Investigation of Gelation Techniques for the Fabrication of Cellulose Aerogels
Abstract
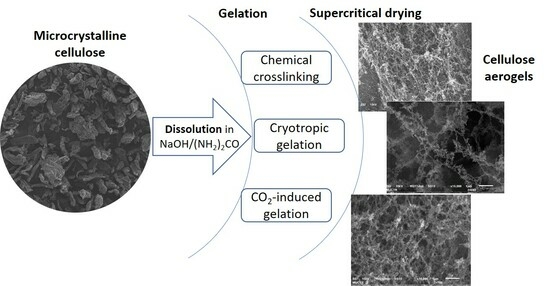
:1. Introduction
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials
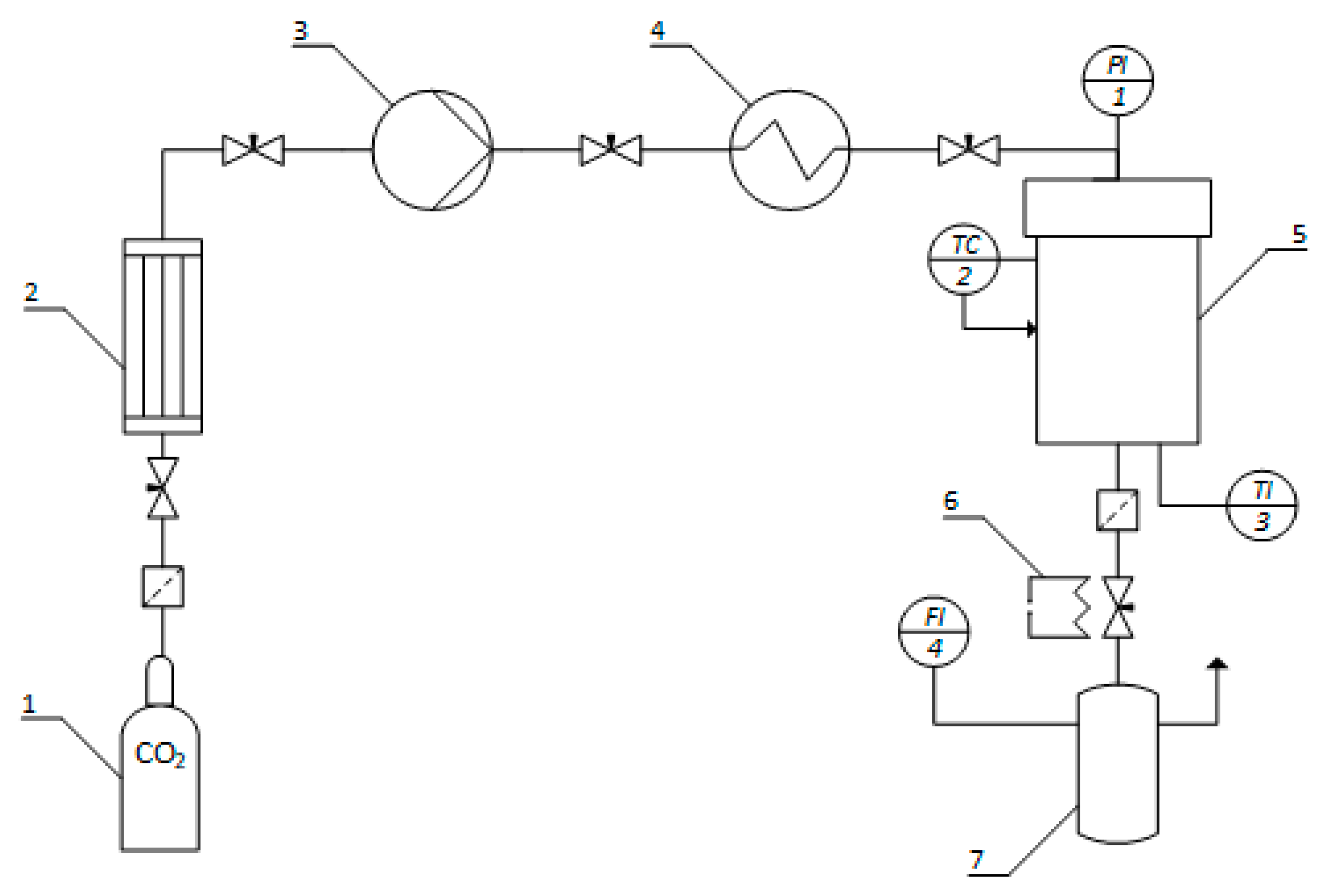
4.2. Methods
4.2.1. Cellulose Dissolution
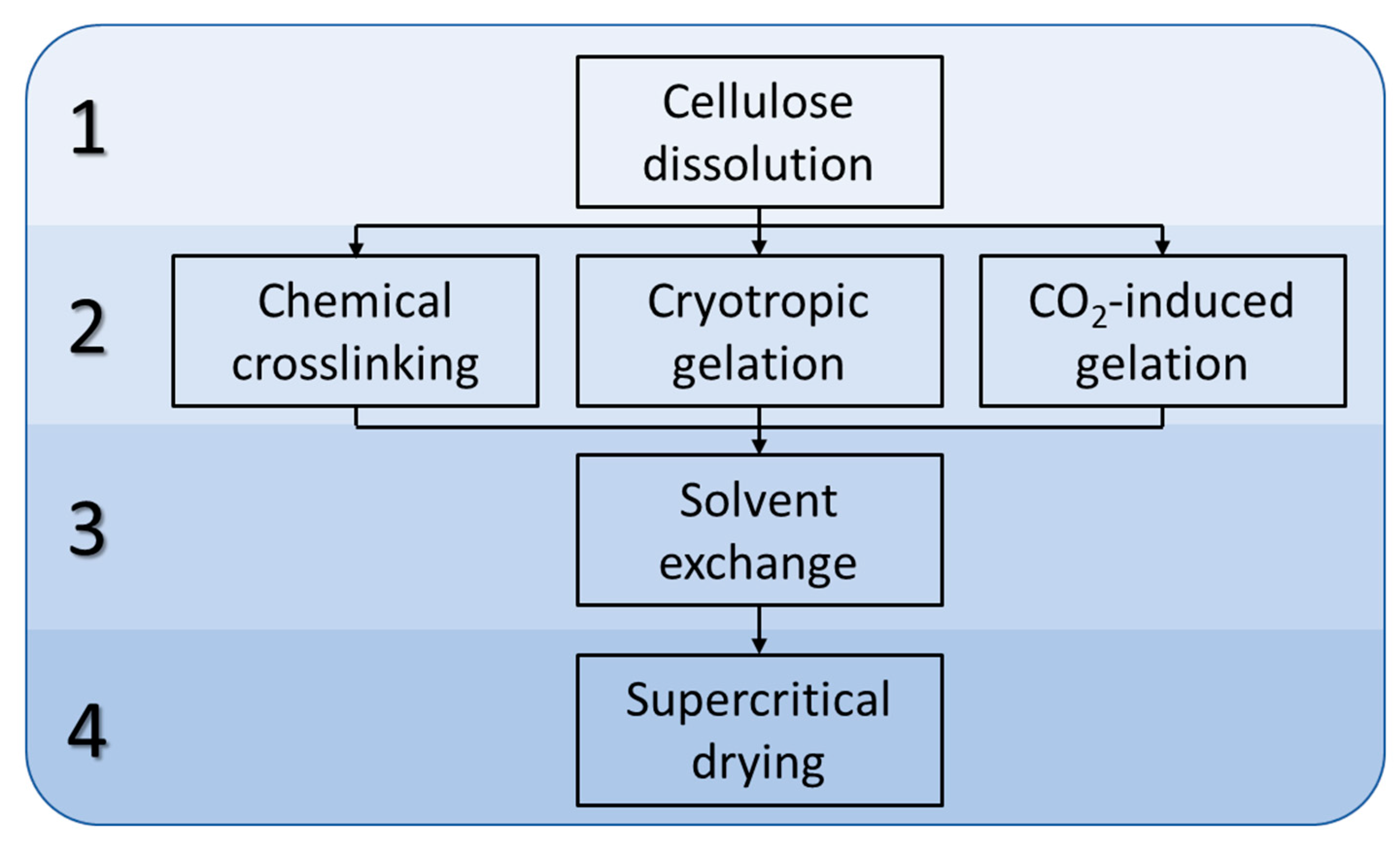
4.2.2. Preparation of Cellulose Hydrogels
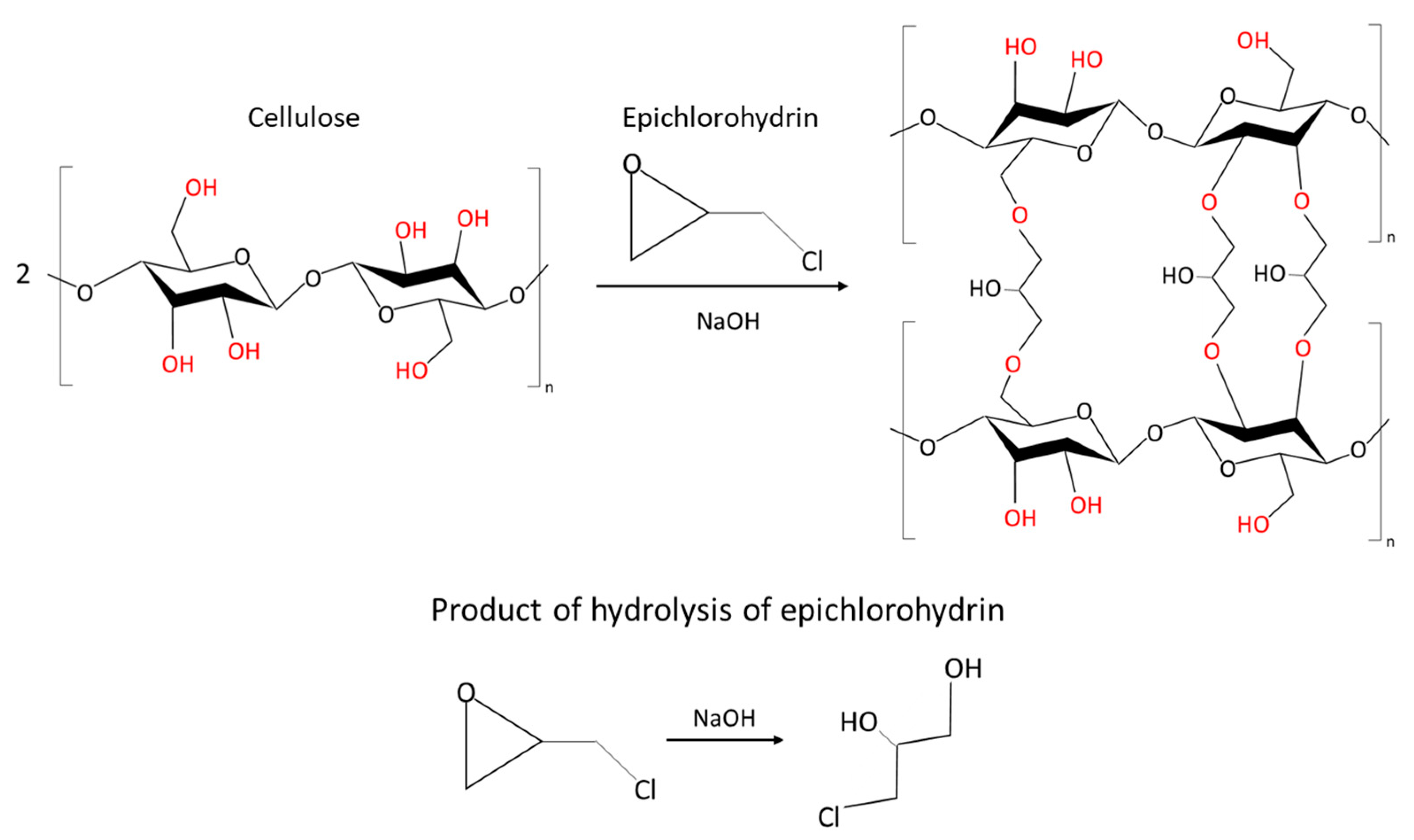
Chemical Cross-Linking Gelation
Cryotropic Gelation
CO2-Induced Gelation
4.2.3. Solvent Exchange
4.2.4. Drying
4.2.5. Characterization
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, G.; Chen, B.; Zeng, X.; Sun, Y.; Tang, X.; Lin, L. Recent advances on sustainable cellulosic materials for pharmaceutical carrier applications. Carbohydr. Polym. 2020, 244, 116492. [Google Scholar] [CrossRef] [PubMed]
- Abdul Khalil, H.P.S.; Adnan, A.S.; Yahya, E.B.; Olaiya, N.G.; Safrida, S.; Hossain, M.S.; Pasquini, D. A review on plant cellulose nanofibre-based aerogels for biomedical applications. Polymers 2020, 12, 1759. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yao, C.; Wang, F.; Li, Z. Cellulose-based nanomaterials for energy applications. Small 2017, 13, 1702240. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, D.; Lee, Y.H.; Chen, W.; Lee, S.Y. Nanocellulose for energy storage systems: Beyond the limits of synthetic materials. Adv. Mater. 2019, 31, 1804826. