Phospholipid-Based Topical Nano-Hydrogel of Mangiferin: Enhanced Topical Delivery and Improved Dermatokinetics
Abstract
1. Introduction
2. Results and Discussion
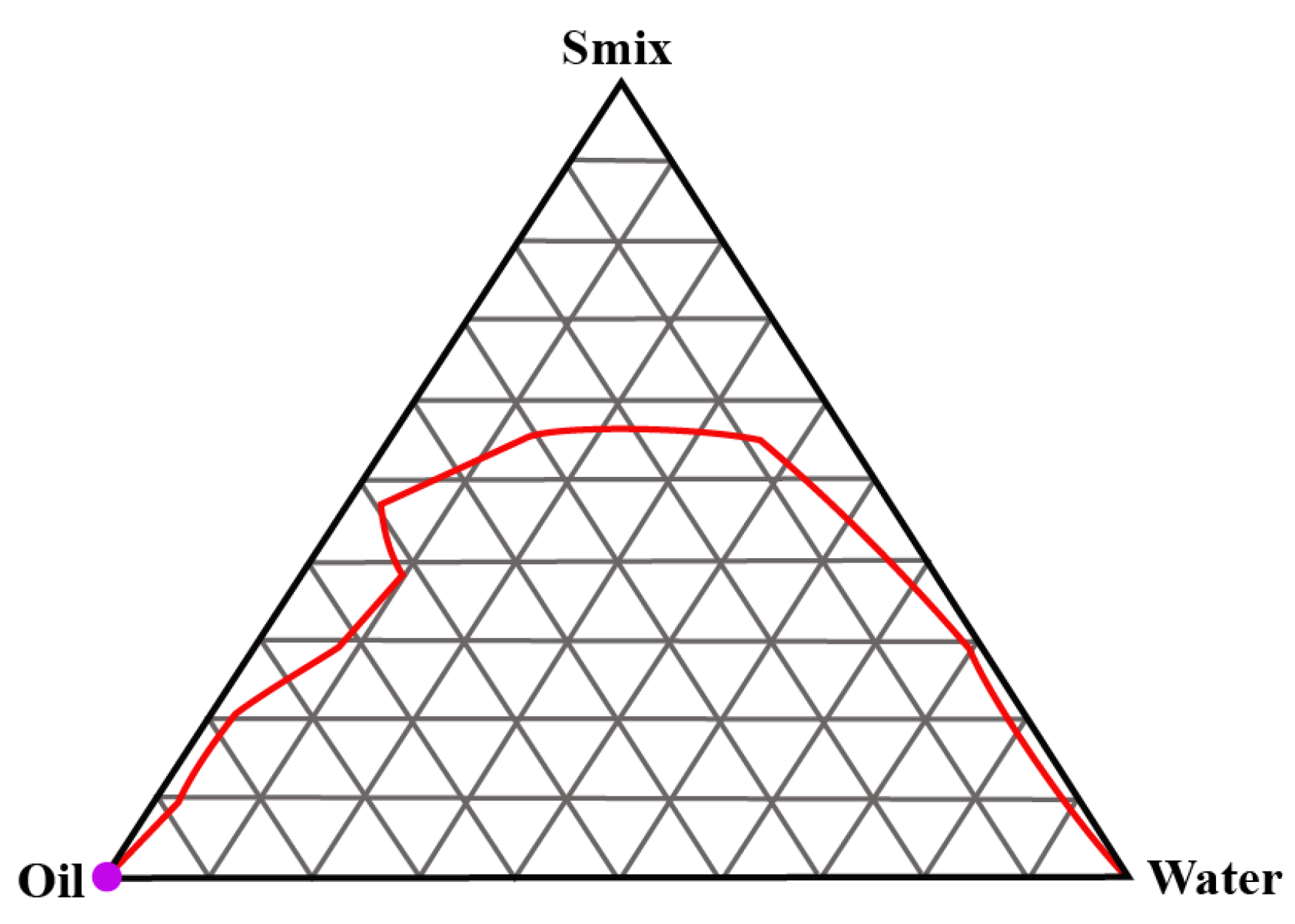
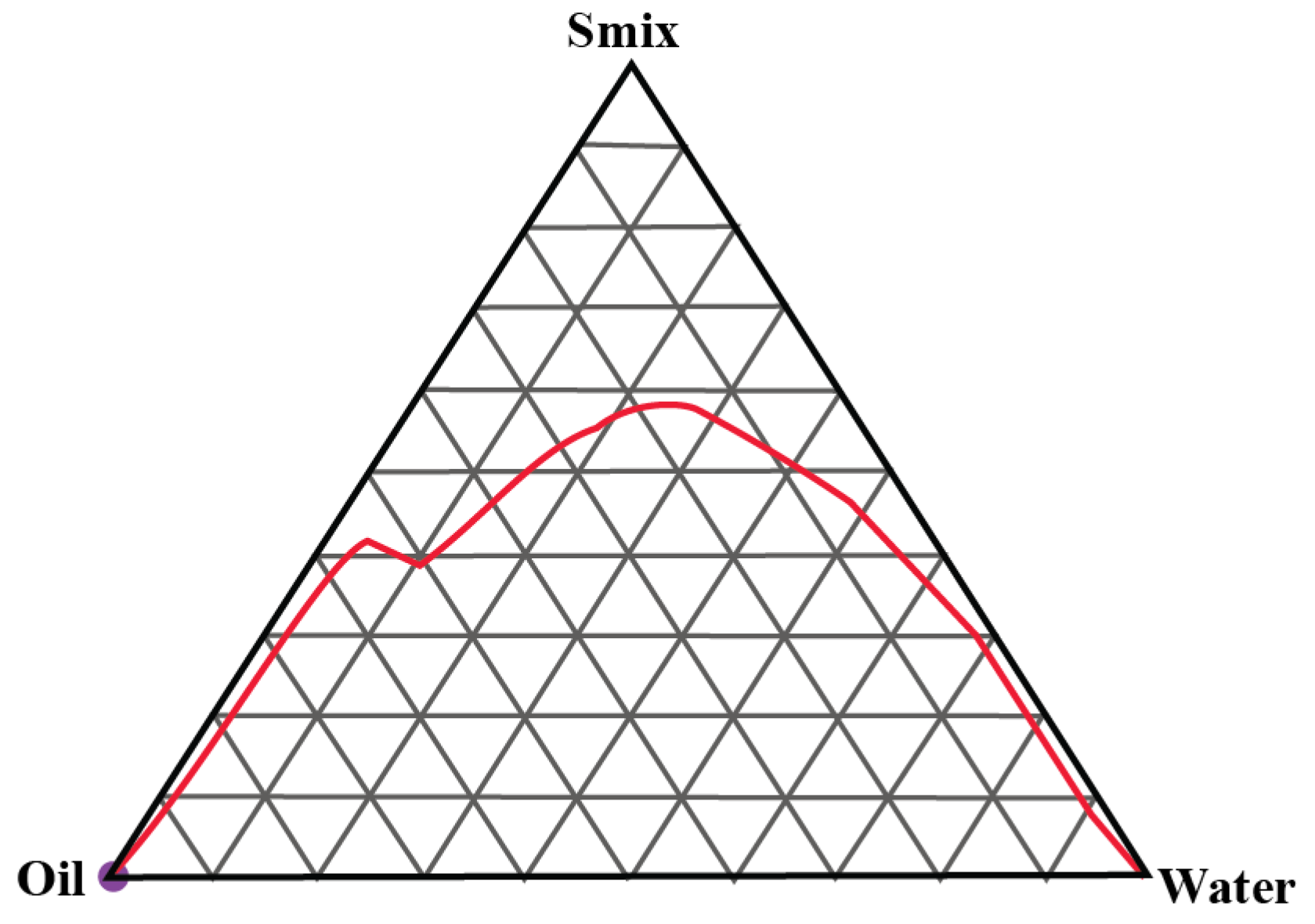
2.1. Construction of Pseudo-Ternary Phase Diagrams
2.2. Optimisation of the Microemulsion Composition
2.3. Polydispersity, pH, Zeta-Potential and Morphology
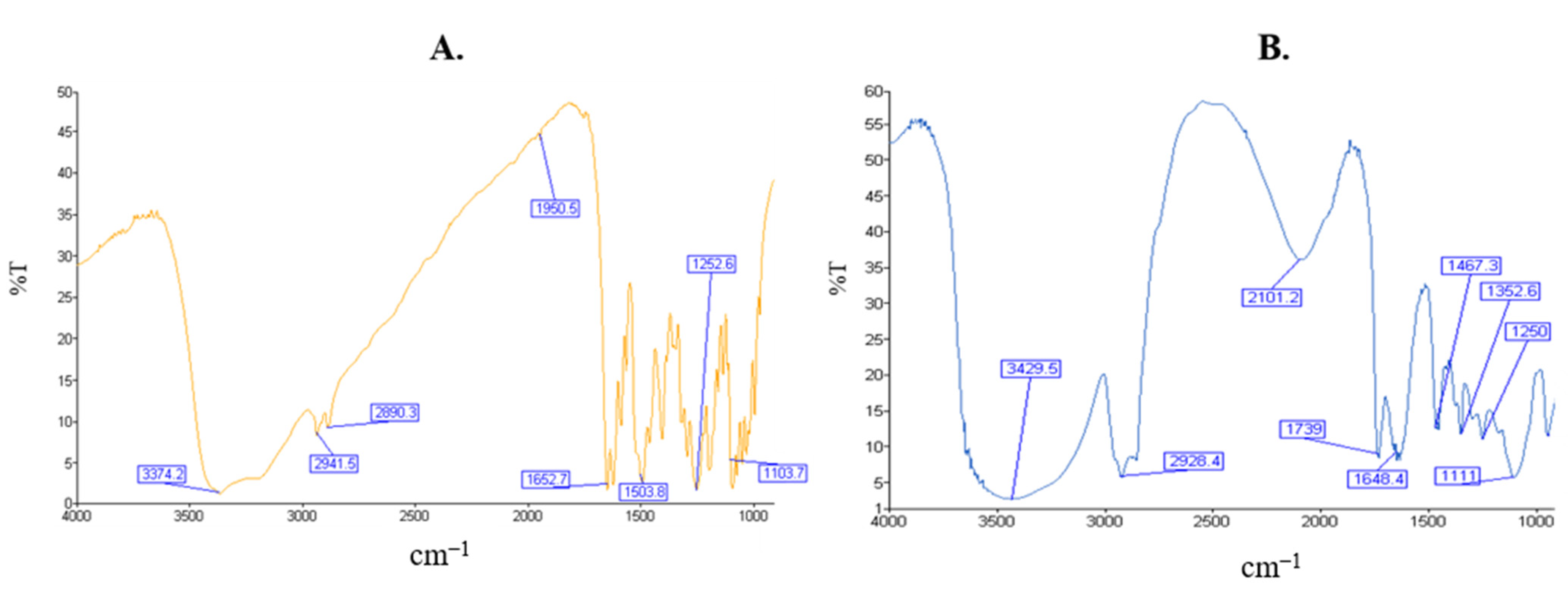
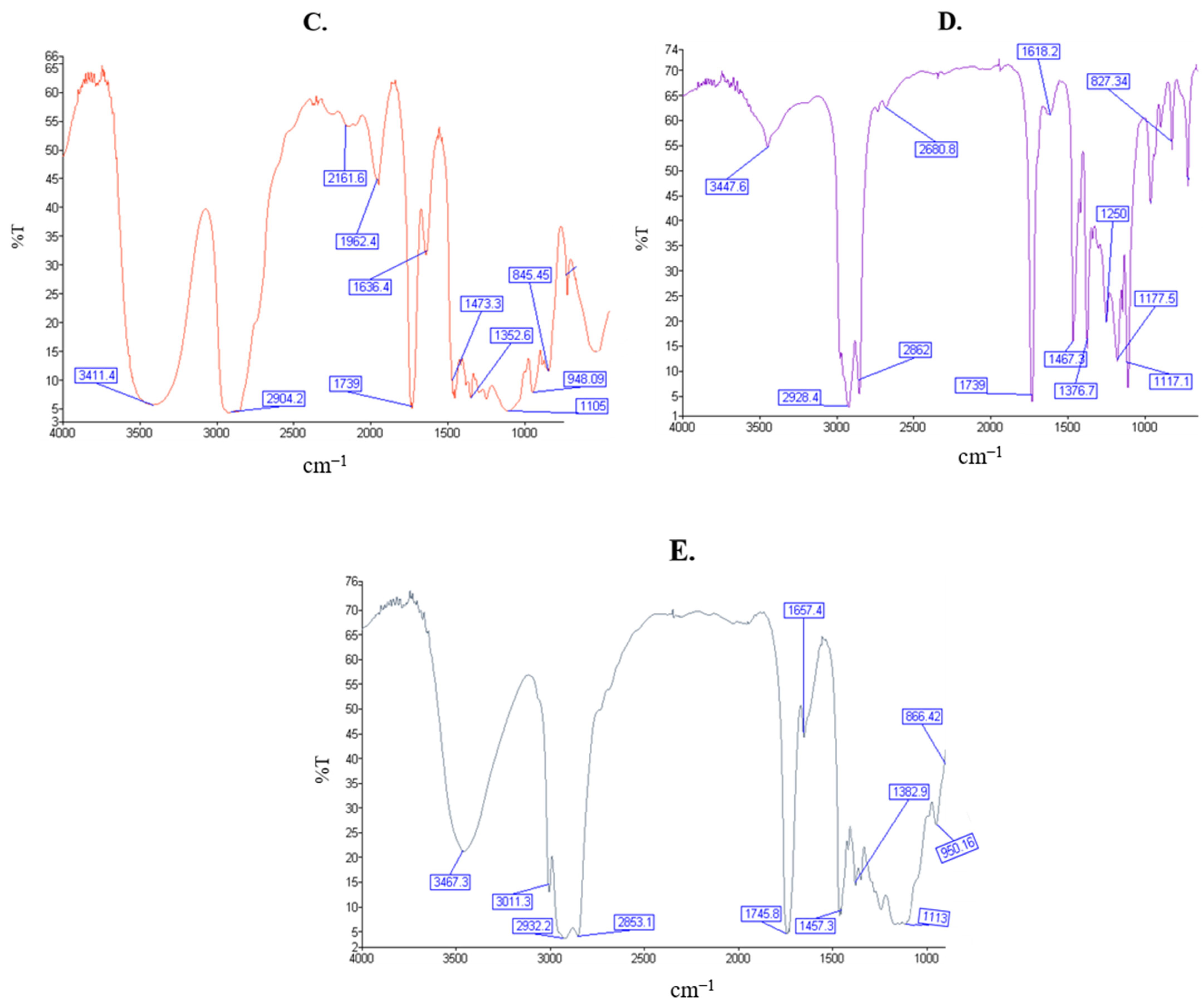
2.4. Fourier Transform Infrared Spectroscopy
2.5. Incorporation in the Hydrogel
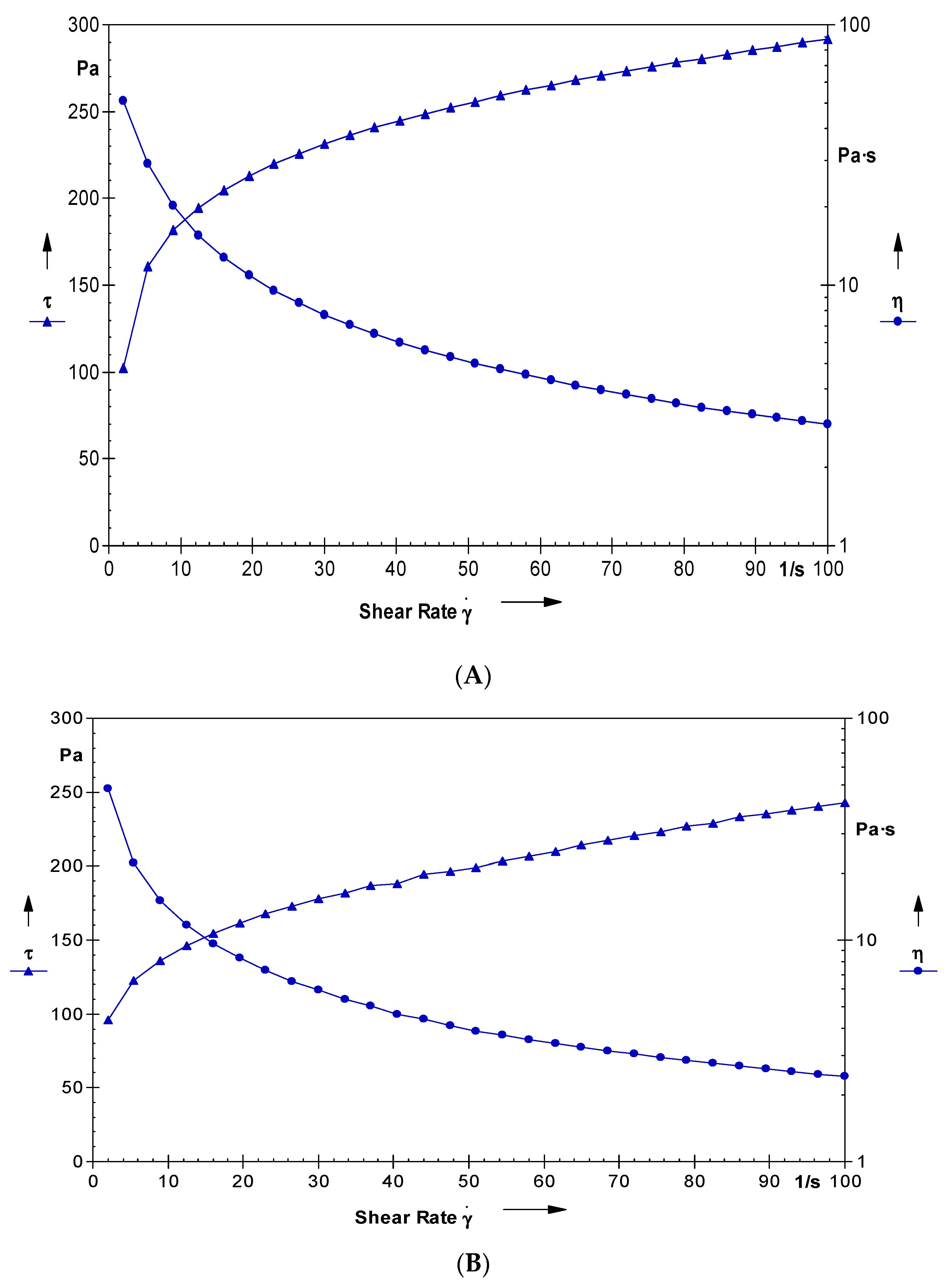
2.6. Rheological Studies of the Nano-Hydrogel
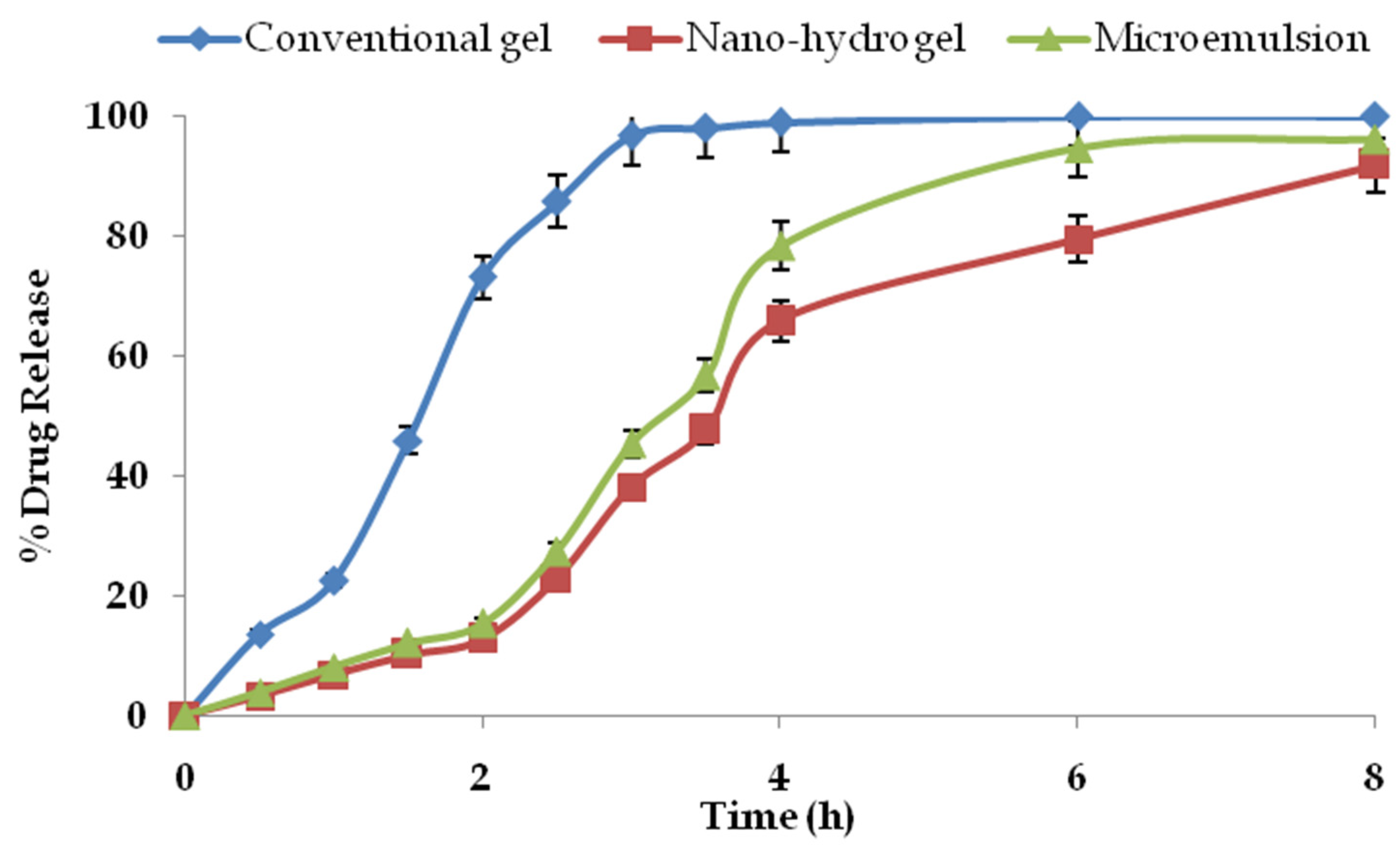
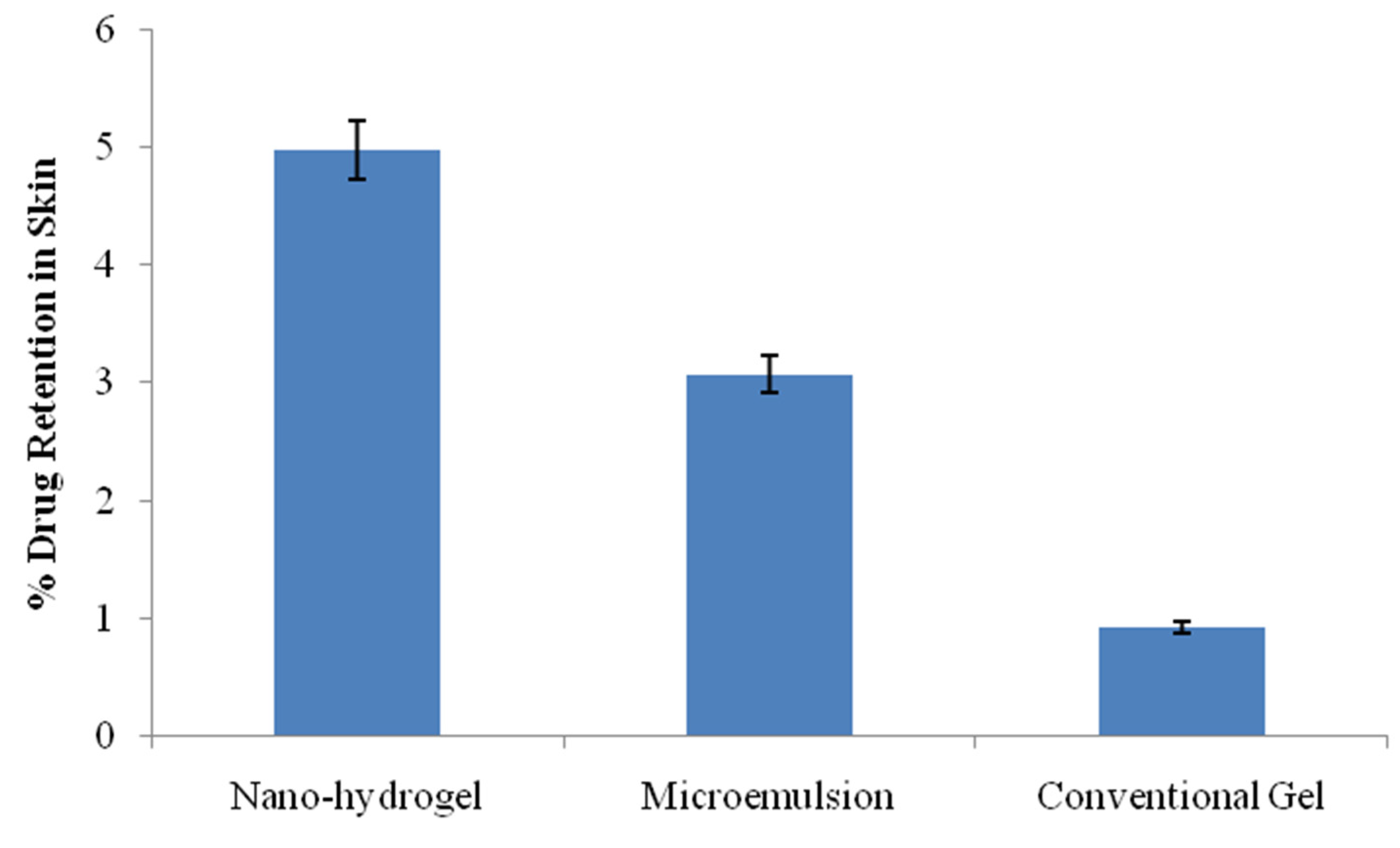
2.7. Drug Permeation and Drug Retention Studies
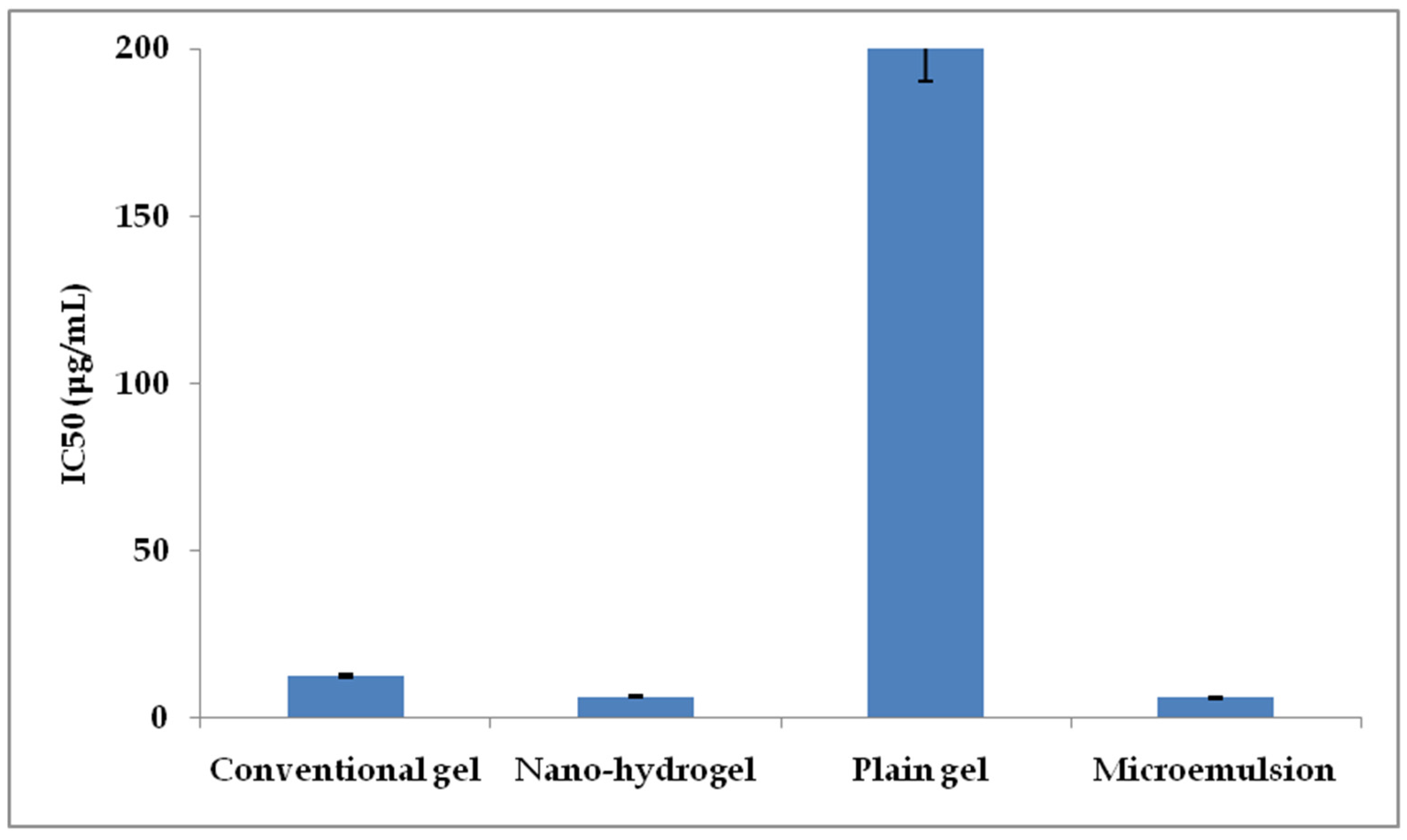
2.8. Cancer Cell Viability
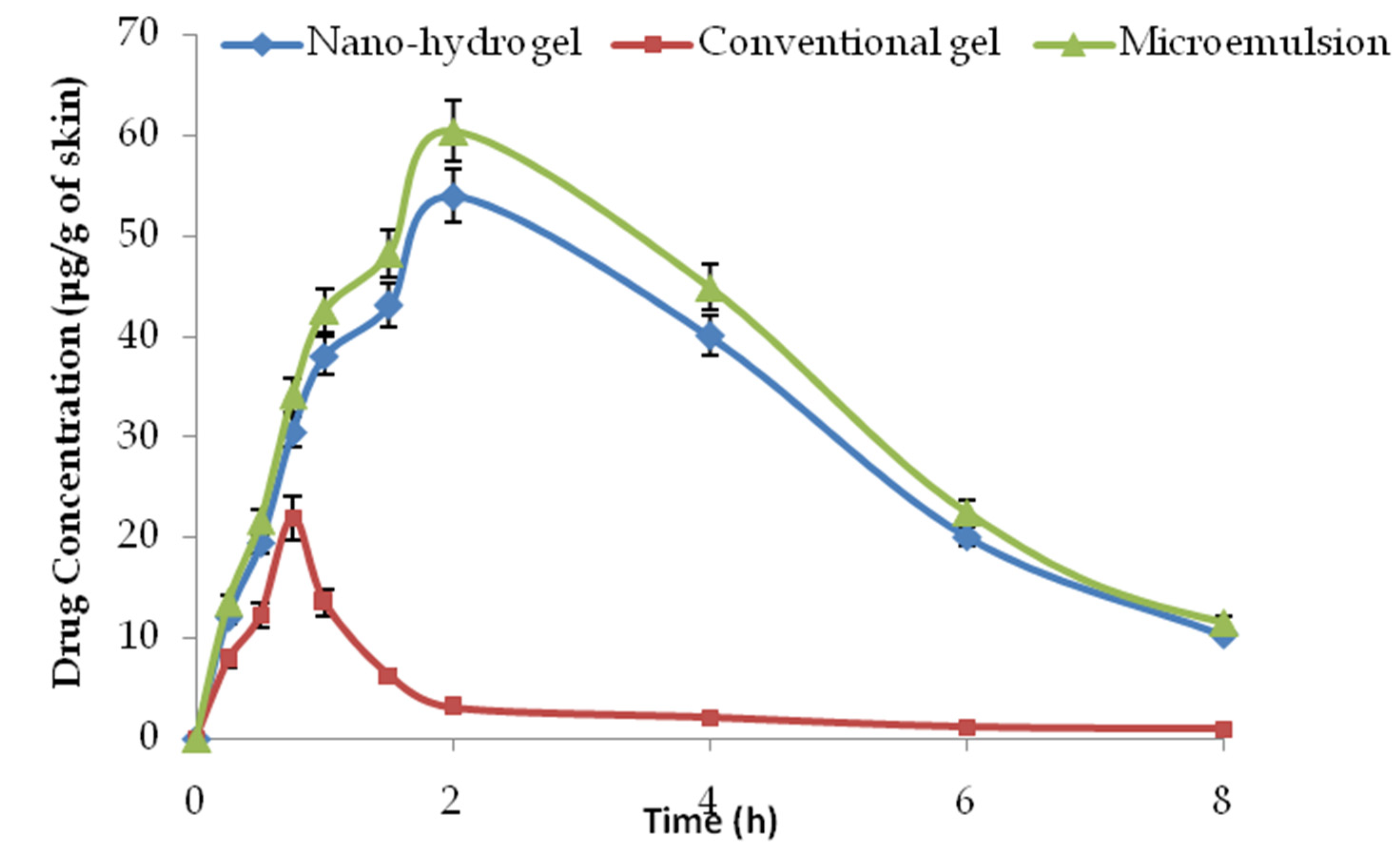
2.9. Dermatokinetic Studies
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Construction of Pseudo-Ternary Phase Diagrams
4.2.2. Optimisation of the Microemulsion Composition
4.2.3. Determination of Drug Entrapment Efficiency and Drug Loading
4.2.4. Micromeritics, pH, Zeta-Potential and Morphology
4.2.5. Fourier Transform Infrared Spectroscopy
4.2.6. Incorporation of the Nanosystem in Hydrogel
4.2.7. Rheology of the Nano-Hydrogel
4.2.8. Ex Vivo Drug Permeation and Drug Retention Studies
4.2.9. Cancer Cell Viability Assay and Normal Cell Safety
4.2.10. Dermal Pharmacokinetic Studies
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akter, S.; Moni, A.; Faisal, G.M.; Uddin, M.R.; Jahan, N.; Hannan, A.; Rahman, A.; Uddin, J. Renoprotective Effects of Mangiferin: Pharmacological Advances and Future Perspectives. Int. J. Environ. Res. Public Health 2022, 19, 1864. [Google Scholar] [CrossRef]
