Applications of Hydrogels in Drug Delivery for Oral and Maxillofacial Diseases
Abstract
1. Introduction
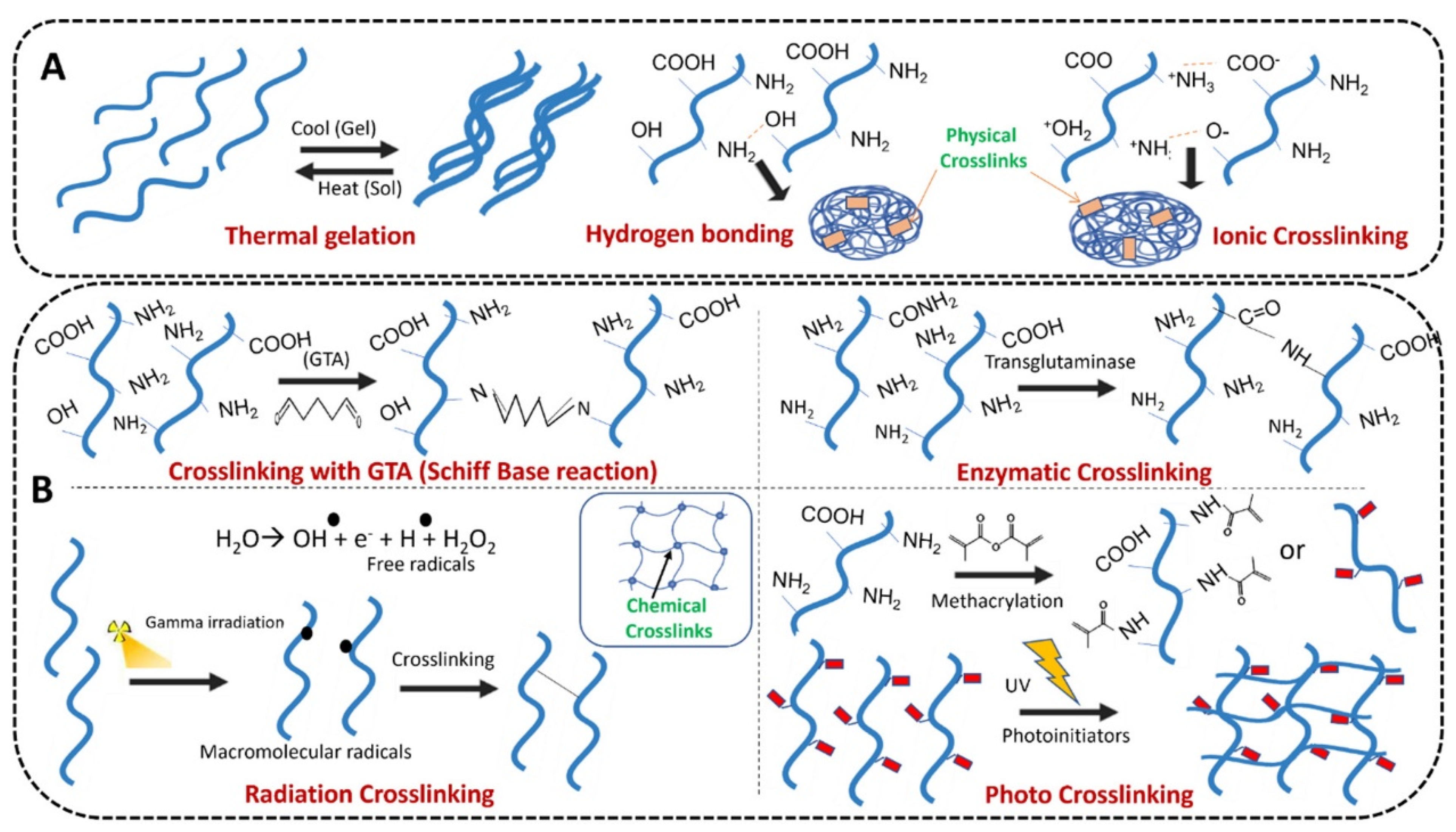
2. Materials and Methods
3. Hydrogels for Oral and Maxillofacial Environment
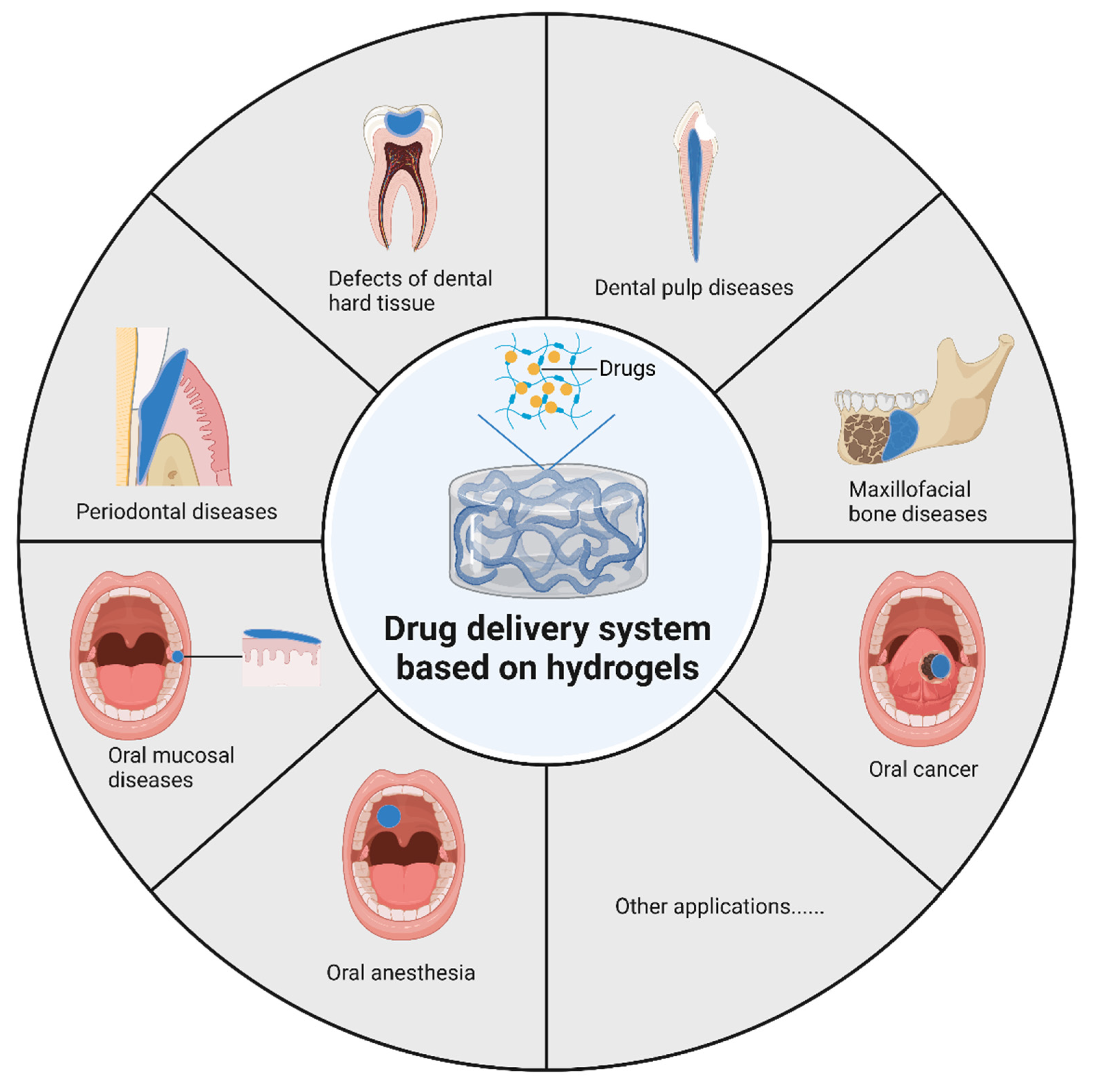
4. Applications in Different Oral and Maxillofacial Diseases
4.1. Defects of Dental Hard Tissue
4.2. Dental Pulp Diseases
4.2.1. Pulp Capping
4.2.2. Root Canal Disinfection
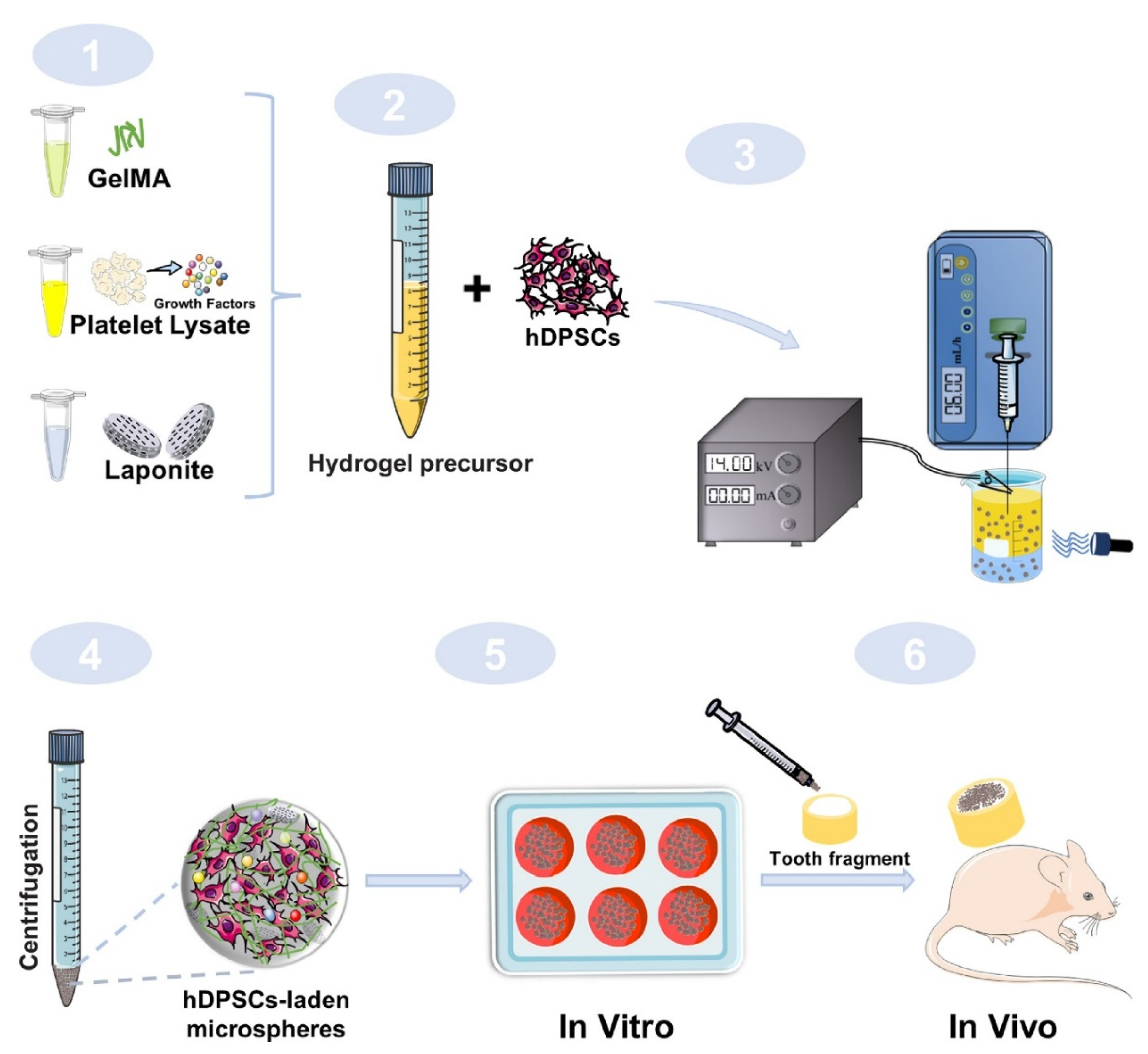
4.2.3. Pulp Regeneration
4.3. Periodontal Disease
4.4. Maxillofacial Bone Diseases
4.4.1. Mandibular Reconstruction
4.4.2. Peri-Implant Diseases
4.4.3. Alveolar Osteitis (Dry Socket)
4.4.4. Osteonecrosis of the Jaw
4.5. Oral Mucosa Diseases
4.6. Oral Cancer
4.7. Oral Anesthesia
4.8. Other Oral Diseases
5. Perspective and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bako, J.; Szepesi, M.; Marton, I.; Borbely, J.; Hegedus, C. Synthesis of nanoparticles for dental drug delivery systems. Fogorv. Szle. 2007, 100, 109–113. [Google Scholar]
- Wang, B.; Booij-Vrieling, H.E.; Bronkhorst, E.M.; Shao, J.; Kouwer, P.H.J.; Jansen, J.A.; Walboomers, X.F.; Yang, F. Antimicrobial and anti-inflammatory thermo-reversible hydrogel for periodontal delivery. Acta Biomater. 2020, 116, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Barber, C.; Powell, R.; Ellis, A.; Hewett, J. Comparing pain control and ability to eat and drink with standard therapy vs Gelclair: A preliminary, double centre, randomised controlled trial on patients with radiotherapy-induced oral mucositis. Support. Care Cancer 2007, 15, 427–440. [Google Scholar]
- Oliva, N.; Conde, J.; Wang, K.; Artzi, N. Designing Hydrogels for On-Demand Therapy. Acc. Chem. Res. 2017, 50, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Salar Amoli, M.; EzEldeen, M.; Jacobs, R.; Bloemen, V. Materials for Dentoalveolar Bioprinting: Current State of the Art. Biomedicines 2021, 10, 71. [Google Scholar] [CrossRef]
- Bollareddy, S.R.; Krishna, V.; Roy, G.; Dasari, D.; Dhar, A.; Venuganti, V.V.K. Transfersome Hydrogel Containing 5-Fluorouracil and Etodolac Combination for Synergistic Oral Cancer Treatment. Aaps. Pharmscitech. 2022, 23, 70. [Google Scholar] [CrossRef]
- Chuang, C.H.; Lin, R.Z.; Melero-Martin, J.M.; Chen, Y.C. Comparison of covalently and physically cross-linked collagen hydrogels on mediating vascular network formation for engineering adipose tissue. Artif. Cells Nanomed. Biotechnol. 2018, 46, S434–S447. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar]
- Buwalda, S.J.; Boere, K.W.M.; Dijkstra, P.J.; Feijen, J.; Vermonden, T.; Hennink, W.E. Hydrogels in a historical perspective: From simple networks to smart materials. J. Control. Release Off. J. Control. Release Soc. 2014, 190, 254–273. [Google Scholar] [CrossRef]
- Mushtaq, F.; Raza, Z.A.; Batool, S.R.; Zahid, M.; Onder, O.C.; Rafique, A.; Nazeer, M.A. Preparation, properties, and applications of gelatin-based hydrogels (GHs) in the environmental, technological, and biomedical sectors. Int. J. Biol. Macromol. 2022, 218, 601–633. [Google Scholar] [CrossRef]
- Dimatteo, R.; Darling, N.J.; Segura, T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv. Drug Deliv. Rev. 2018, 127, 167–184. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, M.; Nazari, B.; Miller, D.W. Injectable hydrogel-based drug delivery systems for local cancer therapy. Drug Discov. Today 2016, 21, 1835–1849. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Huang, Y.; Liu, H.; Tang, Z.; Chen, Y.; Huang, Z.; Xu, S.; Du, J.; Jia, B. Hydrogels for the treatment of oral and maxillofacial diseases: Current research, challenges, and future directions. Biomater. Sci. 2022, 10, 6413–6446. [Google Scholar] [CrossRef]
- Shahini, A.; Yazdimamaghani, M.; Walker, K.J.; Eastman, M.A.; Hatami-Marbini, H.; Smith, B.J.; Ricci, J.L.; Madihally, S.V.; Vashaee, D.; Tayebi, L. 3D conductive nanocomposite scaffold for bone tissue engineering. Int. J. Nanomed. 2014, 9, 167–181. [Google Scholar] [CrossRef]
- Baldino, L.; Zuppolini, S.; Cardea, S.; Diodato, L.; Borriello, A.; Reverchon, E.; Nicolais, L. Production of biodegradable superabsorbent aerogels using a supercritical CO2 assisted drying. J. Supercrit. Fluids 2020, 156, 104681. [Google Scholar] [CrossRef]
- Gorshkova, N.; Brovko, O.; Palamarchuk, I.; Bogolitsyn, K.; Ivakhnov, A. Preparation of bioactive aerogel material based on sodium alginate and chitosan for controlled release of levomycetin. Polym. Adv. Technol. 2021, 32, 3474–3482. [Google Scholar] [CrossRef]
- Kondiah, P.J.; Choonara, Y.E.; Kondiah, P.P.; Marimuthu, T.; Kumar, P.; du Toit, L.C.; Pillay, V. A Review of Injectable Polymeric Hydrogel Systems for Application in Bone Tissue Engineering. Molecules 2016, 21, 1580. [Google Scholar] [CrossRef]
- Xiao, Y.; Gu, Y.; Qin, L.; Chen, L.; Chen, X.; Cui, W.; Li, F.; Xiang, N.; He, X. Injectable thermosensitive hydrogel-based drug delivery system for local cancer therapy. Colloids Surf. B Biointerfaces 2021, 200, 111581. [Google Scholar] [CrossRef]
- Fan, R.; Cheng, Y.; Wang, R.; Zhang, T.; Zhang, H.; Li, J.; Song, S.; Zheng, A. Thermosensitive Hydrogels and Advances in Their Application in Disease Therapy. Polymers 2022, 14, 2379. [Google Scholar] [CrossRef]
- Wang, Q.; Zuo, Z.; Cheung, C.K.C.; Leung, S.S.Y. Updates on thermosensitive hydrogel for nasal, ocular and cutaneous delivery. Int. J. Pharm. 2019, 559, 86–101. [Google Scholar] [CrossRef]
- Chen, J.; Wang, D.; Wang, L.H.; Liu, W.; Chiu, A.; Shariati, K.; Liu, Q.; Wang, X.; Zhong, Z.; Webb, J.; et al. An Adhesive Hydrogel with “Load-Sharing” Effect as Tissue Bandages for Drug and Cell Delivery. Adv. Mater. 2020, 32, 2001628. [Google Scholar] [CrossRef] [PubMed]
- Abebe, M.W.; Appiah-Ntiamoah, R.; Kim, H. Gallic acid modified alginate self-adhesive hydrogel for strain responsive transdermal delivery. Int. J. Biol. Macromol. 2020, 163, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Bekhouche, M.; Bolon, M.; Charriaud, F.; Lamrayah, M.; Da Costa, D.; Primard, C.; Costantini, A.; Pasdeloup, M.; Gobert, S.; Mallein-Gerin, F.; et al. Development of an antibacterial nanocomposite hydrogel for human dental pulp engineering. J. Mater. Chem. B 2020, 8, 8422–8432. [Google Scholar] [CrossRef]
- Silva, C.R.; Babo, P.S.; Gulino, M.; Costa, L.; Oliveira, J.M.; Silva-Correia, J.; Domingues, R.M.A.; Reis, R.L.; Gomes, M.E. Injectable and tunable hyaluronic acid hydrogels releasing chemotactic and angiogenic growth factors for endodontic regeneration. Acta Biomater. 2018, 77, 155–171. [Google Scholar] [CrossRef]
- Kim, J.; Yang, H.J.; Cho, T.H.; Lee, S.E.; Park, Y.D.; Kim, H.M.; Kim, I.S.; Seo, Y.K.; Hwang, S.J.; Kim, S.J. Enhanced regeneration of rabbit mandibular defects through a combined treatment of electrical stimulation and rhBMP-2 application. Med. Biol. Eng. Comput. 2013, 51, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Han, J.J.; Park, Y.D.; Cho, T.H.; Hwang, S.J. Effect of sustained release of rhBMP-2 from dried and wet hyaluronic acid hydrogel carriers compared with direct dip coating of rhBMP-2 on peri-implant osteogenesis of dental implants in canine mandibles. J. Cranio-Maxill. Surg. 2016, 44, 116–125. [Google Scholar] [CrossRef]
