Sustained Release of Voriconazole Using 3D-Crosslinked Hydrogel Rings and Rods for Use in Corneal Drug Delivery
Abstract
1. Introduction
2. Results and Discussion
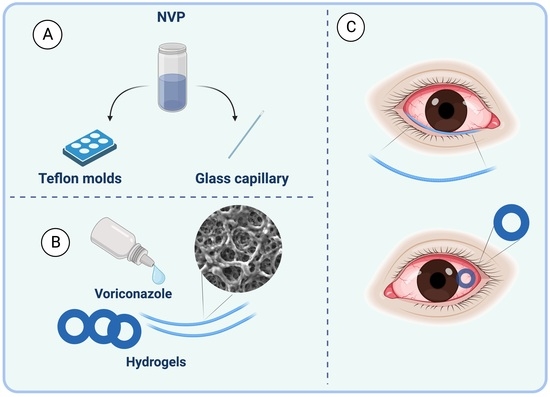
2.1. Preparation of Hydrogel Rings and Rods
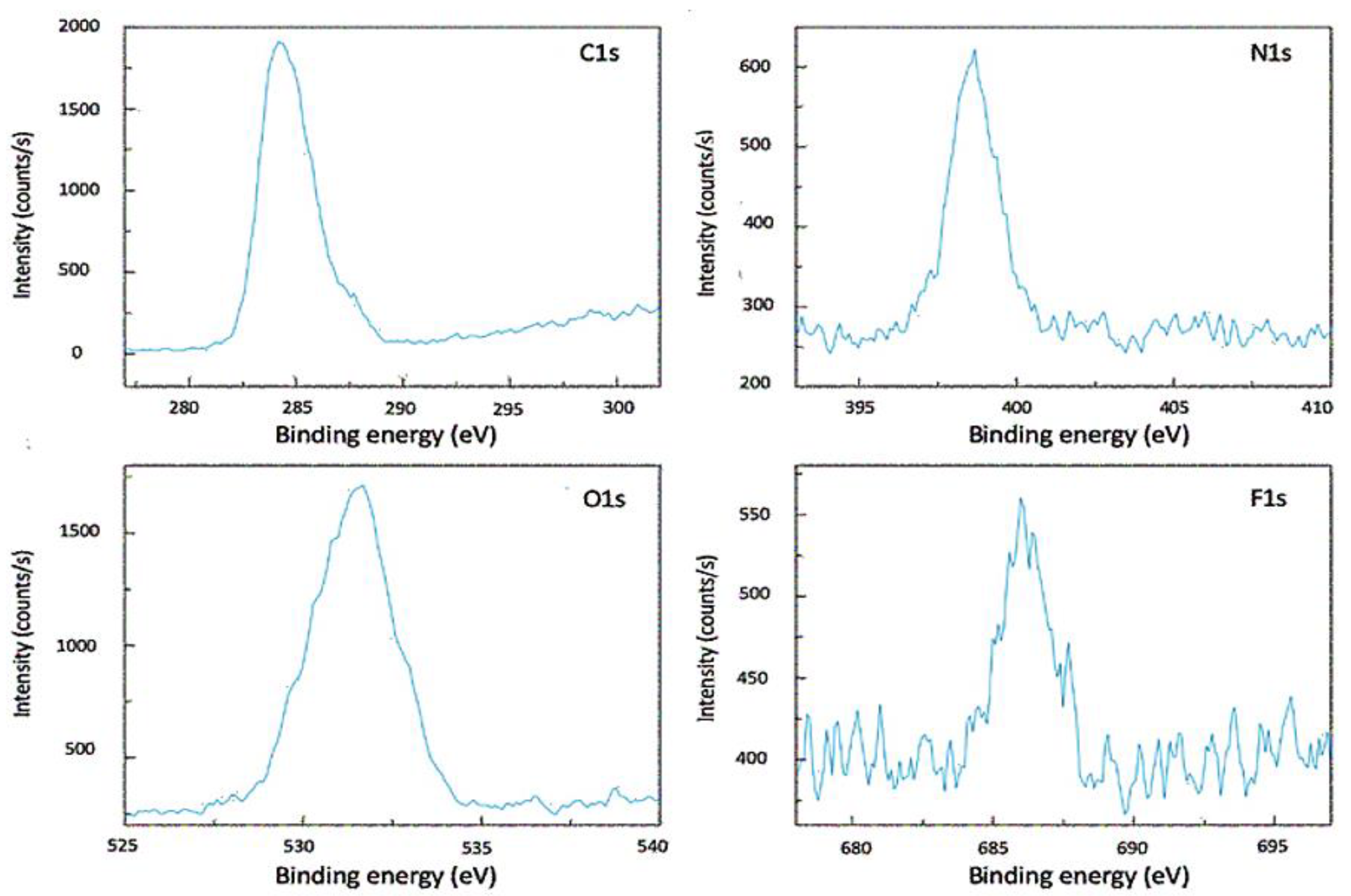
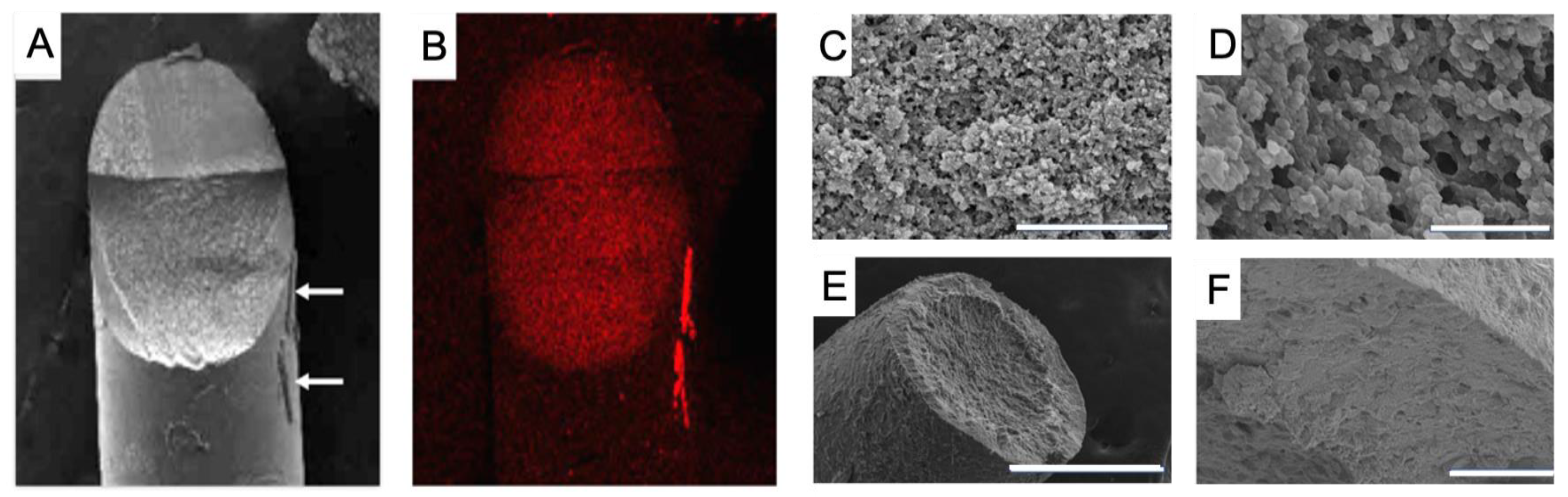
2.2. Material Characterization
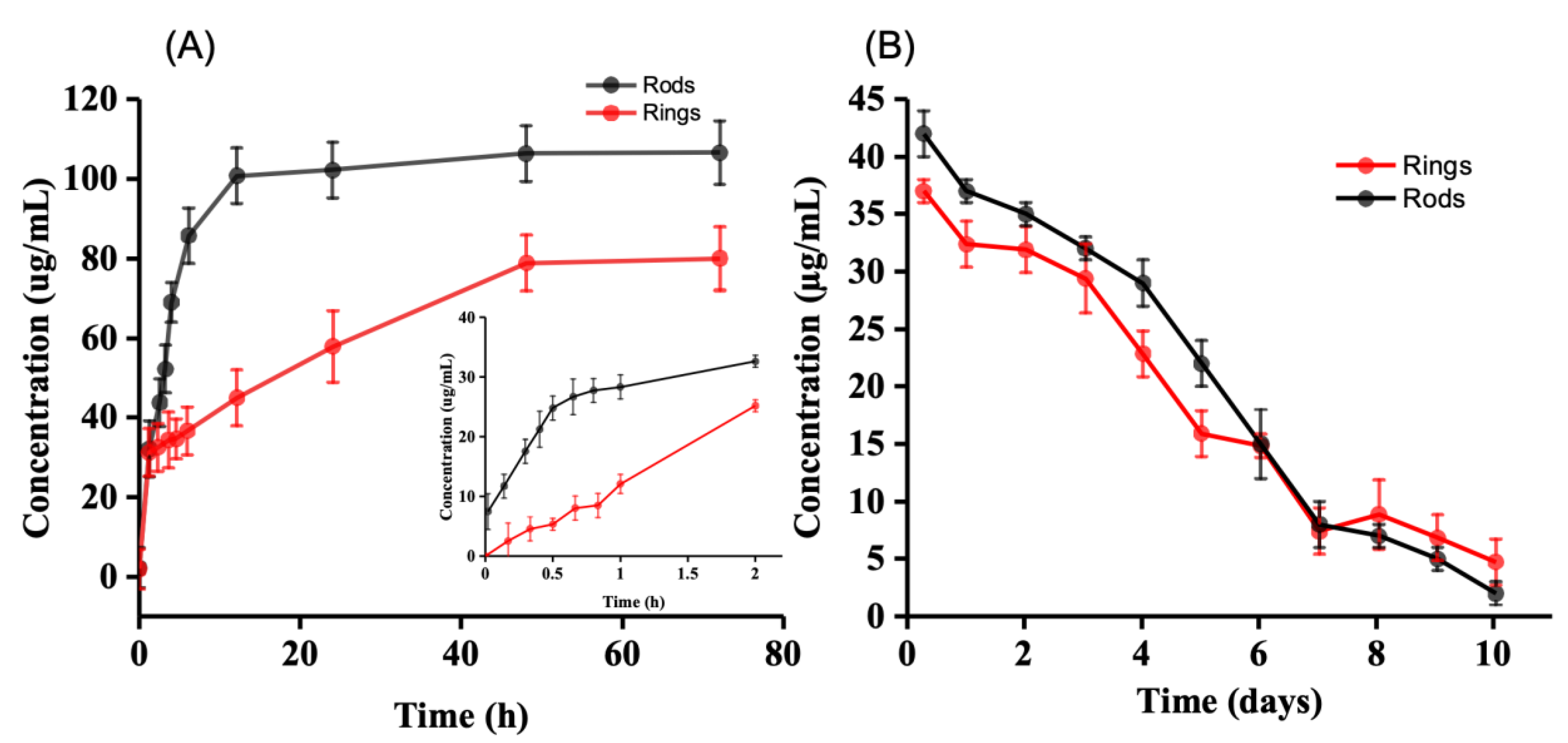
2.3. Drug Release
2.4. Ex-Vivo Assessment with Porcine Eyes
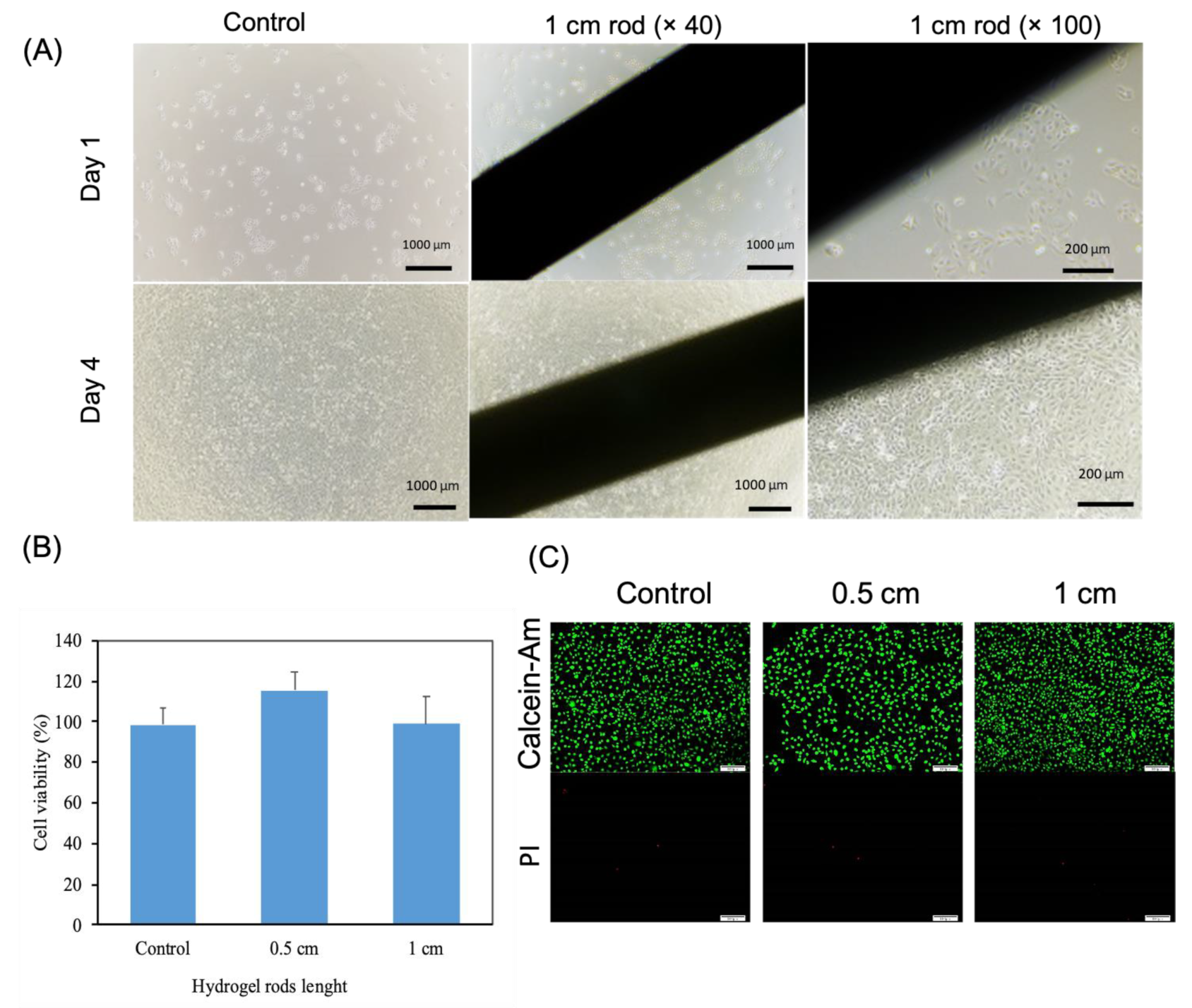
2.5. Cytocompatibility of Hydrogels
3. Conclusions
4. Materials and Methods
4.1. Synthesis of Materials
4.2. Material Characterization
4.3. Drug Release In Vitro
4.4. Ex-Vivo Assessment with Porcine Eyes
4.5. HPLC Analysis
4.6. Cytocompatibility of Hydrogels
4.6.1. Cell Count Kit 8 (CCK-8)
4.6.2. Live-Dead Assay
4.6.3. Cell Proliferation in Direct Contact
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| CCK-8 | Cell Counting Kit-8 |
| DMSO | Dimethyl Sulfoxide |
| HCEC | Human Corneal Epithelial Cells |
| HPLC | High-Performance Liquid Chromatography |
| NVP | No Visible Particles |
| PBS | Phosphate-Buffered Saline |
| PEG1000 | Polyethylene Glycol 1000 |
| PLGA | Polylactic-co-glycolic acid |
| PTFE | Polytetrafluoroethylene |
| PCR | Polymerase Chain Reaction |
| SEM | Scanning Electron Microscope |
| SEM-EDX | Scanning Electron Microscope with Energy Dispersive X-ray Spectroscopy |
| UV-Vis | Ultraviolet-Visible Spectroscopy |
| XPS | X-ray Photoelectron Spectroscopy |
References
- Niu, L.; Liu, X.; Ma, Z.; Yin, Y.; Sun, L.; Yang, L.; Zheng, Y. Fungal keratitis: Pathogenesis, diagnosis and prevention. Microb. Pathog. 2020, 138, 103802. [Google Scholar] [CrossRef] [PubMed]
