Development of Carvedilol Nanoformulation-Loaded Poloxamer-Based In Situ Gel for the Management of Glaucoma
Abstract
:1. Introduction
2. Results and Discussion
2.1. Formulation of Carvedilol-Loaded Spanlastic In Situ Gel (CS-ISG)
2.2. Evaluation of CS-ISGs
2.2.1. Clarity
2.2.2. pH
2.2.3. Drug Content
2.2.4. Gelation Temperature
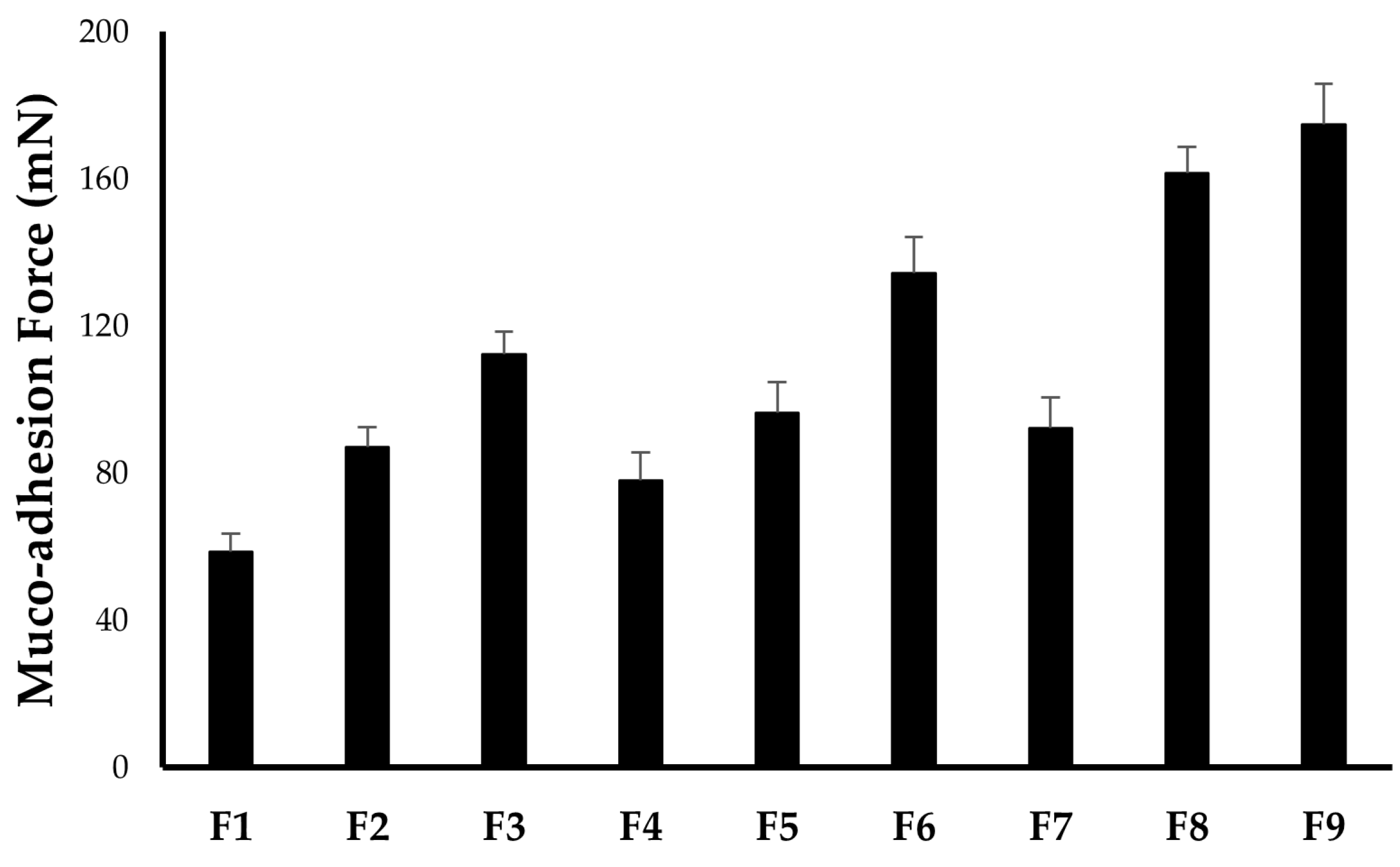
2.2.5. In Vitro Muco-Adhesion Force
2.2.6. Rheological Studies
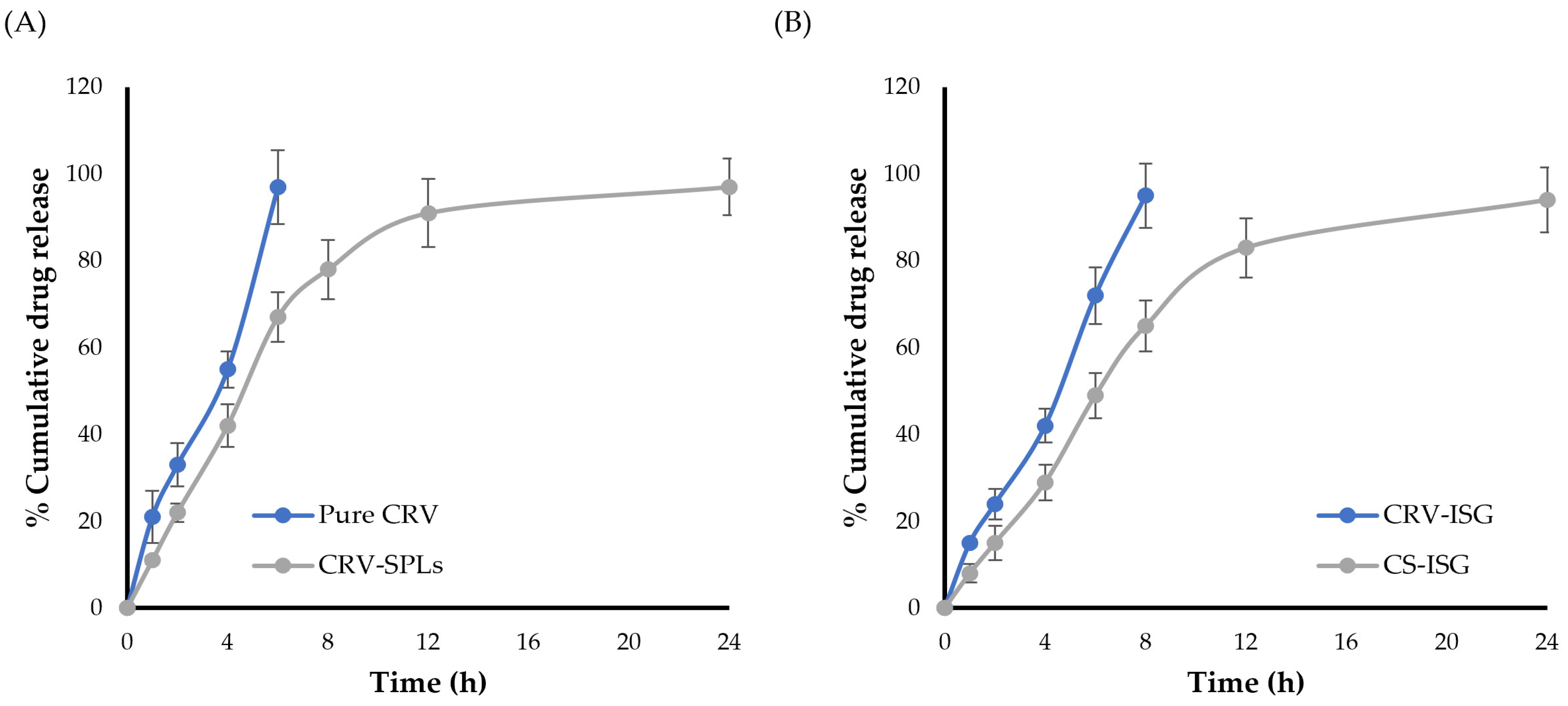
2.3. In Vitro Release Studies
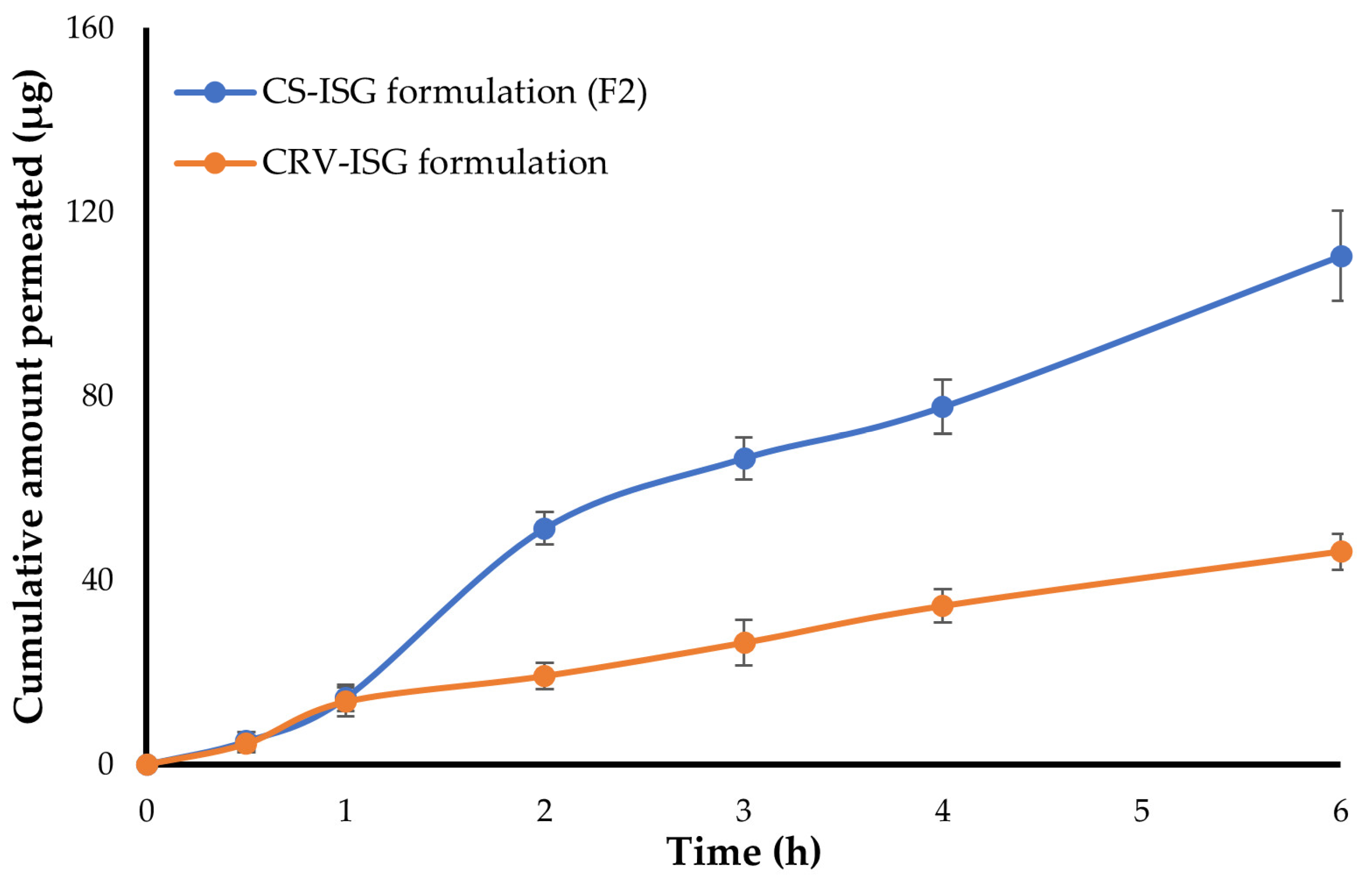
2.4. Ex Vivo Corneal Permeability
2.5. In Vitro Stability Study
2.6. In Vivo Pharmacokinetic Study
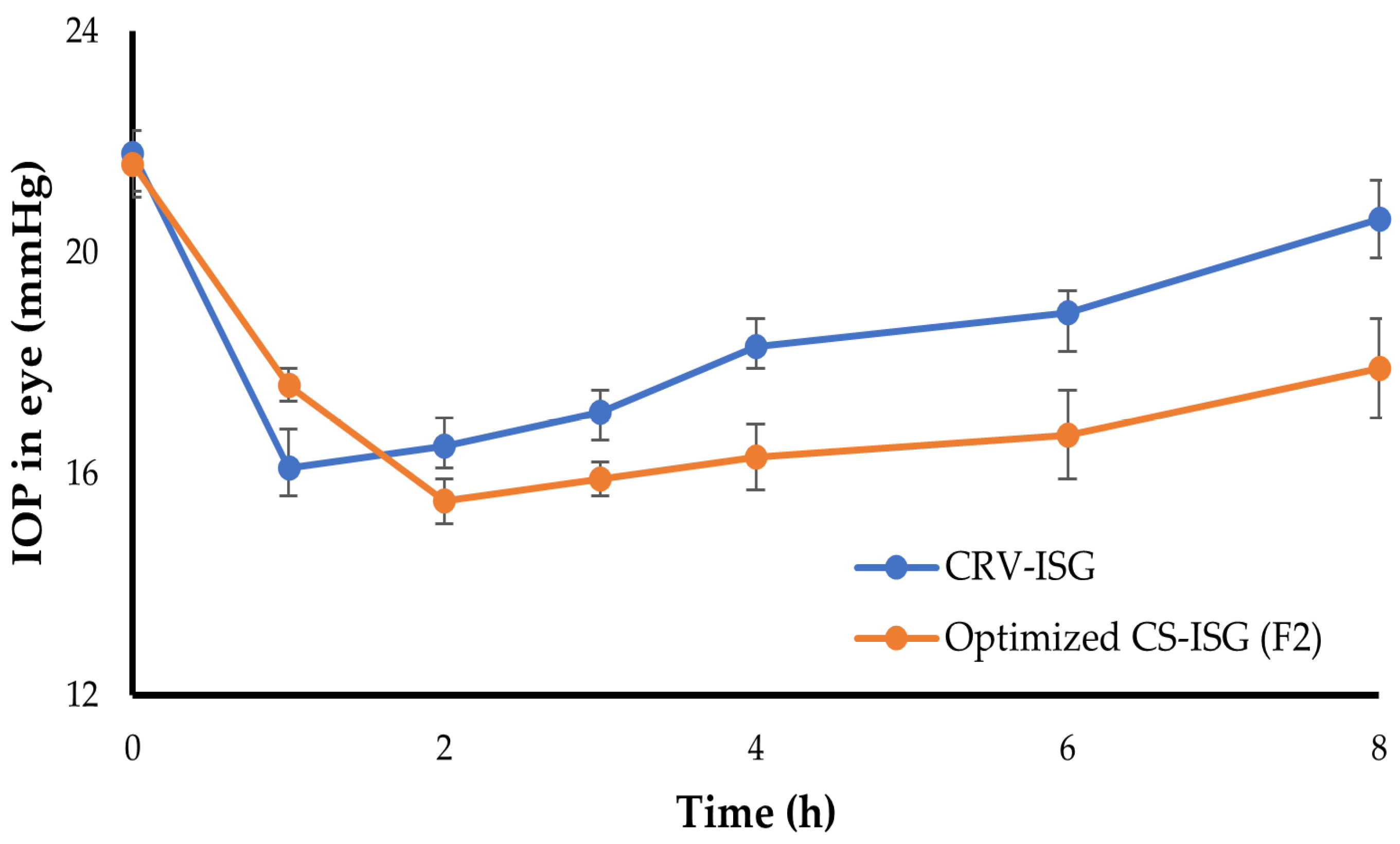
2.7. In Vivo Pharmacodynamic Study
2.8. Ocular Irritation
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Formulation of Carvedilol-Loaded Spanlastics (CRV-SPLs)
4.3. Incorporation of CRV-SPLs into In Situ Gels (CS-ISGs)
4.4. Characterization of CRV-SPLs-Loaded In Situ Gels (CS-ISGs)
4.4.1. Visual Appearance
4.4.2. pH
4.4.3. Drug Content
4.4.4. Determination of Gelation Temperature
4.4.5. Rheological Studies
4.4.6. Measurement of Muco-Adhesion Force
4.5. In Vitro Release Study
4.6. Ex Vivo Corneal Permeability Study
4.7. Stability Studies
4.8. In Vivo Experiments
4.8.1. In Vivo Pharmacokinetics
4.8.2. Pharmacodynamic Study
4.8.3. Assessment of Ocular Irritancy of CS-ISG Formulation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Alamri, A.; Bakri, K.A.; Alqarni, S.M.; Alosaimi, M.N.; Alshehri, E.Y.; Alshahrani, M.S. Knowledge and awareness of glaucoma in a population of Abha, Southern Saudi Arabia. J. Fam. Med. Prim. Care 2022, 11, 6165–6169. [Google Scholar] [CrossRef] [PubMed]
- Sharif, N.A. Therapeutic Drugs and Devices for Tackling Ocular Hypertension and Glaucoma, and Need for Neuroprotection and Cytoprotective Therapies. Front. Pharmacol. 2021, 12, 729249. [Google Scholar] [CrossRef]
