochim. Acta 2011, 56, 9203–9208. [Google Scholar] [CrossRef]
- Maity, S.; Parshi, N.; Prodhan, C.; Chaudhuri, K.; Ganguly, J. Characterization of a fluorescent hydrogel synthesized using chitosan, polyvinyl alcohol and 9-anthraldehyde for the selective detection and discrimination of trace Fe3+ and Fe2+ in water for live-cell imaging. Carbohydr. Polym. 2018, 193, 119–128. [Google Scholar] [CrossRef]
- Shi, Y.; Peng, L.; Yu, G. Nanostructured conducting polymer hydrogels for energy storage applications. Nanoscale 2015, 7, 12796–12806. [Google Scholar] [CrossRef]
- Zhang, W.; Feng, P.; Chen, J.; Zhao, B. Flexible energy storage systems based on electrically conductive hydrogels. Prog. Polym. Sci. 2018, 88, 1–43. [Google Scholar]
- Ismail, Y.A.; Martínez, J.G.; Al Harrasi, A.S.; Kim, S.J.; Otero, T.F. Sensing characteristics of a conducting polymer/hydrogel hybrid microfiber artificial muscle. Sens. Actuators B Chem. 2011, 160, 1180–1190. [Google Scholar] [CrossRef]
- Zhao, Z.; Fang, R.; Rong, Q.; Liu, M. Bioinspired nanocomposite hydrogels with highly ordered structures. Adv. Mater. 2017, 29, 1703045. [Google Scholar] [CrossRef] [PubMed]
- Muya, F.N.; Baker, P.G.; Iwuoha, E.I. Polysulfone hydrogel nanocomposite alkaline phosphatase biosensor for the detection of vanadium. Electrocatalysis 2020, 11, 374–382. [Google Scholar] [CrossRef]
- Zeng, X.; Wei, W.; Li, X.; Zeng, J.; Wu, L. Direct electrochemistry and electrocatalysis of hemoglobin entrapped in semi-interpenetrating polymer network hydrogel based on polyacrylamide and chitosan. Bioelectrochemistry 2007, 71, 135–141. [Google Scholar] [CrossRef]
- Dewan, M.; Adhikari, A.; Dutta, K.; Chattopadhyay, D. Impact of Poly (Vinyl Alcohol) on The Thermogelation Property and Drug Release Profile of Ophthalmic Formulations Based on Poloxamer 407. ChemistrySelect 2023, 8, e202203528. [Google Scholar] [CrossRef]
- Ganguly, S.; Margel, S. 3D printed magnetic polymer composite hydrogels for hyperthermia and magnetic field driven structural manipulation. Prog. Polym. Sci. 2022, 131, 101574. [Google Scholar] [CrossRef]