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhu, Y.; Cheng, W.; Chen, W.; Wu, Y.; Yu, H. Cellulose-based flexible functional materials for emerging intelligent electronics. Adv. Mater. 2021, 33, 2000619. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Hao, N.; Zhang, K.; Xing, C.; Wang, S. Cellulose nanofibrils-based thermally conductive composites for flexible electronics: A mini review. Cellulose 2020, 27, 4173–4187. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, J.; Yao, Q.; Ji, C.; Liu, J.; Zhu, Q. 3D printing with cellulose materials. Cellulose 2018, 25, 4275–4301. [Google Scholar] [CrossRef]
- Mettler, M.S.; Paulsen, A.D.; Vlachos, D.G.; Dauenhauer, P.J. Pyrolytic conversion of cellulose to fuels: Levoglucosan deoxygenation via elimination and cyclization within molten biomass. Energy Environ. Sci. 2012, 5, 7864–7868. [Google Scholar] [CrossRef]
- Bidgoli, H.; Mortazavi, Y.; Khodadadi, A.A. A functionalized nano-structured cellulosic sorbent aerogel for oil spill cleanup: Synthesis and characterization. J. Hazard. Mater. 2019, 366, 229–239. [Google Scholar] [CrossRef]
- Haniffa, M.A.C.M.; Ching, Y.C.; Illias, H.A.; Munawar, K.; Ibrahim, S.; Nguyen, D.H.; Chuah, C.H. Cellulose supported promising magnetic sorbents for magnetic solid-phase extraction: A review. Carbohydr. Polym. 2021, 253, 117245. [Google Scholar] [CrossRef]