- Badian, R.A.; Ekman, L.; Pripp, A.H.; Utheim, T.P.; Englund, E.; Dahlin, L.B.; Rolandsson, O.; Lagali, N. Comparison of Novel Wide-Field In Vivo Corneal Confocal Microscopy with Skin Biopsy for Assessing Peripheral Neuropathy in Type 2 Diabetes. Diabetes 2023, 72, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Goh, J.W.Y.M.; Harrison, R.B.; Hau, S.B.; Alexander, C.L.P.; Tole, D.M.F.; Avadhanam, V.S.M. Comparison of In Vivo Confocal Microscopy, PCR and Culture of Corneal Scrapes in the Diagnosis of Acanthamoeba Keratitis. Cornea 2017, 37, 480–485. [Google Scholar] [CrossRef]
- Watson, S.L.; Cabrera-Aguas, M.; Keay, L.; Khoo, P.; McCall, D.; Lahra, M.M. The clinical and microbiological features and outcomes of fungal keratitis over 9 years in Sydney, Australia. Mycoses 2020, 63, 43–51. [Google Scholar] [CrossRef]
- Hoffman, J.J.; Arunga, S.; Ahmed, A.H.A.M.; Hu, V.H.; Burton, M.J. Management of Filamentous Fungal Keratitis: A Pragmatic Approach. J. Fungi 2022, 8, 1067. [Google Scholar] [CrossRef]
- Jumelle, C.; Gholizadeh, S.; Annabi, N.; Dana, R. Advances and limitations of drug delivery systems formulated as eye drops. J. Control. Release 2020, 321, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Sitnova, A.; Svetozarskiy, S. Modern Technologies in Diagnosis of Fungal Keratitis (Review). Sovrem. Tehnol. Med. 2023, 15, 73–84. [Google Scholar] [CrossRef]
- Reginatto, P.; Agostinetto, G.d.J.; Fuentefria, R.D.N.; Marinho, D.R.; Pizzol, M.D.; Fuentefria, A.M. Eye fungal infections: A mini review. Arch. Microbiol. 2023, 205, 1–26. [Google Scholar] [CrossRef]
