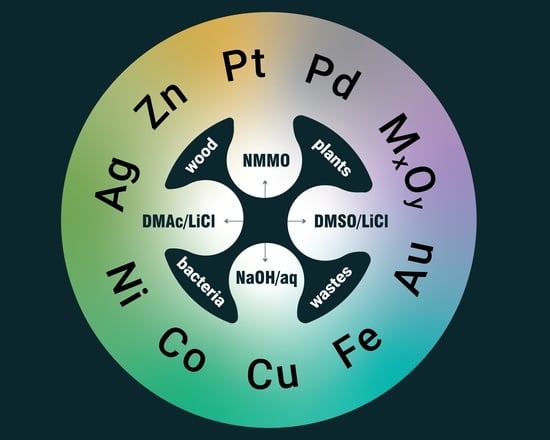
Cellulose-Based Metallogels—Part 1: Raw Materials and Preparation
Abstract
1. Introduction
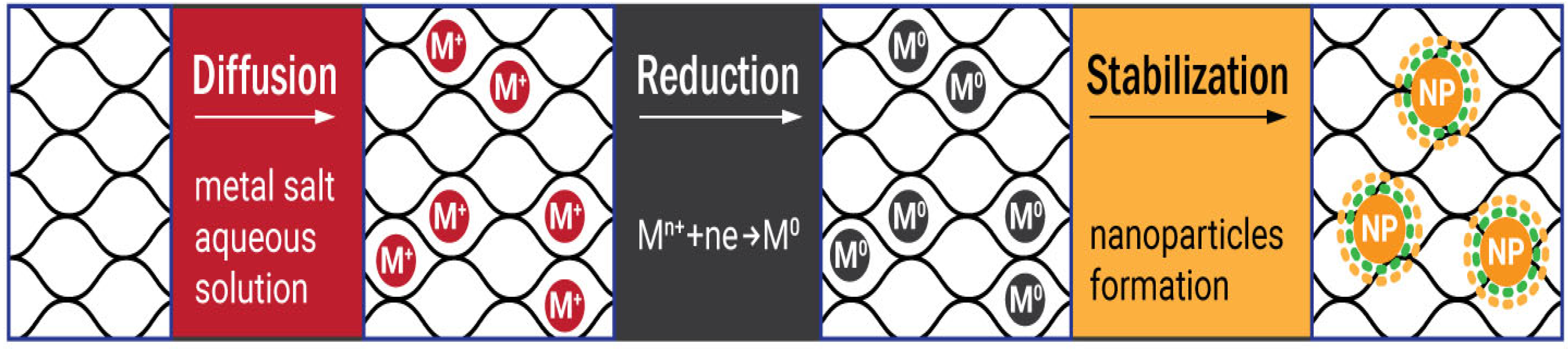
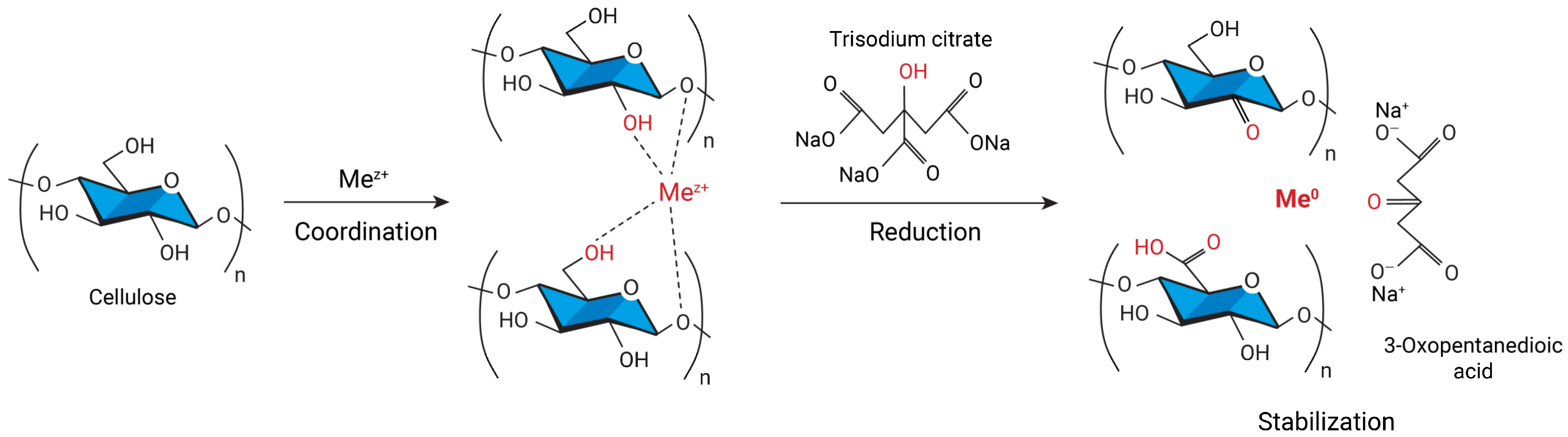
2. Cellulose Metallogels
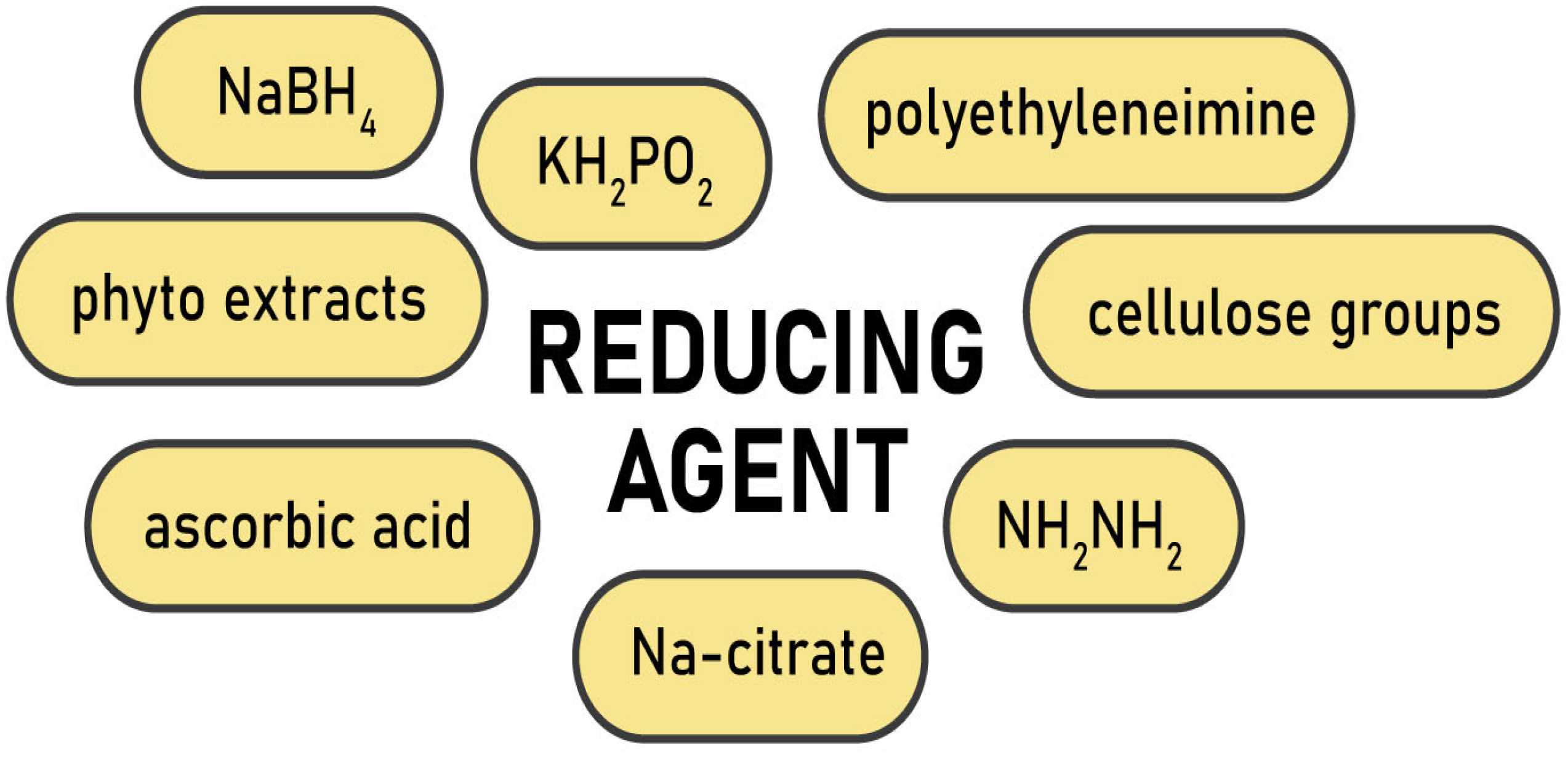
3. Raw Materials
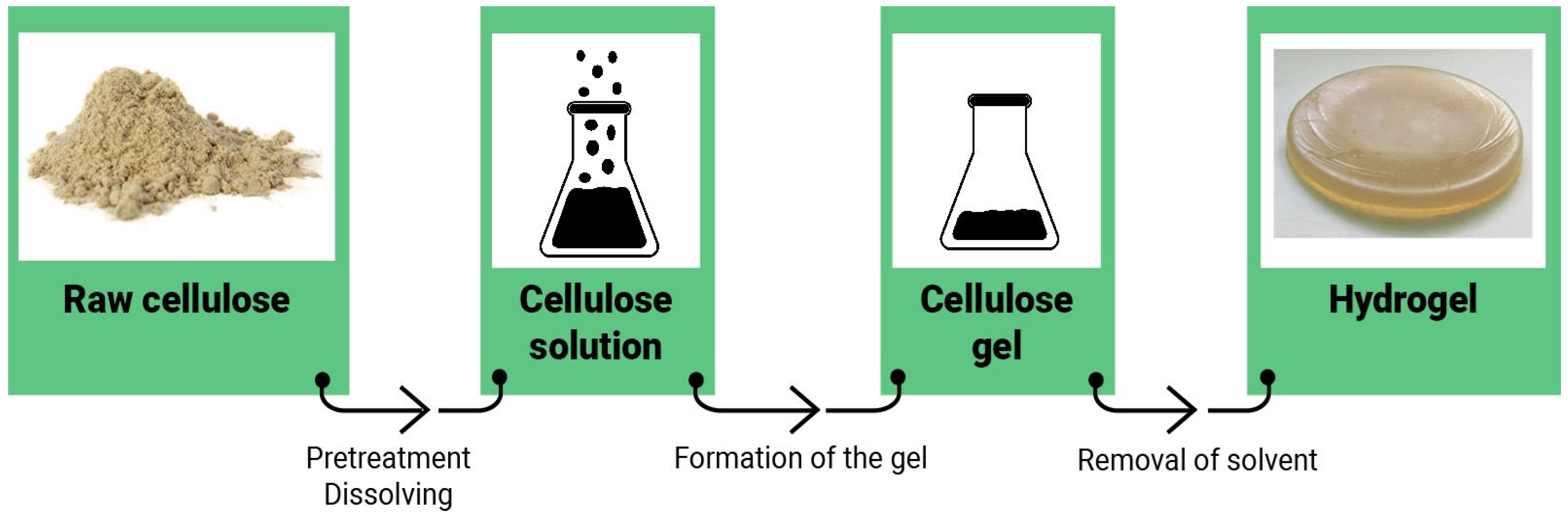
4. Preparation of Cellulose Hydrogels
4.1. N,N-Dimethylacetamide/LiCl
4.2. Dimethyl Sulfoxide/LiCl
4.3. N-Methylmorpholine-N-oxide
4.4. Alkaline Aqueous Systems
- (1)
- Protocols for the production of hydrogels from cellulose are very similar for the three organic solvents considered, namely DMAc/LiCl, DMSO/LiCl, NNMO, while cellulose hydrogels obtained from NaOH-aqueous solutions involve cooling of the system.
- (2)
- The most common solvents are DMAc/LiCl and NaOH; NNMO and DMSO/LiClare used less frequently.
- (3)
- Each solvent has pros and cons (Table 2). Thus, the NaOH-aqueous system is inexpensive and environmentally beneficial; however, there are limitations to the dissolution of some types of cellulose. These drawbacks can be minimized due to extensive pre-treatment of cellulose which is an additional step in the production process. Usually, the hydrogels are synthesized from NaOH-solutions with such additives as urea and/or thiourea and crosslinking with ECH.