- Du, A.; Zhou, B.; Zhang, Z.; Shen, J. A special material or a new state of matter: A review and reconsideration of the aerogel. Materials 2013, 6, 941–968. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Fung, B.M.; Newman, J.K.; Vu, C. Organic aerogels with very high impact strength. Adv. Mater. 2001, 13, 644–646. [Google Scholar] [CrossRef]
- Jin, H.; Nishiyama, Y.; Wada, M.; Kuga, S. Nanofibrillar cellulose aerogels. Colloids Surf. A Physicochem. Eng. Asp. 2004, 240, 63–67. [Google Scholar] [CrossRef]
- Qin, L.; Zhao, X.; He, Y.; Wang, H.; Wei, H.; Zhu, Q.; Zhang, T.; Qin, Y.; Du, A. Preparation, characterization, and in vitro evaluation of resveratrol-loaded cellulose aerogel. Materials 2020, 13, 1624. [Google Scholar] [CrossRef] [PubMed]
- Cui, N.; Xu, Z.; Zhao, X.; Yuan, M.; Pan, L.; Lu, T.; Du, A.; Qin, L. In Vivo Effect of Resveratrol-Cellulose Aerogel Drug Delivery System to Relieve Inflammation on Sports Osteoarthritis. Gels 2023, 8, 544. [Google Scholar] [CrossRef]
- El-Wakil, N.; Kamel, R.; Mahmoud, A.A.; Dufresne, A.; Abouzeid, R.E.; Abo El-Fadl, M.T.; Maged, A. Risedronate-loaded aerogel scaffolds for bone regeneration. Drug Deliv. 2023, 30, 51–63. [Google Scholar] [CrossRef]
- Zhong, S.; Yuan, S.; Zhang, X.; Zhang, J.; Xu, L.; Xu, T.; Zuo, T.; Cai, Y.; Yi, L. Hierarchical Cellulose Aerogel Reinforced with In Situ-Assembled Cellulose Nanofibers for Building Cooling. ACS Appl. Mater. Interfaces 2023, 15, 39807–39817. [Google Scholar] [CrossRef]