- Sarmout, M.; Xiao, Y.; Hu, X.; Rakhmetova, A.; Koole, L.H. A novel approach to achieve semi-sustained drug delivery to the eye through asymmetric loading of soft contact lenses. Heliyon 2023, 9, e16916. [Google Scholar] [CrossRef]
- Kang-Mieler, J.J.; Rudeen, K.M.; Liu, W.; Mieler, W.F. Advances in ocular drug delivery systems. Eye 2020, 34, 1371–1379. [Google Scholar] [CrossRef]
- Mazet, R.; Yameogo, J.B.G.; Wouessidjewe, D.; Choisnard, L.; Geze, A. Recent Advances in the Design of Topical Ophthalmic Delivery Systems in the Treatment of Ocular Surface Inflammation and Their Biopharmaceutical Evaluation. Pharmaceutics 2020, 12, 570. [Google Scholar] [CrossRef] [PubMed]
- Badr, M.Y.; Abdulrahman, N.S.; Schatzlein, A.G.; Uchegbu, I.F. A polymeric aqueous tacrolimus formulation for topical ocular delivery. Int. J. Pharm. 2021, 599, 120364. [Google Scholar] [CrossRef] [PubMed]
- Uwaezuoke, O.; Du Toit, L.C.; Kumar, P.; Ally, N.; Choonara, Y.E. Linoleic Acid-Based Transferosomes for Topical Ocular Delivery of Cyclosporine A. Pharmaceutics 2022, 14, 1695. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.; Yang, X.; Chen, S.; Wang, Q.; Zhang, A.; Tang, B. Cyclodextrin-based host–guest supramolecular hydrogels for local drug delivery. Coord. Chem. Rev. 2021, 454, 214352. [Google Scholar] [CrossRef]
- Lynch, C.R.; Kondiah, P.P.D.; Choonara, Y.E.; du Toit, L.C.; Ally, N.; Pillay, V. Hydrogel Biomaterials for Application in Ocular Drug Delivery. Front. Bioeng. Biotechnol. 2020, 8, 228. [Google Scholar] [CrossRef]
- Arif, Z.U.; Khalid, M.Y.; Noroozi, R.; Hossain, M.; Shi, H.H.; Tariq, A.; Ramakrishna, S.; Umer, R. Additive manufacturing of sustainable biomaterials for biomedical applications. Asian J. Pharm. Sci. 2023, 18, 100812. [Google Scholar] [CrossRef]
