Cryostructuring of Polymeric Systems: 64. Preparation and Properties of Poly(vinyl alcohol)-Based Cryogels Loaded with Antimicrobial Drugs and Assessment of the Potential of Such Gel Materials to Perform as Gel Implants for the Treatment of Infected Wounds †
Abstract
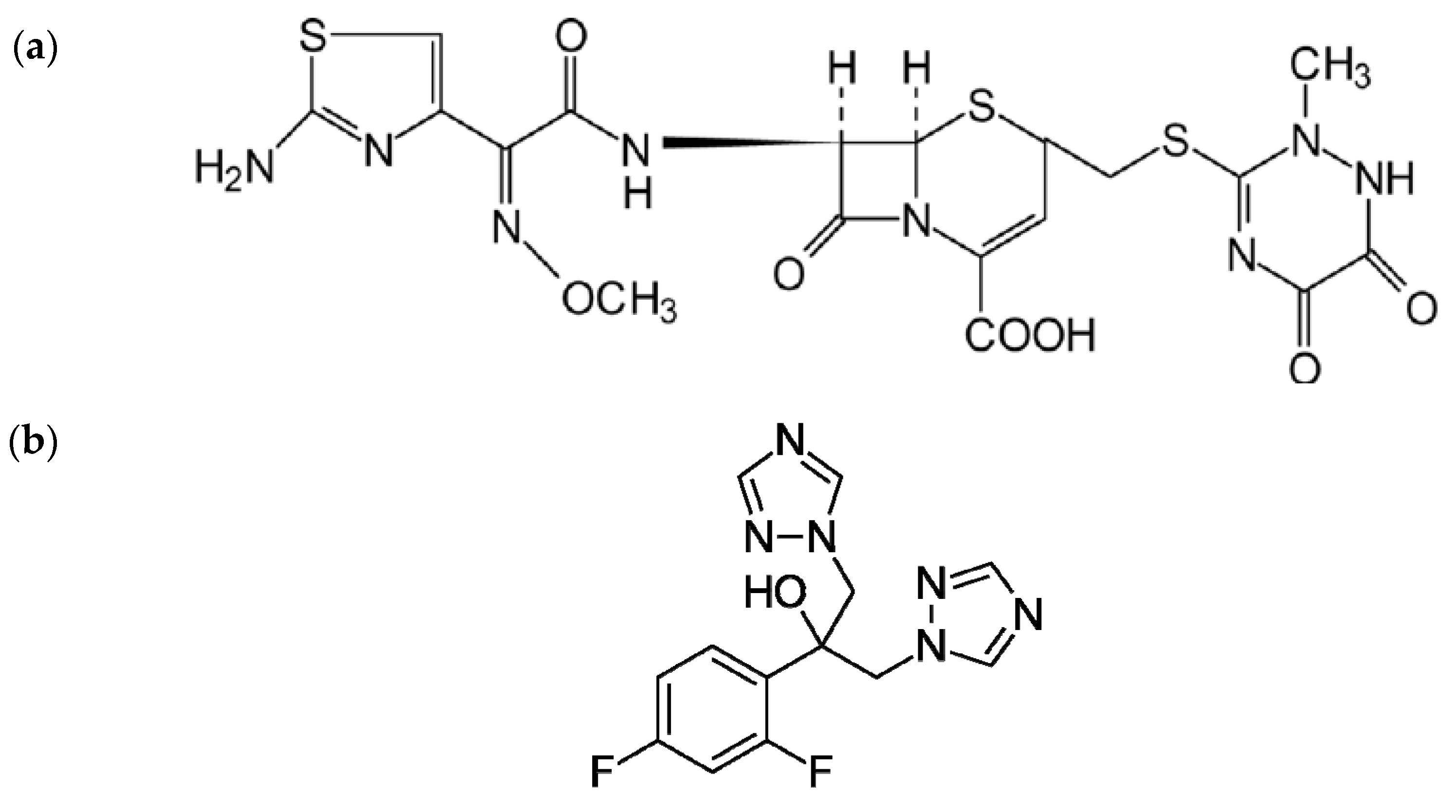
1. Introduction
2. Results and Discussion
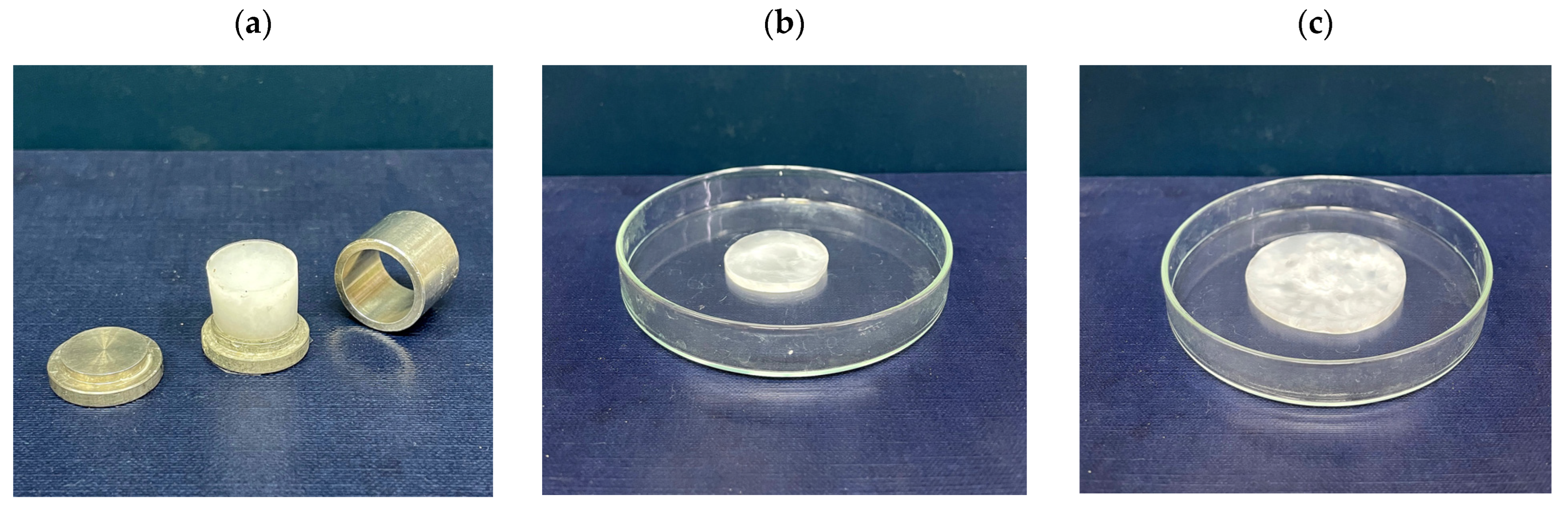
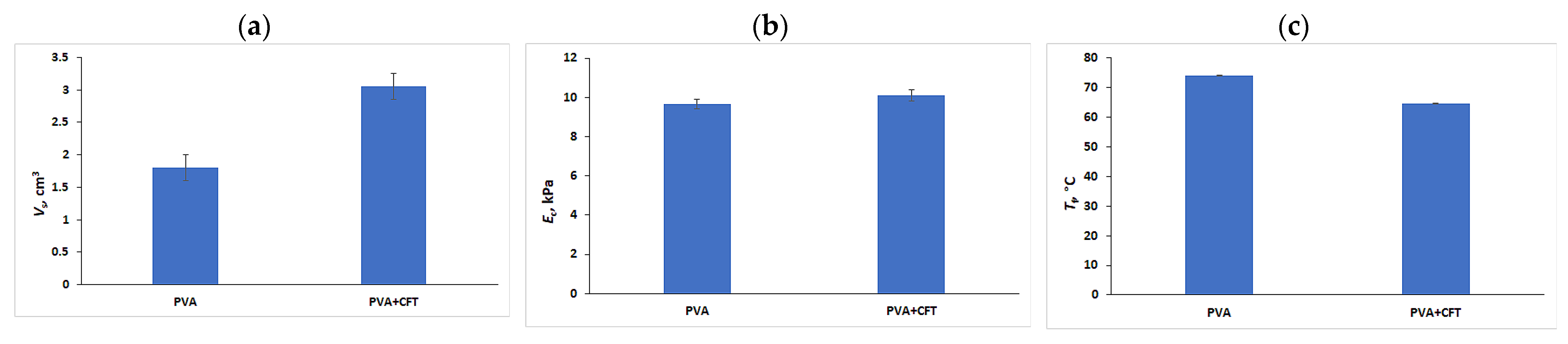
2.1. Preparation and Physicochemical Properties of the Drug-Free and Drug-Loaded PVA Cryogels
- (i)
- The first way is the incorporation of desired medications in the precursor PVA solution followed by its gelation, thus entrapping the target substances in the resultant gel carrier. It is this same method as the preparation of the antibiotic-carrying PVA cryogels used in the known studies [83,84,85,86,87] mentioned in ‘Introduction’.
- (ii)
- The second approach is the initial preparation of the drug-free gel matter, its rinsing, when it is required, from the possible admixtures, and then drug uploading into the gel by its immersion and incubation in the drug-containing solution for the saturation of the carrier with target substances.