| Solvent | Pre-Treatment of Cellulose | Dissolution Process | Gelation Process | Price * | Toxicity |
|---|---|---|---|---|---|
| DMAc/LiCl | solvent exchange (not compulsory) | 25–80 °C | humid ambient condition | 161 GBP/L (+28 GBP/100 g for LiCl) | harmful, flammable |
| DMSO/LiCl | ball-milling complexation | 65–70 °C | ethanol, cross-linking | 89 GBP/L (+28 GBP/100 g for LiCl) | non-toxic, flammable |
| NMMO | - | 110 °C, −0.1 MPa | 110 °C, −0.1 MPa | 1110 GBP/kg | low toxic, non- flammable |
| NaOH/water | hydrothermal, steam explosion, chemical, or enzymatic treatment | (−20)–0 °C, addition of urea/ thiourea | cross-linking (not compulsory), cooling (approximately 0 °C) | 35 GBP/kg | non-toxic, non- flammable |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- De France, K.J.; Hoare, T.; Cranston, E.D. Review of Hydrogels and Aerogels Containing Nanocellulose. Chem. Mater. 2017, 29, 4609–4631. [Google Scholar] [CrossRef]
- Zainal, S.H.; Mohd, N.H.; Suhaili, N.; Anuar, F.H.; Lazim, A.M.; Othaman, R. Preparation of cellulose-based hydrogel: A review. J. Mater. Res. Technol. 2020, 10, 935–952. [Google Scholar] [CrossRef]
- Tholibon, D.; Tharazi, I.; Sulong, A.B.; Muhamad, N.; Ismail, N.F.; Radzi, M.K.F.M.; Radzuan, N.A.M.; Hui, D. Kenaf Fiber Composites: A Review on Synthetic and Biodegradable Polymer Matrix. J. Kejuruter. 2019, 31, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Rico-García, D.; Ruiz-Rubio, L.; Pérez-Alvarez, L.; Hernández-Olmos, S.L.; Guerrero-Ramírez, G.L.; Vilas-Vilela, J.L. Lignin-Based Hydrogels: Synthesis and Applications. Polymers 2020, 12, 81. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Zhang, S.; Huang, L.; Wang, H.; Wang, W.; Ye, Q. Starch-based hydrogel loading with carbendazim for controlled-release and water absorption. Carbohydr. Polym. 2015, 125, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Tabata, Y. Biomaterial technology for tissue engineering applications. J. R. Soc. Interface 2009, 6, S311–S324. [Google Scholar] [CrossRef]
- Kabir, S.M.F.; Sikdar, P.P.; Haque, B.; Bhuiyan, M.A.R.; Ali, A.; Islam, M.N. Cellulose-based hydrogel materials: Chemistry, properties and their prospective applications. Prog. Biomater. 2018, 7, 153–174. [Google Scholar] [CrossRef]
- Huang, K.; Wang, Y. Recent applications of regenerated cellulose films and hydrogels in food packaging. Curr. Opin. Food Sci. 2022, 43, 7–17. [Google Scholar] [CrossRef]
- Fortună, M.E.; Ungureanu, E.; Jităreanu, D.C.; Țopa, D.C.; Harabagiu, V. Effects of Hybrid Polymeric Material Based on Polycaprolactone on the Environment. Materials 2022, 15, 4868. [Google Scholar] [CrossRef]
- Iftime, M.M.; Ailiesei, G.L.; Ungureanu, E.; Marin, L. Designing chitosan based eco-friendly multifunctional soil conditioner systems with urea controlled release and water retention. Carbohydr. Polym. 2019, 223, 115040. [Google Scholar] [CrossRef]
- Fortună, M.E.; Ungureanu, E.; Jitareanu, C.D. Calcium Carbonate–Carboxymethyl Chitosan Hybrid Materials. Materials 2021, 14, 3336. [Google Scholar] [CrossRef]
- López-Velázquez, J.C.; Rodríguez-Rodríguez, R.; Espinosa-Andrews, H.; Qui-Zapata, J.A.; García-Morales, S.; Navarro-López, D.E.; Luna-Bárcenas, G.; Vassallo-Brigneti, E.C.; García-Carvajal, Z.Y. Gelatin–chitosan–PVA hydrogels and their application in agriculture. J. Chem. Technol. Biotechnol. 2019, 94, 3495–3504. [Google Scholar] [CrossRef]
- Fan, S.; Chang, W.; Fei, C.; Zhang, Z.; Hou, B.; Shi, Z.; Wang, H.; Hui, Y. Stretchable and bendable textile matrix based on cellulose fibers for wearable self-powered glucose biosensors. Cellulose 2022, 29, 8919–8935. [Google Scholar] [CrossRef]
- Tarabanko, N.; Baryshnikov, S.V.; Kazachenko, A.S.; Miroshnikova, A.; Skripnikov, A.M.; Lavrenov, A.V.; Taran, O.P.; Kuznetsov, B.N. Hydrothermal hydrolysis of microcrystalline cellulose from birch wood catalyzed by Al2O3-B2O3 mixed oxides. Wood Sci. Technol. 2022, 56, 437–457. [Google Scholar] [CrossRef]
- Del Valle, L.J.; Díaz, A.; Puiggalí, J. Hydrogels for Biomedical Applications: Cellulose, Chitosan, and Protein/Peptide Derivatives. Gels 2017, 3, 27. [Google Scholar] [CrossRef]
- Hu, S.; Zhi, Y.; Shan, S.; Ni, Y. Research progress of smart response composite hydrogels based on nanocellulose. Carbohydr. Polym. 2022, 275, 118741. [Google Scholar] [CrossRef]
- Abeer, M.M.; Amin, M.C.I.M.; Martin, C. A review of bacterial cellulose-based drug delivery systems: Their biochemistry, current approaches and future prospects. J. Pharm. Pharmacol. 2014, 66, 1047–1061. [Google Scholar] [CrossRef]
- Mohite, B.V.; Koli, S.H.; Patil, S.V. Bacterial Cellulose-Based Hydrogels: Synthesis, Properties, and Applications. In Cellulose-Based Superabsorbent Hydrogels; Mondal, M., Ed.; Polymers and Polymeric Composites: A Reference Series; Springer International Publishing: Cham, Switzerland, 2019; pp. 1255–1276. ISBN 978-3-319-77830-3. [Google Scholar]
- Javanbakht, S.; Pooresmaeil, M.; Hashemi, H.; Namazi, H. Carboxymethylcellulose capsulated Cu-based metal-organic framework-drug nanohybrid as a pH-sensitive nanocomposite for ibuprofen oral delivery. Int. J. Biol. Macromol. 2018, 119, 588–596. [Google Scholar] [CrossRef]
- El-Mohdy, H.L.A. Radiation synthesis of nanosilver/poly vinyl alcohol/cellulose acetate/gelatin hydrogels for wound dressing. J. Polym. Res. 2013, 20, 177. [Google Scholar] [CrossRef]