- Cuming, R.S.; Abarca, E.M.; Duran, S.; Wooldridge, A.A.; Stewart, A.J.; Ravis, W.; Babu, R.J.; Lin, Y.-J.; Hathcock, T. Development of a Sustained-Release Voriconazole-Containing Thermogel for Subconjunctival Injection in Horses. Investig. Opthalmol. Vis. Sci. 2017, 58, 2746. [Google Scholar] [CrossRef]
- Okur, N.U.; Yozgatli, V.; Senyigit, Z. Formulation and detailed characterization of voriconazole loaded in situ gels for ocular application. Ank. Univ. Eczaci. Fak. Derg. 2020, 44, 33–49. [Google Scholar] [CrossRef]
- Kim, S.; Lee, H.J.; Jeong, B. Hyaluronic acid-g-PPG and PEG-PPG-PEG hybrid thermogel for prolonged gel stability and sustained drug release. Carbohydr. Polym. 2022, 291, 119559. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Luo, L.J.; Lai, J.Y. Thermogels containing sulfated hyaluronan as novel topical therapeutics for treatment of ocular surface inflammation. Mater. Today Bio 2021, 13, 100183. [Google Scholar] [CrossRef]
- Mora-Pereira, M.; Abarca, E.M.; Duran, S.; Ravis, W.; McMullen, R.J.; Fischer, B.M.; Lee, Y.-H.P.; Wooldridge, A.A. Sustained-release voriconazole-thermogel for subconjunctival injection in horses: Ocular toxicity and in-vivo studies. BMC Vet. Res. 2020, 16, 115. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Lorenzo, C.; Vivero-Lopez, M.; Concheiro, A. Contact lenses that transform gold into nanoparticles for prophylaxis of light-related events and photothermal therapy. Int. J. Pharm. 2023, 641, 123048. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.; de Sousa, H.C.; Gil, M.H.; Santos, L.F.; Oom, M.S.; Alvarez-Lorenzo, C.; Saramago, B.; Serro, A.P. Moxifloxacin-imprinted silicone-based hydrogels as contact lens materials for extended drug release. Eur. J. Pharm. Sci. 2021, 156, 105591. