Linear Polyethyleneimine-Based and Metal Organic Frameworks (DUT-67) Composite Hydrogels as Efficient Sorbents for the Removal of Methyl Orange, Copper Ions, and Penicillin V
Abstract
:1. Introduction
2. Results and Discussion
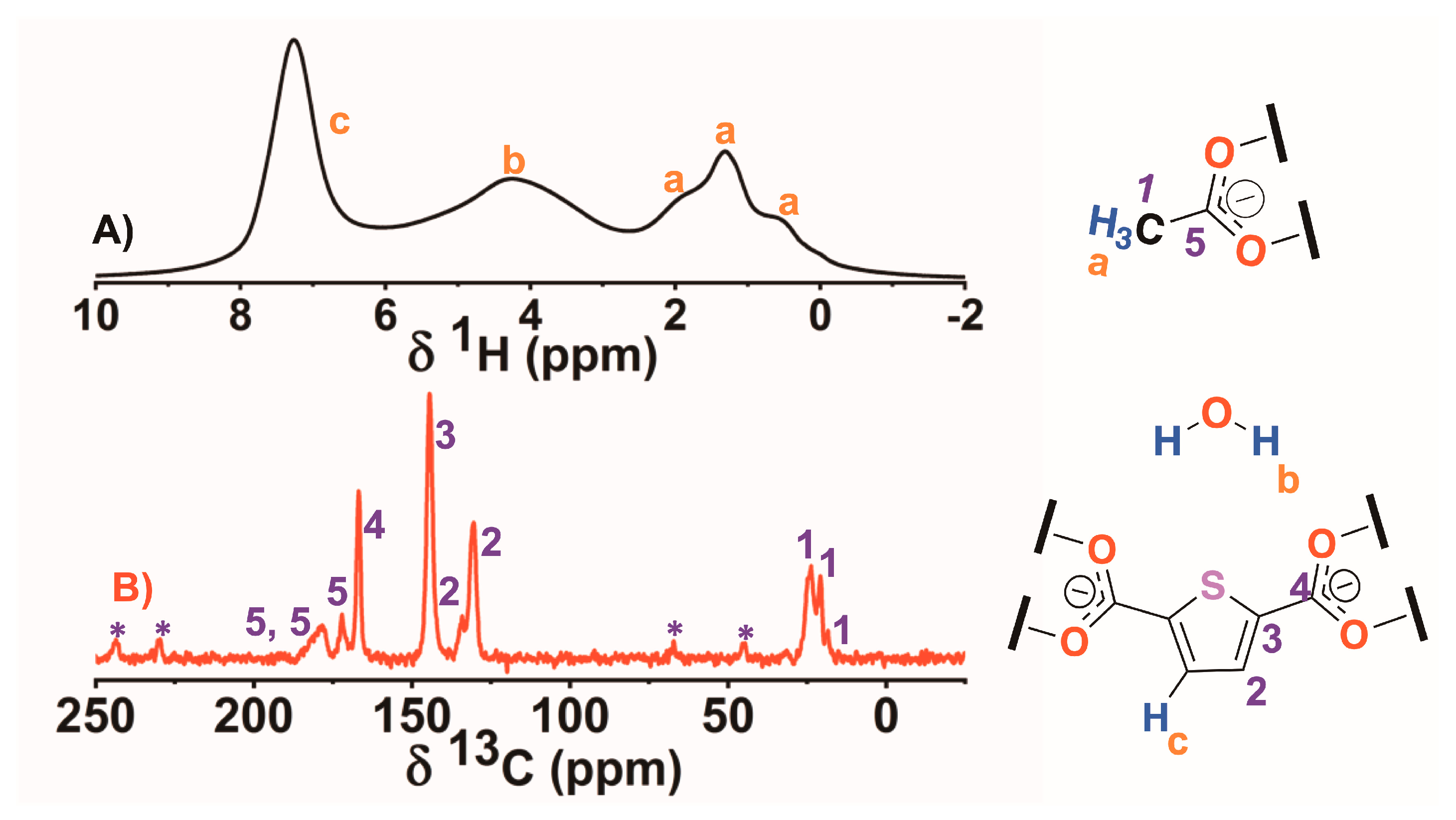
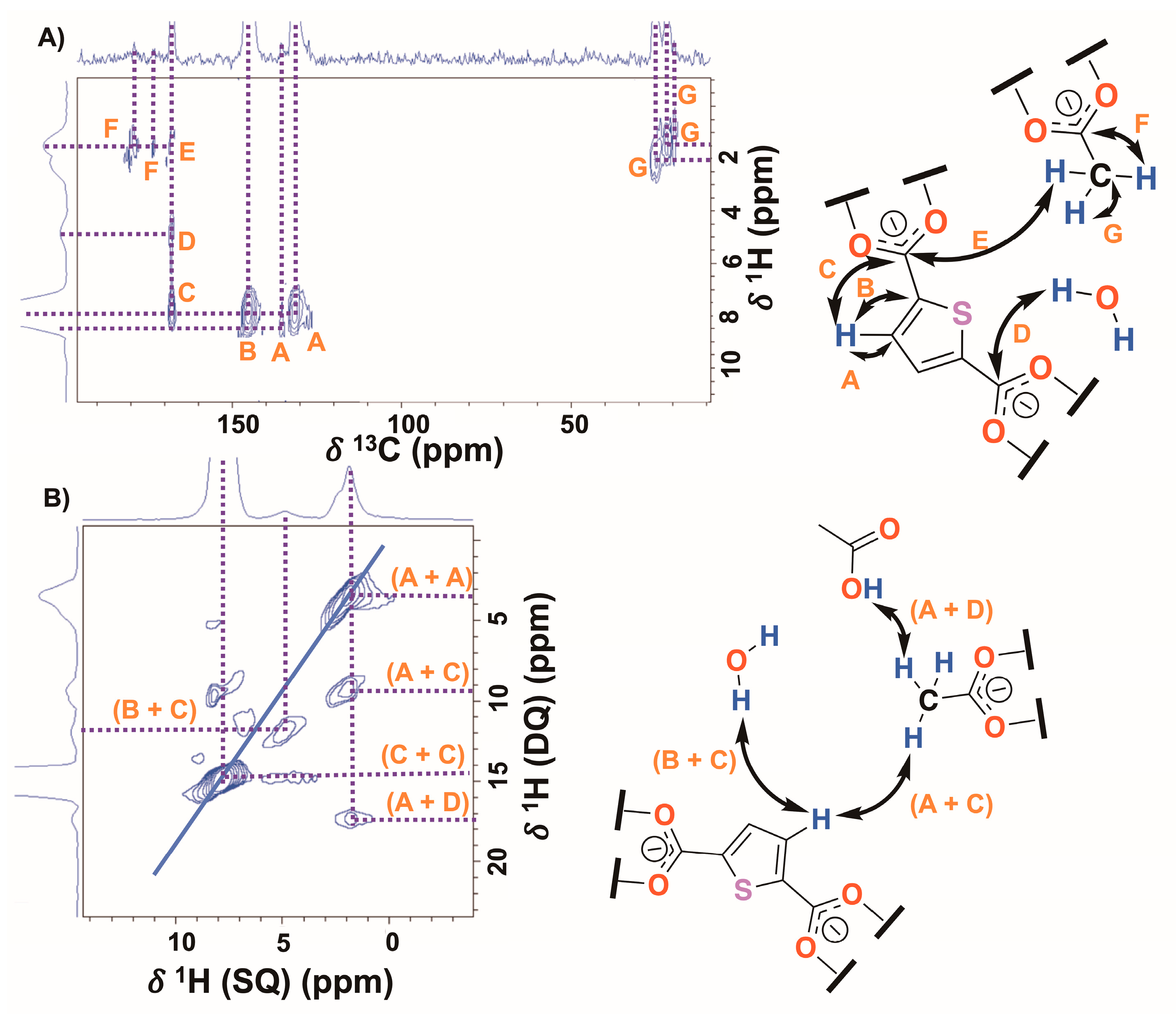
2.1. DUT-67 Synthesis and Characterization