2.2. CFT Release from the Drug-Loaded PVA Cryogels
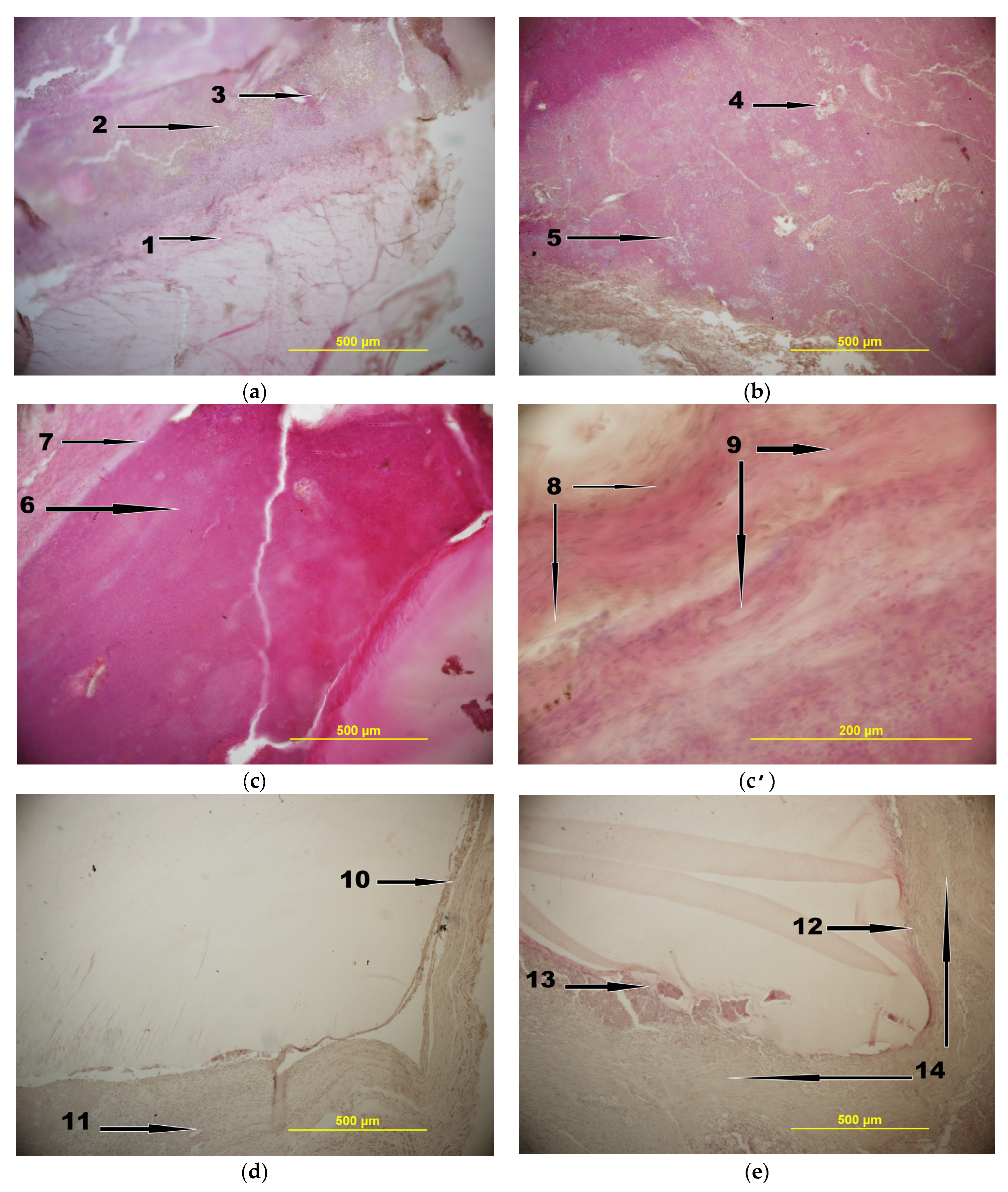
2.3. In Vivo Experiments
3. Conclusions
4. Materials and Methods
4.1. Chemicals
4.2. Preparation of PVA Cryogels
4.3. Loading and Release of Antimicrobial Drugs into and from the PVACGs
4.4. Physical Properties of PVACGs
4.5. Characterization of Antibacterial Activity of the CFT-Containing PVACGs
4.6. In Vivo Experiments
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nambu, M. Rubber-like poly(vinyl alcohol) gel. Kobunshi Ronbunshu 1990, 47, 695–703. (In Japanese) [Google Scholar] [CrossRef]
- Peppas, N.A.; Stauffer, S.R. Reinforced uncrosslinked poly (vinyl alcohol) gels produced by cyclic freezing-thawing processes: A short review. J. Control. Release 1991, 16, 305–310. [Google Scholar] [CrossRef]
- Lozinsky, V.I. Cryotropic gelation of poly(vinyl alcohol) solutions. Russ. Chem. Rev. 1998, 67, 573–586. [Google Scholar] [CrossRef]
- Hassan, C.M.; Peppas, N.A. Structure and applications of poly(vinyl alcohol) hydrogels produced by conventional crosslinking or by freezing/thawing methods. Adv. Polym. Sci. 2000, 153, 37–65. [Google Scholar] [CrossRef]
- Lozinsky, V.I. Cryogels on the basis of natural and synthetic polymers: Preparation, properties and areas of implementation. Russ. Chem. Rev. 2002, 71, 489–511. [Google Scholar] [CrossRef]
- Gutiérrez, M.C.; Aranaz, I.; Ferrer, M.L.; del Monto, F. Production and properties of poly(vinyl alcohol) cryogels: Recent developments. In Macroporous Polymers: Production, Properties and Biological/Biomedical Applications; Mattiasson, B., Kumar, A., Galaev, I., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 83–115. ISBN 978-1-4200-8461-0. [Google Scholar]
- Gun’ko, V.M.; Savina, I.N.; Mikhalovsky, S.V. Cryogels: Morphological, structural and adsorption characterization. Adv. Coll. Interface Sci. 2013, 187–188, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Lozinsky, V.I.; Okay, O. Basic principles of cryotropic gelation. Adv. Polym. Sci. 2014, 263, 49–102. [Google Scholar] [CrossRef]
- Lozinsky, V.I. Cryostructuring of polymeric systems. 55. Retrospective view on the more than 40-years studies performed in the A.N.Nesmeyanov Institute of Organoelement Compounds with respect of the cryostructuring processes in polymeric systems. Gels 2020, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Adelnia, H.; Ensandoost, R.; Moonshi, S.S.; Gavgani, J.N.; Vasafi, E.I.; Ta, H.T. Freeze/thawed polyvinyl alcohol hydrogels: Present, past and future. Eur. Polym. J. 2022, 164, 110974. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Vakula, A.V.; Zubov, A.L. Application of poly(vinyl alcohol) cryogels in biotechnology. IV. Literature data overview. Sov. Biotechnol. 1992, 4, 1–11. [Google Scholar]
- Varfolomeev, S.D.; Rainina, E.I.; Lozinsky, V.I. Cryoimmobilized enzymes and cells in organic synthesis. Pure Appl. Chem. 1992, 64, 1193–1196. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Plieva, F.M. Poly(vinyl alcohol) cryogels employed as matrices for cell immobilization. 3. Overview of recent research and developments. Enzyme Microb. Technol. 1998, 23, 227–242. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Plieva, F.M.; Galaev, I.Y.; Mattiasson, B. The potential of polymeric cryogels in bioseparation. Bioseparation 2001, 10, 163–188. [Google Scholar] [CrossRef]
- Bacheva, A.V.; Plieva, F.M.; Lysogorskaya, E.N.; Filippova, I.Y.; Lozinsky, V.I. Peptide synthesis in organic media with subtil- isin 72 immobilized on poly(vinyl alcohol)-cryogel carrier. Bioorg. Med. Chem. Lett. 2001, 11, 1005–1008. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Galaev, I.Y.; Plieva, F.M.; Savina, I.N.; Jungvid, H.; Mattiasson, B. Polymeric cryogels as promising materials of biotechnological interest. Trends Biotechnol. 2003, 21, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Plieva, F.M.; Galaev, I.Y.; Noppe, W.; Mattiasson, B. Cryogel applications in microbiology. Trends Microbiol. 2008, 16, 543–551. [Google Scholar] [CrossRef]