- Garai, S.; Chatterjee, D.; Mondal, B. Effect of rotation and cross thermal buoyancy on the nanofluidic transport around a circular cylinder. Phys. Fluids 2023, 35, 022011. [Google Scholar] [CrossRef]
- Haleem, A.; Pan, J.M.; Shah, A.; Hussain, H.; He, W.D. A systematic review on new advancement and assessment of emerging polymeric cryogels for environmental sustainability and energy production. Sep. Purif. Technol. 2023, 316, 123678. [Google Scholar] [CrossRef]
- Kleijn, S.E.; Serrano-Bou, B.; Yanson, A.I.; Koper, M.T. Influence of hydrazine-induced aggregation on the electrochemical detection of platinum nanoparticles. Langmuir 2013, 29, 2054–2064. [Google Scholar] [CrossRef]
- Maaloum, M.; Pernodet, N.; Tinland, B. Agarose gel structure using atomic force microscopy: Gel concentration and ionic strength effects. Electrophoresis 1998, 19, 1606–1610. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.J.; Zhou, H.; Fan, F.R.F.; Vorobyev, V.; Zhang, B.; Bard, A.J. Stochastic electrochemistry with electrocatalytic nanoparticles at inert ultramicroelectrodes—Theory and experiments. Phys. Chem. Chem. Phys. 2011, 13, 5394–5402. [Google Scholar] [CrossRef] [PubMed]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods, Fundamentals and Applications, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2001. [Google Scholar]
- Johnson, E.M.; Berk, D.A.; Jain, R.K.; Deen, W.M. Hindered diffusion in agarose gels: Test of effective medium model. Biophys. 1996, 70, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Stellwagen, N.C. Agarose gel pore radii are not dependent on the casting buffer. Electrophoresis 1992, 13, 601–603. [Google Scholar] [CrossRef]
- Narayanan, J.; Xiong, J.Y.; Liu, X.Y. Determination of agarose gel pore size: Absorbance measurements vis a vis other techniques. J. Phys. Conf. Ser. 2006, 28, 83–86. [Google Scholar] [CrossRef]