- Jin, I.G.; Kim, J.H.; Wu, H.G.; Kim, S.K.; Park, Y.; Hwang, S.J. Effect of bone marrow-derived stem cells and bone morphogenetic protein-2 on treatment of osteoradionecrosis in a rat model. J. Cranio-Maxillo-Facial. Surg. 2015, 43, 1478–1486. [Google Scholar]
- Xun, X.; Qiu, J.; Zhang, J.; Wang, H.; Han, F.; Xu, X.; Yuan, R. Triple-functional injectable liposome-hydrogel composite enhances bacteriostasis and osteo/angio-genesis for advanced maxillary sinus floor augmentation. Colloids Surf. B Biointerfaces 2022, 217, 112706. [Google Scholar] [CrossRef]
- Jung, S.W.; Byun, J.H.; Oh, S.H.; Kim, T.H.; Park, J.S.; Rho, G.J.; Lee, J.H. Multivalent ion-based in situ gelling polysaccharide hydrogel as an injectable bone graft. Carbohydr. Polym. 2018, 180, 216–225. [Google Scholar] [CrossRef]
- Sharma, D.; Hamlet, S.; Vaquette, C.; Petcu, E.B.; Ramamurthy, P.; Ivanovski, S. Local delivery of hydrogel encapsulated vascular endothelial growth factor for the prevention of medication-related osteonecrosis of the jaw. Sci. Rep. 2021, 11, 23371. [Google Scholar] [CrossRef]
- Guo, H.; Huang, S.; Yang, X.; Wu, J.; Kirk, T.B.; Xu, J.; Xu, A.; Xue, W. Injectable and Self-Healing Hydrogels with Double-Dynamic Bond Tunable Mechanical, Gel-Sol Transition and Drug Delivery Properties for Promoting Periodontium Regeneration in Periodontitis. Acs Appl. Mater. Interfaces 2021, 13, 61638–61652. [Google Scholar] [CrossRef] [PubMed]
- Mohabatpour, F.; Yazdanpanah, Z.; Papagerakis, S.; Chen, X.; Papagerakis, P. Self-Crosslinkable Oxidized Alginate-Carboxymethyl Chitosan Hydrogels as an Injectable Cell Carrier for In Vitro. J. Funct. Biomater. 2022, 13, 71. [Google Scholar] [CrossRef] [PubMed]
- Osmond, M.J.; Krebs, M.D. Tunable chitosan-calcium phosphate composites as cell-instructive dental pulp capping agents. J. Biomater. Sci.-Polym. Ed. 2021, 32, 1450–1465. [Google Scholar] [CrossRef] [PubMed]
- Abboud, A.R.; Ali, A.M.; Youssef, T. Preparation and characterization of insulin-loaded injectable hydrogels as potential adjunctive periodontal treatment. Dent. Med. Probl. 2020, 57, 377–384. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, Y.; Yu, J.; Ding, R.; Pei, D.; Zhang, Y.; He, G.; Cheng, Y.; Li, A. Injectable hydrogels with high drug loading through B-N coordination and ROS-triggered drug release for efficient treatment of chronic periodontitis in diabetic rats. Biomaterials 2022, 282, 121387. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, J.; Zhu, X.; Wang, F.; Zhou, L. Minocycline-loaded In situ Hydrogel for Periodontitis Treatment. Curr. Drug Deliv. 2018, 15, 664–671. [Google Scholar] [CrossRef]
- Seo, B.B.; Chang, H.I.; Choi, H.; Koh, J.T.; Yun, K.D.; Lee, J.Y.; Song, S.C. New approach for vertical bone regeneration using in situ gelling and sustained BMP-2 releasing poly(phosphazene) hydrogel system on peri-implant site with critical defect in a canine model. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 751–759. [Google Scholar] [CrossRef]
- Wang, B.; Wang, J.; Shao, J.; Kouwer, P.H.J.; Bronkhorst, E.M.; Jansen, J.A.; Walboomers, X.F.; Yang, F. A tunable and injectable local drug delivery system for personalized periodontal application. J. Control. Release 2020, 324, 134–145. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Sanz, C.K.; Münchow, E.A.; Kalra, N.; Dubey, N.; Suárez, C.E.C.; Fenno, J.C.; Lund, R.G.; Bottino, M.C. Photocrosslinkable methacrylated gelatin hydrogel as a cell-friendly injectable delivery system for chlorhexidine in regenerative endodontics. Dent. Mater 2022, 38, 1507–1517. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, T.; Zhang, R.; Liang, X.; Wang, G.; Tian, Y.; Xie, L.; Tian, W. Platelet lysate functionalized gelatin methacrylate microspheres for improving angiogenesis in endodontic regeneration. Acta Biomater. 2021, 136, 441–455. [Google Scholar] [CrossRef]
- Park, J.H.; Gillispie, G.J.; Copus, J.S.; Zhang, W.; Atala, A.; Yoo, J.J.; Yelick, P.C.; Lee, S.J. The effect of BMP-mimetic peptide tethering bioinks on the differentiation of dental pulp stem cells (DPSCs) in 3D bioprinted dental constructs. Biofabrication 2020, 12, 035029. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Sun, Y.; Wang, J.; Wang, B.; Xu, F.; Xie, Z.; Wang, Y. Controlled release of silibinin in GelMA hydrogels inhibits inflammation by inducing M2-type macrophage polarization and promotes vascularization in vitro. RSC Adv. 2020, 12, 13192–13202. [Google Scholar] [CrossRef] [PubMed]
- Alaohali, A.; Salzlechner, C.; Zaugg, L.K.; Suzano, F.; Martinez, A.; Gentleman, E.; Sharpe, P.T. GSK3 Inhibitor-Induced Dentinogenesis Using a Hydrogel. J. Dent. Res. 2022, 101, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Komabayashi, T.; Wadajkar, A.; Santimano, S.; Ahn, C.; Zhu, Q.; Opperman, L.A.; Bellinger, L.L.; Yang, J.; Nguyen, K.T. Preliminary study of light-cured hydrogel for endodontic drug delivery vehicle. J. Investig. Clin. Dent. 2016, 7, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Bako, J.; Toth, F.; Gall, J.; Kovacs, R.; Csík, A.; Varga, I.; Sculean, A.; Zelko, R.; Hegedus, C. Combined Release of Antiseptic and Antibiotic Drugs from Visible Light Polymerized Biodegradable Nanocomposite Hydrogels for Periodontitis Treatment. Pharmaceutics 2022, 14, 957. [Google Scholar] [CrossRef]
- Limjeerajarus, C.N.; Kreua-Ongarjnukool, N.; Seang, S.; Pavasant, P.; Niyomthai, S.T. Characterization of a Thermo-Sensitive Injectable Hydrogel as an Iloprost Delivery System for Dental Use. Key Eng. Mater. 2020, 856, 391–398. [Google Scholar] [CrossRef]
- Pham, D.T.; Phewchan, P.; Navesit, K.; Chokamonsirikun, A.; Khemwong, T.; Tiyaboonchai, W. Development of Metronidazole-loaded In situ Thermosensitive Hydrogel for Periodontitis Treatment. Turk. J. Pharm. Sci. 2021, 18, 510–516. [Google Scholar] [CrossRef]
- Chen, N.; Ren, R.; Wei, X.; Mukundan, R.; Li, G.; Xu, X.; Zhao, G.; Zhao, Z.; Lele, S.M.; Reinhardt, R.A.; et al. Thermoresponsive Hydrogel-Based Local Delivery of Simvastatin for the Treatment of Periodontitis. Mol. Pharm. 2021, 18, 1992–2003. [Google Scholar] [CrossRef]
- Almoshari, Y.; Ren, R.; Zhang, H.; Jia, Z.; Wei, X.; Chen, N.; Li, G.; Ryu, S.; Lele, S.M.; Reinhardt, R.A.; et al. GSK3 inhibitor-loaded osteotropic Pluronic hydrogel effectively mitigates periodontal tissue damage associated with experimental periodontitis. Biomaterials 2020, 261, 120293. [Google Scholar] [CrossRef] [PubMed]
- Al Homsi, R.; Eltahir, S.; Jagal, J.; Ali Abdelkareem, M.; Ghoneim, M.M.; Rawas-Qalaji, M.M.; Greish, K.; Haider, M. Thermosensitive injectable graphene oxide/chitosan-based nanocomposite hydrogels for controlling the in vivo release of bupivacaine hydrochloride. Int. J. Pharm. 2022, 621, 121786. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhou, Y.; Yu, Y.; Zhou, X.; Du, W.; Wan, M.; Fan, Y.; Zhou, X.; Xu, X.; Zheng, L. Evaluation of Chitosan Hydrogel for Sustained Delivery of VEGF for Odontogenic Differentiation of Dental Pulp Stem Cells. Stem. Cells Int. 2019, 2019, 1515040. [Google Scholar] [CrossRef]