- Liao, Z.-X.; Liu, M.-C.; Kempson, I.M.; Fa, Y.-C.; Huang, K.-Y. Light-triggered methylcellulose gold nanoparticle hydrogels for leptin release to inhibit fat stores in adipocytes. Int. J. Nanomed. 2017, 12, 7603–7611. [Google Scholar] [CrossRef]
- Isobe, N.; Chen, X.; Kim, U.-J.; Kimura, S.; Wada, M.; Saito, T.; Isogai, A. TEMPO-oxidized cellulose hydrogel as a high-capacity and reusable heavy metal ion adsorbent. J. Hazard. Mater. 2013, 260, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.D.; Patel, D.K.; Lim, K.-T. Functional cellulose-based hydrogels as extracellular matrices for tissue engineering. J. Biol. Eng. 2019, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- Dastidar, P.; Ganguly, S.; Sarkar, K. Metallogels from Coordination Complexes, Organometallic, and Coordination Polymers. Chem. Asian J. 2016, 11, 2484–2498. [Google Scholar] [CrossRef] [PubMed]
- Sannino, A.; Demitri, C.; Madaghiele, M. Biodegradable Cellulose-based Hydrogels: Design and Applications. Materials 2009, 2, 353–373. [Google Scholar] [CrossRef]
- Ciolacu, D.E.; Nicu, R.; Ciolacu, F. Cellulose-Based Hydrogels as Sustained Drug-Delivery Systems. Materials 2020, 13, 5270. [Google Scholar] [CrossRef]
- Yang, X.; Cranston, E.D. Chemically Cross-Linked Cellulose Nanocrystal Aerogels with Shape Recovery and Superabsorbent Properties. Chem. Mater. 2014, 26, 6016–6025. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, X.; Cranston, E.D.; Zhu, S. Flexible and Porous Nanocellulose Aerogels with High Loadings of Metal-Organic-Framework Particles for Separations Applications. Adv. Mater. 2016, 28, 7652–7657. [Google Scholar] [CrossRef]
- Yang, X.; Shi, K.; Zhitomirsky, I.; Cranston, E.D. Cellulose Nanocrystal Aerogels as Universal 3D Lightweight Substrates for Supercapacitor Materials. Adv. Mater. 2015, 27, 6104–6109. [Google Scholar] [CrossRef]
- Ungureanu, E.; Fortună, M.E.; Țopa, D.C.; Lobiuc, A.; Ungureanu, O.C.; Jităreanu, D.C. Design of Functional Polymer Systems to Optimize the Filler Retention in Obtaining Cellulosic Substrates with Improved Properties. Materials 2023, 16, 1904. [Google Scholar] [CrossRef]
- Pascuta, M.S.; Varvara, R.-A.; Teleky, B.-E.; Szabo, K.; Plamada, D.; Nemeş, S.-A.; Mitrea, L.; Martău, G.A.; Ciont, C.; Călinoiu, L.F.; et al. Polysaccharide-Based Edible Gels as Functional Ingredients: Characterization, Applicability, and Human Health Benefits. Gels 2022, 8, 524. [Google Scholar] [CrossRef]
- Ghosh, S.; Ahmad, R.; Zeyaullah; Khare, S.K. Microbial Nano-Factories: Synthesis and Biomedical Applications. Front. Chem. 2021, 9, 3389. [Google Scholar] [CrossRef]
- De Luciano, G.B.; Panecatl-Bernal, Y.; Soto-Cruz, B.; Méndez-Rojas, M.; López-Salazar, P.; Alcántara-Iniesta, S.; Portillo, M.C.; Romero-López, A.; Mejía-Silva, J.; Alvarado, J.; et al. Controlling Size Distribution of Silver Nanoparticles using Natural Reducing Agents in MCM-41@Ag. Chemistryselect 2022, 7, e202202566. [Google Scholar] [CrossRef]
- Mehrabadi, B.A.T.; Eskandari, S.; Khan, U.; White, R.D.; Regalbuto, J.R. Chapter One—A Review of Preparation Methods for Supported Metal Catalysts. In Advances in Catalysis; Song, C., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 61, pp. 1–35. [Google Scholar]
- Zhao, P.; Li, N.; Astruc, D. State of the art in gold nanoparticle synthesis. Coord. Chem. Rev. 2013, 257, 638–665. [Google Scholar] [CrossRef]
- Das, D.; Kar, T.; Das, P.K. Gel-nanocomposites: Materials with promising applications. Soft Matter 2012, 8, 2348–2365. [Google Scholar] [CrossRef]
- Alahmadi, N.S.; Betts, J.W.; Cheng, F.; Francesconi, M.G.; Kelly, S.M.; Kornherr, A.; Prior, T.J.; Wadhawan, J.D. Synthesis and antibacterial effects of cobalt–cellulose magnetic nanocomposites. RSC Adv. 2017, 7, 20020–20026. [Google Scholar] [CrossRef]
- Acharya, S.; Liyanage, S.; Parajuli, P.; Rumi, S.S.; Shamshina, J.L.; Abidi, N. Utilization of Cellulose to Its Full Potential: A Review on Cellulose Dissolution, Regeneration, and Applications. Polymers 2021, 13, 4344. [Google Scholar] [CrossRef]
- Sánchez-Cid, P.; Jiménez-Rosado, M.; Romero, A.; Pérez-Puyana, V. Novel Trends in Hydrogel Development for Biomedical Applications: A Review. Polymers 2022, 14, 3023. [Google Scholar] [CrossRef]
- Tofanica, B.-M.; Belosinschi, D.; Volf, I. Gels, Aerogels and Hydrogels: A Challenge for the Cellulose-Based Product Industries. Gels 2022, 8, 497. [Google Scholar] [CrossRef]
- Ciolacu, D.E.; Suflet, D.M. Cellulose-Based Hydrogels for Medical/Pharmaceutical Applications. In Biomass as Renewable Raw Material to Obtain Bioproducts of High-Tech Value; Popa, V., Volf, I., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 401–439. ISBN 978-0-444-63774-1. [Google Scholar]
- Rusu, D.; Ciolacu, D.; Simionescu, B.C. Cellulose-Based Hydrogels in Tissue Engineering Applications. Cellul. Chem. Technol. 2019, 53, 907–923. [Google Scholar] [CrossRef]
- Jiang, X.; He, C.; Lin, W. Supramolecular metal-based nanoparticles for drug delivery and cancer therapy. Curr. Opin. Chem. Biol. 2021, 61, 143–153. [Google Scholar] [CrossRef]
- Berret, J.-F.; Graillot, A. Versatile Coating Platform for Metal Oxide Nanoparticles: Applications to Materials and Biological Science. Langmuir 2022, 38, 5323–5338. [Google Scholar] [CrossRef] [PubMed]
- De la Rosa, S.Y.G.; Diaz, R.M.; Gutiérrez, P.T.V.; Patakfalvi, R.; Coronado, G. Functionalized Platinum Nanoparticles with Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 9404. [Google Scholar] [CrossRef] [PubMed]