- Mattiasson, B. Cryogels for biotechnological applications. Adv. Polym. Sci. 2014, 263, 245–282. [Google Scholar] [CrossRef]
- Berillo, D.; Al-Jwaid, A.; Caplin, J. Polymeric materials used for immobilization of bacteria for the bioremediation of contaminants in water. Polymers 2021, 13, 1073. [Google Scholar] [CrossRef]
- Lazzeri, L. Progress in bioartificial polymeric materials. Trends Polym. Sci. 1996, 4, 249–252. [Google Scholar]
- Chu, K.C.; Rutt, B.K. Poly(vinyl alcohol) cryogel: An ideal phantom material for MR studies of arterial flow and elasticity. Magn. Reson. Med. 1997, 37, 314–319. [Google Scholar] [CrossRef]
- Kokabi, M.; Sirousazar, M.; Hassan, Z.M. PVA-clay nanocomposite hydrogels for wound dressing. Eur. Polym. J. 2007, 43, 773–781. [Google Scholar] [CrossRef]
- Pan, Y.; Xiong, D.; Chen, X. Mechanical properties of nanohydroxyapatite reinforced poly(vinyl alcohol) gel composites as biomaterial. J. Mater. Sci. 2007, 42, 5129–5134. [Google Scholar] [CrossRef]
- Hoskins, P.R. Simulation and validation of arterial ultrasound imagining and blood flow. Ultrasound Med. Biol. 2008, 34, 693–717. [Google Scholar] [CrossRef]
- Ghanbari, H.; Viatage, H.; Kidane, A.G.; Burriesci, G.; Tavakoli, M.; Seifalian, A.M. Polymeric heart valves: New materials, emerging hopes. Trends Biotechnol. 2009, 27, 359–367. [Google Scholar] [CrossRef]
- Alves, M.H.; Jensen, B.E.B.; Smith, A.A.A.; Zelikin, A.N. Poly(vinyl alcohol) physical hydrogels: New vista on a long serving biomaterial. Macromol. Biosci. 2011, 11, 1293–1313. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Bae, H.; Chung, N.; Lee, H.; Choi, S.; Hwang, S.; Lee, J. Freezing/thawing processing of PVA in the preparation of structured microspheres for protein drug delivery. Macromol. Res. 2011, 19, 130–136. [Google Scholar] [CrossRef]
- Baker, M.I.; Walsh, S.P.; Schwatz, Z.; Boyan, B.D. A review of polyvinyl alcohol and its uses in cartilage and orthopedic applications. J. Biomed. Mater. Res. B 2012, 100, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Gajra, B.; Pandya, S.S.; Vidyasagar, G.; Rabari, H.; Dedania, R.R.; Rao, S. Poly(vinyl alcohol) hydrogel and its pharmaceutical and biomedical applications: A review. Int. J. Pharm. Res. 2012, 4, 20–26. [Google Scholar]
- Maiolo, A.S.; Amado, M.N.; Gonzalez, J.S.; Alvarez, V.A. Development and characterization of poly(vinyl alcohol) hydrogels for potential use as an articular cartilage replacement. Mater. Sci. Eng. C 2012, 32, 1490–1495. [Google Scholar] [CrossRef]
- Iatridis, J.C.; Nicoll, S.B.; Michalek, A.J.; Walter, B.A.; Gupta, M.S. Role of biomechanics in intervertebral disc degeneration and regenerative therapies: What needs repairing in the disc and what are promising biomaterials for its repair? Spine J. 2013, 13, 243–262. [Google Scholar] [CrossRef]
- Wan, W.; Bannerman, A.D.; Yang, L.; Mak, H. Poly(vinyl alcohol) cryogels for biomedical applications. Adv. Polym. Sci. 2014, 263, 283–321. [Google Scholar] [CrossRef]
- Teixeira, L.S.M.; Patterson, J.; Luyten, F.P. Skeletal tissue regeneration: Where can hydogels play a role? Int. Orthop. 2014, 38, 1861–1876. [Google Scholar] [CrossRef] [PubMed]
- Beddoes, C.M.; Whitehouse, M.R.; Briscoe, W.H.; Su, B. Hydrogels as replacement materials for damaged articular hyaline cartilage. Materials 2016, 9, 443. [Google Scholar] [CrossRef]
- Kumar, A.; Han, S.S. PVA-based hydrogels for tissue engineering: A review. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 159–182. [Google Scholar] [CrossRef]
- Timofejeva, A.; D’Este, M.; Loca, D. Calcium phosphate/polyvinyl alcohol composite hydrogels: A review on the freeze-thaw synthesis approach and applications in regenerative medicine. Eur. Polym. J. 2017, 95, 547–565. [Google Scholar] [CrossRef]
- Teodorescu, M.; Bercea, M.; Morariu, S. Biomaterials of poly(vinyl alcohol) and natural polymers. Polym. Revs. 2018, 58, 247–287. [Google Scholar] [CrossRef]
- Teodorescu, M.; Bercea, M.; Morariu, S. Biomaterials of PVA and PVP in medical and pharmaceutical applications: Perspectives and challenges. Biotechnol. Adv. 2019, 37, 109–131. [Google Scholar] [CrossRef]
