Carbonic Anhydrase Enhanced UV-Crosslinked PEG-DA/PEO Extruded Hydrogel Flexible Filaments and Durable Grids for CO2 Capture
Abstract
1. Introduction
2. Results and Discussion
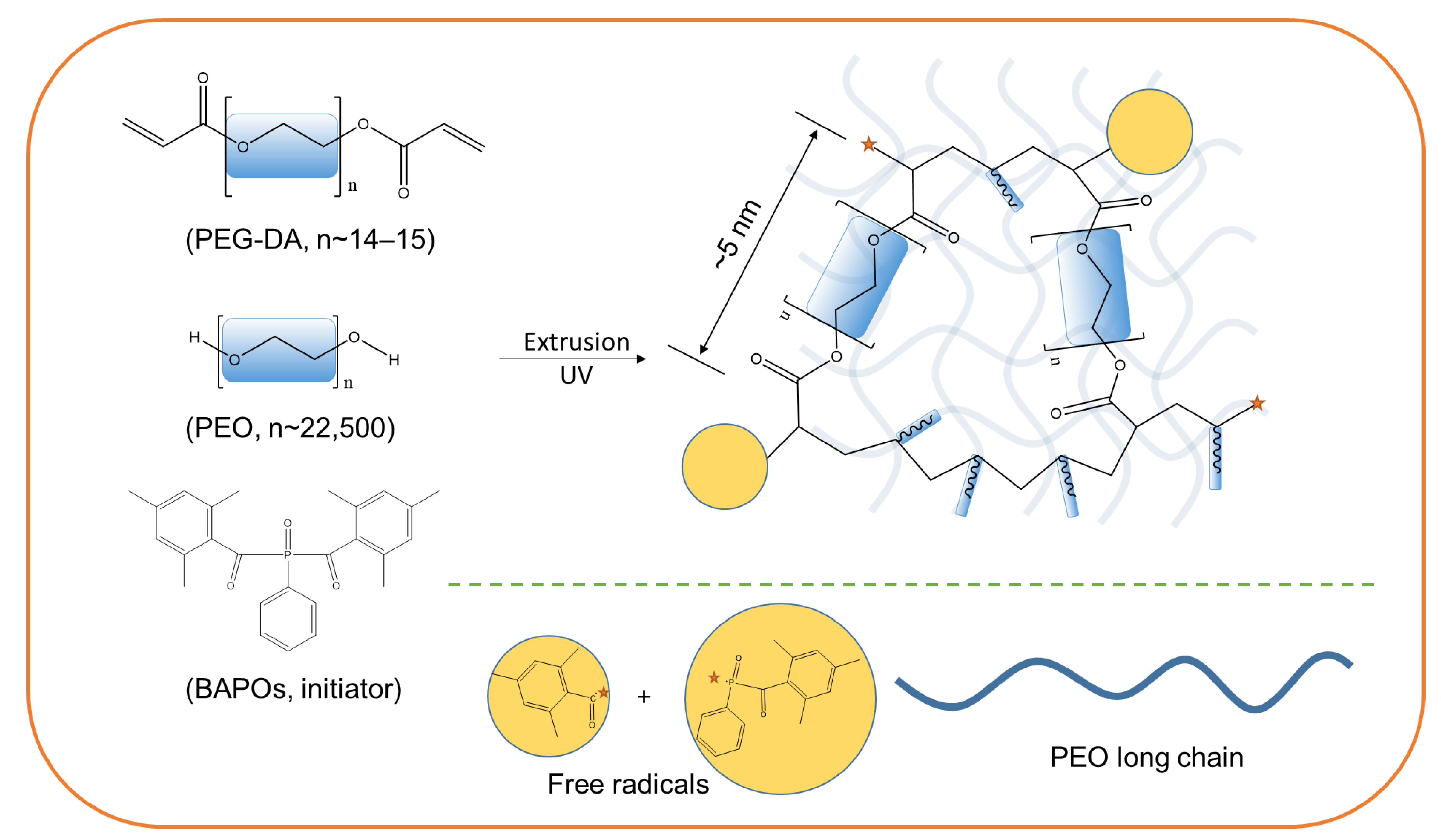
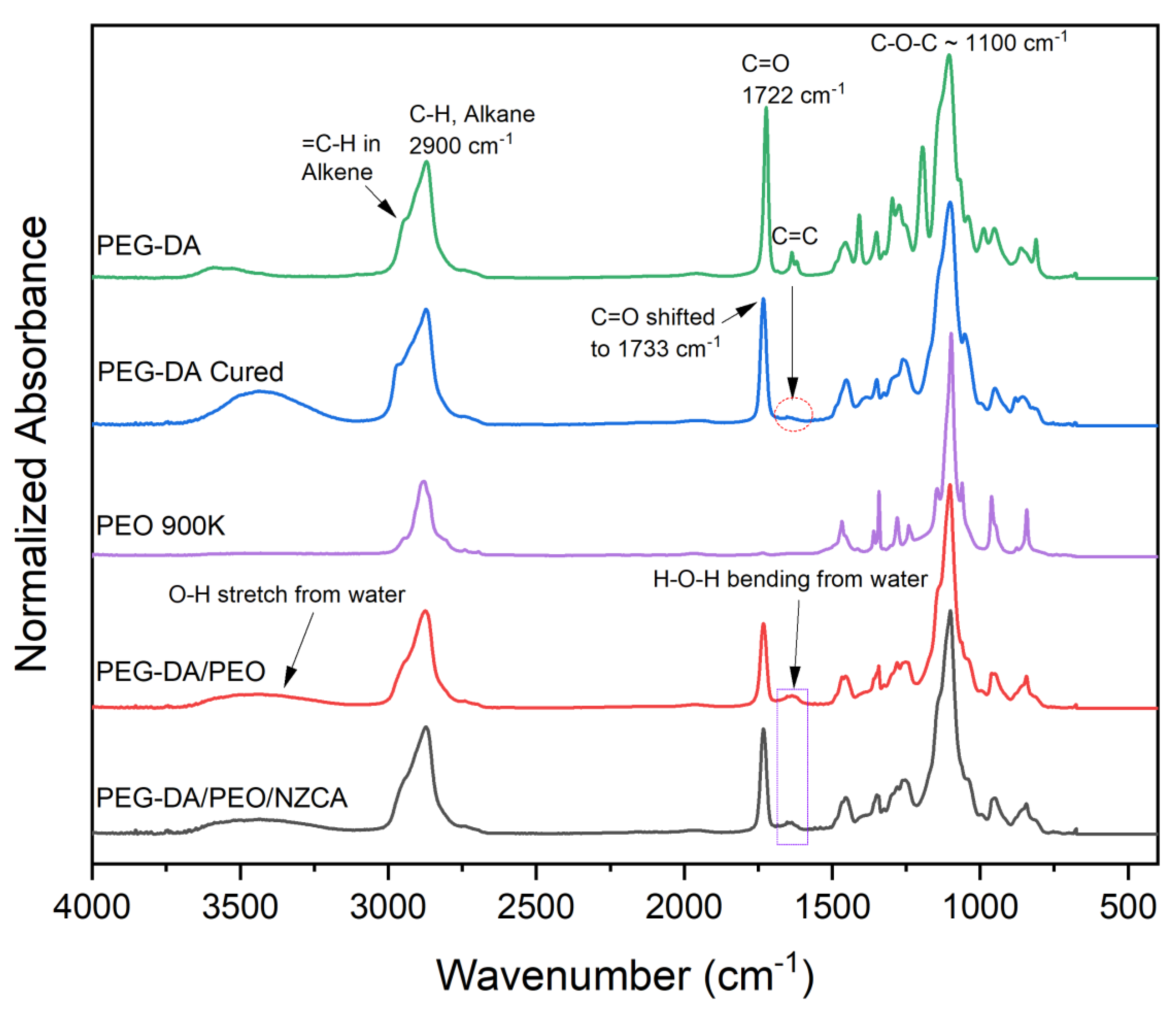
2.1. Chemical Compositions of IPNHs
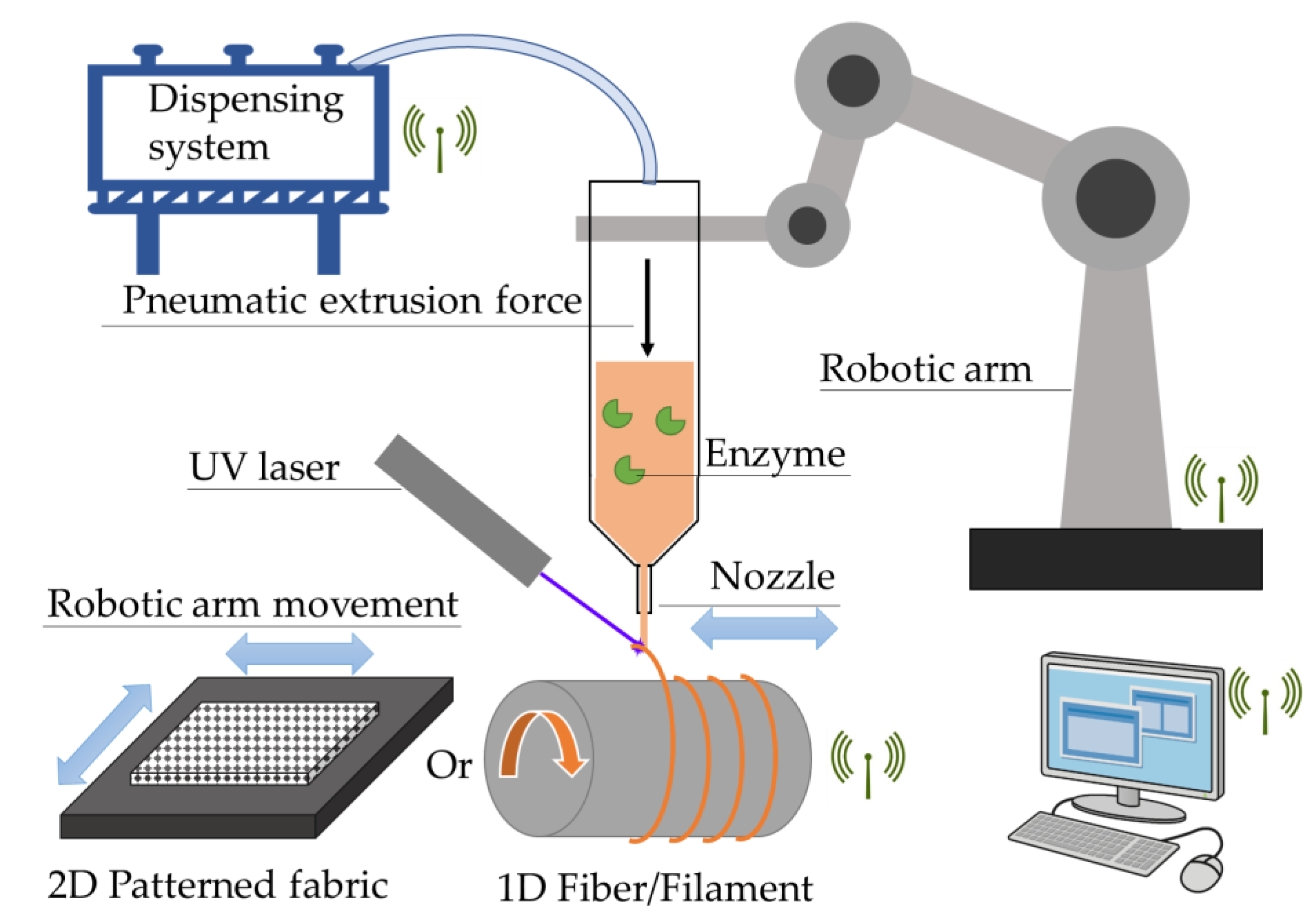
2.2. PEG-DA/PEO IPNH Filament
2.3. PEG-DA/PEO IPNH Grid and Structured Packing
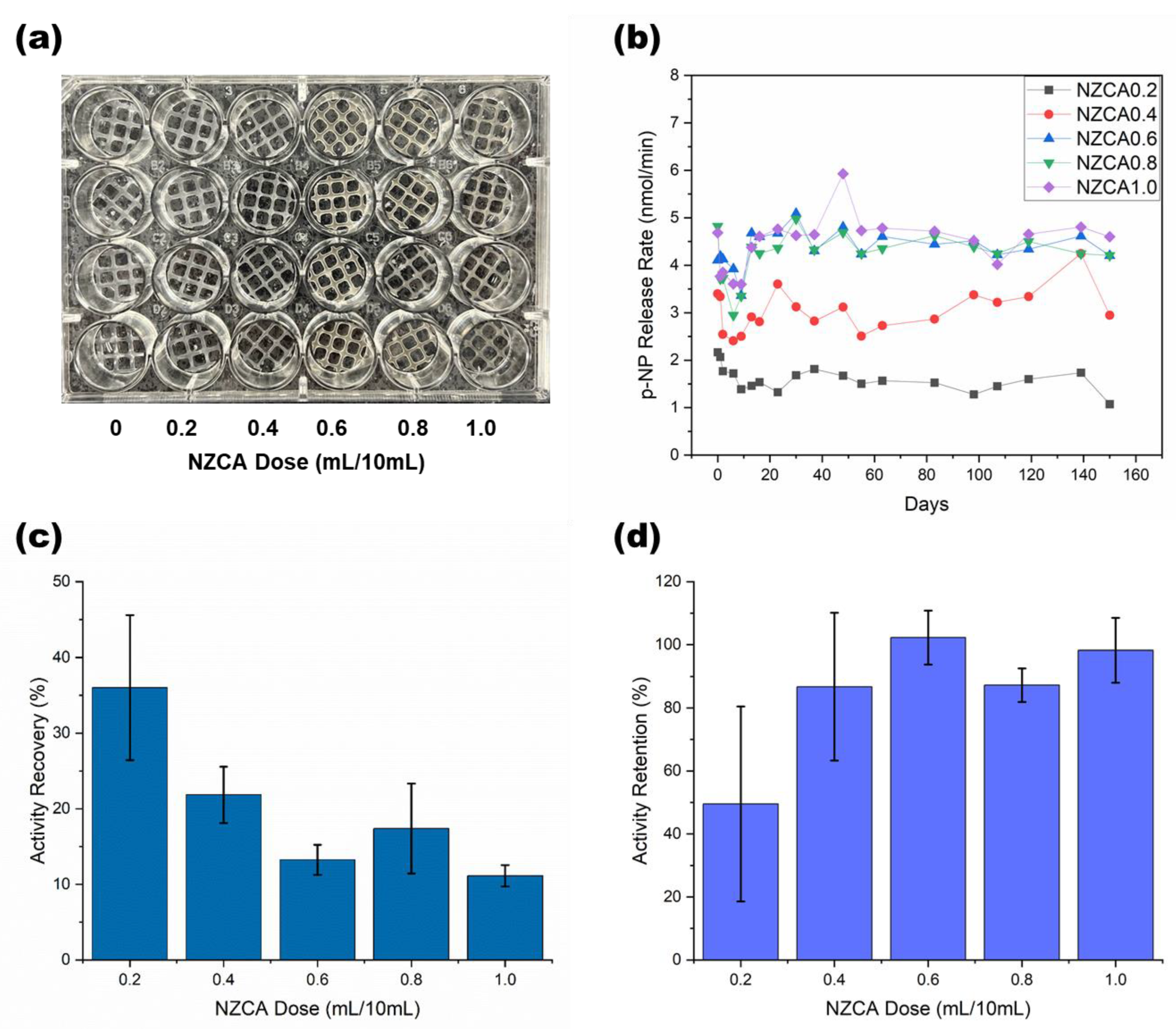
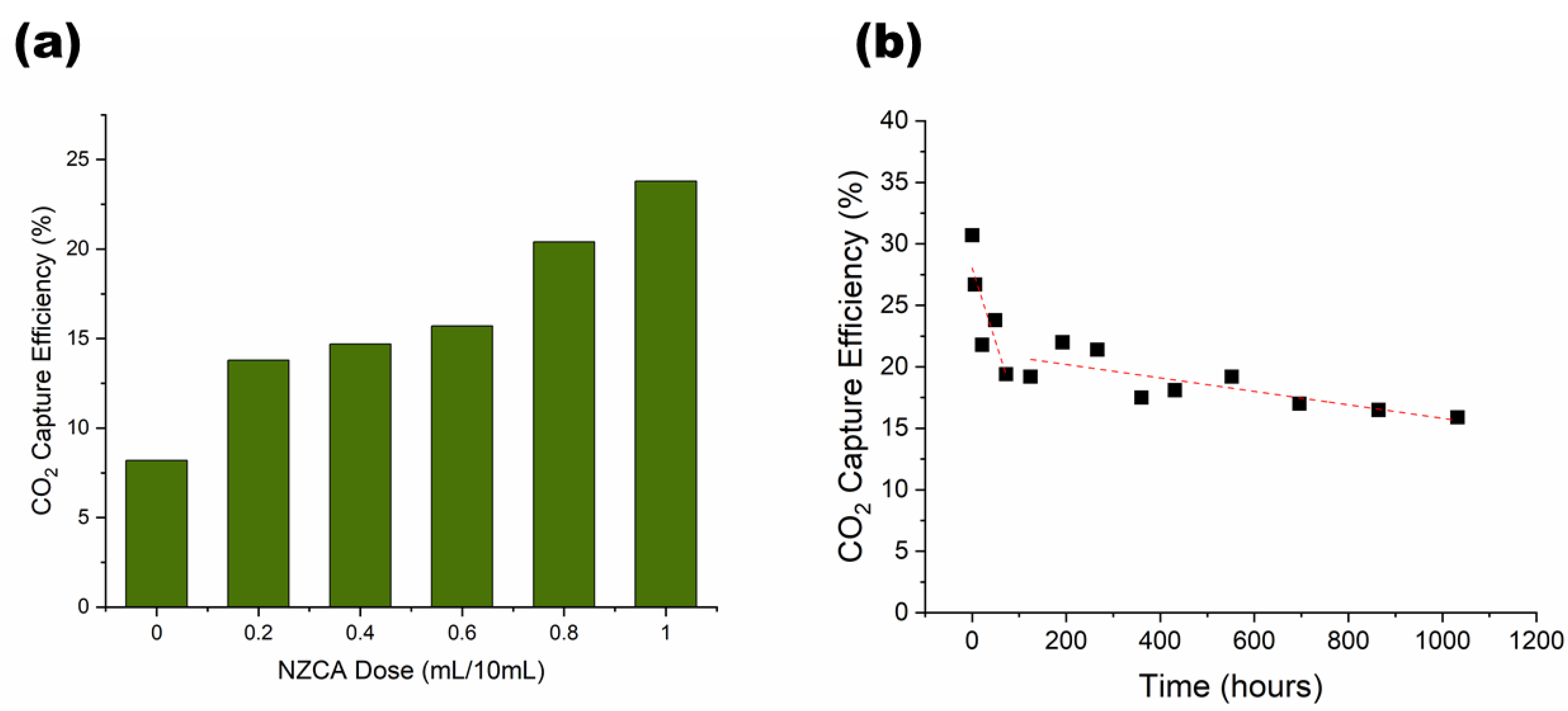
2.4. Esterase Activity Assay of PEG-DA/PEO IPNH Grid
2.5. Laboratory CO2 Scrubber Test of PEG-DA/PEO IPNH Structured Packing
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Extrusion Solutions
4.3. Extrusion Procedures
4.4. Tensile Testing
4.5. Fourier-Transform Infrared Spectroscopy (FTIR)
4.6. Esterase Activity Assay
4.7. Laboratory CO2 Gas Scrubber Test
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lindsey, R. Climate Change: Atmospheric Carbon Dioxide. Available online: https://www.climate.gov/news-features/understanding-climate/climate-change-atmospheric-carbon-dioxide (accessed on 18 July 2022).
- Lenton, T.; Rockström, J.; Gaffney, O.; Rahmstorf, S.; Richardson, K.; Steffen, W.; Shellnhuber, H.J. Climate Tipping Points—Too Risky to Bet Against. Nature 2019, 575, 592–595. [Google Scholar] [CrossRef] [PubMed]
- IEA Greenhouse Gas Emissions from Energy: Overview. Available online: https://www.iea.org/reports/greenhouse-gas-emissions-from-energy-overview (accessed on 23 March 2023).
- Bettenhausen, C.A. Carbon Capture’s Steep Climb. Chem. Eng. News 2021, 99, 28–35. [Google Scholar] [CrossRef]