- Talaat, W.M.; Haider, M.; Kawas, S.A.; Kandil, N.G.; Harding, D.R. Chitosan-Based Thermosensitive Hydrogel for Controlled Drug Delivery to the Temporomandibular Joint. J. Craniofac. Surg. 2016, 27, 735–740. [Google Scholar] [CrossRef]
- Zang, S.; Mu, R.; Chen, F.; Wei, X.; Zhu, L.; Han, B.; Yu, H.; Bi, B.; Chen, B.; Wang, Q.; et al. Injectable chitosan/beta-glycerophosphate hydrogels with sustained release of BMP-7 and ornidazole in periodontal wound healing of class III furcation defects. Mater. Sci. Eng. C 2019, 99, 919–928. [Google Scholar] [CrossRef]
- Arpornmaeklong, P.; Sareethammanuwat, M.; Apinyauppatham, K.; Boonyuen, S. Characteristics and biologic effects of thermosensitive quercetin-chitosan/collagen hydrogel on human periodontal ligament stem cells. J. Biomed. Mater. Res. B Appl Biomater. 2021, 109, 1656–1670. [Google Scholar] [CrossRef] [PubMed]
- Chien, K.H.; Chang, Y.L.; Wang, M.L.; Chuang, J.H.; Yang, Y.C.; Tai, M.C.; Wang, C.Y.; Liu, Y.Y.; Li, H.Y.; Chen, J.T.; et al. Promoting Induced Pluripotent Stem Cell-driven Biomineralization and Periodontal Regeneration in Rats with Maxillary-Molar Defects using Injectable BMP-6 Hydrogel. Sci. Rep. 2018, 8, 114. [Google Scholar] [CrossRef]
- Chen, X.; Chen, D.-R.; Liu, H.; Yang, L.; Zhang, Y.; Bu, L.-L.; Sun, Z.-J.; Cai, L. Local delivery of gambogic acid to improve anti-tumor immunity against oral squamous cell carcinoma. J. Control. Release 2022, 351, 381–393. [Google Scholar] [CrossRef]
- Tan, G.; Zhong, Y.; Yang, L.; Jiang, Y.; Liu, J.; Ren, F. A multifunctional MOF-based nanohybrid as injectable implant platform for drug synergistic oral cancer therapy. Chem. Eng. J. 2020, 390, 124446. [Google Scholar] [CrossRef]
- Shao, W.; Chen, R.; Lin, G.; Ran, K.; Zhang, Y.; Yang, J.; Pan, H.; Shangguan, J.; Zhao, Y.; Xu, H. In situ mucoadhesive hydrogel capturing tripeptide KPV: The anti-inflammatory, antibacterial and repairing effect on chemotherapy-induced oral mucositis. Biomater. Sci. 2021, 10, 227–242. [Google Scholar] [CrossRef]
- Lu, W.; Li, X.; Yang, Y.; Yi, J.; Xie, L.; Zhao, Z.; Li, Y. PTH/PTHrP in controlled release hydrogel enhances orthodontic tooth movement by regulating periodontal bone remodaling. J. Periodontal. Res. 2021, 56, 885–896. [Google Scholar] [CrossRef]
- Lei, L.; Liu, Z.; Yuan, P.; Jin, R.; Wang, X.; Jiang, T.; Chen, X. Injectable colloidal hydrogel with mesoporous silica nanoparticles for sustained co-release of microRNA-222 and aspirin to achieve innervated bone regeneration in rat mandibular defects. J. Mater. Chemistry. B 2019, 7, 2722–2735. [Google Scholar]
- Zhang, L.; Wang, Y.; Wang, C.; He, M.; Wan, J.; Wei, Y.; Zhang, J.; Yang, X.; Zhao, Y.; Zhang, Y. Light-Activable On-Demand Release of Nano-Antibiotic Platforms for Precise Synergy of Thermochemotherapy on Periodontitis. ACS Appl. Mater. Interfaces 2020, 12, 3354–3362. [Google Scholar] [CrossRef]
- Koch, F.; Ekat, K.; Kilian, D.; Hettich, T.; Germershaus, O.; Lang, H.; Peters, K.; Kreikemeyer, B. A Versatile Biocompatible Antibiotic Delivery System Based on Self-Assembling Peptides with Antimicrobial and Regenerative Potential. Adv. Healthc. Mater. 2019, 8, 1900167. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y.N.; Ma, B.; Shao, J.; Liu, H.; Ge, S. Gingipain-Responsive Thermosensitive Hydrogel Loaded with SDF-1 Facilitates In Situ Periodontal Tissue Regeneration. ACS Appl. Mater. Interfaces 2021, 13, 36880–36893. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, F.; Huang, N.; Li, J.; Wu, C.; Tan, B.; Liu, Y.; Li, L.; Yang, C.; Shao, D.; et al. Near-infrared light-responsive hybrid hydrogels for the synergistic chemo-photothermal therapy of oral cancer. Nanoscale 2021, 13, 17168–17182. [Google Scholar] [CrossRef]
- Serra, E.; Saubade, F.; Ligorio, C.; Whitehead, K.; Sloan, A.; Williams, D.W.; Hidalgo-Bastida, A.; Verran, J.; Malic, S. Methylcellulose Hydrogel with Melissa officinalis Essential Oil as a Potential Treatment for Oral Candidiasis. Microorganisms 2020, 8, 215. [Google Scholar] [CrossRef]
- Cubayachi, C.; Couto, R.O.; de Gaitani, C.M.; Pedrazzi, V.; Freitas, O.; Lopez, R.F. Needle-free buccal anesthesia using iontophoresis and amino amide salts combined in a mucoadhesive formulation. Colloids Surf. B Biointerfaces 2015, 136, 1193–1201. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, Z.; Fan, J.; Zhang, L.; Zhang, Z.; Wu, Z.; Shi, Y.; Zheng, H.; Zhang, Z.; Tang, R.; et al. Hydroxypropylmethylcellulose as a film and hydrogel carrier for ACP nanoprecursors to deliver biomimetic mineralization. J. Nanobiotechnol. 2021, 19, 385. [Google Scholar] [CrossRef]
- Zhang, F.; Bao, W.; Li, R.; Zhao, S.; Liu, Y.; Xu, Y.; Liao, L.; Wang, X. Microneedles combined with a sticky and heatable hydrogel for local painless anesthesia. Biomater. Sci. 2019, 7, 4503–4507. [Google Scholar] [CrossRef]
- Muniz, B.V.; Baratelli, D.; Di Carla, S.; Serpe, L.; da Silva, C.B.; Guilherme, V.A.; Ribeiro, L.N.M.; Cereda, C.M.S.; de Paula, E.; Volpato, M.C.; et al. Hybrid Hydrogel Composed of Polymeric Nanocapsules Co-Loading Lidocaine and Prilocaine for Topical Intraoral Anesthesia. Sci. Rep. 2018, 8, 17972. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.-W.; Deng, W.-W.; Song, W.-F.; Wu, C.-C.; Liu, J.; Hong, S.; Zhuang, Z.-N.; Cheng, H.; Sun, Z.-J.; Zhang, X.-Z. Biomaterial-mediated modulation of oral microbiota synergizes with PD-1 blockade in mice with oral squamous cell carcinoma. Nat. Biomed. Eng. 2022, 6, 32–43. [Google Scholar] [CrossRef]
- Raafat, A.I.; Mahmoud, G.A.; Ali, A.E.-H.; Badawy, N.A.; Elshahawy, M.F. In vitro evaluation of mucoadhesive and self-disinfection efficiency of (acrylic acid/polyethylene glycol)-silver nanocomposites for buccal drug delivery. J. Bioact. Compat. Polym. 2018, 33, 95–115. [Google Scholar] [CrossRef]
- Hrib, J.; Sirc, J.; Lesny, P.; Hobzova, R.; Duskova-Smrckova, M.; Michalek, J.; Smucler, R. Hydrogel tissue expanders for stomatology. Part I. Methacrylate-based polymers. J. Mater. Sci.-Mater. Med. 2017, 28, 12. [Google Scholar] [CrossRef]
- Kitagawa, H.; Takeda, K.; Kitagawa, R.; Izutani, N.; Miki, S.; Hirose, N.; Hayashi, M.; Imazato, S. Development of sustained antimicrobial-release systems using poly(2-hydroxyethyl methacrylate)/trimethylolpropane trimethacrylate hydrogels. Acta Biomater. 2014, 10, 4285–4295. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, R.J.; van Houte, J. Dental caries. Annu. Rev. Med. 1975, 26, 121–136. [Google Scholar] [CrossRef]
- Guentsch, A.; Fahmy, M.D.; Wehrle, C.; Nietzsche, S.; Popp, J.; Watts, D.C.; Kranz, S.; Krafft, C.; Sigusch, B.W. Effect of biomimetic mineralization on enamel and dentin: A Raman and EDX analysis. Dent. Mater. 2019, 35, 1300–1307. [Google Scholar] [CrossRef]
- Bertassoni, L.E. Dentin on the nanoscale: Hierarchical organization, mechanical behavior and bioinspired engineering. Dent. Mater. 2017, 33, 637–649. [Google Scholar] [CrossRef]
- Selwitz, R.H.; Ismail, A.I.; Pitts, N.B. Dental caries. Lancet 2007, 369, 51–59. [Google Scholar] [CrossRef]