- Li, Z.; Zhong, L.; Zhang, T.; Qiu, F.; Yue, X.; Yang, D. Sustainable, flexible, and superhydrophobic functionalized cellulose aerogel for selective and versatile oil/water separation. ACS Sustain. Chem. Eng. 2019, 7, 9984–9994. [Google Scholar] [CrossRef]
- Le Moigne, N.; Navard, P. Dissolution mechanisms of wood cellulose fibres in NaOH–water. Cellulose 2010, 17, 31–45. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, L. Rapid dissolution of cellulose in LiOH/urea and NaOH/urea aqueous solutions. Macromol. Biosci. 2005, 5, 539–548. [Google Scholar] [CrossRef]
- Kaya, M. Super absorbent, light, and highly flame retardant cellulose-based aerogel crosslinked with citric acid. J. Appl. Polym. Sci. 2017, 134, 45315. [Google Scholar] [CrossRef]
- Udoetok, I.A.; Dimmick, R.M.; Wilson, L.D.; Headley, J.V. Adsorption properties of cross-linked cellulose-epichlorohydrin polymers in aqueous solution. Carbohydr. Polym. 2016, 136, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Gu, B.; Pennefather, M.P.; Nguyen, T.X.; Phan-Thien, N.; Duong, H.M. Cotton aerogels and cotton-cellulose aerogels from environmental waste for oil spillage cleanup. Mater. Des. 2017, 130, 452–458. [Google Scholar] [CrossRef]