- Reardon, J.; Bucholz, T.; Hulvey, M.; Tuttle, J.; Shaffer, A.; Pulvirenti, D.; Weber, L.; Killian, K.; Zaks, A. Low Energy CO2 Capture Enabled by Biocatalyst Delivery System. Energy Procedia 2014, 63, 301–321. [Google Scholar] [CrossRef]
- Salmon, S.; House, A. Enzyme-Catalyzed Solvents for CO2 Separation. In Novel Materials for Carbon Dioxide Mitigation Technology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 23–86. [Google Scholar]
- Qi, G.; Liu, K.; House, A.; Salmon, S.; Ambedkar, B.; Frimpong, R.A.; Remias, J.E.; Liu, K. Laboratory to Bench-Scale Evaluation of an Integrated CO2 Capture System Using a Thermostable Carbonic Anhydrase Promoted K2CO3 Solvent with Low Temperature Vacuum Stripping. Appl. Energy 2018, 209, 180–189. [Google Scholar] [CrossRef]
- Molina-Fernández, C.; Luis, P. Immobilization of Carbonic Anhydrase for CO2 Capture and Its Industrial Implementation: A Review. J. CO2 Util. 2021, 47, 101475. [Google Scholar] [CrossRef]
- Shen, J.; Salmon, S. Biocatalytic Membranes for Carbon Capture and Utilization. Membranes 2023, 13, 367. [Google Scholar] [CrossRef]
- Rasouli, H.; Nguyen, K.; Iliuta, M.C. Recent Advancements in Carbonic Anhydrase Immobilization and Its Implementation in CO2 Capture Technologies: A Review. Sep. Purif. Technol. 2022, 296, 121299. [Google Scholar] [CrossRef]
- Russo, M.E.; Capasso, C.; Marzocchella, A.; Salatino, P. Immobilization of Carbonic Anhydrase for CO2 Capture and Utilization. Appl. Microbiol. Biotechnol. 2022, 106, 3419–3430. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Yuan, Y.; Salmon, S. Carbonic Anhydrase Immobilized on Textile Structured Packing Using Chitosan Entrapment for CO2 Capture. ACS Sustain. Chem. Eng. 2022, 10, 7772–7785. [Google Scholar] [CrossRef]