- Zahran, M.; Marei, A.H. Innovative natural polymer metal nanocomposites and their antimicrobial activity. Int. J. Biol. Macromol. 2019, 136, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Gholamali, I.; Yadollahi, M. Bio-nanocomposite Polymer Hydrogels Containing Nanoparticles for Drug Delivery: A Review. Regen. Eng. Transl. Med. 2021, 7, 129–146. [Google Scholar] [CrossRef]
- Anžlovar, A.; Žagar, E. Cellulose Structures as a Support or Template for Inorganic Nanostructures and Their Assemblies. Nanomaterials 2022, 12, 1837. [Google Scholar] [CrossRef] [PubMed]
- Oprea, M.; Panaitescu, D.M. Nanocellulose Hybrids with Metal Oxides Nanoparticles for Biomedical Applications. Molecules 2020, 25, 4045. [Google Scholar] [CrossRef]
- Abdellatif, A.A.H.; Alturki, H.N.H.; Tawfeek, H.M. Different cellulosic polymers for synthesizing silver nanoparticles with antioxidant and antibacterial activities. Sci. Rep. 2021, 11, 84. [Google Scholar] [CrossRef]
- Liu, S.; Luo, X.; Zhou, J. Magnetic Responsive Cellulose Nanocomposites and Their Applications. In Cellulose—Medical, Pharmaceutical and Electronic Applications; Van De Ven, T.G.M., Van De Ven, T.G.M., Eds.; InTech: London, UK, 2013; ISBN 978-953-51-1191-7. [Google Scholar]
- Gupta, A.; Briffa, S.M.; Swingler, S.; Gibson, H.; Kannappan, V.; Adamus, G.; Kowalczuk, M.M.; Martin, C.; Radecka, I. Synthesis of Silver Nanoparticles Using Curcumin-Cyclodextrins Loaded into Bacterial Cellulose-Based Hydrogels for Wound Dressing Applications. Biomacromolecules 2020, 21, 1802–1811. [Google Scholar] [CrossRef]
- Kotelnikova, N.; Mikhailidi, A. Hydrate Cellulose Films and Preparation of Samples Modified with Nickel Nano- and Micro-particles. II. Intercalation of Nickel into Hydrate Cellulose Films. Cellul. Chem. Technol. 2012, 46, 27–33. [Google Scholar]
- Mikhailidi, A.M.; Kotel′Nikova, N.E.; Shakhmin, A.L.; Andersson, S.; Saprykina, N.N.; Kudryashov, V.I.; Anan′Eva, E.P.; Martakova, Y.V. Preparation, Characteristics, and Antibacterial Properties of Pulp—Silver Nanocomposites Obtained from DMA/LiCl Solutions. Fibre Chem. 2015, 47, 260–264. [Google Scholar] [CrossRef]
- Pirkkalainen, K.; Leppänen, K.; Vainio, U.; Webb, M.A.; Elbra, T.; Kohout, T.; Nykänen, A.; Ruokolainen, J.; Kotelnikova, N.; Serimaa, R. Nanocomposites of magnetic cobalt nanoparticles and cellulose. Eur. Phys. J. D 2008, 49, 333–342. [Google Scholar] [CrossRef]
- Mikhailidi, A.; Kotelnikova, N. Antibacterial Cellulose Hydrogels with Noble Metals Nanoparticles: Synthesis, Mechanism, and Properties. In Proceedings of the International Conference “Progress in Organic and Macromolecular Compounds”, Iasi, Romania, 7 October 2021; pp. 129–130. [Google Scholar]
- Mikhailidi, A.; Saprykina, N.; Mokeev, M.; Zinchenko, A.; Kotelnikova, N. Highly porous hybrid composite hydrogels based on cellulose and 1,10-phenanthrocyanine of zn(ii): Synthesis and characterization with waxs, ftir, 13c cp/mas nmr and sem. Cellul. Chem. Technol. 2020, 54, 875–888. [Google Scholar] [CrossRef]
- Kotel’nikova, N.E.; Lysenko, E.; Serimaa, R.; Pirkkalainen, K.; Vainio, U.; Lavrentiev, V.; Schakhmin, A.; Saprykina, N.; No-voselov, N.P. Novel Study on Cellulose Matrix as Nanoreactor. Intercalation of Silver, Copper, Platinum, Nickel, and Cobalt Nanoclusters. In Proceedings of the III International Conference “Times of Polymers and Composites”, Ishia, Italy, 18–22 June 2006; p. 180. [Google Scholar]
- Vainio, U.; Pirkkalainen, K.; Kisko, K.; Goerigk, G.; Kotelnikova, N.E.; Serimaa, R. Copper and copper oxide nanoparticles in a cellulose support studied using anomalous small-angle X-ray scattering. Eur. Phys. J. D 2007, 42, 93–101. [Google Scholar] [CrossRef]
- Kotelnikova, N.E.; Paakkari, T.; Serimaa, R.; Wegener, G.; Windeisen, E.; Kotelnikov, V.P.; Demidov, V.N.; Schukarev, A.V. Study of platinum and palladium aggregates intercalation into cellulose by waxs, spectroscopic, and microscopic methods. Macromol. Symp. 1999, 138, 175–180. [Google Scholar] [CrossRef]
- Musa, A.; Ahmad, M.B.; Hussein, M.Z.; Saiman, M.I.; Sani, H.A. Preparation, characterization and catalytic activity of biomaterial-supported copper nanoparticles. Res. Chem. Intermed. 2017, 43, 801–815. [Google Scholar] [CrossRef]
- Yan, W.; Chen, C.; Wang, L.; Zhang, D.; Li, A.-J.; Yao, Z.; Shi, L.-Y. Facile and green synthesis of cellulose nanocrystal-supported gold nanoparticles with superior catalytic activity. Carbohydr. Polym. 2016, 140, 66–73. [Google Scholar] [CrossRef]
- Lustosa, A.K.M.F.; Oliveira, A.C.D.J.; Quelemes, P.V.; Plácido, A.; Da Silva, F.V.; Oliveira, I.S.; De Almeida, M.P.; Amorim, A.D.G.N.; Delerue-Matos, C.; Oliveira, R.D.C.M.D.; et al. In Situ Synthesis of Silver Nanoparticles in a Hydrogel of Carboxymethyl Cellulose with Phthalated-Cashew Gum as a Promising Antibacterial and Healing Agent. Int. J. Mol. Sci. 2017, 18, 2399. [Google Scholar] [CrossRef]
- Li, S.-M.; Jia, N.; Ma, M.-G.; Zhang, Z.; Liu, Q.-H.; Sun, R.-C. Cellulose–silver nanocomposites: Microwave-assisted synthesis, characterization, their thermal stability, and antimicrobial property. Carbohydr. Polym. 2011, 86, 441–447. [Google Scholar] [CrossRef]
- Zhang, S.; Li, F.-X.; Yu, J.-Y.; Hsieh, Y.-L. Dissolution behaviour and solubility of cellulose in NaOH complex solution. Carbohydr. Polym. 2010, 81, 668–674. [Google Scholar] [CrossRef]
- Dong, J.; Carpinone, P.L.; Pyrgiotakis, G.; Demokritou, P.; Moudgil, B.M. Synthesis of Precision Gold Nanoparticles Using Turkevich Method. KONA Powder Part. J. 2020, 37, 224–232. [Google Scholar] [CrossRef]