- Marcì, G.; Mele, G.; Palmisano, L.; Pulito, P.; Sannino, A. Environmentally sustainable production of cellulose-based superabsorbent hydrogels. Green Chem. 2006, 8, 439–444. [Google Scholar] [CrossRef]
- Wang, C.; Okubayashi, S. Polyethyleneimine-crosslinked cellulose aerogel for combustion CO2 capture. Carbohydr. Polym. 2019, 225, 115248. [Google Scholar] [CrossRef] [PubMed]
- Lewis, L.; Hatzikiriakos, S.G.; Hamad, W.Y.; MacLachlan, M.J. Freeze–thaw gelation of cellulose nanocrystals. ACS Macro Lett. 2019, 8, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Preibisch, I.; Ränger, L.M.; Gurikov, P.; Smirnova, I. In Situ Measurement Methods for the CO2-Induced Gelation of Biopolymer Systems. Gels 2020, 6, 28. [Google Scholar] [CrossRef]
- Loodts, V.; Rongy, L.; De Wit, A. Impact of pressure, salt concentration, and temperature on the convective dissolution of carbon dioxide in aqueous solutions. Chaos Interdiscip. J. Nonlinear Sci. 2014, 24, 043120. [Google Scholar] [CrossRef]
- Gurikov, P.; Raman, S.P.; Weinrich, D.; Fricke, M.; Smirnova, I. A novel approach to alginate aerogels: Carbon dioxide induced gelation. RSC Adv. 2015, 5, 7812–7818. [Google Scholar] [CrossRef]
- Floren, M.L.; Spilimbergo, S.; Motta, A.; Migliaresi, C. Carbon dioxide induced silk protein gelation for biomedical applications. Biomacromolecules 2012, 13, 2060–2072. [Google Scholar] [CrossRef]