- Shen, J.; Yuan, Y.; Salmon, S. Durable and Versatile Immobilized Carbonic Anhydrase on Textile Structured Packing for CO2 Capture. Catalysts 2022, 12, 1108. [Google Scholar] [CrossRef]
- Shao, Y.; Liao, Z.; Gao, B.; He, B. Emerging 3D Printing Strategies for Enzyme Immobilization: Materials, Methods, and Applications. ACS Omega 2022, 7, 11530–11543. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhang, S.; Fang, X.; Salmon, S. Advances in 3D Gel Printing for Enzyme Immobilization. Gels 2022, 8, 460. [Google Scholar] [CrossRef]
- Pose-Boirazian, T.; Martínez-Costas, J.; Eibes, G. 3D Printing: An Emerging Technology for Biocatalyst Immobilization. Macromol. Biosci. 2022, 2200110, 2200110. [Google Scholar] [CrossRef] [PubMed]
- Devillard, C.D.; Mandon, C.A.; Lambert, S.A.; Blum, L.J.; Marquette, C.A. Bioinspired Multi-Activities 4D Printing Objects: A New Approach Toward Complex Tissue Engineering. Biotechnol. J. 2018, 13, e1800098. [Google Scholar] [CrossRef] [PubMed]
- Mandon, C.A.; Blum, L.J.; Marquette, C.A. 3D-4D Printed Objects: New Bioactive Material Opportunities. Micromachines 2017, 8, 102. [Google Scholar] [CrossRef]
- Mandon, C.A.; Blum, L.J.; Marquette, C.A. Adding Biomolecular Recognition Capability to 3D Printed Objects. Anal. Chem. 2016, 88, 10767–10772. [Google Scholar] [CrossRef] [PubMed]
- Saadi, M.A.S.R.; Maguire, A.; Pottackal, N.; Thakur, M.S.H.; Ikram, M.M.; Hart, A.J.; Ajayan, P.M.; Rahman, M.M. Direct Ink Writing: A 3D Printing Technology for Diverse Materials. Adv. Mater. 2022, 2108855, 2108855. [Google Scholar] [CrossRef]