- Memic, A.; Colombani, T.; Eggermont, L.J.; Rezaeeyazdi, M.; Steingold, J.; Rogers, Z.J.; Navare, K.J.; Mohammed, H.S.; Bencherif, S.A. Latest advances in cryogel technology for biomedical applications. Adv. Ther. 2019, 2, 1800114. [Google Scholar] [CrossRef]
- Xiang, J.; Shen, L.; Hong, Y. Status and future of hydrogels in wound healing: Synthesis, materials and evaluation. Eur. Polym. J. 2020, 130, 109609. [Google Scholar] [CrossRef]
- Tamer, T.M.; Sabet, M.M.; Omer, A.M.; Abbas, E.; Eid, A.I.; Mohy-Eldin, M.S.; Hassan, M.A. Hemostatic and antibacterial PVA/kaolin composite sponges loaded with penicillin-streptomycin for wound dressing applications. Sci. Rep. 2021, 11, 3428. [Google Scholar] [CrossRef]
- Savina, I.N.; Zoughaib, M.; Yergushov, A.A. Design and assessment of biodegradable macroporous cryogels as advanced tissue engineering and drug carrying materials. Gels 2021, 7, 79. [Google Scholar] [CrossRef]
- Aderibigbe, B.A. Hybrid-based wound dressing: Combination of synthetic and biopolymers. Polymers 2022, 14, 3806. [Google Scholar] [CrossRef]
- Akin, A.; Ozmen, M.M. Antimicrobial cryogel dressings towards effective wound healing. Prog. Biomater. 2022, 11, 331–346. [Google Scholar] [CrossRef]
- Watase, M.; Nishinari, K.; Nambu, M. Rheological properties of an anomalous poly(vinyl alcohol) gel. Polym. Comm. 1983, 24, 52–54. [Google Scholar]
- Rogozhin, S.V.; Lozinsky, V.I.; Vainerman, E.S.; Domotenko, L.V.; Mamtsis, A.M.; Ivanova, S.A.; Shtilʹman, M.I.; Korshak, V.V. Noncovalent cryostructurization in polymer systems. Dokl. Akad. Nauk SSSR 1984, 278, 129–133. (In Russian) [Google Scholar]
- Lozinsky, V.I.; Vainerman, E.S.; Domotenko, L.V.; Mamtsis, A.M.; Titova, E.F.; Belavtseva, E.M.; Rogozhin, S.V. Study of cryostructurization of polymer systems. VII. Structure formation under freezing of poly(vinyl alcohol) aqueous solutions. Colloid Polym. Sci. 1986, 264, 19–24. [Google Scholar] [CrossRef]
- Yokoyama, F.; Masada, I.; Shimamura, K.; Ikawa, T.; Monobe, K. Morphology and structure of highly elastic poly(vinyl alcohol) hydrogel prepared by repeated freezing-and-melting. Colloid Polym. Sci. 1986, 264, 595–601. [Google Scholar] [CrossRef]
- Domotenko, L.V.; Lozinsky, V.I.; Vainerman, E.S.; Rogozhin, S.V. Influence of freezing and thawing conditions of poly(vinyl alcohol) aqueous solutions on the properties of obtained cryogels. Polymer Sci. USSR Ser. A 1988, 30, 1758–1764. [Google Scholar] [CrossRef]
- Watase, M.; Nishinari, K. Thermal and rheological properties of polyvinyl alcohol hydrogels prepared by repeated cycles of freezing and thawing. Macromol. Chem. 1988, 189, 871–880. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Domotenko, L.V.; Zubov, A.L.; Simenel, I.A. Study of cryostructuration of polymer systems. 12. Poly(vnyl alcohol) cryogels: Influence of low-molecular electrolytes. J. Appl. Polym. Sci. 1996, 61, 1991–1998. [Google Scholar] [CrossRef]
- Mori, Y.; Tokura, H.; Yoshikawa, M. Properties of hydrogels synthesized by freezing and thawing aqueous poly(vinyl alcohol) solutions and their applications. J. Mater. Sci. 1997, 32, 491–496. [Google Scholar] [CrossRef]
- Willcox, P.J.; Howie, D.W.; Schmidt-Rohr, K.; Hoagland, D.A.; Gido, S.P.; Pudjijanto, S.; Kleiner, W.; Venkatraman, S. Microstructure of poly(vinyl alcohol) hydrogels produced by freeze/thaw cycling. J. Polym. Sci. B. Polym. Phys. 1999, 37, 3438–3454. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Damshkaln, L.G.; Shaskol’skii, B.L.; Babushkina, T.A.; Kurochkin, I.N.; Kurochkin, I.I. Study of cryostructuring of polymer systems. 27. Physicochemical properties of poly(vinyl alcohol) cryogels and features of their macroporous morphology. Colloid J. 2007, 69, 747–764. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Damshkaln, L.G.; Kurochkin, I.N.; Kurochkin, I.I. Study of cryostructuring of polymer systems. 28. Physicochemical properties and morphology of poly(vinyl alcohol) cryogels formed by multiple freezing-thawing. Colloid J. 2008, 70, 189–198. [Google Scholar] [CrossRef]