- Ji, C.; Annabi, N.; Khademhosseini, A.; Dehghani, F. Fabrication of porous chitosan scaffolds for soft tissue engineering using dense gas CO2. Acta Biomater. 2011, 7, 1653–1664. [Google Scholar] [CrossRef]
- Zubal, L.; Bonani, W.; Maniglio, D.; Ceccato, R.; Renciuk, D.; Hampl, A.; Vojtova, L. Soluble collagen dissolution and assembling in pressurized carbon dioxide water solutions. Express Polym. Lett. 2018, 12, 159–170. [Google Scholar] [CrossRef]
- Alam, M.N.; Islam, M.S.; Christopher, L.P. Sustainable production of cellulose-based hydrogels with superb absorbing potential in physiological saline. ACS Omega 2019, 4, 9419–9426. [Google Scholar] [CrossRef] [PubMed]
- Hospodarova, V.; Singovszka, E.; Stevulova, N. Characterization of cellulosic fibers by FTIR spectroscopy for their further implementation to building materials. Am. J. Anal. Chem. 2018, 9, 303–310. [Google Scholar] [CrossRef]
- Abidi, N.; Cabrales, L.; Haigler, C.H. Changes in the cell wall and cellulose content of developing cotton fibers investigated by FTIR spectroscopy. Carbohydr. Polym. 2014, 100, 9–16. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]

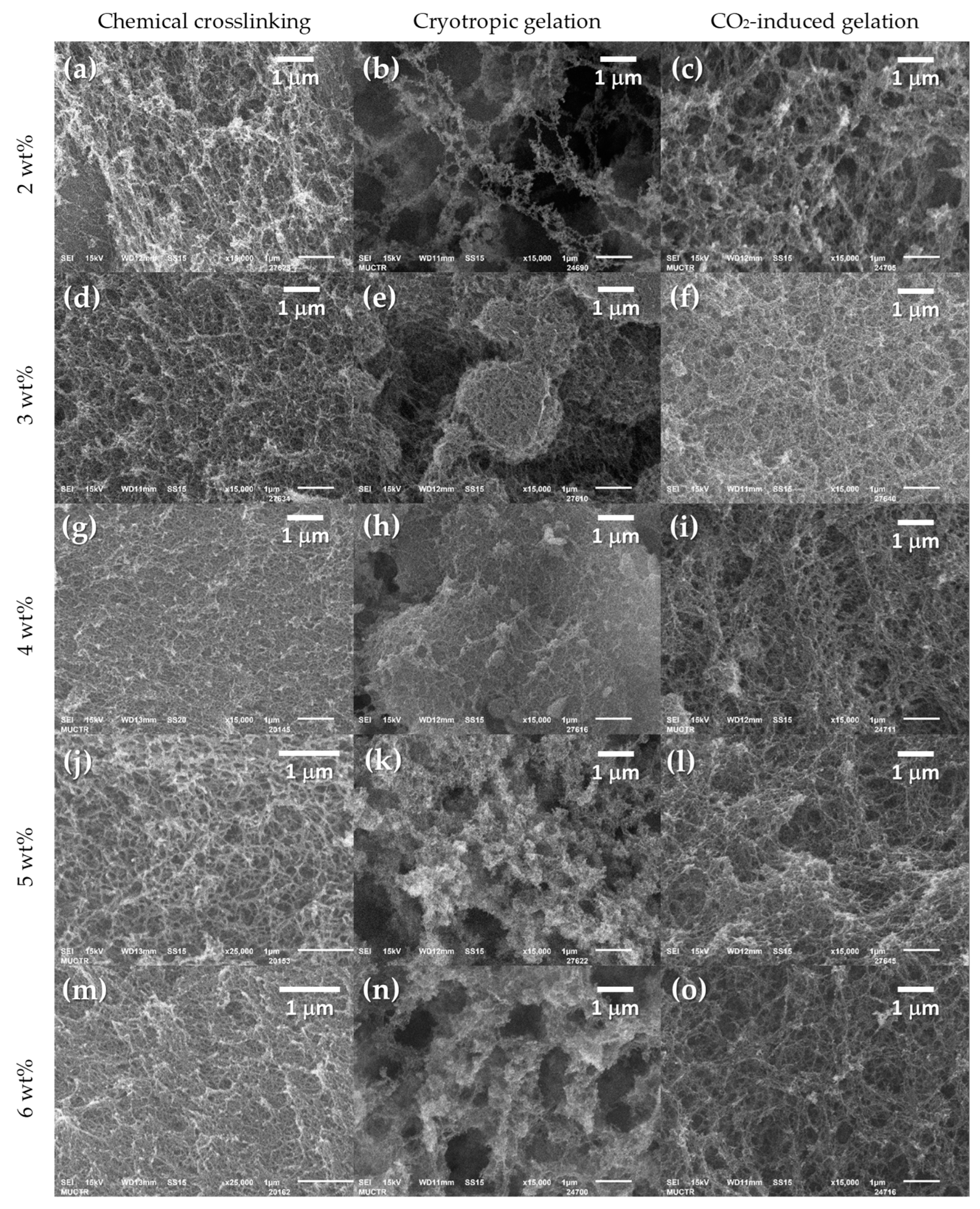


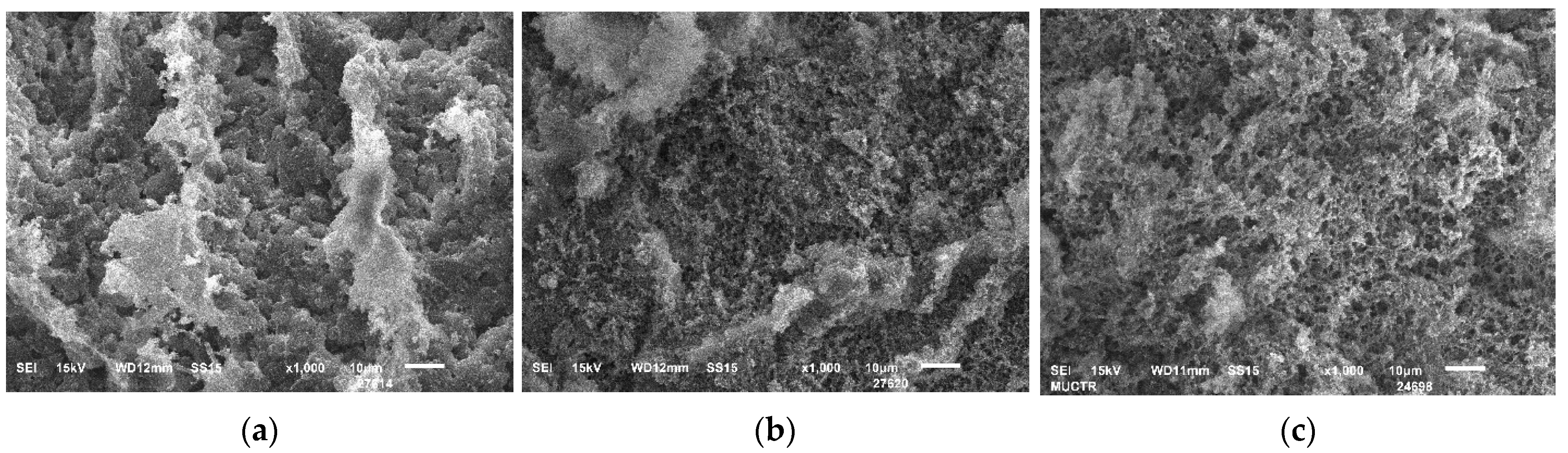
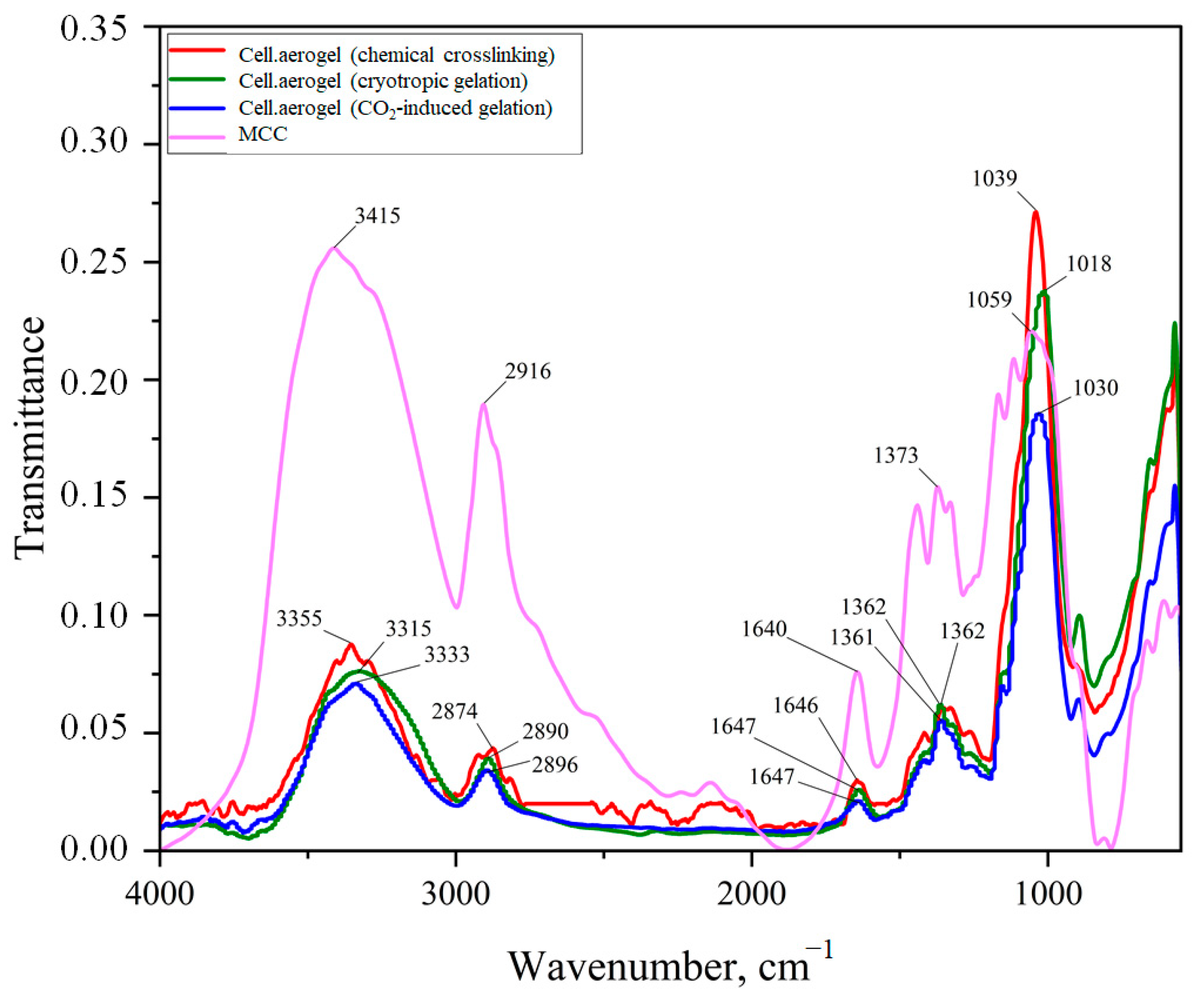
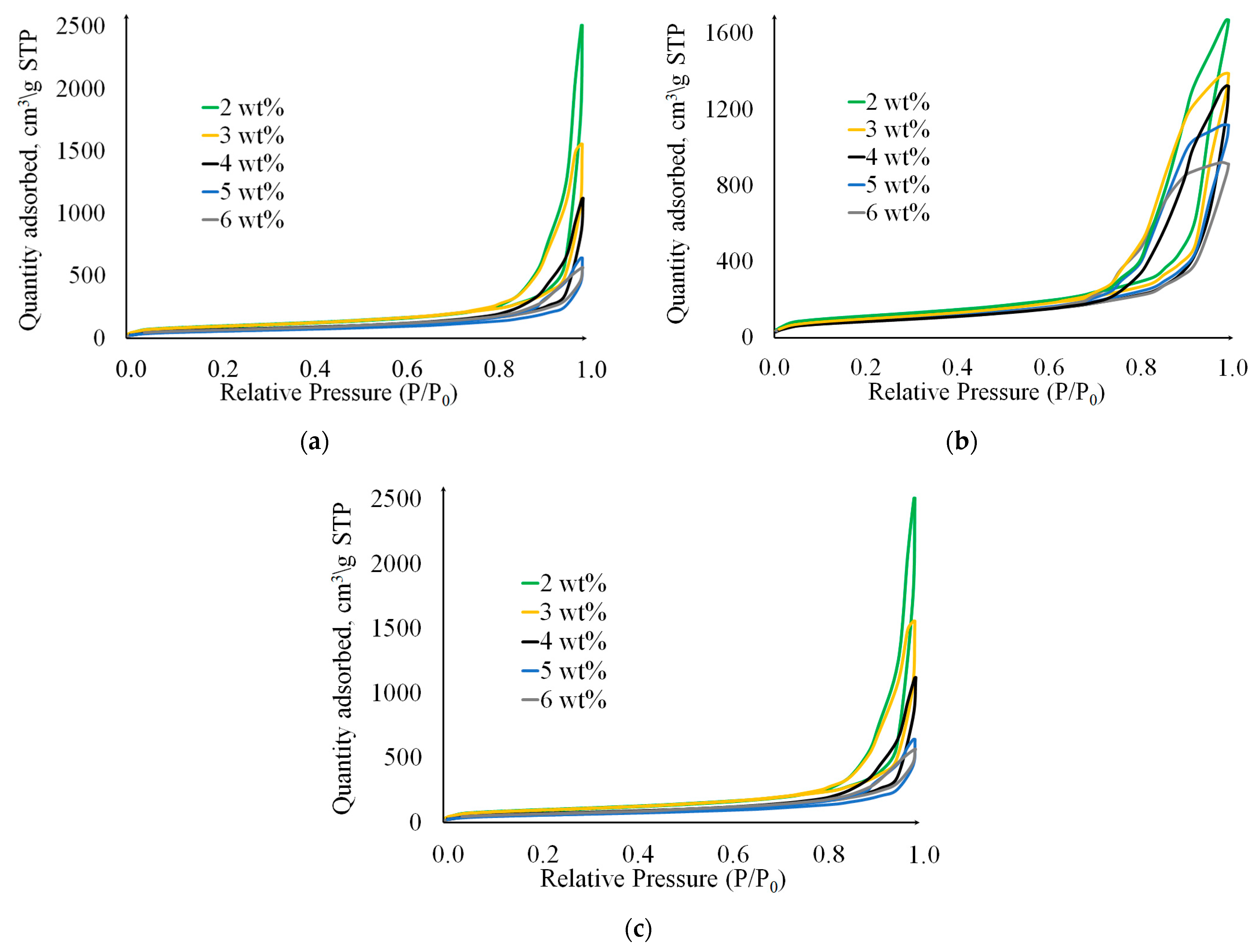
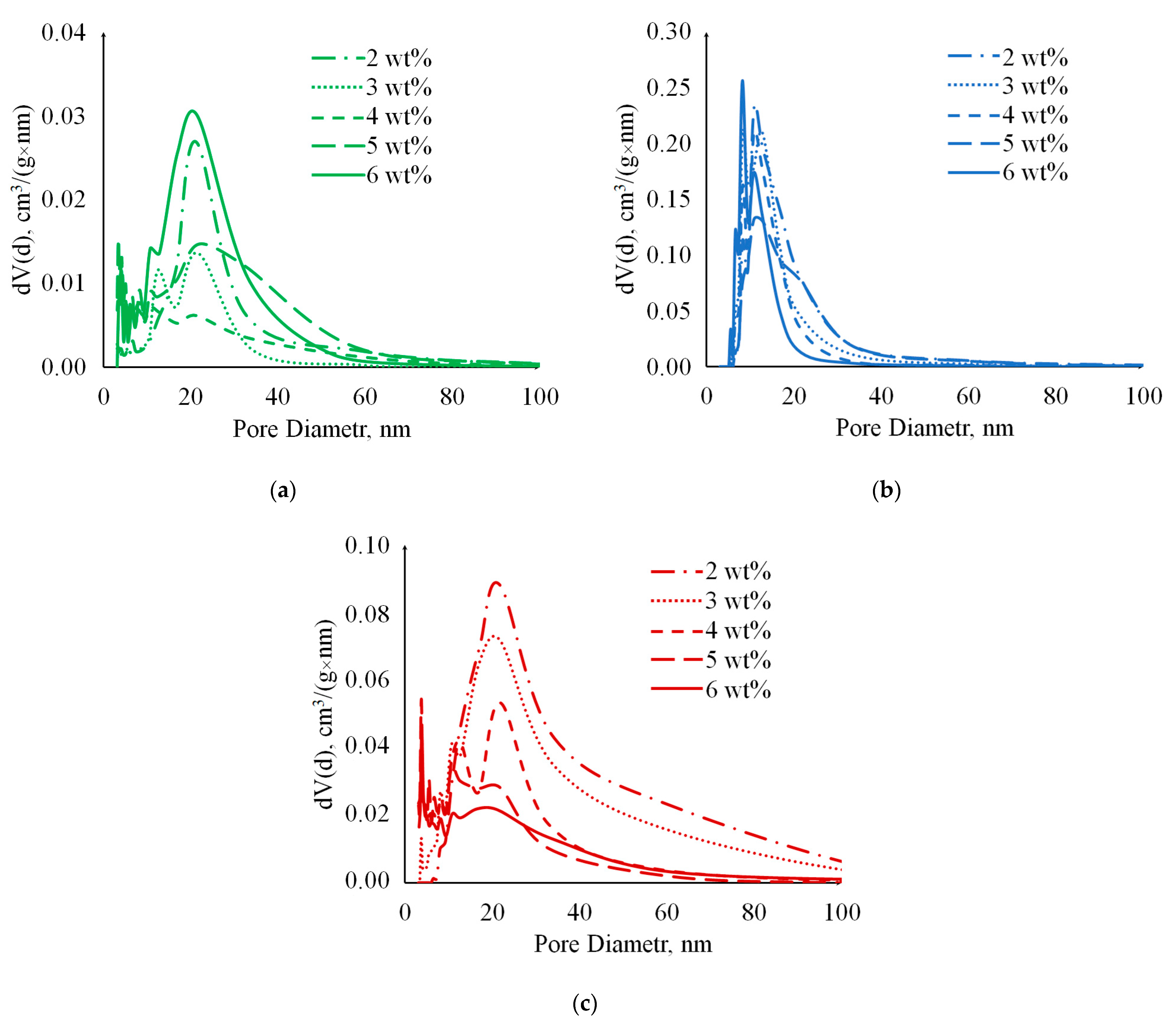
| № | Gelation Method | , wt% | , % | , % | , g/cm3 | , g/cm3 | , % |
|---|---|---|---|---|---|---|---|