- Wang, M.; Liang, Y.; Chen, K.; Wang, M.; Long, X.; Liu, H.; Sun, Y.; He, B. The management of diabetes mellitus by mangiferin: Advances and prospects. Nanoscale 2022, 14, 2119–2135. [Google Scholar] [CrossRef] [PubMed]
- Yap, K.M.; Sekar, M.; Seow, L.J.; Gan, S.H.; Bonam, S.R.; Rani, N.N.I.M.; Lum, P.T.; Subramaniyan, V.; Wu, Y.S.; Fuloria, N.K.; et al. Mangifera indica (Mango): A Promising Medicinal Plant for Breast Cancer Therapy and Understanding Its Potential Mechanisms of Action. Breast Cancer Targets Ther. 2021, 13, 471–503. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Arshad, M.S.; Butt, M.S.; Kwon, J.-H.; Arshad, M.U.; Sultan, M.T. Mangiferin: A natural miracle bioactive compound against lifestyle related disorders. Lipids Health Dis. 2017, 16, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.; Alqarni, M.H.; Foudah, A.I.; Raish, M.; Salkini, M.A. Babchi Oil-Based Nanoemulsion Hydrogel for the Management of Psoriasis: A Novel Energy Economic Approach Employing Biosurfactants. Gels 2022, 8, 761. [Google Scholar] [CrossRef]
- Alam, A.; Foudah, A.I.; Alqarni, M.H.; Yusufoglu, H.S. Microwave-assisted and chemically tailored chlorogenic acid-functionalized silver nanoparticles of Citrus sinensis in gel matrix aiding QbD design for the treatment of acne. J. Cosmet. Dermatol. 2023. [CrossRef]
- Singh, R.S.P.; Paul, R.K.; Raza, K.; Mukker, J.K. Pharmacokinetics and pharmacodynamics of nanopharmaceuticals. In Multifunctional Nanocarriers; Elsevier: Amsterdam, The Netherlands, 2022; pp. 443–459. [Google Scholar] [CrossRef]