- Cuccia, N.L.; Pothineni, S.; Wu, B.; Méndez Harper, J.; Burton, J.C. Pore-size dependence and slow relaxation of hydrogel friction on smooth surfaces. Proc. Natl. Acad. Sci. USA 2020, 117, 11247–11256. [Google Scholar] [CrossRef]
- Jayawardena, I.; Turunen, P.; Garms, B.C.; Rowan, A.; Corrie, S.; Grøndahl, L. Evaluation of techniques used for visualisation of hydrogel morphology and determination of pore size distributions. Mater. Adv. 2023, 4, 669–682. [Google Scholar] [CrossRef]
- Sathawong, S.; Sridach, W.; Techato, K.A. Recovery of Kraft lignin from OPEFB and using for lignin–agarose hydrogel. J. Polym. Environ. 2018, 26, 3307–3315. [Google Scholar] [CrossRef]
- Mehta, G.K.; Kondaveeti, S.; Siddhanta, A.K. Facile synthesis of agarose-l-phenylalanine ester hydrogels. Polym. Chem. 2011, 2, 2334–2340. [Google Scholar] [CrossRef]
- Xiao, X.; Fan, F.R.F.; Zhou, J.; Bard, A.J. Current transients in single nanoparticle collision events. J. Am. Chem. Soc. 2008, 130, 16669–16677. [Google Scholar] [CrossRef]