- Öztürk, K.; Arslan, F.B.; Öztürk, S.C.; Çalış, S. Mixed micelles formulation for carvedilol delivery: In-vitro characterization and in-vivo evaluation. Int. J. Pharm. 2022, 611, 121294. [Google Scholar] [CrossRef] [PubMed]
- Abdelmonem, R.; Elhabal, S.F.; Abdelmalak, N.S.; El-Nabarawi, M.A.; Teaima, M.H. Formulation and Characterization of Acetazolamide/Carvedilol Niosomal Gel for Glaucoma Treatment: In Vitro, and In Vivo Study. Pharmaceutics 2021, 13, 221. [Google Scholar] [CrossRef] [PubMed]
- Hassan, D.H.; Abdelmonem, R.; Abdellatif, M.M. Formulation and Characterization of Carvedilol Leciplex for Glaucoma Treatment: In-Vitro, Ex-Vivo and In-Vivo Study. Pharmaceutics 2018, 10, 197. [Google Scholar] [CrossRef] [PubMed]
- Irimia, T.; Ghica, M.V.; Popa, L.; Anuţa, V.; Arsene, A.L.; Dinu-Pîrvu, C.E. Strategies for Improving Ocular Drug Bioavailability and Corneal Wound Healing with Chitosan-Based Delivery Systems. Polymers 2018, 10, 1221. [Google Scholar] [CrossRef]
- Rawat, P.S.; Ravi, P.R.; Mir, S.I.; Khan, M.S.; Kathuria, H.; Katnapally, P.; Bhatnagar, U. Design, Characterization and Pharmacokinetic–Pharmacodynamic Evaluation of Poloxamer and Kappa-Carrageenan-Based Dual-Responsive In Situ Gel of Nebivolol for Treatment of Open-Angle Glaucoma. Pharmaceutics 2023, 15, 405. [Google Scholar] [CrossRef]
- null, A.; Ali, J.; Fazil, M.; Qumbar, M.; Khan, N.; Ali, A. Colloidal drug delivery system: Amplify the ocular delivery. Drug Deliv. 2016, 23, 700–716. [Google Scholar] [CrossRef]
- Ahmed, S.; Amin, M.M.; Sayed, S. Ocular Drug Delivery: A Comprehensive Review. AAPS PharmSciTech 2023, 24, 66. [Google Scholar] [CrossRef]
- Silva, B.; São Braz, B.; Delgado, E.; Gonçalves, L. Colloidal nanosystems with mucoadhesive properties designed for ocular topical delivery. Int. J. Pharm. 2021, 606, 120873. [Google Scholar] [CrossRef] [PubMed]