| 1 | Chemical crosslinking | 2.0 | 21 ± 3.0 | 16 ± 1.6 | 0.067 ± 0.004 | 1.589 ± 0.021 | 95.8 |
| 2 | 3.0 | 33 ± 3.6 | 8 ± 1.4 | 0.189 ± 0.007 | 1.488 ± 0.013 | 87.3 | |
| 3 | 4.0 | 31 ± 1.1 | 11 ± 0.9 | 0.201 ± 0.011 | 1.592 ± 0.017 | 87.4 | |
| 4 | 5.0 | 30 ± 1.0 | 7 ± 0.7 | 0.214 ± 0.013 | 1.632 ± 0.007 | 86.9 | |
| 5 | 6.0 | 31 ± 0.9 | 11 ± 0.8 | 0.349 ± 0.017 | 1.530 ± 0.009 | 77.2 | |
| 6 | Cryotropic gelation | 2.0 | 24 ± 0.4 | 9 ± 1.0 | 0.047 ± 0.007 | 1.856 ± 0.004 | 97.5 |
| 7 | 3.0 | 24 ± 0.7 | 11 ± 0.8 | 0.084 ± 0.005 | 1.870 ± 0.007 | 95.5 | |
| 8 | 4.0 | 25 ± 0.4 | 5 ± 0.8 | 0.094 ± 0.003 | 1.648 ± 0.011 | 94.3 | |
| 9 | 5.0 | 24 ± 0.3 | 7 ± 1.3 | 0.126 ± 0.005 | 1.737 ± 0.014 | 92.8 | |
| 10 | 6.0 | 22 ± 0.4 | 2 ± 0.9 | 0.131 ± 0.001 | 1.565 ± 0.008 | 91.6 | |
| 11 | CO2-induced gelation | 2.0 | 16 ± 0.5 | 9 ± 0.5 | 0.045 ± 0.002 | 1.634 ± 0.010 | 97.2 |
| 12 | 3.0 | 17 ± 0.9 | 4 ± 0.9 | 0.059 ± 0.000 | 2.176 ± 0.005 | 97.3 | |
| 13 | 4.0 | 17 ± 0.4 | 9 ± 0.4 | 0.073 ± 0.000 | 1.603 ± 0.016 | 95.5 | |
| 14 | 5.0 | 17 ± 0.2 | 5 ± 0.5 | 0.101 ± 0.000 | 1.961 ± 0.004 | 94.9 | |
| 15 | 6.0 | 15 ± 0.2 | 7 ± 0.6 | 0.108 ± 0.000 | 1.606 ± 0.013 | 93.3 |
| № | Gelation Method | , wt% | , м2/г | , cм3/г |
|---|---|---|---|---|
| 1 | Chemical crosslinking | 2.0 | 63 ± 7 | 0.34 ± 0.06 |
| 2 | 3.0 | 33 ± 7 | 0.23 ± 0.03 | |
| 3 | 4.0 | 58 ± 11 | 0.18 ± 0.07 | |