- Schmieg, B.; Schimek, A.; Franzreb, M. Development and Performance of a 3D-Printable Poly(Ethylene Glycol) Diacrylate Hydrogel Suitable for Enzyme Entrapment and Long-Term Biocatalytic Applications. Eng. Life Sci. 2018, 18, 659–667. [Google Scholar] [CrossRef]
- Schmieg, B.; Döbber, J.; Kirschhöfer, F.; Pohl, M.; Franzreb, M. Advantages of Hydrogel-Based 3D-Printed Enzyme Reactors and Their Limitations for Biocatalysis. Front. Bioeng. Biotechnol. 2019, 6, 211. [Google Scholar] [CrossRef]
- Steier, A.; Schmieg, B.; Irtel von Brenndorff, Y.; Meier, M.; Nirschl, H.; Franzreb, M.; Lahann, J. Enzyme Scaffolds with Hierarchically Defined Properties via 3D Jet Writing. Macromol. Biosci. 2020, 20, e2000154. [Google Scholar] [CrossRef]
- Shen, X.; Yang, M.; Cui, C.; Cao, H. In Situ Immobilization of Glucose Oxidase and Catalase in a Hybrid Interpenetrating Polymer Network by 3D Bioprinting and Its Application. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 568, 411–418. [Google Scholar] [CrossRef]
- Liu, J.; Shen, X.; Zheng, Z.; Li, M.; Zhu, X.; Cao, H.; Cui, C. Immobilization of Laccase by 3D Bioprinting and Its Application in the Biodegradation of Phenolic Compounds. Int. J. Biol. Macromol. 2020, 164, 518–525. [Google Scholar] [CrossRef]
- Sperling, L.H.; Hu, R. Interpenetrating Polymer Networks. In Polymer Blends Handbook; Springer: Berlin/Heidelberg, Germany, 2014; pp. 677–724. [Google Scholar] [CrossRef]
- Farooq, U.; Teuwen, J.; Dransfeld, C. Toughening of Epoxy Systems with Interpenetrating Polymer Network (IPN): A Review. Polymers 2020, 12, 1908. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bowman, J.; Tu, S.; Nykypanchuk, D.; Kuksenok, O.; Minko, S. Polyethylene Glycol Crowder’s Effect on Enzyme Aggregation, Thermal Stability, and Residual Catalytic Activity. Langmuir 2021, 37, 8474–8485. [Google Scholar] [CrossRef] [PubMed]