- Shukr, M.H.; Ismail, S.; El-Hossary, G.G.; El-Shazly, A.H. Spanlastics nanovesicular ocular insert as a novel ocular delivery of travoprost: Optimization using Box–Behnken design and in vivo evaluation. J. Liposome Res. 2022, 32, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, S.; Kaur, I.P. Spanlastics—A novel nanovesicular carrier system for ocular delivery. Int. J. Pharm. 2011, 413, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Rathod, S.; Arya, S.; Shukla, R.; Ray, D.; Aswal, V.K.; Bahadur, P.; Tiwari, S. Investigations on the role of edge activator upon structural transitions in Span vesicles. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127246. [Google Scholar] [CrossRef]
- Abdelbari, M.A.; El-Mancy, S.S.; Elshafeey, A.H.; Abdelbary, A.A. Implementing Spanlastics for Improving the Ocular Delivery of Clotrimazole: In vitro Characterization, Ex vivo Permeability, Microbiological Assessment and In vivo Safety Study. Int. J. Nanomed. 2021, 16, 6249–6261. [Google Scholar] [CrossRef]
- Shymborska, Y.; Budkowski, A.; Raczkowska, J.; Donchak, V.; Melnyk, Y.; Vasiichuk, V.; Stetsyshyn, Y. Switching it Up: The Promise of Stimuli-Responsive Polymer Systems in Biomedical Science. Chem. Rec. 2023, e202300217. [Google Scholar] [CrossRef]
- Nagai, N.; Minami, M.; Deguchi, S.; Otake, H.; Sasaki, H.; Yamamoto, N. An in situ Gelling System Based on Methylcellulose and Tranilast Solid Nanoparticles Enhances Ocular Residence Time and Drug Absorption Into the Cornea and Conjunctiva. Front. Bioeng. Biotechnol. 2020, 8, 764. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Y.; Li, X.; Kebebe, D.; Zhang, B.; Ren, J.; Lu, J.; Li, J.; Du, S.; Liu, Z. Research progress of in-situ gelling ophthalmic drug delivery system. Asian J. Pharm. Sci. 2019, 14, 1–15. [Google Scholar] [CrossRef]