- Shao, C.; Jin, B.; Mu, Z.; Lu, H.; Zhao, Y.; Wu, Z.; Yan, L.; Zhang, Z.; Zhou, Y.; Pan, H.; et al. Repair of tooth enamel by a biomimetic mineralization frontier ensuring epitaxial growth. Sci. Adv. 2019, 5, eaaw9569. [Google Scholar] [CrossRef]
- Pandya, M.; Diekwisch, T.G.H. Enamel biomimetics-fiction or future of dentistry. Int. J. Oral. Sci. 2019, 11, 8. [Google Scholar] [CrossRef]
- Hanafy, R.A.; Mostafa, D.; Abd El-Fattah, A.; Kandil, S. Biomimetic chitosan against bioinspired nanohydroxyapatite for repairing enamel surfaces. Bioinspired Biomim. Nanobiomater. 2020, 9, 85–94. [Google Scholar] [CrossRef]
- Mukherjee, K.; Ruan, Q.C.; Liberman, D.; White, S.N.; Moradian-Oldak, J. Repairing human tooth enamel with leucine-rich amelogenin peptide-chitosan hydrogel. J. Mater. Res. 2016, 31, 556–563. [Google Scholar] [CrossRef]
- Campodoni, E.; Dozio, S.M.; Panseri, S.; Montesi, M.; Tampieri, A.; Sandri, M. Mimicking Natural Microenvironments: Design of 3D-Aligned Hybrid Scaffold for Dentin Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 836. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Ding, L.; Li, Z.; Wang, X.; Wang, K.; Han, S.; Li, W.; Zhou, X.; Zhang, L. Chitosan hydrogel containing amelogenin-derived peptide: Inhibition of cariogenic bacteria and promotion of remineralization of initial caries lesions. Arch. Oral. Biol. 2019, 100, 42–48. [Google Scholar] [CrossRef]
- Takeda, K.; Kitagawa, H.; Tsuboi, R.; Kiba, W.; Sasaki, J.; Hayashi, M.; Imazato, S. Effectiveness of non-biodegradable poly(2-hydroxyethyl methacrylate)-based hydrogel particles as a fibroblast growth factor-2 releasing carrier. Dent. Mater. 2015, 31, 1406–1414. [Google Scholar] [CrossRef] [PubMed]
- Theyse, L.F. Endodontic treatment. Vet. Q. 1998, 20 (Suppl. 1), S32. [Google Scholar] [CrossRef]
- Schmalz, G.; Widbiller, M.; Galler, K.M. Clinical Perspectives of Pulp Regeneration. J. Endod. 2020, 46, S161–S174. [Google Scholar] [CrossRef]
- Matoug-Elwerfelli, M.; Nazzal, H.; Duggal, M.; El-Gendy, R. What the future holds for regenerative endodontics: Novel antimicrobials and regenerative strategies. Eur. Cells Mater. 2021, 41, 811–833. [Google Scholar] [CrossRef]
- Samiei, M.; Fathi, M.; Barar, J.; Fathi, N.; Amiryaghoubi, N.; Omidi, Y. Bioactive hydrogel-based scaffolds for the regeneration of dental pulp tissue. J. Drug Deliv. Sci. Technol. 2021, 64, 102600. [Google Scholar] [CrossRef]
- Whitehouse, L.L.; Thomson, N.H.; Do, T.; Feichtinger, G.A. Bioactive molecules for regenerative pulp capping. Eur. Cell Mater. 2021, 42, 415–437. [Google Scholar] [CrossRef] [PubMed]
- Brizuela, C.; Ormeño, A.; Cabrera, C.; Cabezas, R.; Silva, C.I.; Ramírez, V.; Mercade, M. Direct Pulp Capping with Calcium Hydroxide, Mineral Trioxide Aggregate, and Biodentine in Permanent Young Teeth with Caries: A Randomized Clinical Trial. J. Endod. 2017, 43, 1776–1780. [Google Scholar] [CrossRef]
- Meire, M.A.; van der Waal, S.V. A critical analysis of research methods and experimental models to study intracanal medicaments. Int. Endod. J. 2022, 55 (Suppl. 2), 330–345. [Google Scholar] [CrossRef] [PubMed]
- Haseeb, R.; Lau, M.; Sheah, M.; Montagner, F.; Quiram, G.; Palmer, K.; Stefan, M.C.; Rodrigues, D.C. Synthesis and Characterization of New Chlorhexidine-Containing Nanoparticles for Root Canal Disinfection. Materials 2016, 9, 452. [Google Scholar] [CrossRef]
- AlSaeed, T.; Nosrat, A.; Melo, M.A.; Wang, P.; Romberg, E.; Xu, H.; Fouad, A.E. Antibacterial Efficacy and Discoloration Potential of Endodontic Topical Antibiotics. J. Endod. 2018, 44, 1110–1114. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Münchow, E.A.; Bordini, E.A.F.; Rodrigues, N.S.; Dubey, N.; Sasaki, H.; Fenno, J.C.; Schwendeman, S.; Bottino, M.C. Engineering of Injectable Antibiotic-laden Fibrous Microparticles Gelatin Methacryloyl Hydrogel for Endodontic Infection Ablation. Int. J. Mol. Sci. 2022, 23, 971. [Google Scholar] [CrossRef]
- Diogenes, A.; Ruparel, N.B. Regenerative Endodontic Procedures: Clinical Outcomes. Dent. Clin. North Am. 2017, 61, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Iwaya, S.I.; Ikawa, M.; Kubota, M. Revascularization of an immature permanent tooth with apical periodontitis and sinus tract. Dent. Traumatol. 2001, 17, 185–187. [Google Scholar] [CrossRef]
- Banchs, F.; Trope, M. Revascularization of immature permanent teeth with apical periodontitis: New treatment protocol? J. Endod. 2004, 30, 196–200. [Google Scholar] [CrossRef]
- Zaky, S.H.; Shehabeldin, M.; Ray, H.; Sfeir, C. The role of inflammation modulation in dental pulp regeneration. Eur. Cells Mater. 2021, 41, 184–193. [Google Scholar] [CrossRef]
- Yang, J.; Yuan, G.; Chen, Z. Pulp Regeneration: Current Approaches and Future Challenges. Front. Physiol. 2016, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, G.L.; Sarra, G.; Schroter, G.T.; Gomes Silva, L.S.R.; Kubo Ariga, S.K.; Goncalves, F.; Caballero-Flores, H.V.; Moreira, M.S. Pro-angiogenic potential of a functionalized hydrogel scaffold as a secretome delivery platform: An innovative strategy for cell homing-based dental pulp tissue engineering. J. Tissue Eng. Regen. Med. 2022, 16, 472–483. [Google Scholar] [CrossRef] [PubMed]
- Soares, D.G.; Anovazzi, G.; Bordini, E.A.F.; Zuta, U.O.; Silva Leite, M.L.A.; Basso, F.G.; Hebling, J.; de Souza Costa, C.A. Biological Analysis of Simvastatin-releasing Chitosan Scaffold as a Cell-free System for Pulp-dentin Regeneration. J. Endod. 2018, 44, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Pulyodan, M.K.; Paramel Mohan, S.; Valsan, D.; Divakar, N.; Moyin, S.; Thayyil, S. Regenerative Endodontics: A Paradigm Shift in Clinical Endodontics. J. Pharm. Bioallied. Sci. 2020, 12, S20–S26. [Google Scholar] [CrossRef]
- Anitua, E.; Zalduendo, M.; Troya, M.; Erezuma, I.; Lukin, I.; Hernaez-Moya, R.; Orive, G. Composite alginate-gelatin hydrogels incorporating PRGF enhance human dental pulp cell adhesion, chemotaxis and proliferation. Int. J. Pharm. 2022, 617, 121631. [Google Scholar] [CrossRef] [PubMed]
- Loesche, W.J.; Grossman, N.S. Periodontal disease as a specific, albeit chronic, infection: Diagnosis and treatment. Clin. Microbiol. Rev. 2001, 14, 727–752. [Google Scholar] [CrossRef]
- Kinane, D.F. Causation and pathogenesis of periodontal disease. Periodontol 2000 2001, 25, 8–20. [Google Scholar] [CrossRef]
- Slots, J. Periodontitis: Facts, fallacies and the future. Periodontol 2000 2017, 75, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Azari, G.; Aghayan, S.; Seyedjafari, E. Sustained Release of Risedronate from PLGA Microparticles Embedded in Alginate Hydrogel for Treatment of Bony Lesions. Iran. Biomed. J. 2022, 26, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Aminu, N.; Chan, S.-Y.; Yam, M.-F.; Toh, S.-M. A dual-action chitosan-based nanogel system of triclosan and flurbiprofen for localised treatment of periodontitis. Int. J. Pharm. 2019, 570, 118659. [Google Scholar] [CrossRef]
- Isik, G.; Hasirci, N.; Tezcaner, A.; Kiziltay, A. Multifunctional periodontal membrane for treatment and regeneration purposes. J. Bioact. Compat. Polym. 2020, 35, 117–138. [Google Scholar] [CrossRef]