- Paul, R.K.; Kesharwani, P.; Raza, K. Recent update on nano-phytopharmaceuticals in the management of diabetes. J. Biomater. Sci. Polym. Ed. 2021, 32, 2046–2068. [Google Scholar] [CrossRef]
- Liu, M.; Liu, Y.; Ge, Y.; Zhong, Z.; Wang, Z.; Wu, T.; Zhao, X.; Zu, Y. Solubility, Antioxidation, and Oral Bioavailability Improvement of Mangiferin Microparticles Prepared Using the Supercritical Antisolvent Method. Pharmaceutics 2020, 12, 90. [Google Scholar] [CrossRef]
- Harsha, P.; Thotakura, N.; Kumar, M.; Sharma, S.; Mittal, A.; Khurana, R.K.; Singh, B.; Negi, P.; Raza, K. A novel PEGylated carbon nanotube conjugated mangiferin: An explorative nanomedicine for brain cancer cells. J. Drug Deliv. Sci. Technol. 2019, 53, 101186. [Google Scholar] [CrossRef]
- Zhu, Y.; Ye, J.; Zhang, Q. Self-emulsifying Drug Delivery System Improve Oral Bioavailability: Role of Excipients and Physico-chemical Characterization. Pharm. Nanotechnol. 2020, 8, 290–301. [Google Scholar] [CrossRef]
- Mao, X.; Liu, L.; Cheng, L.; Cheng, R.; Zhang, L.; Deng, L.; Sun, X.; Zhang, Y.; Sarmento, B.; Cui, W. Adhesive nanoparticles with inflammation regulation for promoting skin flap regeneration. J. Control. Release 2019, 297, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Cheng, R.; Zhang, H.; Bae, J.; Cheng, L.; Zhang, L.; Deng, L.; Cui, W.; Zhang, Y.; Santos, H.A.; et al. Self-Healing and Injectable Hydrogel for Matching Skin Flap Regeneration. Adv. Sci. 2018, 6, 1801555. [Google Scholar] [CrossRef] [PubMed]