- Abbas, M.N.; Khan, S.A.; Sadozai, S.K.; Khalil, I.A.; Anter, A.; Fouly, M.E.; Osman, A.H.; Kazi, M. Nanoparticles Loaded Thermoresponsive In Situ Gel for Ocular Antibiotic Delivery against Bacterial Keratitis. Polymers 2022, 14, 1135. [Google Scholar] [CrossRef]
- Aldawsari, M.F.; Moglad, E.H.; Alotaibi, H.F.; Alkahtani, H.M.; Khafagy, E.-S. Ophthalmic Bimatoprost-Loaded Niosomal In Situ Gel: Preparation, Optimization, and In Vivo Pharmacodynamics Study. Polymers 2023, 15, 4336. [Google Scholar] [CrossRef]
- Sallam, N.M.; Sanad, R.A.B.; Ahmed, M.M.; Khafagy, E.L.S.; Ghorab, M.; Gad, S. Impact of the mucoadhesive lyophilized wafer loaded with novel carvedilol nano-spanlastics on biochemical markers in the heart of spontaneously hypertensive rat models. Drug Deliv. Transl. Res. 2021, 11, 1009–1036. [Google Scholar] [CrossRef]
- Abelson, M.B.; Udell, I.J.; Weston, J.H. Normal human tear pH by direct measurement. Arch. Ophthalmol. 1981, 99, 301. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Ramsey, J.D.; Samadi, A.; Atoufi, Z.; Yazdi, M.K.; Ganjali, M.R.; Amirabad, L.M.; Zangene, E.; Farokhi, M.; Formela, K.; et al. Poloxamer: A versatile tri-block copolymer for biomedical applications. Acta Biomater. 2020, 110, 37–67. [Google Scholar] [CrossRef]
- Liu, S.; Bao, H.; Li, L. Role of PPO–PEO–PPO triblock copolymers in phase transitions of a PEO–PPO–PEO triblock copolymer in aqueous solution. Eur. Polym. J. 2015, 71, 423–439. [Google Scholar] [CrossRef]
- Russo, E.; Villa, C. Poloxamer Hydrogels for Biomedical Applications. Pharmaceutics 2019, 11, 671. [Google Scholar] [CrossRef]
- Abdeltawab, H.; Svirskis, D.; Hill, A.G.; Sharma, M. Increasing the Hydrophobic Component of Poloxamers and the Inclusion of Salt Extend the Release of Bupivacaine from Injectable In Situ Gels, While Common Polymer Additives Have Little Effect. Gels 2022, 8, 484. [Google Scholar] [CrossRef]