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Kanani, P.A.; Jadav, H.J.; Desai, B.V.; Desai, D.T.; Patel, H.P.; Shetty, K.H.; Shah, D.O.; Willcox, M.D. Timolol-eluting graphene oxide laden silicone contact lens: Control release profile with improved critical lens properties. J. Drug Deliv. Sci. Technol. 2022, 69, 103134. [Google Scholar] [CrossRef]
- Al-Kinani, A.A.; Zidan, G.; Elsaid, N.; Seyfoddin, A.; Alani, A.W.G.; Alany, R.G. Ophthalmic gels: Past, present and future. Adv. Drug Deliv. Rev. 2018, 126, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Pijls, R.T.; Cruysberg, L.P.; Nuijts, R.M.M.A.; Dias, A.A.; Koole, L.H. Capacity and tolerance of a new device for ocular drug delivery. Int. J. Pharm. 2007, 341, 152–161. [Google Scholar] [CrossRef]
- Noroozi, R.; Arif, Z.U.; Taghvaei, H.; Khalid, M.Y.; Sahbafar, H.; Hadi, A.; Sadeghianmaryan, A.; Chen, X. 3D and 4D Bioprinting Technologies: A Game Changer for the Biomedical Sector? Ann. Biomed. Eng. 2023, 51, 1683–1712. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Koole, L.H.; Gao, C.; Yang, D.; Yang, L.; Zhang, C.; Li, H. The potential utility of hybrid photo-crosslinked hydrogels with non-immunogenic component for cartilage repair. NPJ Regen. Med. 2021, 6, 54. [Google Scholar] [CrossRef] [PubMed]
- Arif, Z.U.; Khalid, M.Y.; Noroozi, R.; Sadeghianmaryan, A.; Jalalvand, M.; Hossain, M. Recent advances in 3D-printed polylactide and polycaprolactone-based biomaterials for tissue engineering applications. Int. J. Biol. Macromol. 2022, 218, 930–968. [Google Scholar] [CrossRef]