- Khurana, R.K.; Gaspar, B.L.; Welsby, G.; Katare, O.P.; Singh, K.K.; Singh, B. Improving the biopharmaceutical attributes of mangiferin using vitamin E-TPGS co-loaded self-assembled phosholipidic nano-mixed micellar systems. Drug Deliv. Transl. Res. 2018, 8, 617–632. [Google Scholar] [CrossRef] [PubMed]
- Santonocito, D.; Vivero-Lopez, M.; Lauro, M.R.; Torrisi, C.; Castelli, F.; Sarpietro, M.G.; Puglia, C. Design of Nanotechnological Carriers for Ocular Delivery of Mangiferin: Preformulation Study. Molecules 2022, 27, 1328. [Google Scholar] [CrossRef]
- Katare, O.P.; Raza, K.; Singh, B.; Dogra, S. Novel drug delivery systems in topical treatment of psoriasis: Rigors and vigors. Indian J. Dermatol. Venereol. Leprol. 2010, 76, 612–621. [Google Scholar] [CrossRef]
- Raza, K.; Kumar, M.; Kumar, P.; Malik, R.; Sharma, G.; Kaur, M.; Katare, O.P. Topical Delivery of Aceclofenac: Challenges and Promises of Novel Drug Delivery Systems. BioMed Res. Int. 2014, 2014, 406731. [Google Scholar] [CrossRef]
- Raza, K.; Negi, P.; Takyar, S.; Shukla, A.; Amarji, B.; Katare, O.P. Novel dithranol phospholipid microemulsion for topical application: Development, characterization and percutaneous absorption studies. J. Microencapsul. 2011, 28, 190–199. [Google Scholar] [CrossRef]
- Patel, M.R.; Patel, R.; Parikh, J.R.; Patel, B.G. Novel isotretinoin microemulsion-based gel for targeted topical therapy of acne: Formulation consideration, skin retention and skin irritation studies. Appl. Nanosci. 2015, 6, 539–553. [Google Scholar] [CrossRef]
- Pleguezuelos-Villa, M.; Nacher, A.; Hernández, M.J.; Buso, M.O.V.; Sauri, A.R.; Díez-Sales, O. Mangiferin nanoemulsions in treatment of inflammatory disorders and skin regeneration. Int. J. Pharm. 2019, 564, 299–307. [Google Scholar] [CrossRef]