- Nagai, K.; Ideguchi, H.; Kajikawa, T.; Li, X.; Chavakis, T.; Cheng, J.; Messersmith, P.B.; Heber-Katz, E.; Hajishengallis, G. An injectable hydrogel-formulated inhibitor of prolyl-4-hydroxylase promotes T regulatory cell recruitment and enhances alveolar bone regeneration during resolution of experimental periodontitis. Faseb. J. 2020, 34, 13726–13740. [Google Scholar] [CrossRef]
- Pakzad, Y.; Ganji, F. Thermosensitive hydrogel for periodontal application: In vitro drug release, antibacterial activity and toxicity evaluation. J. Biomater. Appl. 2016, 30, 919–929. [Google Scholar] [CrossRef]
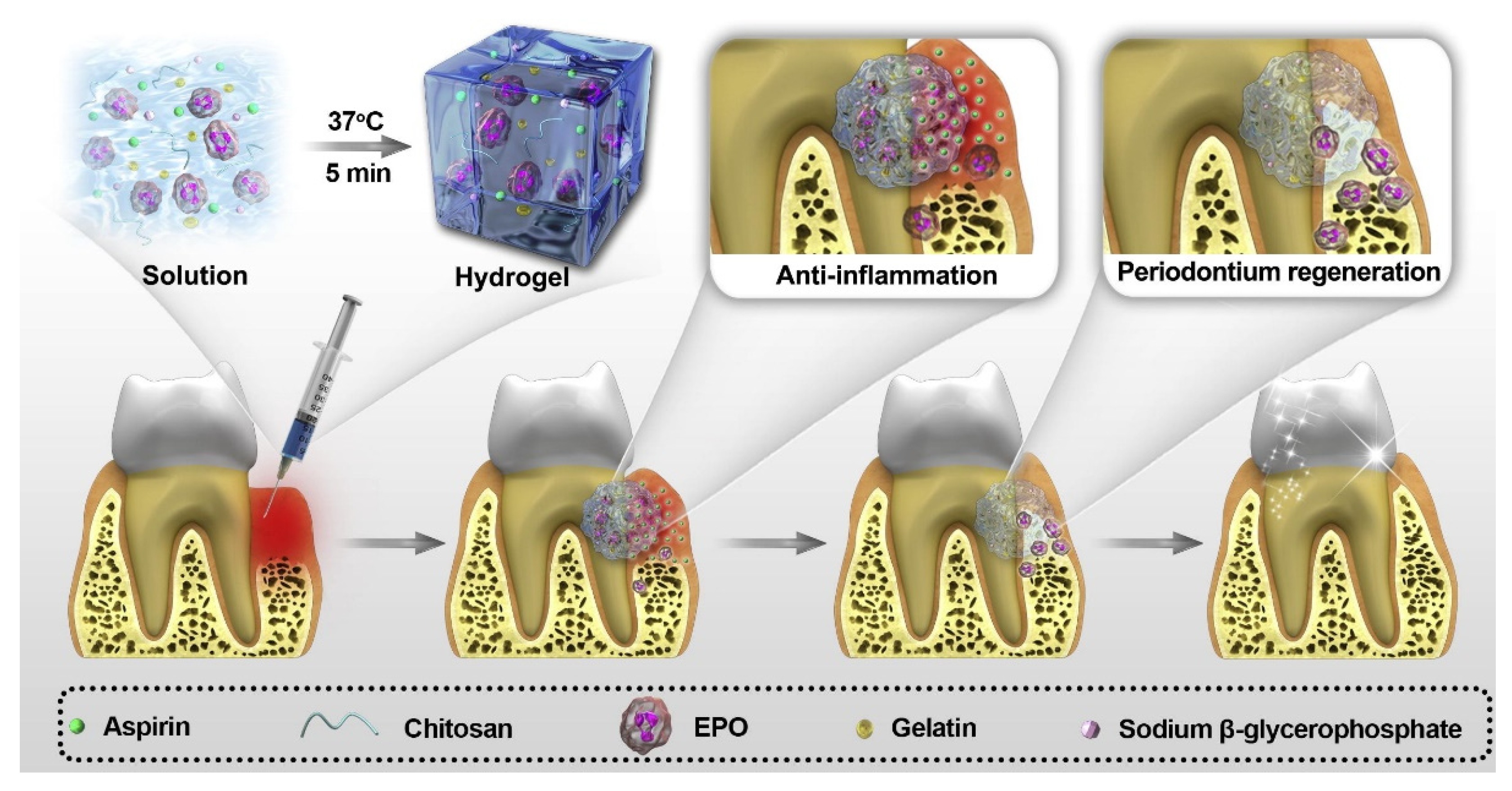
- Xu, X.; Gu, Z.; Chen, X.; Shi, C.; Liu, C.; Liu, M.; Wang, L.; Sun, M.; Zhang, K.; Liu, Q.; et al. An injectable and thermosensitive hydrogel: Promoting periodontal regeneration by controlled-release of aspirin and erythropoietin. Acta Biomater. 2019, 86, 235–246. [Google Scholar] [CrossRef]
- Chang, P.C.; Chao, Y.C.; Hsiao, M.H.; Chou, H.S.; Jheng, Y.H.; Yu, X.H.; Lee, N.; Yang, C.; Liu, D.M. Inhibition of Periodontitis Induction Using a Stimuli-Responsive Hydrogel Carrying Naringin. J. Periodontol. 2017, 88, 190–196. [Google Scholar] [CrossRef]
- Yu, M.C.; Chang, C.Y.; Chao, Y.C.; Jheng, Y.H.; Yang, C.; Lee, N.; Yu, S.H.; Yu, X.H.; Liu, D.M.; Chang, P.C. pH-Responsive Hydrogel With an Anti-Glycation Agent for Modulating Experimental Periodontitis. J. Periodontol. 2016, 87, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Bakó, J.; Vecsernyés, M.; Ujhelyi, Z.; Kovácsné, I.B.; Borbíró, I.; Bíró, T.; Borbély, J.; Hegedűs, C. Composition and characterization of in situ usable light cured dental drug delivery hydrogel system. J. Mater. Sci. Mater. Med. 2013, 24, 659–666. [Google Scholar] [CrossRef]
- Li, Z.; Li, G.; Xu, J.; Li, C.; Han, S.; Zhang, C.; Wu, P.; Lin, Y.; Wang, C.; Zhang, J.; et al. Hydrogel Transformed from Nanoparticles for Prevention of Tissue Injury and Treatment of Inflammatory Diseases. Adv. Mater. 2022, 34, 2109178. [Google Scholar] [CrossRef]
- Lin, J.; He, Z.; Liu, F.; Feng, J.; Huang, C.; Sun, X.; Deng, H. Hybrid Hydrogels for Synergistic Periodontal Antibacterial Treatment with Sustained Drug Release and NIR-Responsive Photothermal Effect. Int. J. Nanomed. 2020, 15, 5377–5387. [Google Scholar] [CrossRef]
- Hayden, R.E.; Mullin, D.P.; Patel, A.K. Reconstruction of the segmental mandibular defect: Current state of the art. Curr. Opin. Otolaryngol. Head. Neck. Surg. 2012, 20, 231–236. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, W.; Wu, S.; Kuss, M.; Jiang, X.; Untrauer, J.B.; Reid, S.P.; Duan, B. 3D printed composite scaffolds with dual small molecule delivery for mandibular bone regeneration. Biofabrication 2020, 12, 035020. [Google Scholar]
- Cao, J.; Wang, L.; Lei, D.L.; Liu, Y.P.; Du, Z.J.; Cui, F.Z. Local injection of nerve growth factor via a hydrogel enhances bone formation during mandibular distraction osteogenesis. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2012, 113, 48–53. [Google Scholar] [CrossRef]
- Srouji, S.; Rachmiel, A.; Blumenfeld, I.; Livne, E. Mandibular defect repair by TGF-beta and IGF-1 released from a biodegradable osteoconductive hydrogel. J. Cranio-maxillofac. Surg. 2005, 33, 79–84. [Google Scholar] [CrossRef]
- Tatakis, D.N.; Koh, A.; Jin, L.; Wozney, J.M.; Rohrer, M.D.; Wikesjo, U.M. Peri-implant bone regeneration using recombinant human bone morphogenetic protein-2 in a canine model: A dose-response study. J. Periodontal. Res. 2002, 37, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Kuhn, L.; Charles, L.; Pendrys, D.; Shafer, D.; Freilich, M. Comparison of bone morphogenetic protein-2 delivery systems to induce supracrestal bone guided by titanium implants in the rabbit mandible. Clin. Oral. Implants Res. 2016, 27, 676–685. [Google Scholar] [CrossRef]
- Gruber, R.M.; Krohn, S.; Mauth, C.; Dard, M.; Molenberg, A.; Lange, K.; Perske, C.; Schliephake, H. Mandibular reconstruction using a calcium phosphate/polyethylene glycol hydrogel carrier with BMP-2. J. Clin. Periodontol. 2014, 41, 820–826. [Google Scholar] [CrossRef]
- Sculean, A.; Gruber, R.; Bosshardt, D.D. Soft tissue wound healing around teeth and dental implants. J. Clin. Periodontol. 2014, 41 (Suppl. 15), S6–S22. [Google Scholar] [CrossRef]
- Al-Assaf, D.A.; Bede, S.Y. The Effects of Local Alendronate With or Without Recombinant Human Bone Morphogenetic Protein 2 on Dental Implant Stability and Marginal Bone Level: A Randomized Controlled Study. J. Craniofac. Surg. 2022, 33, 1003–1007. [Google Scholar] [CrossRef]
- Akagawa, Y.; Kubo, T.; Koretake, K.; Hayashi, K.; Doi, K.; Matsuura, A.; Morita, K.; Takeshita, R.; Yuan, Q.; Tabata, Y. Initial bone regeneration around fenestrated implants in Beagle dogs using basic fibroblast growth factor-gelatin hydrogel complex with varying biodegradation rates. J. Prosthodont. Res. 2009, 53, 41–47. [Google Scholar] [CrossRef]
- Asbi, T.; Hussein, H.A.; Horwitz, J.; Gabay, E.; Machtei, E.E.; Giladi, H.Z. A single application of chlorhexidine gel reduces gingival inflammation and interleukin 1-beta following one-stage implant placement: A randomized controlled study. Clin. Implant. Dent. Relat. Res. 2021, 23, 726–734. [Google Scholar] [CrossRef]
- Bowe, D.D.C. The management of dry socket/alveolar osteitis. J. Ir. Dent. Assoc. 2011, 57, 305–310. [Google Scholar] [PubMed]
- Lone, P.A.; Ahmed, S.w.; Prasad, V.; Ahmed, B. Role of turmeric in management of alveolar osteitis (dry socket): A randomised clinical study. J. Oral Biol. Craniofacial Res. 2018, 8, 44–47. [Google Scholar] [CrossRef]
- Davis-Searles, P.R.; Nakanishi, Y.; Kim, N.-C.; Graf, T.N.; Oberlies, N.H.; Wani, M.C.; Wall, M.E.; Agarwal, R.; Kroll, D.J. Milk thistle and prostate cancer: Differential effects of pure flavonolignans from Silybum marianum on antiproliferative end points in human prostate carcinoma cells. Cancer Res. 2005, 65, 4448–4457. [Google Scholar] [CrossRef] [PubMed]
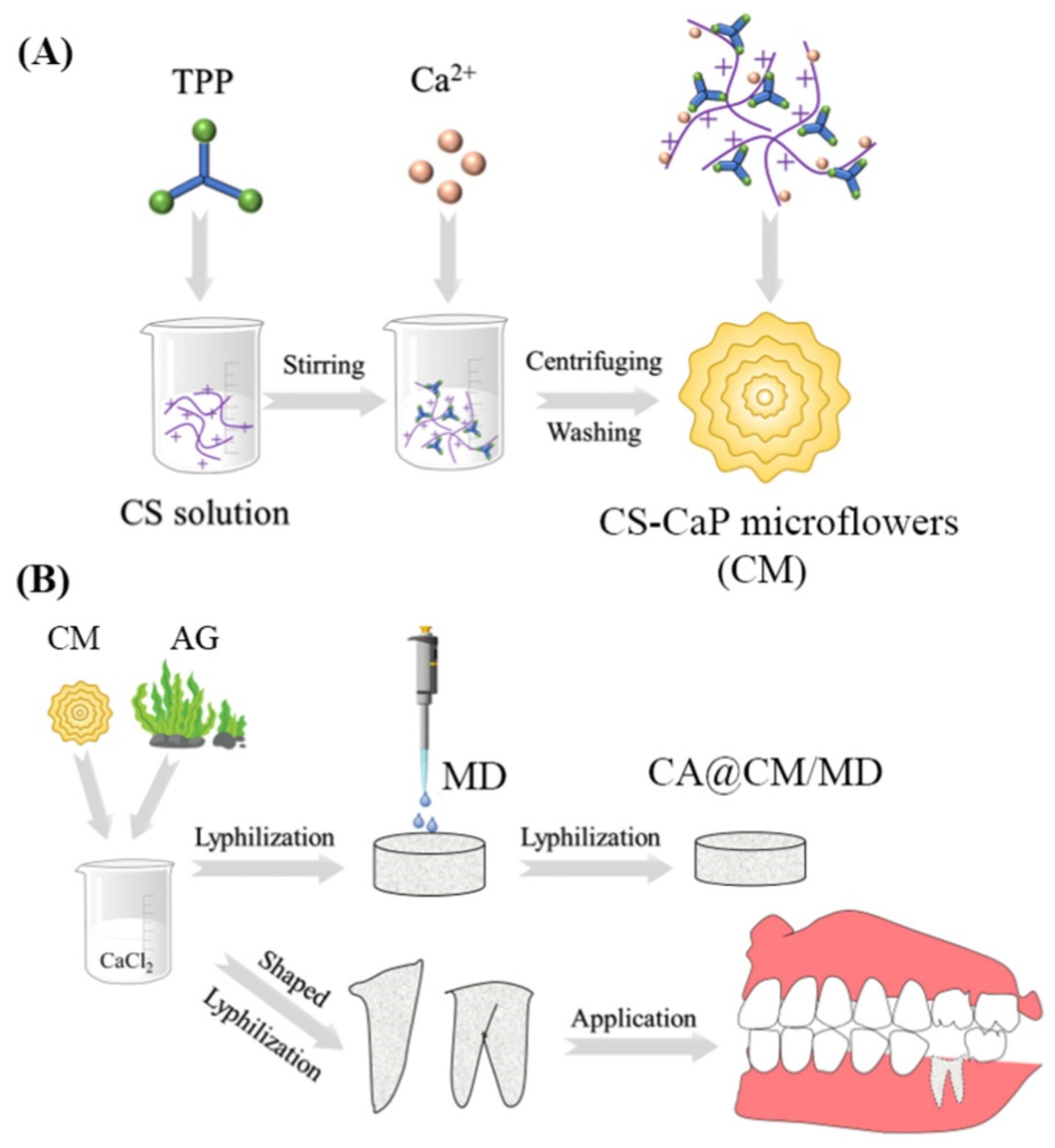
- Yan, M.; Pan, Y.; Lu, S.; Li, X.; Wang, D.; Shao, T.; Wu, Z.; Zhou, Q. Chitosan-CaP microflowers and metronidazole loaded calcium alginate sponges with enhanced antibacterial, hemostatic and osteogenic properties for the prevention of dry socket after tooth removal. Int. J. Biol. Macromol. 2022, 212, 134–145. [Google Scholar]
- Otto, S.; Pautke, C.; Van den Wyngaert, T.; Niepel, D.; Schiødt, M. Medication-related osteonecrosis of the jaw: Prevention, diagnosis and management in patients with cancer and bone metastases. Cancer Treat. Rev. 2018, 69, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Frankart, A.J.; Frankart, M.J.; Cervenka, B.; Tang, A.L.; Krishnan, D.G.; Takiar, V. Osteoradionecrosis: Exposing the Evidence Not the Bone. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 1206–1218. [Google Scholar] [CrossRef]
- Malmgren, B.; Aström, E.; Söderhäll, S. No osteonecrosis in jaws of young patients with osteogenesis imperfecta treated with bisphosphonates. J. Oral. Pathol. Med. Off. Publ. Int. Assoc. Oral. Pathol. Am. Acad. Oral. Pathol. 2008, 37, 196–200. [Google Scholar] [CrossRef]