- Patachia, S.; Florea, C.; Friedrich, C.; Thomann, Y. Tailoring of poly(vinyl alcohol) cryogels properties by salts addition. Express Polym. Lett. 2009, 3, 320–331. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, F.; Wu, J. Physically crosslinked hydrogels prepared by freeze-thaw technique. React. Func. Polym. 2013, 73, 923–928. [Google Scholar] [CrossRef]
- Lozinsky, V.I. A breif history of polymeric cryogels. Adv. Polym. Sci. 2014, 263, 1–48. [Google Scholar] [CrossRef]
- Masri, C.; Chagnon, G.; Favier, D. Influence of processing parameters on the macroscopic mechanical behavior of PVA hydrogels. Mater. Sci. Eng. C 2017, 75, 769–776. [Google Scholar] [CrossRef]
- Chee, B.S.; de Lima, G.G.; Devine, D.M.; Nugent, M.J.D. Investigation of the effects of orientation on freeze/thaw polyvinyl alcohol hydrogel properties. Mater. Today Commun. 2018, 17, 82–93. [Google Scholar] [CrossRef]
- Kolosova, O.Y.; Kurochkin, I.N.; Kurochkin, I.I.; Lozinsky, V.I. Cryostructuring of polymeric systems. 48. Influence of organic non-ionic and ionic chaotropes or kosmotropes on the cryotropic gel-formation of aqueous poly(vinyl alcohol) solutions, as well as on the properties and microstructure of the resultant cryogels. Eur. Polym. J. 2018, 102, 169–177. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Kolosova, O.Y.; Michurov, D.A.; Dubovik, A.S.; Vasil’ev, V.G.; Grinberg, V.Y. Cryostructuring of polymeric systems. 49. Unexpected “kosmotropic-like” impact of organic chaotropes on the PVA freeze-thaw-induced gelation in DMSO. Gels 2018, 4, 81. [Google Scholar] [CrossRef]
- Kurochkin, I.I.; Kurochkin, I.N.; Kolosova, O.Y.; Lozinsky, V.I. Cryostructuring of polymeric systems. 56. Application of deep neural networks for the classification of structural features peculiar to macroporous poly(vinyl alcohol) cryogels prepared witout and with the additives of chaotropes or kosmotropes. Molecules 2020, 25, 4480. [Google Scholar] [CrossRef]
- Joshi, N.; Suman, K.; Joshi, Y.M. Rheological behavior of aqueous poly(vinyl alcohol) solution during freeze-thaw gelation process. Macromolecules 2020, 53, 3452–3463. [Google Scholar] [CrossRef]
- Solomennyi, A.M. Mathematical substantiation of the technology of creating a pharmaceutical composition in the form of cryogel. Pharmacophore 2021, 12, 98–105. [Google Scholar] [CrossRef]
- Michurov, D.A.; Kolosova, O.Y.; Lozinsky, V.I. Cryostructuring of polymeric systems. 61. Physicochemical properties of poly(vinyl alcohol) cryogels prepared on the basis of urea-containing DMSO-solutions of the polymer and evaluation of the resultant gel materials as potential drug carriers. Bull. Univ. Karaganda—Chem. 2022, 107, 75–86. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Lu, J.; Ding, M.; Chen, Y. Synthesis and properties of poly(vinyl alcohol) hydrogels with high strength and toughness. Polym. Test. 2022, 108, 107516. [Google Scholar] [CrossRef]
- Rivera-Hernández, G.; Antunes-Ricardo, M.; Martínez-Morales, P.; Sánchez, L. Poly(vinyl alcohol)-based drug delivery systems for cancer treatment. Internat. J. Pharm. 2021, 600, 120478. [Google Scholar] [CrossRef] [PubMed]
- Oustadi, F.; Nazarpak, M.H.; Mansouri, M.; Ketabat, F. Preparation, characterization, and drug release study of ibuprofen-loaded poly(vinyl alcohol)/poly(vinyl pyrrolidone) bilayer antibacterial membrane. Internat. J. Polym. Mater. Polym. Biomater. 2022, 71, 14–23. [Google Scholar] [CrossRef]
- Demir, D.; Özdemir, S.; Gonca, S.; Bölgen, N. Novel styrax liquidus loaded chitosan/polyvinyl alcohol cryogels with antioxidant and antimicrobial properties. J. Appl. Polym. Sci. 2022, 139, 52033. [Google Scholar] [CrossRef]
- Chandika, P.; Kim, M.-S.; Khan, F.; Kim, Y.-M.; Heo, S.-Y.; Oh, G.-W.; Kim, N.G.; Jung, W.-K. Wound healing properties of triple cross-linked poly(vinyl alcohol)/methacrylate kappa-carrageenan/chitooligosaccharide hydrogel. Carbohydr. Polym. 2021, 269, 118272. [Google Scholar] [CrossRef]