- Cao, F.; Zhang, X.; Ping, Q. New method for ophthalmic delivery of azithromycin by poloxamer/carbopol-based in situ gelling system. Drug Deliv. 2010, 17, 500–507. [Google Scholar] [CrossRef]
- Al-Kassas, R.S.; El-Khatib, M.M. Ophthalmic controlled release in situ gelling systems for ciprofloxacin based on polymeric carriers. Drug Deliv. 2009, 16, 145–152. [Google Scholar] [CrossRef]
- Al Khateb, K.; Ozhmukhametova, E.K.; Mussin, M.N.; Seilkhanov, S.K.; Rakhypbekov, T.K.; Lau, W.M.; Khutoryanskiy, V.V. In situ gelling systems based on Pluronic F127/Pluronic F68 formulations for ocular drug delivery. Int. J. Pharm. 2016, 502, 70–79. [Google Scholar] [CrossRef]
- Jaipal, A.; Pandey, M.M.; Charde, S.Y.; Raut, P.P.; Prasanth, K.V.; Prasad, R.G. Effect of HPMC and mannitol on drug release and bioadhesion behavior of buccal discs of buspirone hydrochloride: In-vitro and in-vivo pharmacokinetic studies. Saudi Pharm. J. 2015, 23, 315–326. [Google Scholar] [CrossRef]
- Gupta, H.; Jain, S.; Mathur, R.; Mishra, P.; Mishra, A.K.; Velpandian, T. Sustained ocular drug delivery from a temperature and pH triggered novel in situ gel system. Drug Deliv. 2007, 14, 507–515. [Google Scholar] [CrossRef]
- Kurniawansyah, I.S.; Rusdiana, T.; Sopyan, I.; Ramoko, H.; Wahab, H.A.; Subarnas, A. In situ ophthalmic gel forming systems of poloxamer 407 and hydroxypropyl methyl cellulose mixtures for sustained ocular delivery of chloramphenicole: Optimization study by factorial design. Heliyon 2020, 6, e05365. [Google Scholar] [CrossRef]
- Ranch, K.M.; Maulvi, F.A.; Naik, M.J.; Koli, A.R.; Parikh, R.K.; Shah, D.O. Optimization of a novel in situ gel for sustained ocular drug delivery using Box-Behnken design: In vitro, ex vivo, in vivo and human studies. Int. J. Pharm. 2019, 554, 264–275. [Google Scholar] [CrossRef]