- Khosla, S.; Burr, D.; Cauley, J.; Dempster, D.W.; Ebeling, P.R.; Felsenberg, D.; Gagel, R.F.; Gilsanz, V.; Guise, T.; Koka, S.; et al. Bisphosphonate-associated osteonecrosis of the jaw: Report of a task force of the American Society for Bone and Mineral Research. J. Bone. Miner. Res. Off. J. Am. Soc. Bone. Miner. Res. 2007, 22, 1479–1491. [Google Scholar] [CrossRef]
- Brierly, G.I.; Ren, J.; Baldwin, J.; Saifzadeh, S.; Theodoropoulos, C.; Tsurkan, M.V.; Lynham, A.; Hsu, E.; Nikolarakos, D.; Werner, C.; et al. Investigation of Sustained BMP Delivery in the Prevention of Medication-Related Osteonecrosis of the Jaw (MRONJ) in a Rat Model. Macromol. Biosci. 2019, 19, e1900226. [Google Scholar] [CrossRef]
- Imada, M.; Yagyuu, T.; Ueyama, Y.; Maeda, M.; Yamamoto, K.; Kurokawa, S.; Jo, J.I.; Tabata, Y.; Tanaka, Y.; Kirita, T. Prevention of tooth extraction-triggered bisphosphonate-related osteonecrosis of the jaws with basic fibroblast growth factor: An experimental study in rats. PLoS ONE 2019, 14, e0211928. [Google Scholar] [CrossRef]
- Chrcanovic, B.R.; Reher, P.; Sousa, A.A.; Harris, M. Osteoradionecrosis of the jaws—A current overview—Part 1: Physiopathology and risk and predisposing factors. Oral. Maxillofac. Surg. 2010, 14, 3–16. [Google Scholar] [CrossRef]
- O’Dell, K.; Sinha, U. Osteoradionecrosis. Oral. Maxillofac. Surg. Clin. North Am. 2011, 23, 455–464. [Google Scholar]
- Springer, I.N.G.; Niehoff, P.; Açil, Y.; Marget, M.; Lange, A.; Warnke, P.H.; Pielenz, H.; Roldán, J.C.; Wiltfang, J. BMP-2 and bFGF in an irradiated bone model. J. Cranio-Maxillofac. Surg. 2008, 36, 210–217. [Google Scholar] [CrossRef]
- Lin, D.; Yang, L.; Wen, L.; Lu, H.; Chen, Q.; Wang, Z. Crosstalk between the oral microbiota, mucosal immunity, and the epithelial barrier regulates oral mucosal disease pathogenesis. Mucosal. Immunol. 2021, 14, 1247–1258. [Google Scholar] [CrossRef]
- Xing, J.; Ding, Y.; Zheng, X.; Yu, P.; Qin, M.; Qiu, R.; Li, Y.; Shang, S.; Xie, J.; Li, J. Barnacle-Inspired robust and aesthetic Janus patch with instinctive wet adhesive for oral ulcer treatment. Chem. Eng. J. 2022, 444, 136580. [Google Scholar] [CrossRef]
- Ryu, J.H.; Choi, J.S.; Park, E.; Eom, M.R.; Jo, S.; Lee, M.S.; Kwon, S.K.; Lee, H. Chitosan oral patches inspired by mussel adhesion. J. Control. Release Off. J. Control. Release Soc. 2020, 317, 57–66. [Google Scholar]
- Ding, T.; Zou, J.; Qi, J.; Dan, H.; Tang, F.; Zhao, H.; Chen, Q. Mucoadhesive Nucleoside-Based Hydrogel Delays Oral Leukoplakia Canceration. J. Dent. Res. 2022, 101, 921–930. [Google Scholar] [CrossRef]
- Singh, S.; Fatima, Z.; Hameed, S. Predisposing factors endorsing Candida infections. Infez. Med. 2015, 23, 211–223. [Google Scholar]
- Sultan, A.S.; Vila, T.; Hefni, E.; Karlsson, A.J.; Jabra-Rizk, M.A. Evaluation of the Antifungal and Wound-Healing Properties of a Novel Peptide-Based Bioadhesive Hydrogel Formulation. Antimicrob. Agents Chemother. 2019, 63, e00888-19. [Google Scholar] [CrossRef]
- D’Souza, S.; Addepalli, V. Preventive measures in oral cancer: An overview. Biomed. Pharmacother. 2018, 107, 72–80. [Google Scholar] [CrossRef]
- Baselga, J.; Trigo, J.M.; Bourhis, J.; Tortochaux, J.; Cortés-Funes, H.; Hitt, R.; Gascón, P.; Amellal, N.; Harstrick, A.; Eckardt, A. Phase II multicenter study of the antiepidermal growth factor receptor monoclonal antibody cetuximab in combination with platinum-based chemotherapy in patients with platinum-refractory metastatic and/or recurrent squamous cell carcinoma of the head and neck. J. Clin. Oncol. 2005, 23, 5568–5577. [Google Scholar] [CrossRef]
- Abbasi, M.M.; Jahanban-Esfahlan, R.; Monfaredan, A.; Seidi, K.; Hamishehkar, H.; Khiavi, M.M. Oral and IV dosages of doxorubicin-methotrexate loaded-nanoparticles inhibit progression of oral cancer by down-regulation of matrix Methaloproteinase 2 expression in vivo. Asian Pac. J. Cancer Prev. 2014, 15, 10705–10711. [Google Scholar] [CrossRef]
- Szturz, P.; Vermorken, J.B. Management of recurrent and metastatic oral cavity cancer: Raising the bar a step higher. Oral. Oncol. 2020, 101, 104492. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liang, J.; Yang, Y.; Liang, H.; Jia, H.; Li, D. Current Trends of Targeted Drug Delivery for Oral Cancer Therapy. Front. Bioeng. Biotechnol. 2020, 8, 618931. [Google Scholar] [CrossRef] [PubMed]
- Shtenberg, Y.; Goldfeder, M.; Prinz, H.; Shainsky, J.; Ghantous, Y.; Abu El-Naaj, I.; Schroeder, A.; Bianco-Peled, H. Mucoadhesive alginate pastes with embedded liposomes for local oral drug delivery. Int. J. Biol. Macromol. 2018, 111, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, Y.; Xu, M.; Yang, D. Current trends of targeted therapy for oral squamous cell carcinoma. J. Cancer Res. Clin. Oncol. 2022, 148, 2169–2186. [Google Scholar] [CrossRef]
- Cheng, C.T.; Castro, G.; Liu, C.H.; Lau, P. Advanced nanotechnology: An arsenal to enhance immunotherapy in fighting cancer. Clin. Chim. Acta 2019, 492, 12–19. [Google Scholar] [CrossRef]
- Shi, Y.; Xie, T.X.; Leach, D.G.; Wang, B.; Young, S.; Osman, A.A.; Sikora, A.G.; Ren, X.; Hartgerink, J.D.; Myers, J.N.; et al. Local Anti-PD-1 Delivery Prevents Progression of Premalignant Lesions in a 4NQO-Oral Carcinogenesis Mouse Model. Cancer Prev. Res. 2021, 14, 767–778. [Google Scholar] [CrossRef]
- Leach, D.G.; Dharmaraj, N.; Lopez-Silva, T.L.; Venzor, J.R.; Pogostin, B.H.; Sikora, A.G.; Hartgerink, J.D.; Young, S. Biomaterial-Facilitated Immunotherapy for Established Oral Cancers. ACS Biomater. Sci. Eng. 2021, 7, 415–421. [Google Scholar] [CrossRef]
- Alamzadeh, Z.; Beik, J.; Mirrahimi, M.; Shakeri-Zadeh, A.; Ebrahimi, F.; Komeili, A.; Ghalandari, B.; Ghaznavi, H.; Kamrava, S.K.; Moustakis, C. Gold nanoparticles promote a multimodal synergistic cancer therapy strategy by co-delivery of thermo-chemo-radio therapy. Eur. J. Pharm. Sci. 2020, 145, 105235. [Google Scholar] [CrossRef]
- Franz-Montan, M.; de Morais Ribeiro, L.N.; Volpato, M.C.; Saia Cereda, C.M.; Groppo, F.C.; Tofoli, G.R.; de Araujo, D.R.; Santi, P.; Padula, C.; de Paula, E. Recent advances and perspectives in topical oral anesthesia. Expert. Opin. Drug Deliv. 2017, 14, 673–684. [Google Scholar] [CrossRef]
- Calixto, G.M.F.; Muniz, B.V.; Castro, S.R.; de Araujo, J.S.M.; de Souza Amorim, K.; Ribeiro, L.N.M.; Ferreira, L.E.N.; de Araujo, D.R.; de Paula, E.; Franz-Montan, M. Mucoadhesive, thermoreversible hydrogel, containing tetracaine-loaded nanostructured lipid carriers for topical, intranasal needle-free anesthesia. Pharmaceutics 2021, 13, 1760. [Google Scholar]
- Nguyen, S.; Hiorth, M. Advanced drug delivery systems for local treatment of the oral cavity. Ther. Deliv. 2015, 6, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Mihalache, C.; Rata, D.M.; Cadinoiu, A.N.; Patras, X.; Sindilar, E.V.; Bacaita, S.E.; Popa, M.; Atanase, L.I.; Daraba, O.M. Bupivacaine-loaded chitosan hydrogels for topical anesthesia in dentistry. Polym. Int. 2020, 69, 1152–1160. [Google Scholar] [CrossRef]
- Ribeiro, L.N.M.; Franz-Montan, M.; Breitkreitz, M.C.; Rodrigues da Silva, G.H.; de Castro, S.R.; Guilherme, V.A.; de Araújo, D.R.; de Paula, E. Nanohybrid hydrogels designed for transbuccal anesthesia. Int. J. Nanomed. 2018, 13, 6453–6463. [Google Scholar] [CrossRef]