- Negi, J.S. Nanolipid Materials for Drug Delivery Systems: A Comprehensive Review. In Characterization and Biology of Nanomaterials for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 137–163. [Google Scholar] [CrossRef]
- Panigrahi, K.C.; Patra, C.N.; Jena, G.K.; Ghose, D.; Jena, J.; Panda, S.K.; Sahu, M. Gelucire: A versatile polymer for modified release drug delivery system. Futur. J. Pharm. Sci. 2018, 4, 102–108. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Wang, P.-W.; Alalaiwe, A.; Lin, Z.-C.; Fang, J.-Y. Use of Lipid Nanocarriers to Improve Oral Delivery of Vitamins. Nutrients 2019, 11, 68. [Google Scholar] [CrossRef] [PubMed]
- Jianxian, C.; Saleem, K.; Ijaz, M.; Ur-Rehman, M.; Murtaza, G.; Asim, M.H. Development and in vitro Evaluation of Gastro-protective Aceclofenac-loaded Self-emulsifying Drug Delivery System. Int. J. Nanomed. 2020, 15, 5217–5226. [Google Scholar] [CrossRef] [PubMed]
- Lukić, M.; Pantelić, I.; Savić, S. Towards Optimal pH of the Skin and Topical Formulations: From the Current State of the Art to Tailored Products. Cosmetics 2021, 8, 69. [Google Scholar] [CrossRef]
- Raza, K.; Singh, B.; Singal, P.; Wadhwa, S.; Katare, O.P. Systematically optimized biocompatible isotretinoin-loaded solid lipid nanoparticles (SLNs) for topical treatment of acne. Colloids Surf. B Biointerfaces 2013, 105, 67–74. [Google Scholar] [CrossRef]
- Negi, P.; Sharma, I.; Hemrajani, C.; Rathore, C.; Bisht, A.; Raza, K.; Katare, O.P. Thymoquinone-loaded lipid vesicles: A promising nanomedicine for psoriasis. BMC Complement. Altern. Med. 2019, 19, 334. [Google Scholar] [CrossRef]