- Kotel’Nikova, N.E.; Demidov, V.N.; Wegener, G.; Windeisen, E. Mechanisms of Diffusion-Reduction Interaction of Microcrystalline Cellulose and Silver Ions. Russ. J. Gen. Chem. 2003, 73, 427–433. [Google Scholar] [CrossRef]
- Das, A.; Kumar, A.; Patil, N.B.; Viswanathan, C.; Ghosh, D. Preparation and characterization of silver nanoparticle loaded amorphous hydrogel of carboxymethylcellulose for infected wounds. Carbohydr. Polym. 2015, 130, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Hamrayev, H.; Shameli, K.; Korpayev, S. Green Synthesis of Zinc Oxide Nanoparticles and Its Biomedical Applications: A Review. J. Res. Nanosci. Nanotechnol. 2021, 1, 62–74. [Google Scholar] [CrossRef]
- George, D.; Maheswari, P.U.; Begum, K.M.S. Chitosan-cellulose hydrogel conjugated with L-histidine and zinc oxide nanoparticles for sustained drug delivery: Kinetics and in-vitro biological studies. Carbohydr. Polym. 2020, 236, 116101. [Google Scholar] [CrossRef] [PubMed]
- George, D.; Maheswari, P.U.; Begum, K.M.S. Synergic formulation of onion peel quercetin loaded chitosan-cellulose hydrogel with green zinc oxide nanoparticles towards controlled release, biocompatibility, antimicrobial and anticancer activity. Int. J. Biol. Macromol. 2019, 132, 784–794. [Google Scholar] [CrossRef]
- Thi, T.U.D.; Nguyen, T.T.; Thi, Y.D.; Thi, K.H.T.; Phan, B.T.; Pham, K.N. Green synthesis of ZnO nanoparticles using orange fruit peel extract for antibacterial activities. RSC Adv. 2020, 10, 23899–23907. [Google Scholar] [CrossRef]
- Dulta, K.; Ağçeli, G.K.; Chauhan, P.; Jasrotia, R. Ecofriendly Synthesis of Zinc Oxide Nanoparticles by Carica papaya Leaf Extract and Their Applications. J. Clust. Sci. 2022, 33, 603–617. [Google Scholar] [CrossRef]
- Anagha, B.; George, D.; Maheswari, P.U.; Begum, K.M.M.S. Biomass Derived Antimicrobial Hybrid Cellulose Hydrogel with Green ZnO Nanoparticles for Curcumin Delivery and its Kinetic Modelling. J. Polym. Environ. 2019, 27, 2054–2067. [Google Scholar] [CrossRef]
- Dong, H.; Snyder, J.F.; Tran, D.T.; Leadore, J.L. Hydrogel, aerogel and film of cellulose nanofibrils functionalized with silver nanoparticles. Carbohydr. Polym. 2013, 95, 760–767. [Google Scholar] [CrossRef]
- Zander, N.E.; Dong, H.; Steele, J.; Grant, J.T. Metal Cation Cross-Linked Nanocellulose Hydrogels as Tissue Engineering Substrates. ACS Appl. Mater. Interfaces 2014, 6, 18502–18510. [Google Scholar] [CrossRef]
- Puițel, A.C.; Tofanica, B.M.; Gavrilescu, D.A. Fibrous Raw Materials from Agricultural Residues. In Pulp Production and Processing. High-Tech Applications; Popa, V., Ed.; De Gruyter: Berlin, Germany, 2020; pp. 49–72. ISBN 978-3-11-065883-5. [Google Scholar]
- Gavrilescu, D.; Tofanica, B.M.; Puitel, A.C. Environmental Friendly Pulping and Bleaching of Rapeseed Stalk Fibers. Environ. Eng. Manag. J. 2012, 11, 681–686. [Google Scholar] [CrossRef]
- Tofanica, B.M.; Puitel, A.C. Optimization and design of alkaline pulping of rapeseed (Brassica napus) stalks. Chem. Eng. Commun. 2019, 206, 378–386. [Google Scholar] [CrossRef]
- Tofanica, B.M. Rapeseed—A Valuable Renewable Bioresource. Cellul. Chem. Technol. 2019, 53, 837–849. [Google Scholar] [CrossRef]
- Cheşcă, A.M.; Nicu, R.; Tofănică, B.M.; Puiţel, A.C.; Gavrilescu, D. Optimization of Soda Pulping Process of Corn Stalks by Response Surface Modelling. Cellul. Chem. Technol. 2018, 52, 823–831. [Google Scholar]
- Chesca, A.M.; Nicu, R.; Tofănică, B.M.; Puiţel, A.C.; Vlase, R.; Gavrilescu, D. Pulping of Corn Stalks—Assessment in Bio-Based Packaging Materials. Cellul. Chem. Technol. 2018, 52, 645–653. [Google Scholar]
- Gavrilescu, D.; Chesca, A.M.; Tofanica, B.M.; Puitel, A.C.; Nicu, R. Environmentally friendly cellulosic fibers from corn stalks. Environ. Eng. Manag. J. 2018, 17, 1765–1771. [Google Scholar] [CrossRef]
- Moisei, N.; Puitel, A.C.; Tofanica, B.M.; Gavrilescu, D. Turning wheat straw in a sustainable raw material for paper industry. Environ. Eng. Manag. J. 2017, 16, 1027–1032. [Google Scholar] [CrossRef]
- Li, S.; Chen, G. Agricultural waste-derived superabsorbent hydrogels: Preparation, performance, and socioeconomic impacts. J. Clean. Prod. 2019, 251, 119669. [Google Scholar] [CrossRef]
- Mikhailidi, A.; Saurov, S.K.; Anderson, S.; Kotelnikova, N. Lignocellulose fibers elaborating super-swollen three-dimensional cellulose hydrogels from solution in N,N-dimethylacetamide/lithium chloride. TAPPI J. 2018, 17, 81–88. [Google Scholar] [CrossRef]
- Cho, C.; Aye, T.; Khaing, A.; Kobayashi, T. Comparative Study of Cellulose Hydrogel Films Prepared from Various Biomass Wastes. In Cellulose Science and Derivatives; Sand, A., Banga, S., Eds.; IntechOpen: London, UK, 2021; ISBN 978-1-83969-579-7. [Google Scholar]
- Cui, X.; Lee, J.J.L.; Chen, W.N. Eco-friendly and biodegradable cellulose hydrogels produced from low cost okara: Towards non-toxic flexible electronics. Sci. Rep. 2019, 9, 18166. [Google Scholar] [CrossRef]
- Truong, T.T.C.; Vo, N.T.T.; Nguyen, K.D.; Bui, H.M. Preparation of cellulose-based hydrogel derived from tea. Cellul. Chem. Technol. 2019, 53, 573–582. [Google Scholar] [CrossRef]
- Jampi, A.L.W.; Chin, S.-F.; Wasli, M.E.; Chia, C.-H. Preparation of cellulose hydrogel from sago pith waste as a medium for seed germination. J. Phys. Sci. 2021, 32, 13–26. [Google Scholar] [CrossRef]