- Özbaş, Z.; Özkahraman, B.; Bayrak, G.; Süloğlu, A.K.; Perçin, I.; Boran, F.; Tamahkar, E. Poly (vinyl alcohol)/(hyaluronic acid-g-kappa-carrageenan) hydrogel as antibiotic-releasing wound dressing. Chem. Pap. 2021, 75, 6591–6600. [Google Scholar] [CrossRef]
- Xiong, S.; Li, R.; Ye, S.; Ni, P.; Shan, J.; Yuan, T.; Liang, J.; Fan, Y.; Zhang, X. Vanilin enhances the antibacterial and antioxidant properties of polyvinyl alcohol-chtosan hydrohel dressings. Internat. J. Biol. Macromol. 2022, 220, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Branco, A.C.; Oliveira, A.; Monteiro, I.; Nolasco, P.; Silva, D.C.; Figueiredo-Pina, C.G.; Colaço, R.; Serro, A.P. PVA-based hydrogels loaded with diclofenac for cartilage replacement. Gels 2022, 8, 143. [Google Scholar] [CrossRef] [PubMed]
- Bercea, M.; Gradinaru, L.-M.; Morariu, S.; Plugariu, I.-A.; Gradinaru, R.V. Tailoring the properties of PVA/HPC/BSA hydrogels for wound dressing applications. React. Func. Polym. 2022, 170, 105094. [Google Scholar] [CrossRef]
- Xiang, D.; Cui, Y.; Wan, Z.; Wang, S.; Peng, L.; Liao, Z.; Chen, C.; Liu, W. Study on swelling, compression properties and degradation stability of PVA composite hydrogels for artificial nucleus pulposus. J. Mech. Behav. Biomed. Mater. 2022, 136, 105496. [Google Scholar] [CrossRef]
- Jalageri, M.B.; Kumar, G.C. Hydroxyapatite reinforced polyvinyl alcohol/polyvinyl pyrrolidone based hydrogel for cartilage replacement. Gels 2022, 8, 555. [Google Scholar] [CrossRef]
- Crolla, J.P.; Britton, M.M.; Espino, D.M.; Thomas-Seale, L.E.J. The dynamic viscoelastic characterisation and magnetic resonance imaging of poly(vinyl alcohol) cryogel: Identifying new attributes and opportunities. Mater. Sci. Eng. C. 2021, 129, 112383. [Google Scholar] [CrossRef]
- Antoniou, A.; Damianou, C. MR relaxation properties of tissue-mimicking phantoms. Ultrasonics 2022, 119, 106600. [Google Scholar] [CrossRef]
- Villa, E.; Arteaga-Marrero, N.; González-Fernández, J.; Ruiz-Alzola, J. Bimodal microwave and ultrasound phantoms for non-invasive clinical imaging. Sci. Rep. 2020, 10, 20401. [Google Scholar] [CrossRef]
- Al-Mutairi, F.F.; Chung, E.M.L.; Moran, C.M.; Ramnarine, K.V. A novel elastography phantom prototype for assessment of ultrasound elastography performance. Ultrasound Med. Biol. 2021, 47, 2749–2758. [Google Scholar] [CrossRef]
- Bonanthaya, K.; Panneerselvam, E.; Manuel, S.; Kumar, V.V.; Rain, A. (Eds.) Oral and Maxillofacial Surgery for the Clinician; Springer Nature Singapore Ltd.: Singapore, 2021; 1965p, ISBN 978-981-15-1345-9. [Google Scholar]
- Shaikhaliev, A.I.; Korshakov, E.V.; Kolosova, O.Y.; Krasnov, M.S.; Lozinsky, V.I. Temporary Implant for the Patients with Infected Defects in the Maxillofacial Region and a Method for Treating Them Using such an Implant. Russian Patent 2,729,929, 13 August 2020. [Google Scholar]
- Moretto, A.; Tesolin, L.; Marsilio, F.; Schiavon, M.; Berna, M.; Veronese, F.M. Slow release of two antibiotics of veterinary interest from PVA hydrogels. Il Farmaco 2004, 59, 1–5. [Google Scholar] [CrossRef]
- Martinez, Y.N.; Piñuel, L.; Castro, G.R.; Breccia, J.D. Polyvinyl alcohol–pectin cryogel films for controlled release of enrofloxacin. Appl. Biochem. Biotechnol. 2012, 167, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Lotfipour, F.; Alami-Milani, M.; Salatin, M.; Hadavi, A.; Jelvehgari, M. Freeze-thaw-induced cross-linked PVA/chitosan for oxytetracycline-loaded wound dressing: The experimental design and optimization. Res. Pharm. Sci. 2019, 14, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Tamahkar, E. Bacterial cellulose/poly vinyl alcohol based wound dressings with sustained antibiotic delivery. Chem. Pap. 2021, 75, 3979–3987. [Google Scholar] [CrossRef]
- Foye, W.O. Foye’s Principles of Medicinal Chemistry, 7th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; 1500p, ISBN 9781609133450. [Google Scholar]
- Available online: https://en.wikipedia.org/wiki/Ceftriaxone (accessed on 27 January 2023).