- Chapman, R.; Stenzel, M.H. All Wrapped up: Stabilization of Enzymes within Single Enzyme Nanoparticles. J. Am. Chem. Soc. 2019, 141. [Google Scholar] [CrossRef] [PubMed]
- Pérez, B.; Coletta, A.; Pedersen, J.N.; Petersen, S.V.; Periole, X.; Pedersen, J.S.; Sessions, R.B.; Guo, Z.; Perriman, A.; Schiøtt, B. Insight into the Molecular Mechanism behind PEG-Mediated Stabilization of Biofluid Lipases. Sci. Rep. 2018, 8, 12293. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Domach, M.; Auger, R.; Yang, F.X.; Russell, A.J. Polyethylene Glycol-Induced Stabilization of Subtilisin. Enzyme Microb. Technol. 1996, 18, 82–89. [Google Scholar] [CrossRef]
- Krishnamurthy, V.M.; Kaufman, G.K.; Urbach, A.R.; Gitlin, I.; Gudiksen, K.L.; Weibel, D.B.; Whitesides, G.M. Carbonic Anhydrase as a Model for Biophysical and Physical-Organic Studies of Proteins and Protein−Ligand Binding. Chem. Rev. 2008, 108, 946–1051. [Google Scholar] [CrossRef] [PubMed]
- Pramono, E.; Utomo, S.B.; Wulandari, V.; Clegg, F. FTIR Studies on the Effect of Concentration of Polyethylene Glycol on Polimerization of Shellac. J. Phys. Conf. Ser. 2016, 776, 012053. [Google Scholar] [CrossRef]
- Askari, F.; Zandi, M.; Shokrolahi, P.; Tabatabaei, M.H.; Hajirasoliha, E. Reduction in Protein Absorption on Ophthalmic Lenses by PEGDA Bulk Modification of Silicone Acrylate-Based Formulation. Prog. Biomater. 2019, 8, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Worzakowska, M. TG/DSC/FTIR/QMS Studies on the Oxidative Decomposition of Terpene Acrylate Homopolymers. J. Therm. Anal. Calorim. 2017, 127, 2025–2035. [Google Scholar] [CrossRef]