- Abdullah, A.-S.H.; Mohammed, A.S.; Abdullah, R.; Mirghani, M.E.S.; Al-Qubaisi, M. Cytotoxic effects of Mangifera indica L. kernel extract on human breast cancer (MCF-7 and MDA-MB-231 cell lines) and bioactive constituents in the crude extract. BMC Complement. Altern. Med. 2014, 14, 199. [Google Scholar] [CrossRef]
- Tian, X.; Gao, Y.; Xu, Z.; Lian, S.; Ma, Y.; Guo, X.; Hu, P.; Li, Z.; Huang, C. Pharmacokinetics of mangiferin and its metabolite-Norathyriol, Part 1: Systemic evaluation of hepatic first-pass effect in vitro and in vivo. Biofactors 2016, 42, 533–544. [Google Scholar] [CrossRef]
- Rao, B.; Vidyadhara, S.; Sasidhar, R.; Chowdary, Y. Formulation and Evaluation of Liquid Loaded Tablets Containing Docetaxel-Self Nano Emulsifying Drug Delivery Systems. Trop. J. Pharm. Res. 2015, 14, 567–573. [Google Scholar] [CrossRef]
- Lwin, O.M.; Giribabu, N.; Kilari, E.K.; Salleh, N. Topical administration of mangiferin promotes healing of the wound of streptozotocin-nicotinamide-induced type-2 diabetic male rats. J. Dermatol. Treat. 2021, 32, 1039–1048. [Google Scholar] [CrossRef]
- Pavia, L.P.; Lampman, G.M.; Kriz, G.S.; James, R.V. Introduction to Spectrocopy, 5th ed.; Cengage Publications: Singapore, 2019; pp. 15–106. [Google Scholar]
- Raza, K.; Singh, B.; Lohan, S.; Sharma, G.; Negi, P.; Yachha, Y.; Katare, O.P. Nano-lipoidal carriers of tretinoin with enhanced percutaneous absorption, photostability, biocompatibility and anti-psoriatic activity. Int. J. Pharm. 2013, 456, 65–72. [Google Scholar] [CrossRef]