- Available online: https://en.wikipedia.org/wiki/Fluconazole (accessed on 27 January 2023).
- Langer, R.; Peppas, N.A. Advances in biomaterials, drug delivery, and bionanotechnology. AIChE J. 2003, 49, 2990–3006. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Domotenko, L.V.; Vainerman, E.S.; Mamtsis, A.M.; Rogozhin, S.V. On the possibility of mechanodestruction of poly(vinyl alcohol) molecules under moderate freezing of its concentrated water solutions. Polym. Bull. 1986, 15, 333–340. [Google Scholar] [CrossRef]
- Nishinari, K.; Watase, M.; Tanaka, F. Structure of junction zones in poly(vinyl alcohol) gels by rheological and thermal studies. J. Chim. Phys. Phys. Chim. Biol. 1996, 93, 880–886. [Google Scholar] [CrossRef]
- Masuda, K.; Horii, F. CP/MAS 13C NMR analyses of the chain conformation and hydrogen bonding for frozen poly(vinyl alcohol) solutions. Macromolecules 1998, 31, 5810–5817. [Google Scholar] [CrossRef]
- Eldridge, J.E.; Ferry, J.D. Studies of the cross-linking process in gelatin gels. III. Dependence of melting point on concentra- tion and molecular weight. J. Phys. Chem. 1954, 58, 992–995. [Google Scholar] [CrossRef]
- Tako, M.; Nakamura, S. Gelation mechanism of agarose. Carbohydr. Res. 1988, 180, 277–284. [Google Scholar] [CrossRef]
- Papadopoulou, V.; Kosmidis, K.; Vlachou, M.; Macheras, P. On the use of the Weibull function for the discernment of drug release mechanisms. Int. J. Pharm. 2006, 309, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Narasimhan, B. Mathematical models in drug delivery: How modeling has shaped the way we design new drug delivery systems. J. Control. Release 2014, 190, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Kosmidis, K.; Argyrakis, P.; Macheras, P. A reappraisal of drug release laws using Monte Carlo simulations: The prevalence of the Weibull function. Pharm. Res. 2003, 20, 988–995. [Google Scholar] [CrossRef]
- Bonev, B.; Hooper, J.; Parisot, J. Principles of assessing bacterial susceptibility to antibiotics using the agar diffusion method. J. Antimicrob. Chemother. 2008, 61, 1295–1301. [Google Scholar] [CrossRef]
- Barwick, R. Rabbit nutrition. Vet. Nurs. J. 2000, 15, 94–100. [Google Scholar] [CrossRef]
- Chiang, P.J.; Tseng, M.J.; He, Z.S.; Li, C.H. Automated counting of bacterial colonies by image analysis. J. Microbiol. Methods 2015, 108, 74–82. [Google Scholar] [CrossRef]


| Bacterial | GIZ Diameter (mm) after Cell Cultivation for Definite Time | ||
|---|---|---|---|
| Strain | 24 h | 48 h | 72 h |
| Staphylococcus aureus | 47 ± 2 | 52 ± 2 | 65 ± 2 |
| Escherichia coli | 48 ± 2 | 48 ± 2 | 44 ± 2 |
| Pseudomonas fluorescens | 18 ± 1 | 22 ± 1 | 25 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolosova, O.Y.; Shaikhaliev, A.I.; Krasnov, M.S.; Bondar, I.M.; Sidorskii, E.V.; Sorokina, E.V.; Lozinsky, V.I. Cryostructuring of Polymeric Systems: 64. Preparation and Properties of Poly(vinyl alcohol)-Based Cryogels Loaded with Antimicrobial Drugs and Assessment of the Potential of Such Gel Materials to Perform as Gel Implants for the Treatment of Infected Wounds. Gels 2023, 9, 113. https://doi.org/10.3390/gels9020113
Kolosova OY, Shaikhaliev AI, Krasnov MS, Bondar IM, Sidorskii EV, Sorokina EV, Lozinsky VI. Cryostructuring of Polymeric Systems: 64. Preparation and Properties of Poly(vinyl alcohol)-Based Cryogels Loaded with Antimicrobial Drugs and Assessment of the Potential of Such Gel Materials to Perform as Gel Implants for the Treatment of Infected Wounds. Gels. 2023; 9(2):113. https://doi.org/10.3390/gels9020113
Chicago/Turabian StyleKolosova, Olga Yu., Astemir I. Shaikhaliev, Mikhail S. Krasnov, Ivan M. Bondar, Egor V. Sidorskii, Elena V. Sorokina, and Vladimir I. Lozinsky. 2023. "Cryostructuring of Polymeric Systems: 64. Preparation and Properties of Poly(vinyl alcohol)-Based Cryogels Loaded with Antimicrobial Drugs and Assessment of the Potential of Such Gel Materials to Perform as Gel Implants for the Treatment of Infected Wounds" Gels 9, no. 2: 113. https://doi.org/10.3390/gels9020113
APA StyleKolosova, O. Y., Shaikhaliev, A. I., Krasnov, M. S., Bondar, I. M., Sidorskii, E. V., Sorokina, E. V., & Lozinsky, V. I. (2023). Cryostructuring of Polymeric Systems: 64. Preparation and Properties of Poly(vinyl alcohol)-Based Cryogels Loaded with Antimicrobial Drugs and Assessment of the Potential of Such Gel Materials to Perform as Gel Implants for the Treatment of Infected Wounds. Gels, 9(2), 113. https://doi.org/10.3390/gels9020113