- Tijink, M.; Janssen, J.; Timmer, M.; Austen, J.; Aldenhoff, Y.; Kooman, J.; Koole, L.; Damoiseaux, J.; van Oerle, R.; Henskens, Y.; et al. Development of novel membranes for blood purification therapies based on copolymers of N-vinylpyrrolidone and n-butylmethacrylate. J. Mater. Chem. B 2013, 1, 6066. [Google Scholar] [CrossRef][Green Version]
- Ladetto, M.F.; Lázaro-Martínez, J.M.; Devoto, T.B.; Briceño, V.J.; Castro, G.R.; Cuestas, M.L. Quantitative determination of voriconazole by thionine reduction and its potential application in a pharmaceutical and clinical setting. Anal. Methods 2023, 15, 1230–1240. [Google Scholar] [CrossRef] [PubMed]
- Pijls, R.T.; Sonderkamp, T.; Daube, G.W.; Krebber, R.; Hanssen, H.H.; Nuijts, R.M.M.A.; Koole, L.H. Studies on a new device for drug delivery to the eye. Eur. J. Pharm. Biopharm. 2005, 59, 283–288. [Google Scholar] [CrossRef]
- Datta, D.; Roy, G.; Garg, P.; Venuganti, V.V.K. Ocular delivery of cyclosporine A using dissolvable microneedle contact lens. J. Drug Deliv. Sci. Technol. 2022, 70, 103211. [Google Scholar] [CrossRef]
- Morgan, S.R.; Pilia, N.; Hewitt, M.; Moses, R.L.; Moseley, R.; Lewis, P.N.; Morrison, P.W.; Kelly, S.L.; Parker, J.E.; Whitaker, D.; et al. Controlled in vitro delivery of voriconazole and diclofenac to the cornea using contact lenses for the treatment of Acanthamoeba keratitis. Int. J. Pharm. 2020, 579, 119102. [Google Scholar] [CrossRef]