- Allaw, M.; Pleguezuelos-Villa, M.; Manca, M.L.; Caddeo, C.; Aroffu, M.; Nacher, A.; Diez-Sales, O.; Saurí, A.R.; Ferrer, E.E.; Fadda, A.M.; et al. Innovative strategies to treat skin wounds with mangiferin: Fabrication of transfersomes modified with glycols and mucin. Nanomedicine 2020, 15, 1671–1685. [Google Scholar] [CrossRef]
- Sharma, G.; Goyal, H.; Thakur, K.; Raza, K.; Katare, O.P. Novel elastic membrane vesicles (EMVs) and ethosomes-mediated effective topical delivery of aceclofenac: A new therapeutic approach for pain and inflammation. Drug Deliv. 2016, 23, 3135–3145. [Google Scholar] [CrossRef] [PubMed]
- Raza, K.; Singh, B.; Singla, S.; Wadhwa, S.; Garg, B.; Chhibber, S.; Katare, O.P. Nanocolloidal Carriers of Isotretinoin: Antimicrobial Activity against Propionibacterium acnes and Dermatokinetic Modeling. Mol. Pharm. 2013, 10, 1958–1963. [Google Scholar] [CrossRef] [PubMed]
- Thotakura, N.; Kumar, P.; Wadhwa, S.; Raza, K.; Katare, P. Dermatokinetics as an Important Tool to Assess the Bioavailability of Drugs by Topical Nanocarriers. Curr. Drug Metab. 2017, 18, 404–411. [Google Scholar] [CrossRef] [PubMed]



| Code | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 |
|---|---|---|---|---|---|---|---|---|---|
| Size (nm) | 286.59 | 254.35 | 278.20 | 185.73 | 179.34 | 210.62 | 118.55 | 100.7 | 114.91 |
| % EE | 78.29 | 82.33 | 82.17 | 76.87 | 81.75 | 79.94 | 76.09 | 83.43 | 82.18 |
| % DL | 11.74 | 12.41 | 12.32 | 11.53 | 12.26 | 11.99 | 11.41 | 12.51 | 12.32 |
| Dermatokinetic Parameter | Conventional Gel | Microemulsion | Nano-Hydrogel |
|---|---|---|---|
| Cmax (μg/g of skin) | 21.97 ± 1.98 | 60.45 ± 4.11 | 53.98 ± 5.07 |
| Tmax (h) | 0.75 | 2 | 2 |
| (μg·mL−1·h) | 41.55 ± 9.03 | 361.31 ± 36.13 | 328.46 ± 23.18 |