- Seo, M.; Seo, M.; Choi, S.-E.; Shin, K.; Lee, J.B.; Yang, D.-Y.; Kim, J.W. Cellulose nanofiber-multilayered fruit peel-mimetic gelatin hydrogel microcapsules for micropackaging of bioactive ingredients. Carbohydr. Polym. 2020, 229, 115559. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Lee, J.; Ng, K.R.; Chen, W.N. Food Waste Durian Rind-Derived Cellulose Organohydrogels: Toward Anti-Freezing and Antimicrobial Wound Dressing. ACS Sustain. Chem. Eng. 2021, 9, 1304–1312. [Google Scholar] [CrossRef]
- Liu, Z.; Cao, Y.; Wang, Z.; Xia, J.; Su, C. Preparation and Characterization of Lignocellulose Gel from Soybean Stem/LiCl/DMSO Solution. Chem. Ind. For. Prod. 2016, 36, 81–88. [Google Scholar]
- Madramootoo, C.A.; Jain, A.; Oliva, C.; Wang, Y.; Abbasi, N.A. Growth and yield of tomato on soil amended with waste paperbased hydrogels. Sci. Hortic. 2023, 310, 111752. [Google Scholar] [CrossRef]
- Mikhailidi, A.M.; Kotel’nikova, N.Y. Functional Materials from Paper Wastes: II–Cellulose Hydrogels with High Water Retention Capacity Obtained from Solutions of Waste Paper in DMAc/LiCl. Russ. J. Bioorg. Chem. 2022, 48, 1486–1497. [Google Scholar] [CrossRef]
- Wu, Q.; Ma, C.; Chen, L.; Sun, Y.; Wei, X.; Ma, C.; Zhao, H.; Yang, X.; Ma, X.; Zhang, C.; et al. A Tissue Paper/Hydrogel Composite Light-Responsive Biomimetic Actuator Fabricated by In Situ Polymerization. Polymers 2022, 14, 5454. [Google Scholar] [CrossRef]
- Abdelaziz, R.M.; El-Maghraby, A.; Sadik, W.A.-A.; El-Demerdash, A.-G.M.; Fadl, E.A. Biodegradable cellulose nanocrystals hydrogels for removal of acid red 8 dye from aqueous solutions. Sci. Rep. 2022, 12, 6424. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, L.; Li, Y.; Liu, Y.; Chen, Y.; Li, H.; Li, M.; Xu, F.; Liu, Y. Preparation of a double-network hydrogel based on wastepaper and its application in the treatment of wastewater containing copper(ii) and methylene blue. RSC Adv. 2021, 11, 18131–18143. [Google Scholar] [CrossRef]
- Liu, M.; Tong, S.; Tong, Z.; Guan, Y.; Sun, Y. A strong, biodegradable and transparent cellulose-based bioplastic stemmed from waste paper. J. Appl. Polym. Sci. 2023, 140, e53671. [Google Scholar] [CrossRef]
- Wu, C.; McClements, D.J.; He, M.; Fan, Z.; Li, Y.; Teng, F. Preparation of okara cellulose hydrogels using ionic liquids: Structure, properties, and performance. J. Mol. Liq. 2021, 331, 115744. [Google Scholar] [CrossRef]
- Shen, X.; Shamshina, J.L.; Berton, P.; Bandomir, J.; Wang, H.; Gurau, G.; Rogers, R.D. Comparison of Hydrogels Prepared with Ionic-Liquid-Isolated vs Commercial Chitin and Cellulose. ACS Sustain. Chem. Eng. 2016, 4, 471–480. [Google Scholar] [CrossRef]
- Baharin, K.W.; Zakaria, S.; Ellis, A.V.; Talip, N.; Kaco, H.; Gan, S.; Zailan, F.D.; Hashim, S.N.A.S. Factors Affecting Cellulose Dissolution of Oil Palm Empty Fruit Bunch and Kenaf Pulp in NaOH/Urea Solvent. Sains Malays. 2018, 47, 377–386. [Google Scholar]
- Kotelnikova, N.; Bykhovtsova, Y.; Mikhailidi, A.; Mokeev, M.; Lavrent’ev, V.; Vlasova, E.; Saprykina, N. Solubility of Ligno-cellulose in N, N-Dimethylacetamide/Lithium Chloride. WAXS, 13C CP/MAS NMR, FTIR and SEM Studies of Samples Re-generated from the Solutions. Cellul. Chem. Technol. 2014, 48, 643–651. [Google Scholar]
- Tovar-Carrillo, K.L.; Tagaya, M.; Kobayashi, T. Bamboo Fibers Elaborating Cellulose Hydrogel Films for Medical Applications. J. Mater. Sci. Chem. Eng. 2013, 1, 7–12. [Google Scholar] [CrossRef]
- Zhang, S.; Shan, S.; Zhang, H.; Gao, X.; Tang, X.; Chen, K. Antimicrobial cellulose hydrogels preparation with RIF loading from bamboo parenchyma cells: A green approach towards wound healing. Int. J. Biol. Macromol. 2022, 203, 1–9. [Google Scholar] [CrossRef]
- Cruz-Medina, R.; Ayala-Hernández, D.A.; Vega-Rios, A.; López-Martínez, E.I.; Mendoza-Duarte, M.E.; Estrada-Monje, A.; Zaragoza-Contreras, E.A. Curing of Cellulose Hydrogels by UV Radiation for Mechanical Reinforcement. Polymers 2021, 13, 2342. [Google Scholar] [CrossRef]
- Nakasone, K.; Kobayashi, T. Cytocompatible cellulose hydrogels containing trace lignin. Mater. Sci. Eng. C 2016, 64, 269–277. [Google Scholar] [CrossRef]
- Strlič, M.; Kolar, J. Size exclusion chromatography of cellulose in LiCl/N,N-dimethylacetamide. J. Biochem. Biophys. Methods 2003, 56, 265–279. [Google Scholar] [CrossRef]
- Chrapava, S.; Touraud, D.; Rosenau, T.; Potthast, A.; Kunz, W. The investigation of the influence of water and temperature on the LiCl/DMAc/cellulose system. Phys. Chem. Chem. Phys. 2003, 5, 1842–1847. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Ayoub, A.; Daystar, J.S.; Venditti, R.A.; Pawlak, J.J. Enhanced Absorbent Products Incorporating Cellulose and Its Derivatives: A Review. Bioresources 2013, 8, 6556–6629. [Google Scholar] [CrossRef]
- Ishii, D.; Tatsumi, D.; Matsumoto, T.; Murata, K.; Hayashi, H.; Yoshitani, H. Investigation of the Structure of Cellulose in LiCl/DMAc Solution and Its Gelation Behavior by Small-Angle X-Ray Scattering Measurements. Macromol. Biosci. 2006, 6, 293–300. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, R.; Xiang, J.; Kang, H.; Liu, Z.; Huang, Y. Dissolution Mechanism of Cellulose in N,N-Dimethylacetamide/Lithium Chloride: Revisiting through Molecular Interactions. J. Phys. Chem. B 2014, 118, 9507–9514. [Google Scholar] [CrossRef]
- WHMIS Classification for N,N-Dimethylacetamide—CNESST. Available online: https://reptox.cnesst.gouv.qc.ca/en/Pages/information-sheet-whmis.aspx?langue=a&no_produit=3251 (accessed on 27 February 2023).