- Xing, J.Z.; Lu, L.; Unsworth, L.D.; Major, P.W.; Doschak, M.R.; Kaipatur, N.R. RANKL release from self-assembling nanofiber hydrogels for inducing osteoclastogenesis in vitro. Acta Biomater. 2017, 49, 306–315. [Google Scholar] [CrossRef]
- Gauer, R.L.; Semidey, M.J. Diagnosis and treatment of temporomandibular disorders. Am. Fam. Physician 2015, 91, 378–386. [Google Scholar]
- Mountziaris, P.M.; Kramer, P.R.; Mikos, A.G. Emerging intra-articular drug delivery systems for the temporomandibular joint. Methods 2009, 47, 134–140. [Google Scholar] [CrossRef]
- Fox, R.I. Sjögren’s syndrome. Lancet 2005, 366, 321–331. [Google Scholar] [CrossRef]
- Al Hamad, A.; Lodi, G.; Porter, S.; Fedele, S.; Mercadante, V. Interventions for dry mouth and hyposalivation in Sjögren’s syndrome: A systematic review and meta-analysis. Oral. Dis. 2019, 25, 1027–1047. [Google Scholar] [CrossRef]
- Cha, S.; Kim, H.K.; Kho, H.S.; Park, Y.S. The Sustained Effects on Tear Volume of Pilocarpine Hydrochloride in Gelatin by Hydrogel Administered by An Implant-mediated Drug Delivery System. Curr. Drug Deliv. 2017, 14, 581–586. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Bordini, E.A.F.; Ferreira, J.A.; Mei, L.; Dubey, N.; Fenno, J.C.; Piva, E.; Lund, R.G.; Schwendeman, A.; Bottino, M.C. Injectable MMP-Responsive Nanotube-Modified Gelatin Hydrogel for Dental Infection Ablation. ACS Appl. Mater. Interfaces 2020, 12, 16006–16017. [Google Scholar] [CrossRef]
- Chotitumnavee, J.; Parakaw, T.; Srisatjaluk, R.L.; Pruksaniyom, C.; Pisitpipattana, S.; Thanathipanont, C.; Amarasingh, T.; Tiankhum, N.; Chimchawee, N.; Ruangsawasdi, N. In vitro evaluation of local antibiotic delivery via fibrin hydrogel. J. Dent. Sci. 2019, 14, 7–14. [Google Scholar] [CrossRef] [PubMed]
| Classification | Hydrogels | Crosslinking Method | Drug-Loading Ways | Active Ingredients | Application | Characteristic | References |
|---|---|---|---|---|---|---|---|
| Injectable | Fibrin | Physical crosslinking | Poly (d,l) Lactic Acid nanoparticles | Clindamycin | Regenerative endodontics and antibacterial | Excellent cytocompatibility; physiological degradation kinetics; non-toxicity of degradation products; replacement with cell-derived ECM within a few days. | [23] |
| Hyaluronic acid (HA) | Chemical crosslinking | / | rhBMP-2; rMSCs and BMP-2; platelet lysate, chemotactic and pro-angiogenic growth factors (PDGF and VEGF) | Regenerative endodontics; Mandibular reconstruction; Peri-implant osteogenesis | Controlled release of drugs; supportive matrix for cell culture, recruitment, and revascularization induction. | [24,25,26,27] | |
| Alginate | Chemical crosslinking | / | Vancomycin/deferoxamine/dexamethasone (Van/DFO/Dex) | Maxillofacial bone regeneration | Locally sustained release property; prominent biological functions. | [28] | |
| Alginate (ALG)/hyaluronic acid (HA) | Chemical crosslinking | / | BMP-2 | Mandibular reconstruction | In situ gelling hydrogel with a controllable gelation rate using CaSO4 as a crosslinking agent and Na2HPO4 as a crosslinking retardation agent. | [29] | |
| Gelatin-hyaluronic acid hydrogel | Chemical crosslinking | / | Vascular endothelial growth factor (VEGF) | medication-related ONJ (MRONJ) | Assists bone healing and prevents MRONJ via a pro-angiogenic and immunomodulatory mechanism. | [30] | |
| −CHO inaldehyde-modified hyaluronic acid (HA-CHO) and −NH2 in glycol chitosan (GC), Fe3+ | Chemical crosslinking | / | Ginsenoside Rg1 and amelogenin | Periodontal disease | Injectable and self-healing hydrogels with double-dynamic bond tunable mechanical, gel-sol transition, and drug delivery properties. | [31] | |
| Oxidized alginate/carboxymethyl chitosan | Chemical crosslinking | / | A dental epithelial cell line, HAT-7 | Dental Enamel Regeneration | The Self-Crosslinkable hydrogels could be used as an injectable cell carrier for dental enamel tissue engineering applications. | [32] | |
| Carboxymethyl-chitosan and a diglycidyl ether | Chemical Crosslinking | / | Calcium phosphate nanoparticles | Pulp capping | These composites have moduli up to 3 MPa, and support the culture of dental pulp stem cells for more than 3 weeks. | [33] | |
| The chitosan hydrogel as well as blends of polyvinyl pyrrolidone (PVP), polyvinyl alcohol (PVA) and PEG | Chemical crosslinking | / | Insulin | Periodontal bone regeneration | A linear hydrogel that is injectable into periodontal pockets, and is able to carry a small insulin load through physical bonds and provide sustained release. | [34] | |
| Oxidized dextran (OD) and phenylboronic acid-functionalized poly (ethylene imine) (PBA-PEI) | Chemical crosslinking | / | Doxycycline and metformin | Periodontal antibacterial, anti-inflammatory and bone regeneration | Simultaneously improving drug loading efficiency (doxycycline and metformin) through B−N coordination and achieve ROS triggered drug release locally. | [35] | |
| poly(lactide-co-glycolide) (PLGA) and N-methylpyrrolidone (NMP) | Chemical crosslinking | / | Minocycline (MCL) | Periodontal antibacterial | Exhibited the characteristic of Newton fluid with acceptable syringeability. Drug release could last for more than 48 h with an acceptable “burst release”. | [36] | |
| poly(phosphazene) | Physical crosslinking | / | BMP-2 | Peri-implant osteogenesis | Vertical bone regeneration and higher osseointegration levels. | [37] | |
| polyisocyanopeptide (PIC) | Physical crosslinking | PLGA microspheres | Doxycycline and lipoxin | Periodontal anti-inflammatory and antibacterial | Appropriate injectability; long-term structural stability; the release profiles of drugs could be manipulated by adjusting the loaded mass ratio of acid- and ester- terminated PLGA microspheres in the PIC gels. | [2,38] | |
| Gelatin methacrylate (GelMA) | Chemical crosslinking (Photocrosslinking) | (Au NBPs@SiO (2)) or none | Chlorhexidine (CHX); pro-angiogenic growth factors (GFs); BMP-mimetic peptide; minocycline; silibinin | Regenerative endodontics; Periodontal antibacterial; Prevention of dry sockets | Cytocompatible; biodegradable; provides sustained release of drugs. | [39,40,41,42] | |
| MA-HA/di-thiol PEG | Chemical crosslinking (Photocrosslinking) | / | A novel, small-molecule noncompetitive adenosine triphosphate (ATP) drug: NP928, belongs to the thiadiazolidinone (TDZD) family | Dentin Regeneration | Biodegradable; gelling in situ upon dental blue light exposure. | [43] | |
| Polyethylene glycol-maleate-citrate (PEGMC) | Chemical crosslinking (Photocrosslinking) | / | Calcium hydroxide | Direct pulp capping | The light-curing time for hydrogel is comparable to composite resin. Controlled Ca2+ release was obtained. | [44] | |
| Methacrylated-poly-γ-glutamic acid (MPGA) polymer | Chemical crosslinking (Photocrosslinking) | Methacrylated-poly-γ-glutamic acid nanoparticles (PGA-MNP) | Metronidazole and CHX | Periodontal antibacterial | It is a pH-sensitive drug delivery system which used blue-light photopolymerization for preparation. | [45] | |