| Kp (h−1) | 1.91 | 5.01 | 4.67 |
| K(h−1) | 0.43 | 0.28 | 0.29 |
| T1/2 (h−1) | 1.61 | 2.48 | 2.38 |
| Formulation Code | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 |
|---|---|---|---|---|---|---|---|---|---|
| Oil (%) | 12.5 | 15.78 | 22.28 | 12.5 | 15.78 | 22.28 | 12.5 | 15.78 | 22.28 |
| Ternary Phase (Smix) | 1:1 | 1:1 | 1:1 | 2:1 | 2:1 | 2:1 | 3:1 | 3:1 | 3:1 |
| Smix (%) | 43.75 | 42.10 | 38.88 | 58.33 | 56.15 | 51.85 | 65.62 | 63.16 | 58.33 |
| Water (%) | q.s. to 100 | q.s. to 100 | q.s. to 100 | q.s. to 100 | q.s. to 100 | q.s. to 100 | q.s. to 100 | q.s. to 100 | q.s. to 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkholifi, F.K.; Alam, A.; Foudah, A.I.; Yusufoglu, H.S. Phospholipid-Based Topical Nano-Hydrogel of Mangiferin: Enhanced Topical Delivery and Improved Dermatokinetics. Gels 2023, 9, 178. https://doi.org/10.3390/gels9030178
Alkholifi FK, Alam A, Foudah AI, Yusufoglu HS. Phospholipid-Based Topical Nano-Hydrogel of Mangiferin: Enhanced Topical Delivery and Improved Dermatokinetics. Gels. 2023; 9(3):178. https://doi.org/10.3390/gels9030178
Chicago/Turabian StyleAlkholifi, Faisal K., Aftab Alam, Ahmed I. Foudah, and Hasan S. Yusufoglu. 2023. "Phospholipid-Based Topical Nano-Hydrogel of Mangiferin: Enhanced Topical Delivery and Improved Dermatokinetics" Gels 9, no. 3: 178. https://doi.org/10.3390/gels9030178
APA StyleAlkholifi, F. K., Alam, A., Foudah, A. I., & Yusufoglu, H. S. (2023). Phospholipid-Based Topical Nano-Hydrogel of Mangiferin: Enhanced Topical Delivery and Improved Dermatokinetics. Gels, 9(3), 178. https://doi.org/10.3390/gels9030178