- Gilani, S.J.; Jumah, M.N.B.; Zafar, A.; Imam, S.S.; Yasir, M.; Khalid, M.; Alshehri, S.; Ghuneim, M.M.; Albohairy, F.M. Formulation and Evaluation of Nano Lipid Carrier-Based Ocular Gel System: Optimization to Antibacterial Activity. Gels 2022, 8, 255. [Google Scholar] [CrossRef]
- Nair, A.B.; Shah, J.; Jacob, S.; Al-Dhubiab, B.E.; Sreeharsha, N.; Morsy, M.A.; Gupta, S.; Attimarad, M.; Shinu, P.; Venugopala, K.N. Experimental design, formulation and in vivo evaluation of a novel topical in situ gel system to treat ocular infections. PLoS ONE 2021, 16, e0248857. [Google Scholar] [CrossRef]
- Huang, J.; Peng, T.; Li, Y.; Zhan, Z.; Zeng, Y.; Huang, Y.; Pan, X.; Wu, C.Y.; Wu, C. Ocular Cubosome Drug Delivery System for Timolol Maleate: Preparation, Characterization, Cytotoxicity, Ex Vivo, and In Vivo Evaluation. AAPS PharmSciTech 2017, 18, 2919–2926. [Google Scholar] [CrossRef]
- Ban, J.; Zhang, Y.; Huang, X.; Deng, G.; Hou, D.; Chen, Y.; Lu, Z. Corneal permeation properties of a charged lipid nanoparticle carrier containing dexamethasone. Int. J. Nanomed. 2017, 12, 1329–1339. [Google Scholar] [CrossRef]
- Natarajan, J.V.; Ang, M.; Darwitan, A.; Chattopadhyay, S.; Wong, T.T.; Venkatraman, S.S. Nanomedicine for glaucoma: Liposomes provide sustained release of latanoprost in the eye. Int. J. Nanomed. 2012, 7, 123–131. [Google Scholar] [CrossRef]
- Leonardi, A.; Bucolo, C.; Drago, F.; Salomone, S.; Pignatello, R. Cationic solid lipid nanoparticles enhance ocular hypotensive effect of melatonin in rabbit. Int. J. Pharm. 2015, 478, 180–186. [Google Scholar] [CrossRef]
- Brambilla, E.; Locarno, S.; Gallo, S.; Orsini, F.; Pini, C.; Farronato, M.; Thomaz, D.V.; Lenardi, C.; Piazzoni, M.; Tartaglia, G. Poloxamer-Based Hydrogel as Drug Delivery System: How Polymeric Excipients Influence the Chemical-Physical Properties. Polymers 2022, 14, 3624. [Google Scholar] [CrossRef]