- Wen, J.; Li, Y.; Chen, Y.; He, S.; Qiu, X. Dimethylacetamide (DMAC) Recycling Device and Method. Patent CN102787395A, 21 November 2012. [Google Scholar]
- Xia, J.; Liu, Z.; Chen, Y.; Cao, Y.; Wang, Z. Effect of lignin on the performance of biodegradable cellulose aerogels made from wheat straw pulp-LiCl/DMSO solution. Cellulose 2019, 27, 879–894. [Google Scholar] [CrossRef]
- Wang, Z.; Yokoyama, T.; Matsumoto, Y. Dissolution of Ethylenediamine Pretreated Pulp with High Lignin Content in LiCl/DMSO without Milling. J. Wood Chem. Technol. 2010, 30, 219–229. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, S.; Matsumoto, Y.; Kuga, S. Cellulose gel and aerogel from LiCl/DMSO solution. Cellulose 2012, 19, 393–399. [Google Scholar] [CrossRef]
- Xia, J.; Liu, Z.; Chen, Y.; Wang, Z.; Cao, Y. Fabrication of thermo-sensitive lignocellulose hydrogels with switchable hydrophilicity and hydrophobicity through an SIPN strategy. RSC Adv. 2019, 9, 29600–29608. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Q.; Yu, J.; Ma, J.; Wang, Z.; Fan, Y.; Kuga, S. Strengthened cellulosic gels by the chemical gelation of cellulose via crosslinking with TEOS. Cellulose 2019, 26, 9819–9829. [Google Scholar] [CrossRef]
- WHMIS Classification for Dimethyl Sulfoxide—CNESST. Available online: https://reptox.cnesst.gouv.qc.ca/en/Pages/information-sheet-whmis.aspx?langue=a&no_produit=7708 (accessed on 27 February 2023).
- Fink, H.-P.; Weigel, P.; Purz, H.; Ganster, J. Structure formation of regenerated cellulose materials from NMMO-solutions. Prog. Polym. Sci. 2001, 26, 1473–1524. [Google Scholar] [CrossRef]
- Zhou, L.; Xie, F.; Li, H.; Li, W.; Li, Y.W.; Li, W.J. Preparation and properties of regenerated cellulose hydrogels. IOP Conf. Series Mater. Sci. Eng. 2017, 170, 012038. [Google Scholar] [CrossRef]
- Krysztof, M.; Olejnik, K.; Kulpinski, P.; Stanislawska, A.; Khadzhynova, S. Regenerated cellulose from N-methylmorpholine N-oxide solutions as a coating agent for paper materials. Cellulose 2018, 25, 3595–3607. [Google Scholar] [CrossRef]
- Isogai, A.; Atalla, R.H. Dissolution of Cellulose in Aqueous NaOH Solutions. Cellulose 1998, 5, 309–319. [Google Scholar] [CrossRef]
- Song, S.; Liu, Z.; Abubaker, M.A.; Ding, L.; Zhang, J.; Yang, S.; Fan, Z. Antibacterial polyvinyl alcohol/bacterial cellulose/nano-silver hydrogels that effectively promote wound healing. Mater. Sci. Eng. C 2021, 126, 112171. [Google Scholar] [CrossRef]
- Lin, X.; Han, X.; Wang, J. In situ synthesis of easily separable Au nanoparticles catalysts based on cellulose hydrogels. Polym. J. 2018, 50, 495–501. [Google Scholar] [CrossRef]
- El Bouazzaoui, Y.; Habsaoui, A.; Touhami, M.E. Hydrogel synthesis using extracted cellulose from Opuntia Ficus indica seeds and its application in methylene blue dye removal. Chem. Data Collect. 2022, 41, 100918. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikhailidi, A.; Volf, I.; Belosinschi, D.; Tofanica, B.-M.; Ungureanu, E. Cellulose-Based Metallogels—Part 1: Raw Materials and Preparation. Gels 2023, 9, 390. https://doi.org/10.3390/gels9050390
Mikhailidi A, Volf I, Belosinschi D, Tofanica B-M, Ungureanu E. Cellulose-Based Metallogels—Part 1: Raw Materials and Preparation. Gels. 2023; 9(5):390. https://doi.org/10.3390/gels9050390
Chicago/Turabian StyleMikhailidi, Aleksandra, Irina Volf, Dan Belosinschi, Bogdan-Marian Tofanica, and Elena Ungureanu. 2023. "Cellulose-Based Metallogels—Part 1: Raw Materials and Preparation" Gels 9, no. 5: 390. https://doi.org/10.3390/gels9050390
APA StyleMikhailidi, A., Volf, I., Belosinschi, D., Tofanica, B.-M., & Ungureanu, E. (2023). Cellulose-Based Metallogels—Part 1: Raw Materials and Preparation. Gels, 9(5), 390. https://doi.org/10.3390/gels9050390