- Herrera-González, A.M.; Caldera-Villalobos, M.; Pérez-Mondragón, A.A.; Cuevas-Suárez, C.E.; González-López, J.A. Analysis of Double Bond Conversion of Photopolymerizable Monomers by FTIR-ATR Spectroscopy. J. Chem. Educ. 2019, 96, 1786–1789. [Google Scholar] [CrossRef]
- Peirce, S.; Perfetto, R.; Russo, M.E.; Capasso, C.; Rossi, M.; Salatino, P.; Marzocchella, A. Characterization of Technical Grade Carbonic Anhydrase as Biocatalyst for CO2 Capture in Potassium Carbonate Solutions. Greenh. Gases Sci. Technol. 2018, 8, 279–291. [Google Scholar] [CrossRef]
- Seki, T.; Chiang, K.Y.; Yu, C.C.; Yu, X.; Okuno, M.; Hunger, J.; Nagata, Y.; Bonn, M. The Bending Mode of Water: A Powerful Probe for Hydrogen Bond Structure of Aqueous Systems. J. Phys. Chem. Lett. 2020, 11, 8459–8469. [Google Scholar] [CrossRef] [PubMed]
- Medders, G.R.; Paesani, F. Infrared and Raman Spectroscopy of Liquid Water through “First-Principles” Many-Body Molecular Dynamics. J. Chem. Theory Comput. 2015, 11, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Sadat, A.; Joye, I.J. Peak Fitting Applied to Fourier Transform Infrared and Raman Spectroscopic Analysis of Proteins. Appl. Sci. 2020, 10, 5918. [Google Scholar] [CrossRef]
- Hua, J.; Ng, P.F.; Fei, B. High-Strength Hydrogels: Microstructure Design, Characterization and Applications. J. Polym. Sci. Part B Polym. Phys. 2018, 56, 1325–1335. [Google Scholar] [CrossRef]
- Xiang, C.; Zhang, X.; Zhang, J.; Chen, W.; Li, X.; Wei, X.; Li, P. A Porous Hydrogel with High Mechanical Strength and Biocompatibility for Bone Tissue Engineering. J. Funct. Biomater. 2022, 13, 140. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Kim, J. Recent Strategies for Strengthening and Stiffening Tough Hydrogels. Adv. NanoBiomed Res. 2021, 1, 2100026. [Google Scholar] [CrossRef]