- Balasubramaniam, J.; Pandit, J.K. Ion-activated in situ gelling systems for sustained ophthalmic delivery of ciprofloxacin hydrochloride. Drug Deliv. 2003, 10, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Ali, J.; Khar, R.; Ahuja, A.; Kalra, R. Buccoadhesive erodible disk for treatment of oro-dental infections: Design and characterisation. Int. J. Pharm. 2002, 238, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Bíró, T.; Bocsik, A.; Jurišić Dukovski, B.; Gróf, I.; Lovrić, J.; Csóka, I.; Deli, M.A.; Aigner, Z. New Approach in Ocular Drug Delivery: In vitro and ex vivo Investigation of Cyclodextrin-Containing, Mucoadhesive Eye Drop Formulations. Drug Des. Devel Ther. 2021, 15, 351–360. [Google Scholar] [CrossRef] [PubMed]

| Formula | Poloxamer 407 | Poloxamer 188 |
|---|---|---|
| F1 | 20 | 5 |
| F2 | 22 | 5 |
| F3 | 25 | 5 |
| F4 | 20 | 7.5 |
| F5 | 22 | 7.5 |
| F6 | 25 | 7.5 |
| F7 | 20 | 10 |
| F8 | 22 | 10 |
| F9 | 25 | 10 |
| Formula | pH | Drug Content (%) | Gelation Temperature (TG; °C) | Muco-Adhesion Force (mN) | Viscosity (cp) | |
|---|---|---|---|---|---|---|
| At 25 °C | At 37 °C | |||||
| F1 | 6.48 ± 0.04 | 99.3 ± 0.4 | 36.8 ± 0.3 | 58.7 ± 4.9 | 58.6 ± 4.2 | 276.3 ± 13.8 |
| F2 | 6.94 ± 0.03 | 99.1 ± 0.5 | 32.4 ± 0.5 | 87.2 ± 5.2 | 83.6 ± 5.9 | 369.3 ± 10.9 |
| F3 | 7.13 ± 0.07 | 98.6 ± 0.7 | 28.8 ± 0.6 | 112.3 ± 6.1 | 112.8 ± 9.3 | 424.3 ± 13.7 |
| F4 | 6.71 ± 0.06 | 98.4 ± 0.4 | 37.9 ± 0.5 | 78.1 ± 7.6 | 76.3 ± 4.4 | 303.5 ± 10.8 |
| F5 | 7.03 ± 0.05 | 98.3 ± 0.6 | 34.9 ± 0.7 | 96.4 ± 8.3 | 103.3 ± 6.1 | 363.7 ± 18.8 |
| F6 | 7.29 ± 0.08 | 97.9 ± 0.9 | 31.5 ± 0.4 | 134.5 ± 9.7 | 146.4 ± 11.5 | 485.3 ± 43.9 |
| F7 | 6.87 ± 0.08 | 98.2 ± 0.7 | 40.5 ± 0.8 | 92.3 ± 8.2 | 96.1 ± 6.5 | 362.7 ± 11.7 |