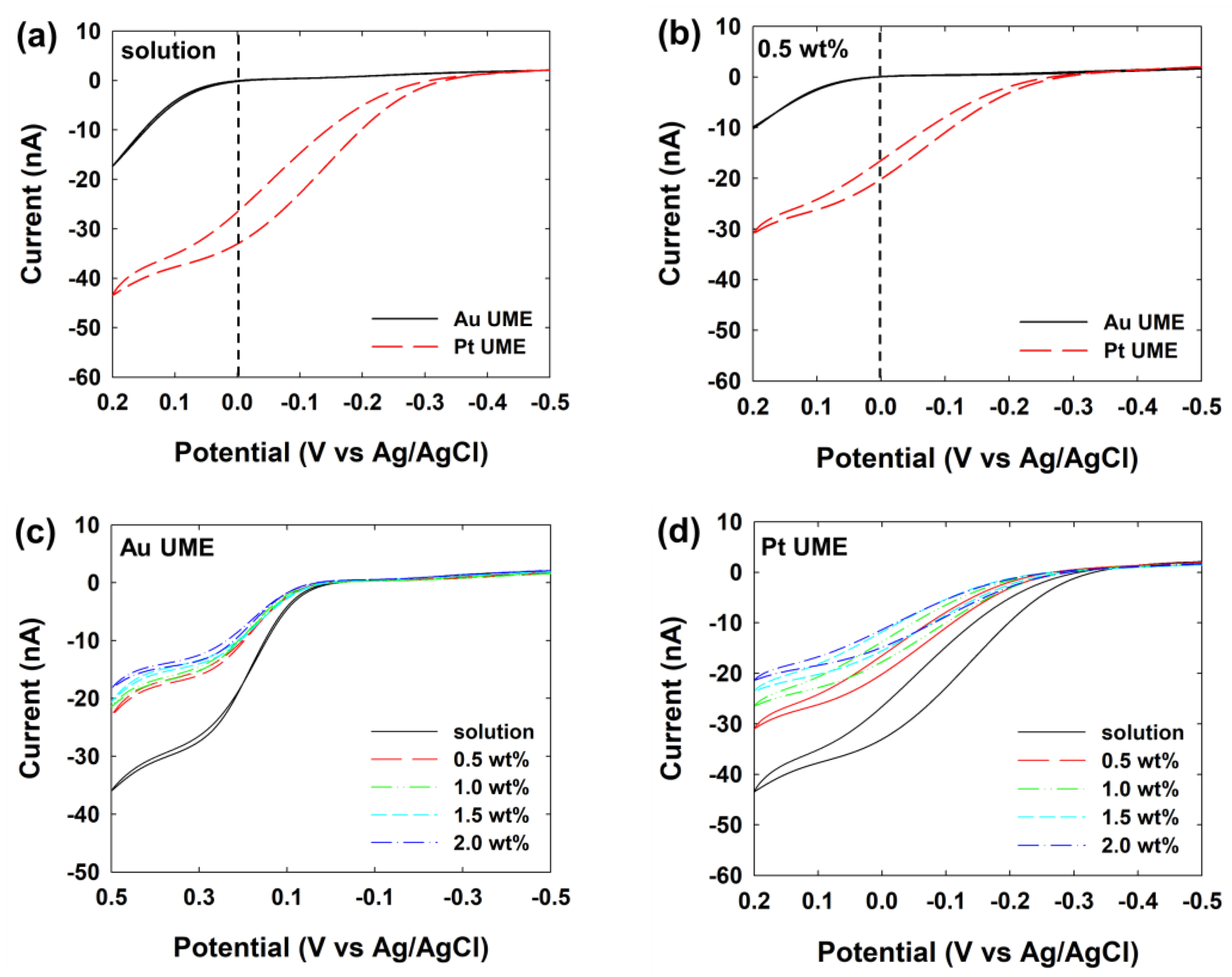

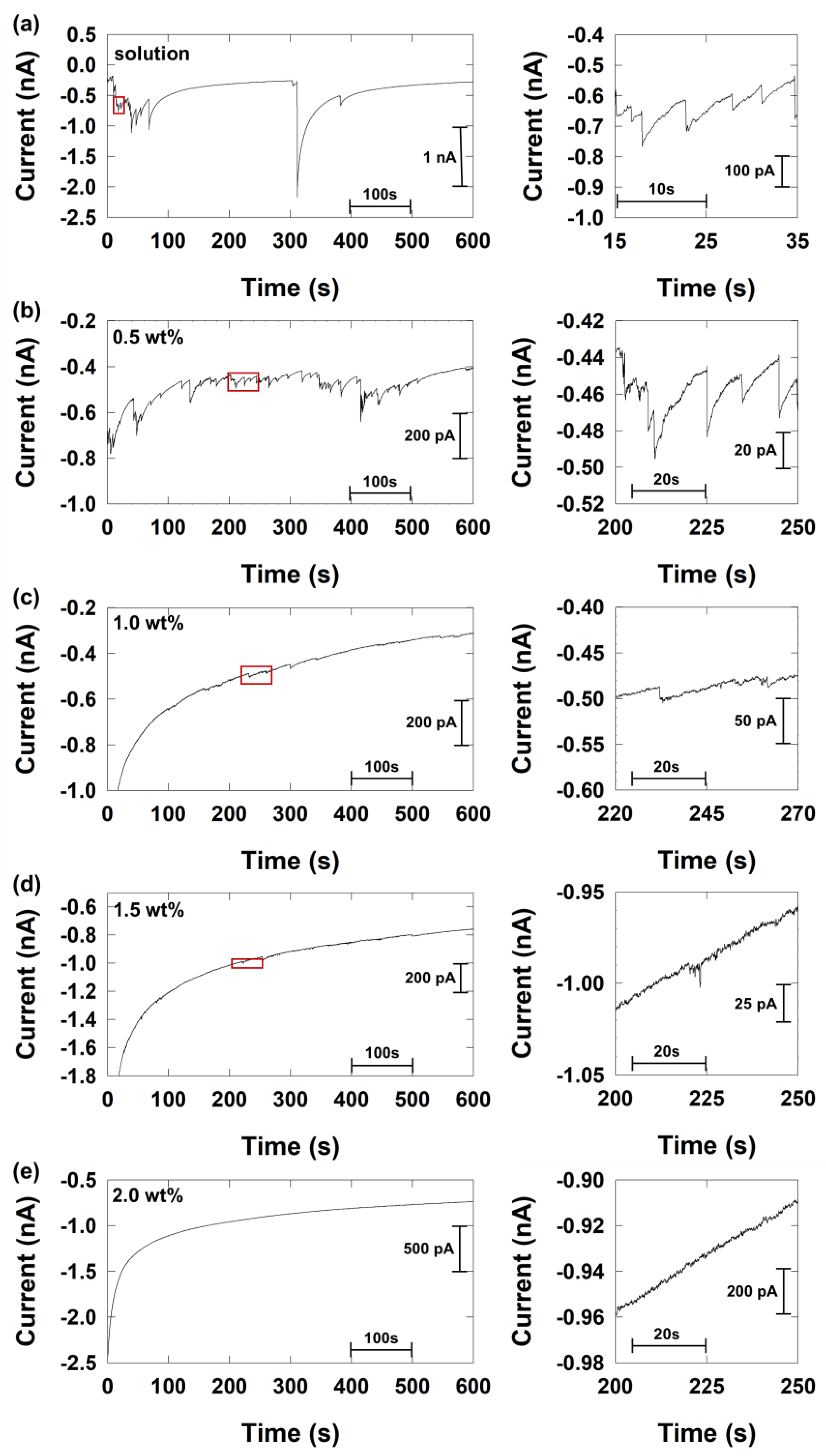
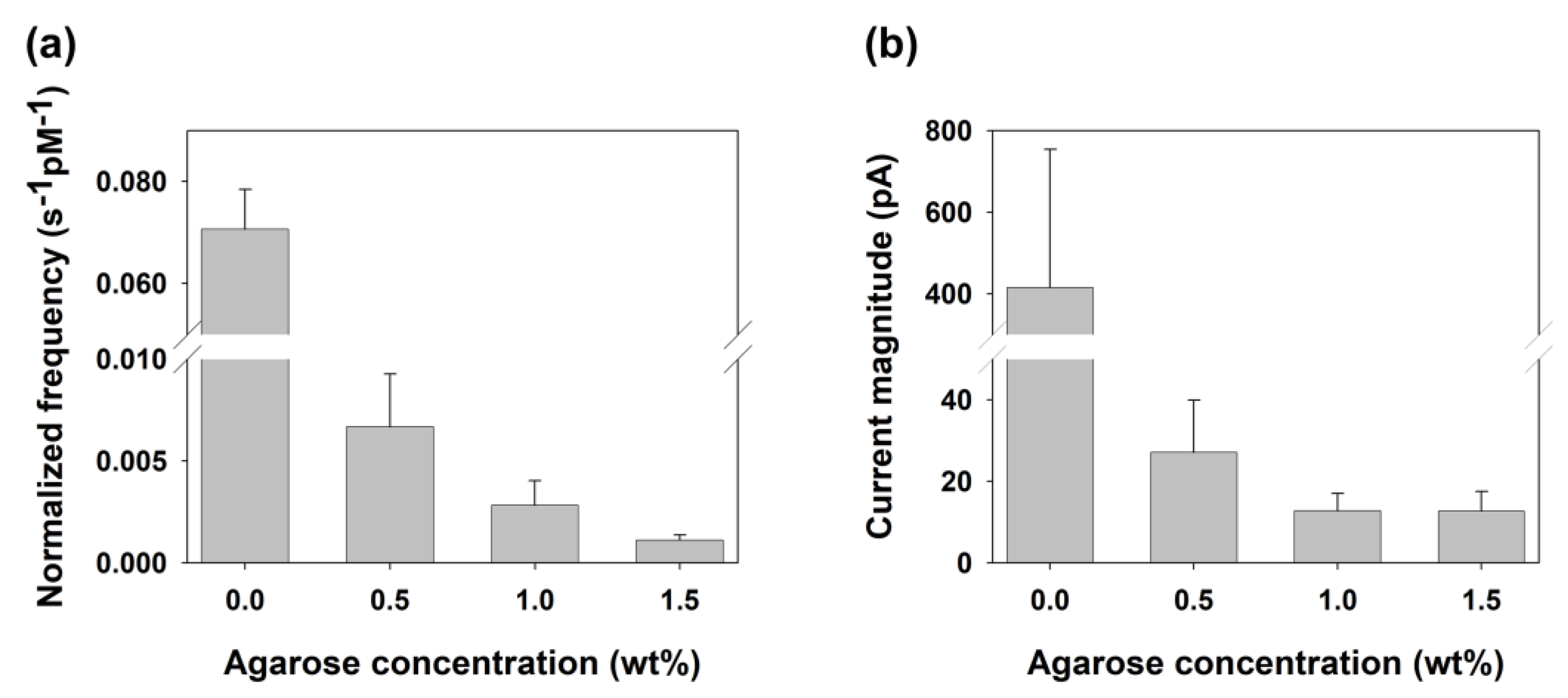
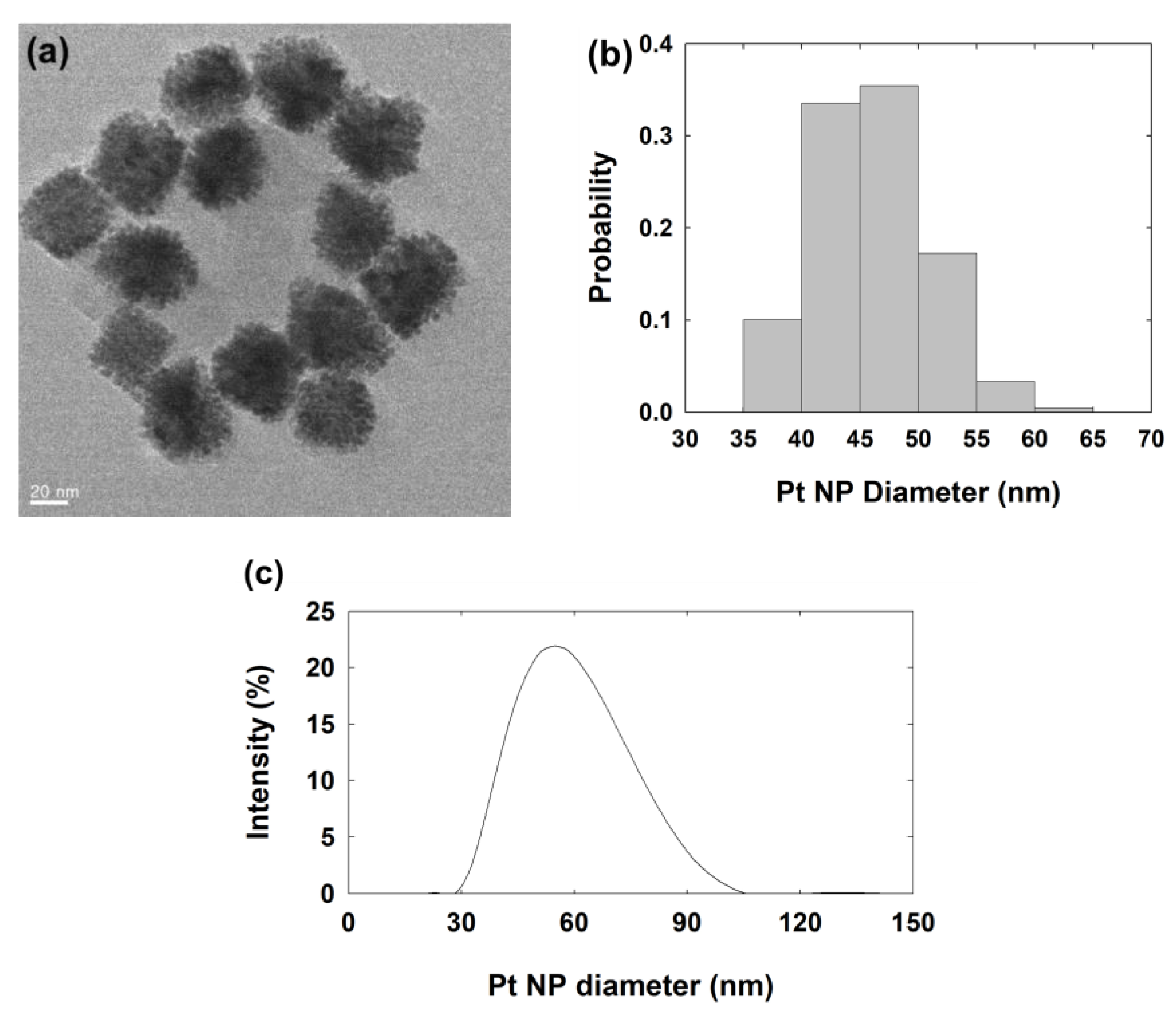
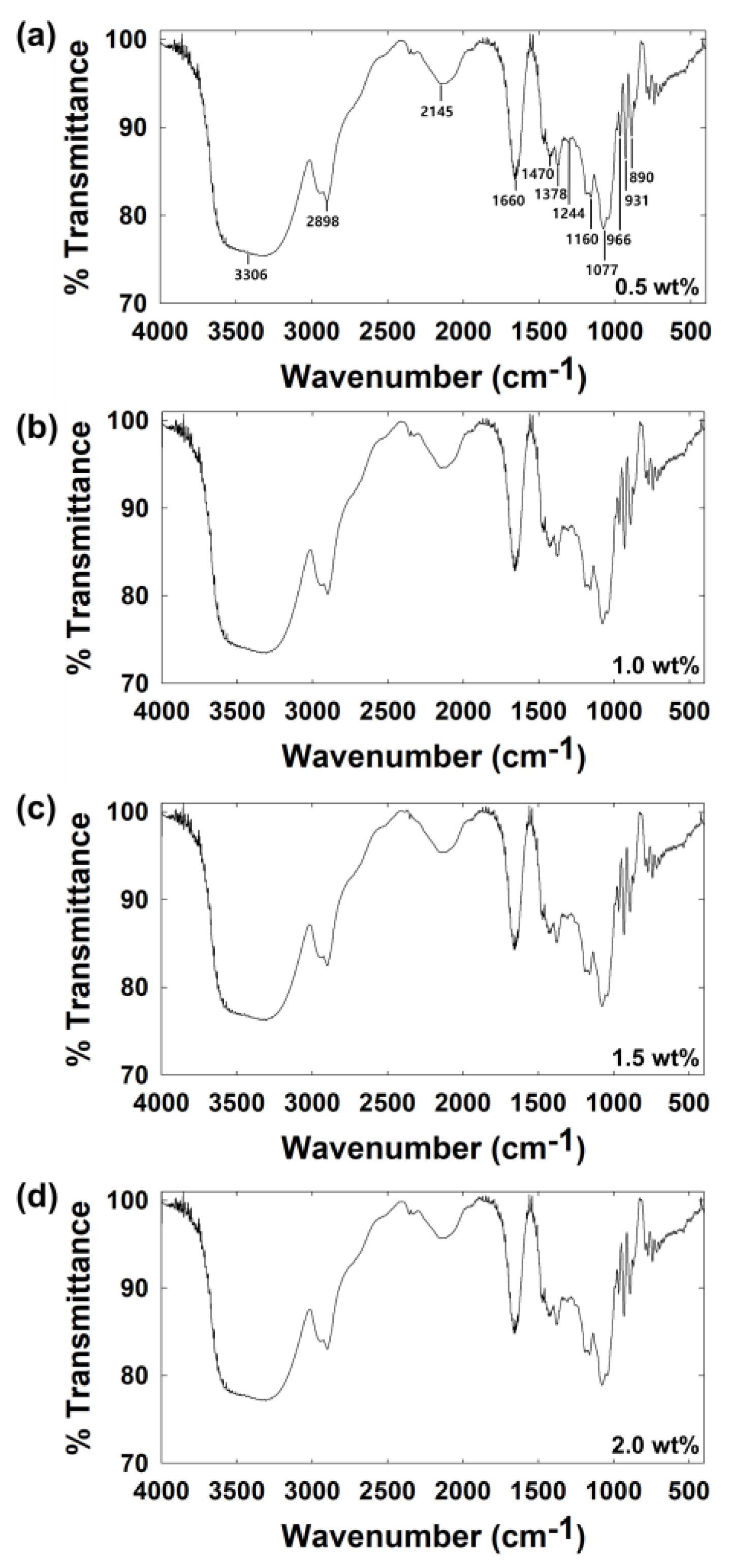
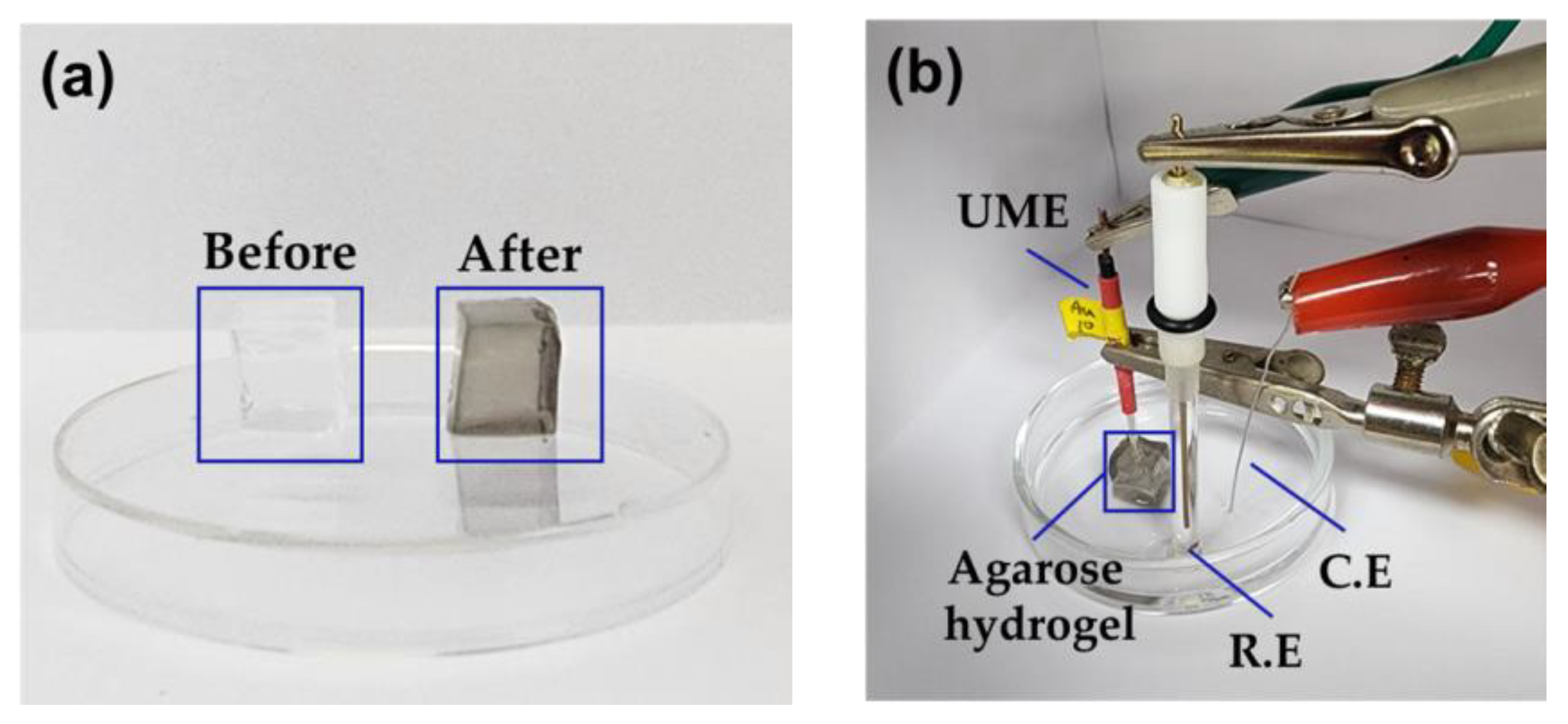
| Concentration of Agarose Hydrogel (wt%) | Steady-State Current (nA) | Calculated Diffusion Coefficient of Hydrazine a (×10−6 cm2/s) |
|---|---|---|
| 0 (solution) | 33.8 | 8.75 |
| 0.5 | 28.4 | 7.37 |
| 1.0 | 25.5 | 6.59 |
| 1.5 | 20.3 | 5.25 |
| 2.0 | 18.5 | 4.79 |
| Concentration of Agarose Hydrogel (wt%) | Calculated Current Magnitude a (pA) | Experimental Current Magnitude (pA) (±SD) | Calculated Frequency b (s−1) | Experimental Frequency (s−1) (±SD) | Normalized Frequency (s−1·pM−1) (±SD) |
|---|---|---|---|---|---|
| 0 (solution) | 330 | 495 (±338) | 0.104 | 0.028 (±0.003) c | 0.035 (±0.004) |
| 0.5 | 278 | 27 (±13) | - | 0.066 (±0.026) d | 0.003 (±0.001) |
| 1.0 | 249 | 13 (±4) | - | 0.028 (±0.012) d | 0.001 (±0.001) |