- Sun, J.; Wang, C.; Wang, Y.; Ji, S.; Liu, W. Immobilization of Carbonic Anhydrase on Polyethylenimine/Dopamine Codeposited Membranes. J. Appl. Polym. Sci. 2019, 136, 47784. [Google Scholar] [CrossRef]
- Cui, J.; Feng, Y.; Jia, S. Silica Encapsulated Catalase@metal-Organic Framework Composite: A Highly Stable and Recyclable Biocatalyst. Chem. Eng. J. 2018, 351, 506–514. [Google Scholar] [CrossRef]
- Park, J.-M.; Kim, M.; Park, H.-S.; Jang, A.; Min, J.; Kim, Y.-H. Immobilization of Lysozyme-CLEA onto Electrospun Chitosan Nanofiber for Effective Antibacterial Applications. Int. J. Biol. Macromol. 2013, 54, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Li, C.; Tan, Z.; Hou, Y.; Jia, S.; Cui, J. Carbonic Anhydrase@ZIF-8 Hydrogel Composite Membrane with Improved Recycling and Stability for Efficient CO2 Capture. J. Agric. Food Chem. 2019, 67, 3372–3379. [Google Scholar] [CrossRef]
- Sahoo, P.C.; Kumar, M.; Singh, A.; Singh, M.P.; Puri, S.K.; Ramakumar, S.S.V. Accelerated CO2 Capture in Hybrid Solvent Using Co-Immobilized Enzyme/Complex on a Hetero-Functionalized Support. J. CO2 Util. 2017, 21, 77–81. [Google Scholar] [CrossRef]
- Hou, J.; Dong, G.; Xiao, B.; Malassigne, C.; Chen, V. Preparation of Titania Based Biocatalytic Nanoparticles and Membranes for CO2 Conversion. J. Mater. Chem. A 2015, 3, 3332–3342. [Google Scholar] [CrossRef]
- Fabbricino, S.; Del Prete, S.; Russo, M.E.; Capasso, C.; Marzocchella, A.; Salatino, P. In Vivo Immobilized Carbonic Anhydrase and Its Effect on the Enhancement of CO2 Absorption Rate. J. Biotechnol. 2021, 336, 41–49. [Google Scholar] [CrossRef]