- Soe, H.M.S.H.; Kerdpol, K.; Rungrotmongkol, T.; Pruksakorn, P.; Autthateinchai, R.; Wet-Osot, S.; Loftsson, T.; Jansook, P. Voriconazole Eye Drops: Enhanced Solubility and Stability through Ternary Voriconazole/Sulfobutyl Ether β-Cyclodextrin/Polyvinyl Alcohol Complexes. Int. J. Mol. Sci. 2023, 24, 2343. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.; Gober, M.D.; Bashir, H.; O’Day, C.; Blair, I.A.; Mesaros, C.; Weng, L.; Huang, A.; Chen, A.; Tang, R.; et al. Voriconazole enhances UV-induced DNA damage by inhibiting catalase and promoting oxidative stress. Exp. Dermatol. 2020, 29, 29–38. [Google Scholar] [CrossRef]
- Martos, A.I.; Martin-Mazuelos, E.; Romero, A.; Serrano, C.; Gonzalez, T.; Almeida, C.; Puche, B.; Canton, E.; Peman, J.; Espinel-Ingroff, A. Evaluation of disk diffusion method compared to broth microdilution for antifungal susceptibility testing of 3 echinocandins against Aspergillus spp. Diagn. Microbiol. Infect. Dis. 2012, 73, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, M.G.; Morrison, P.W.J.; Boostrom, H.M.; Morgan, S.R.; Fallon, M.; Lewis, P.N.; Whitaker, D.; Brancale, A.; Varricchio, C.; Quantock, A.J.; et al. In Vitro Topical Delivery of Chlorhexidine to the Cornea: Enhancement Using Drug-Loaded Contact Lenses and β-Cyclodextrin Complexation, and the Importance of Simulating Tear Irrigation. Mol. Pharm. 2020, 17, 1428–1441. [Google Scholar] [CrossRef]
- Salètes, M.; Vartin, M.; Mocquot, C.; Chevalier, C.; Grosgogeat, B.; Colon, P.; Attik, N. Mesoporous Bioactive Glasses Cytocompatibility Assessment: A Review of In Vitro Studies. Biomimetics 2021, 6, 9. [Google Scholar] [CrossRef]
- Lee, C.; O’Connell, C.D.; Onofrillo, C.; Choong, P.F.M.; Di Bella, C.; Duchi, S. Human articular cartilage repair: Sources and detection of cytotoxicity and genotoxicity in photo-crosslinkable hydrogel bioscaffolds. Stem Cells Transl. Med. 2020, 9, 302–315. [Google Scholar] [CrossRef]