| Injectable and thermosensitive | Pluronic F127 (PF127) | Chemical crosslinking (thermal crosslinking) | / | Iloprost; Simvastatin; glycogen synthase kinase 3 beta inhibitor (BIO); Metronidazole | Regenerative endodontics; Periodontal anti-inflammatory and osteogenic | Controlled drug release; could adhere to hard tissue and gradually release. | [46,47,48,49] |
| chitosan (CS)/β-glycerophosphate (GP) | Physical crosslinking | Graphene oxide (GO) nanosheets or none | Bupivacaine hydrochloride (BH); VEGF; hyaluronic acid; bone morphogenetic protein-7 (BMP-7) and ornidazole (ORN); Naringin; aspirin and erythropoietin (EPO); quercetin | Topical anesthesia; Pulp capping; Temporomandibular disorders; Periodontal anti-inflammatory and tissue regeneration | Prolonged drug release time; a stable and sustained drug release system. | [50,51,52,53,54] | |
| Chitosan/gelatin/glycerol phosphate | Chemical crosslinking | / | BMP-6 | Periodontal tissue regeneration | Provide a 3D environment for transplanted stem cells and to enhance stem cell delivery and engraftment. | [55] | |
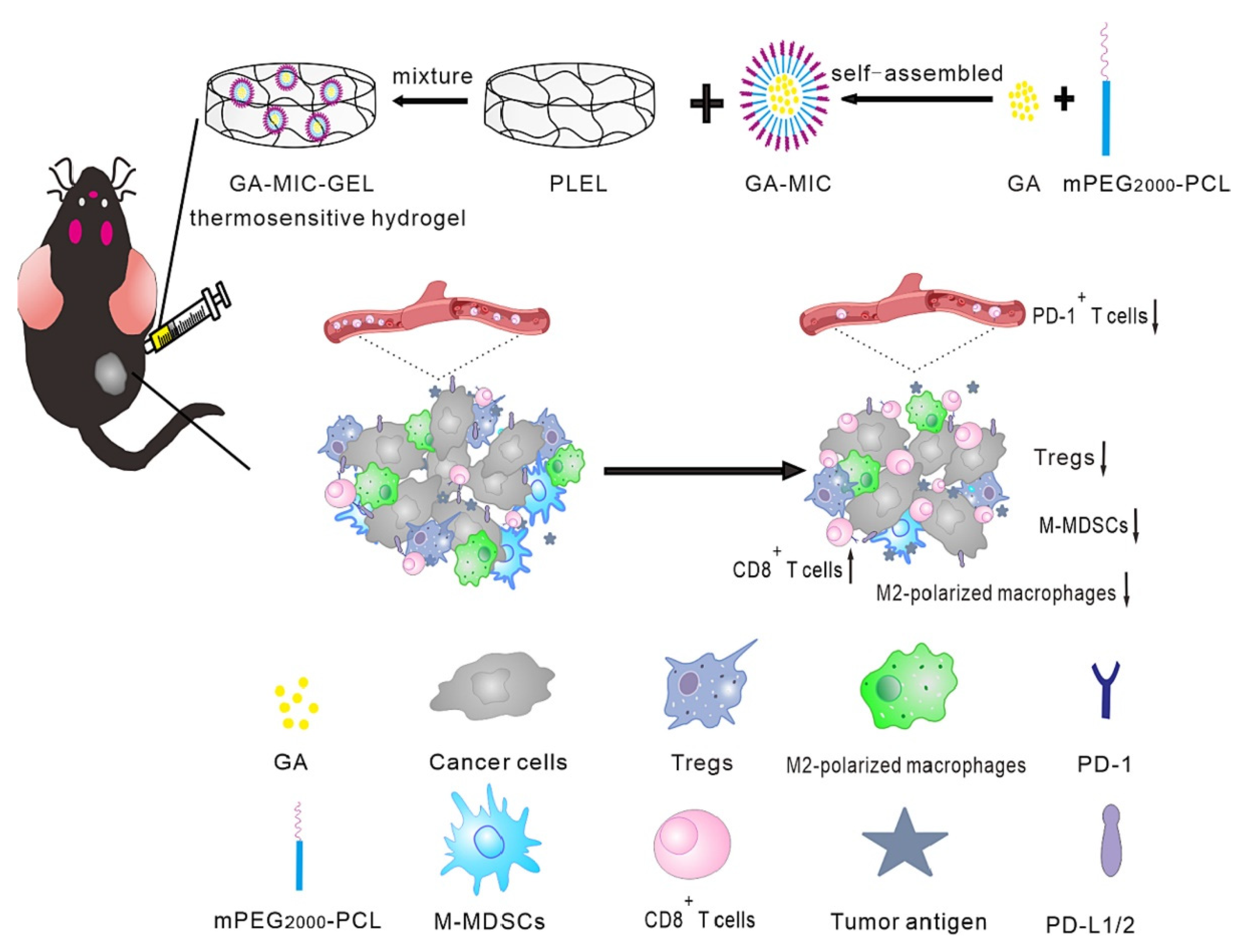
| poly(D, L-lactide)-poly(ethylene glycol)-poly(D, L-lactide) (PLEL) | Chemical crosslinking | mPEG2000-PCL micelles | Gambogic acid (GA) | Oral cancer | The thermosensitive GA-MIC-GEL with sensitive sol-gel transition characteristics could form hydrogel at 37 °C within 24 s, facilitating the local delivery and sustained GA release. | [56] | |
| poly(D,L-lactide-coglycolide)-poly(ethy-lene glycol)-poly(D,L-lactide-coglycolide) triblock copolymers (PLGA-PEG-PLGA) | Chemical crosslinking | / | Doxorubicin (DOX) and celecoxib | Oral cancer | pH-responsiveness; biocompatibility; simultaneously release hydrophobic and hydrophilic drugs at the oral tumor site. | [57] | |
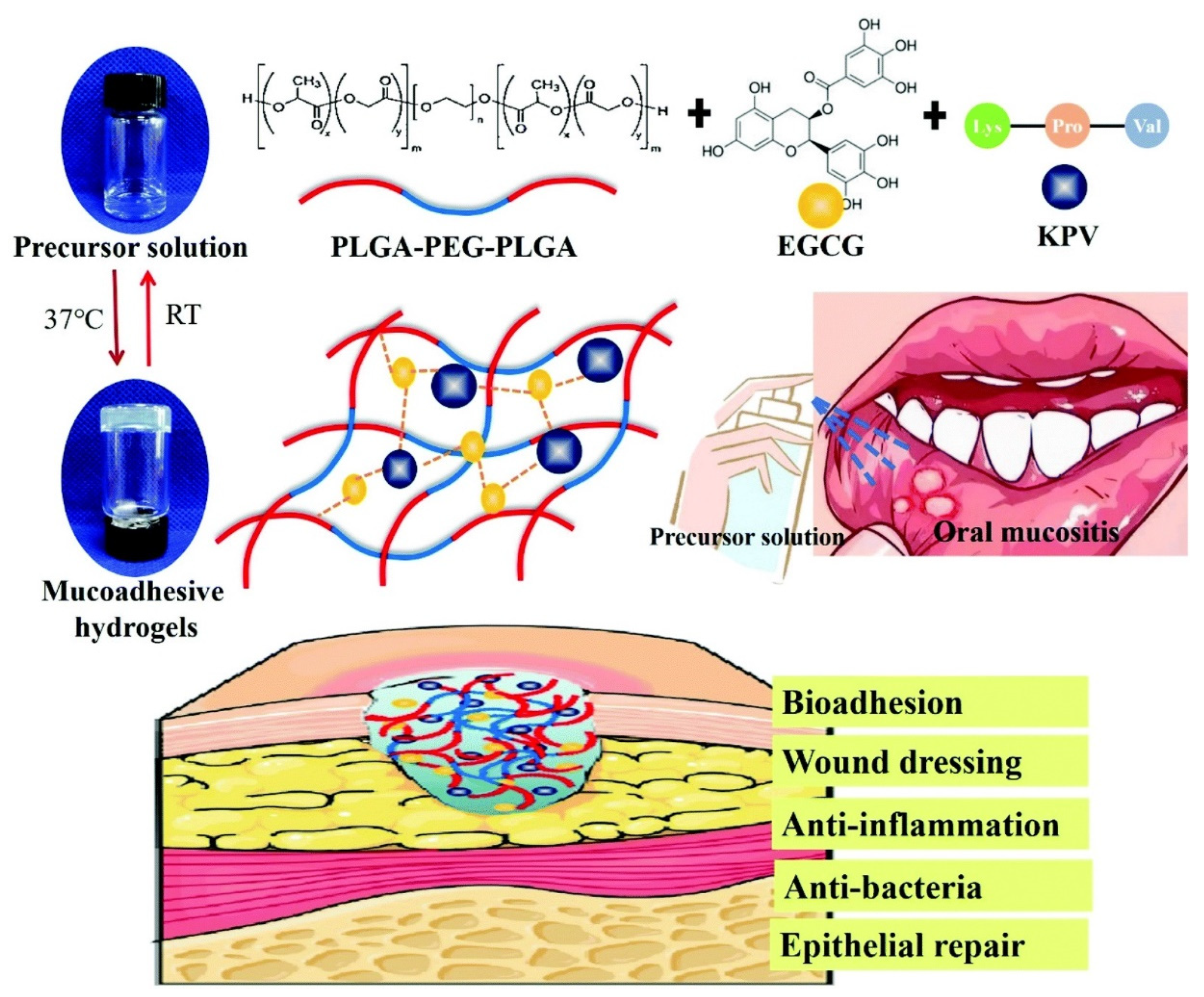
| PLGA-PEG-PLGA (PPP)/epigallocatechin-3-gallate (EGCG) | Chemical crosslinking | / | Tripeptide KPV | Oral mucosal disease | In situ mucoadhesive; anti-inflammatory, antibacterial and repairing effect on chemotherapy-induced oral mucositis. | [58] | |
| poly(ethylene glycol)-poly(ε- caprolactone)-poly(ethylene glycol) (PEG-PCL-PEG, PECE) | Chemical crosslinking | / | Parathyroid hormone (PTH) or parathyroid hormone-related protein (PTHrP) | Orthodontic tooth movement | Aqueous solution of PECE copolymers changed from the “sol” phase to the “gel” phase with the increase in temperature. | [59] | |
| poly(ethylene glycol)-6-poly(lactic-co-glycolic acid)-6-po(y(N-isopropy!acrylamide) (PEG-PLGA-PNIPAM) hydrogel | Chemical crosslinking | Mesoporous silica nanoparticle (MSN)-embedded core-shell structure | MicroRNA-222 and ASP | Mandibular reconstruction | Injectable colloidal hydrogel with mesoporous silica nanoparticles for sustained co-release of microRNA-222 and aspirin | [60] | |
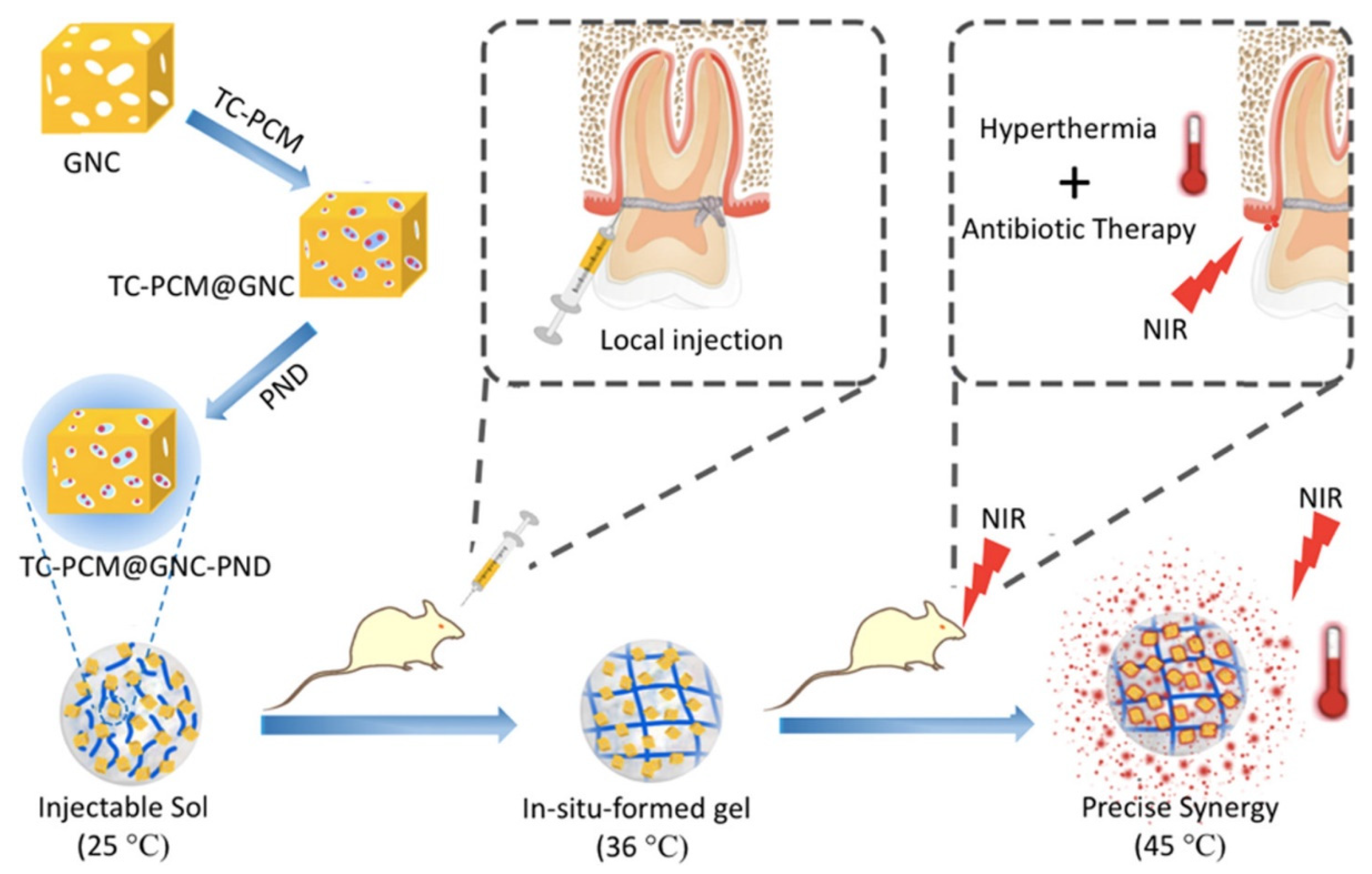
| poly(N-isopropylacrylamide-co-diethylaminoethyl methacrylate) (PND) | Chemical crosslinking | Gold nanocages (GNC) | Tetracycline(TC) | Periodontal antibacterial | Near infrared light (NIR) light controlling drug release through the dual thermosensitive interaction of liquid-solid transition of PCM and coil-granule transition of PND. | [61] | |
| Self-assembling peptides (SAP) hydrogel (P11-4 and P11-28/29) | Physical crosslinking | / | Tetracycline, ciprofloxacin, and doxycycline | Periodontal tissue regeneration and antibacterial | Biocompatibility; cargo-loading capacity; tunable physicochemical and mechanical properties. | [62] | |
| Polyethylene glycol diacrylate (PEG-DA) based scaffolds, dithiothreitol (DTT), and a novel designed functional peptide module (FPM) | Chemical crosslinking | / | Stromal cell derived factor-1 (SDF-1) | Periodontal tissue regeneration and antibacterial | PEGPD@SDF-1 hydrogel exhibited preferable biocompatibility and could promote the proliferation, migration, osteogenic differentiation of periodontal ligament stem cells (PDLSCs) and inhibit the growth of Porphyromonas gingivalis. | [63] | |
| Adhesive | Methylcellulose | Physical crosslinking | Mesoporous silica nanoparticles (MSNs) or none | DOX; Melissa officinalis oil | Oral cancer; Oral mucosal disease | Biocompatibility; controllable mechanical performance; thermosensitive and injectable characteristics. | [64,65] |
| Hydroxypropyl methylcellulose (HPMC) | Chemical crosslinking | Transfersomes or none | Prilocaine hydrochloride and lidocaine hydrochloride; 5-Fluorouracil and Etodolac; polyaspartic acid-stabilized amorphous calcium phosphate (PAsp-ACP) nanoparticles | Topical anesthesia; Oral cancer; Biomimetic mineralization | HPMC can be desiccated to form a dry film. In a moist environment, this film gradually changes into a gel. | [6,66,67] | |
| PAM-PDA | Physical crosslinking | AuNPs | Medical anesthetic | Topical anesthesia | This hydrogel with microneedle resulted in reduced pain, higher anesthetic accuracy and faster recovery. | [68] | |
| Carbopol | Physical crosslinking | Poly(ε-caprolactone) nanocapsules | Lidocaine and prilocaine | Topical anesthesia | Non-Newtonian pseudoplastic flows; satisfactory mucoadhesive strength; non-cytotoxicity; slow permeation across oral mucosa. | [69] | |
| Polyaldehyde dextran and chitosan | Chemical crosslinking | / | Silver nanoparticles | Oral cancer | Antitumor responses were enhanced by the subcutaneous delivery of an adhesive hydrogel incorporating silver nanoparticles (which inhibited the growth of bacteria competing with Peptostreptococcus). | [70] | |
| Acrylic acid/polyethylene glycol | Chemical crosslinking | / | Silver nanoparticles and propranolol HCl | Antibacterial | The nanocomposites show a promising self-disinfection property and mucoadhesive strength. | [71] | |
| Expansive | N-vinylpyrrolidone(NVP), 2-hydroxyethyl methacrylate (HEMA), and glycerolmonomethacrylate (GMMA) monomers with methacrylicacid (MA). | Chemical crosslinking | / | Benzocaine | Anesthesia of tissue expanders | Tissue expanders based on the controlled rate expansive hydrogels. Most of the drug (90%) was released within 48 h. | [72] |
| Nondegradable | 2-hydroxyethyl methacrylate (HEMA) and trimethylolpropane trimethacrylate (TMPT) | Chemical crosslinking | / | Cetylpyridinium chloride (CPC) | Antibacterial | Applying a non-biodegradable hydrogel to resin-based materials as a reservoir for water-soluble antimicrobials | [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Wu, D.; Tu, H.; Cao, M.; Li, M.; Peng, L.; Yang, J. Applications of Hydrogels in Drug Delivery for Oral and Maxillofacial Diseases. Gels 2023, 9, 146. https://doi.org/10.3390/gels9020146
Liu L, Wu D, Tu H, Cao M, Li M, Peng L, Yang J. Applications of Hydrogels in Drug Delivery for Oral and Maxillofacial Diseases. Gels. 2023; 9(2):146. https://doi.org/10.3390/gels9020146
Chicago/Turabian StyleLiu, Lijia, Dan Wu, Heng Tu, Mengjiao Cao, Mengxin Li, Li Peng, and Jing Yang. 2023. "Applications of Hydrogels in Drug Delivery for Oral and Maxillofacial Diseases" Gels 9, no. 2: 146. https://doi.org/10.3390/gels9020146
APA StyleLiu, L., Wu, D., Tu, H., Cao, M., Li, M., Peng, L., & Yang, J. (2023). Applications of Hydrogels in Drug Delivery for Oral and Maxillofacial Diseases. Gels, 9(2), 146. https://doi.org/10.3390/gels9020146