| 1.5 | 198 | 12 (±5) | - | 0.011 (±0.003) d | 0.0005 (±0.0001) |
| 2.0 | 181 | - | - | 0 d | 0 |
| Method Based on | Pore Diameter (nm) | Reference | |||
|---|---|---|---|---|---|
| 0.5 wt% | 1.0 wt% | 1.5 wt% | 2.0 wt% | ||
| DNA mobilities change | 250 | 100 | 60 | 40 | [55] |
| absorbance change | 450 | 150 | 70 | 45 | [56] |
| surface fraction change | 280 | 85 | 71 | 6 | [57] |
| Cryo-SEM | 330 | 230 | 190 | 150 | [58] |
| Time Range (s) | 0 wt% (Solution) | 0.5 wt% | ||
|---|---|---|---|---|
| Experimental Current Magnitude (pA) (±SD) | Experimental Frequency (s−1) | Experimental Current Magnitude (pA) (±SD) | Experimental Frequency (s−1) | |
| 0~200 | 330 (±180) | 0.075 | 31.8 (±15) | 0.13 |
| 200–400 | 1050 (±1140) | 0.015 | 32.2 (±18) | 0.12 |
| Concentration of Agarose Hydrogel (wt%) | Experimental Current Magnitude (pA) | Noise Level a (pA) | Signal to Noise Ratio (S/N Ratio) |
|---|---|---|---|
| 0 (solution) | 495 | 53 | 7.8 |
| 0.5 | 27 | 0.65 | 42 |
| 1.0 | 13 | 0.79 | 16 |
| 1.5 | 12 | 0.85 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Na, J.; Park, K.; Kwon, S.J. Single-Entity Electrochemistry in the Agarose Hydrogel: Observation of Enhanced Signal Uniformity and Signal-to-Noise Ratio. Gels 2023, 9, 537. https://doi.org/10.3390/gels9070537
Na J, Park K, Kwon SJ. Single-Entity Electrochemistry in the Agarose Hydrogel: Observation of Enhanced Signal Uniformity and Signal-to-Noise Ratio. Gels. 2023; 9(7):537. https://doi.org/10.3390/gels9070537
Chicago/Turabian StyleNa, Jaedo, Kyungsoon Park, and Seong Jung Kwon. 2023. "Single-Entity Electrochemistry in the Agarose Hydrogel: Observation of Enhanced Signal Uniformity and Signal-to-Noise Ratio" Gels 9, no. 7: 537. https://doi.org/10.3390/gels9070537
APA StyleNa, J., Park, K., & Kwon, S. J. (2023). Single-Entity Electrochemistry in the Agarose Hydrogel: Observation of Enhanced Signal Uniformity and Signal-to-Noise Ratio. Gels, 9(7), 537. https://doi.org/10.3390/gels9070537