| F8 | 7.18 ± 0.06 | 97.8 ± 1.1 | 36.9 ± 0.9 | 161.5 ± 7.3 | 114.7 ± 9.6 | 454.9 ± 11.4 |
| F9 | 7.32 ± 0.05 | 97.3 ± 0.9 | 33.6 ± 0.7 | 174.8 ± 11.2 | 164.3 ± 12.0 | 549.8 ± 15.2 |
| Formula | Jss (μg/h) | Papp (cm/s) × 10−6 | Q6h (μg) |
|---|---|---|---|
| Plain CRV-ISG | 8.38 ± 0.95 | 2.67 | 46.2 ± 3.9 |
| CS-ISG formulation (F2) | 22.37 ± 2.24 | 6.39 | 110.4 ± 9.8 |
| Time | Visual Appearance | pH | Drug Content | Gelling Capacity |
|---|---|---|---|---|
| 0 | Clear | 6.94 ± 0.03 | 99.1 ± 0.5 | +++ |
| 4th week | Clear | 7.05 ± 0.10 | 98.3 ± 1.0 | +++ |
| 8th week | Clear | 7.21 ± 0.09 | 97.5 ± 1.3 | +++ |
| Pharmacokinetic Parameter | CRV-ISG | CS-ISG (F2) |
|---|---|---|
| Cmax (ng/mL) | 485.7 ± 52.9 | 781.4 ± 69.4 |
| tmax (h) | 1 | 2 |
| t1/2(h) | 1.01 ± 0.2 | 2.21 ± 0.4 |
| AUC0–6h (ng·h/mL) | 1161.3 ± 98.6 | 2494.5 ± 113.7 |
| MRT (h) | 2.15 ± 0.3 | 4.11 ± 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almutairy, B.K.; Khafagy, E.-S.; Abu Lila, A.S. Development of Carvedilol Nanoformulation-Loaded Poloxamer-Based In Situ Gel for the Management of Glaucoma. Gels 2023, 9, 952. https://doi.org/10.3390/gels9120952
Almutairy BK, Khafagy E-S, Abu Lila AS. Development of Carvedilol Nanoformulation-Loaded Poloxamer-Based In Situ Gel for the Management of Glaucoma. Gels. 2023; 9(12):952. https://doi.org/10.3390/gels9120952
Chicago/Turabian StyleAlmutairy, Bjad K., El-Sayed Khafagy, and Amr Selim Abu Lila. 2023. "Development of Carvedilol Nanoformulation-Loaded Poloxamer-Based In Situ Gel for the Management of Glaucoma" Gels 9, no. 12: 952. https://doi.org/10.3390/gels9120952
APA StyleAlmutairy, B. K., Khafagy, E.-S., & Abu Lila, A. S. (2023). Development of Carvedilol Nanoformulation-Loaded Poloxamer-Based In Situ Gel for the Management of Glaucoma. Gels, 9(12), 952. https://doi.org/10.3390/gels9120952