- Leimbrink, M.; Nikoleit, K.G.; Spitzer, R.; Salmon, S.; Bucholz, T.; Górak, A.; Skiborowski, M. Enzymatic Reactive Absorption of CO2 in MDEA by Means of an Innovative Biocatalyst Delivery System. Chem. Eng. J. 2018, 334, 1195–1205. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, Y.; Bilheux, H.; Salmon, S. Biocatalytic Yarn for Peroxide Decomposition with Controlled Liquid Transport. Adv. Mater. Interfaces 2021, 8, 2002104. [Google Scholar] [CrossRef]
- Hou, J.; Ji, C.; Dong, G.; Xiao, B.; Ye, Y.; Chen, V. Biocatalytic Janus Membranes for CO2 Removal Utilizing Carbonic Anhydrase. J. Mater. Chem. A 2015, 3, 17032–17041. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, J.; Zhang, S.; Fang, X.; Salmon, S. Carbonic Anhydrase Enhanced UV-Crosslinked PEG-DA/PEO Extruded Hydrogel Flexible Filaments and Durable Grids for CO2 Capture. Gels 2023, 9, 341. https://doi.org/10.3390/gels9040341
Shen J, Zhang S, Fang X, Salmon S. Carbonic Anhydrase Enhanced UV-Crosslinked PEG-DA/PEO Extruded Hydrogel Flexible Filaments and Durable Grids for CO2 Capture. Gels. 2023; 9(4):341. https://doi.org/10.3390/gels9040341
Chicago/Turabian StyleShen, Jialong, Sen Zhang, Xiaomeng Fang, and Sonja Salmon. 2023. "Carbonic Anhydrase Enhanced UV-Crosslinked PEG-DA/PEO Extruded Hydrogel Flexible Filaments and Durable Grids for CO2 Capture" Gels 9, no. 4: 341. https://doi.org/10.3390/gels9040341
APA StyleShen, J., Zhang, S., Fang, X., & Salmon, S. (2023). Carbonic Anhydrase Enhanced UV-Crosslinked PEG-DA/PEO Extruded Hydrogel Flexible Filaments and Durable Grids for CO2 Capture. Gels, 9(4), 341. https://doi.org/10.3390/gels9040341