| 4 | 5.0 | 87 ± 46 | 0.31 ± 0.21 | |
| 5 | 6.0 | 78 ± 34 | 0.36 ± 0.23 | |
| 6 | Cryotropic gelation | 2.0 | 406 ± 8 | 2.64 ± 0.12 |
| 7 | 3.0 | 379 ± 7 | 2.21 ± 0.16 | |
| 8 | 4.0 | 332 ± 5 | 2.08 ± 0.11 | |
| 9 | 5.0 | 326 ± 5 | 1.95 ± 0.15 | |
| 10 | 6.0 | 312 ± 8 | 1.54 ± 0.10 | |
| 11 | CO2-induced gelation | 2.0 | 343 ± 11 | 3.70 ± 0.26 |
| 12 | 3.0 | 325 ± 21 | 2.19 ± 0.24 | |
| 13 | 4.0 | 281 ± 14 | 1.88 ± 0.13 | |
| 14 | 5.0 | 232 ± 19 | 1.13 ± 0.14 | |
| 15 | 6.0 | 225 ± 13 | 0.82 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menshutina, N.; Fedotova, O.; Trofimova, K.; Tsygankov, P. Investigation of Gelation Techniques for the Fabrication of Cellulose Aerogels. Gels 2023, 9, 919. https://doi.org/10.3390/gels9120919
Menshutina N, Fedotova O, Trofimova K, Tsygankov P. Investigation of Gelation Techniques for the Fabrication of Cellulose Aerogels. Gels. 2023; 9(12):919. https://doi.org/10.3390/gels9120919
Chicago/Turabian StyleMenshutina, Natalia, Olga Fedotova, Kseniya Trofimova, and Pavel Tsygankov. 2023. "Investigation of Gelation Techniques for the Fabrication of Cellulose Aerogels" Gels 9, no. 12: 919. https://doi.org/10.3390/gels9120919
APA StyleMenshutina, N., Fedotova, O., Trofimova, K., & Tsygankov, P. (2023). Investigation of Gelation Techniques for the Fabrication of Cellulose Aerogels. Gels, 9(12), 919. https://doi.org/10.3390/gels9120919