| Hydrogel Rings (after 2 h), µg | ||
| Sample | Dry form | Wet form |
| 1 | 40.232 | 55.92 |
| 2 | 36.296 | 57.43 |
| 3 | 44.716 | 66.406 |
| 4 | 43.748 | 56.034 |
| 5 | 36.512 | 52.828 |
| 6 | 48.324 | 64.72 |
| 7 | 45.084 | 59.43 |
| 8 | 48.522 | 69.734 |
| Mean ± SD | 42.93 ± 4.8 | 60.31 ± 6.0 |
| Bioavailability | 25.1 ± 2.5% | 35.8 ± 3.2% |
| 1% Voriconazole eye drops (after 2 h), µg [34] | ||
| Drug amount | Bioavailability | |
| Mean ± SD | 6 ± 1.5 | 3.9 ± 1.0% |
| Mold # | Outer Diameter, mm | Inner Diameter, mm | Depth, mm |
|---|---|---|---|
| 1 | 10.0 | 6.0 | 1.0 |
| 2 | 10.0 | 6.0 | 0.8 |
| 3 | 9.0 | 5.0 | 1.0 |
| 4 | 9.0 | 5.0 | 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakhmetova, A.; Yi, Z.; Sarmout, M.; Koole, L.H. Sustained Release of Voriconazole Using 3D-Crosslinked Hydrogel Rings and Rods for Use in Corneal Drug Delivery. Gels 2023, 9, 933. https://doi.org/10.3390/gels9120933
Rakhmetova A, Yi Z, Sarmout M, Koole LH. Sustained Release of Voriconazole Using 3D-Crosslinked Hydrogel Rings and Rods for Use in Corneal Drug Delivery. Gels. 2023; 9(12):933. https://doi.org/10.3390/gels9120933
Chicago/Turabian StyleRakhmetova, Aiym, Zhiqi Yi, Malake Sarmout, and Leo H. Koole. 2023. "Sustained Release of Voriconazole Using 3D-Crosslinked Hydrogel Rings and Rods for Use in Corneal Drug Delivery" Gels 9, no. 12: 933. https://doi.org/10.3390/gels9120933
APA StyleRakhmetova, A., Yi, Z., Sarmout, M., & Koole, L. H. (2023). Sustained Release of Voriconazole Using 3D-Crosslinked Hydrogel Rings and Rods for Use in Corneal Drug Delivery. Gels, 9(12), 933. https://doi.org/10.3390/gels9120933