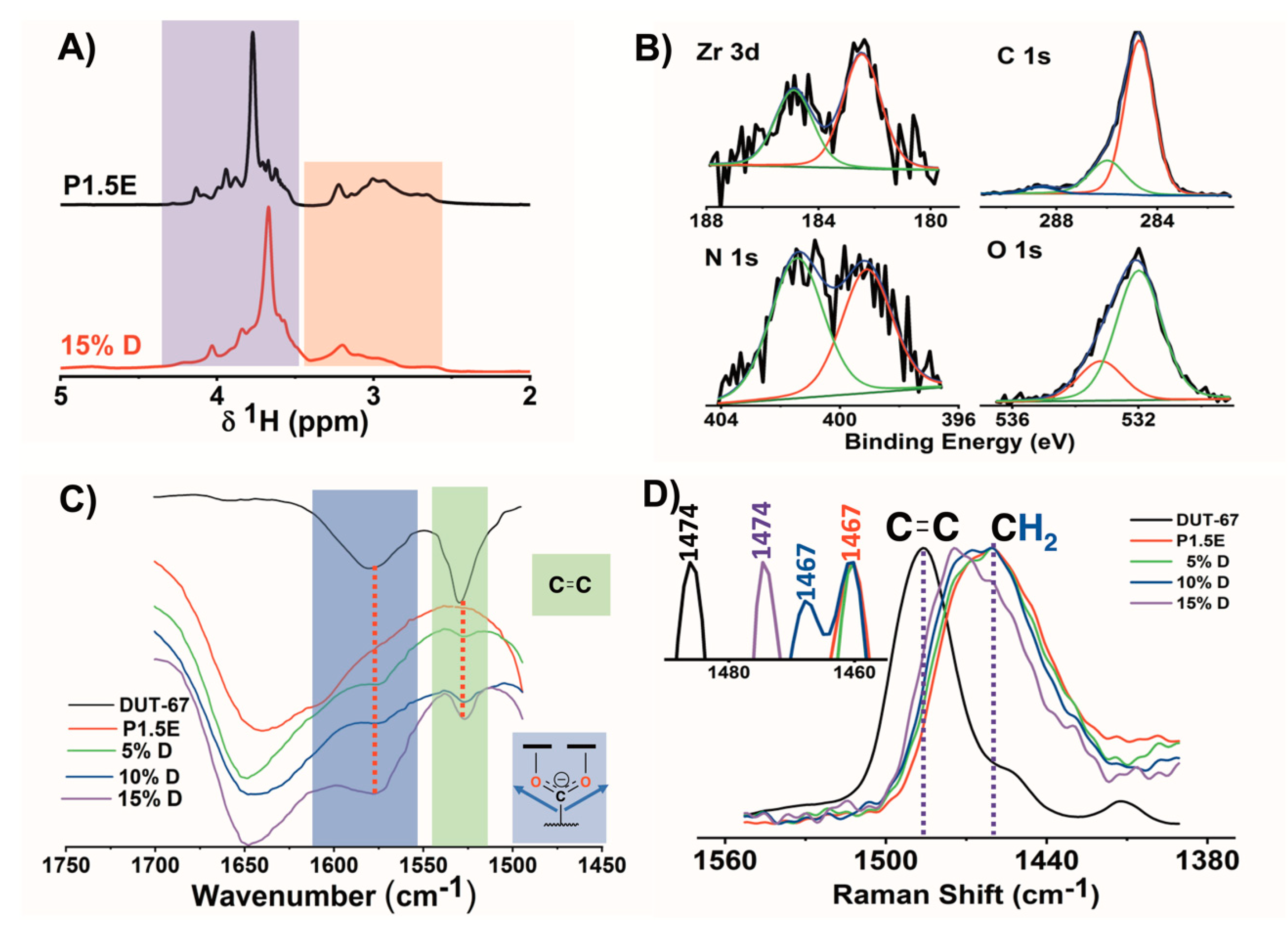
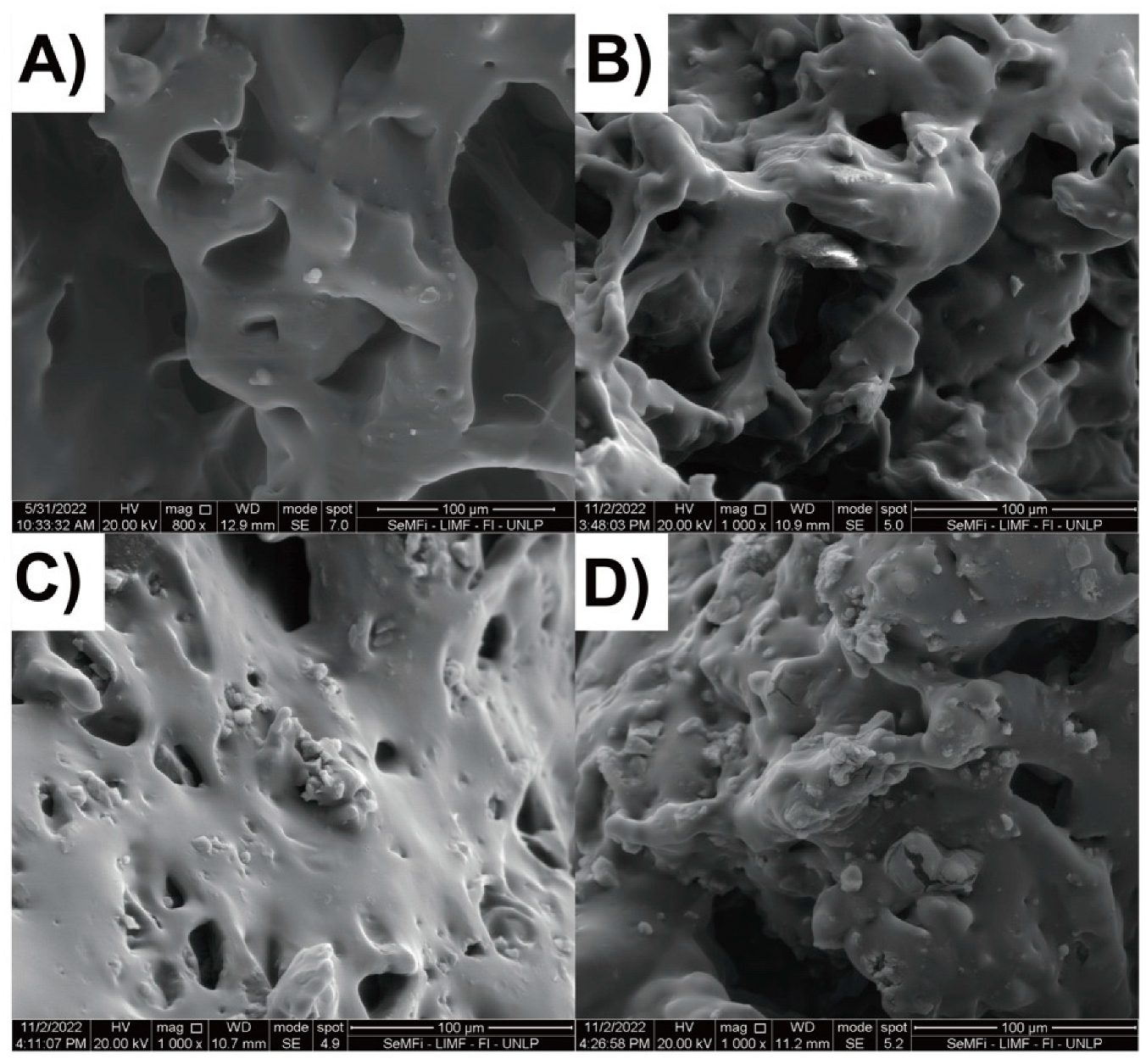
2.2. Composite Hydrogels Synthesis and Characterization
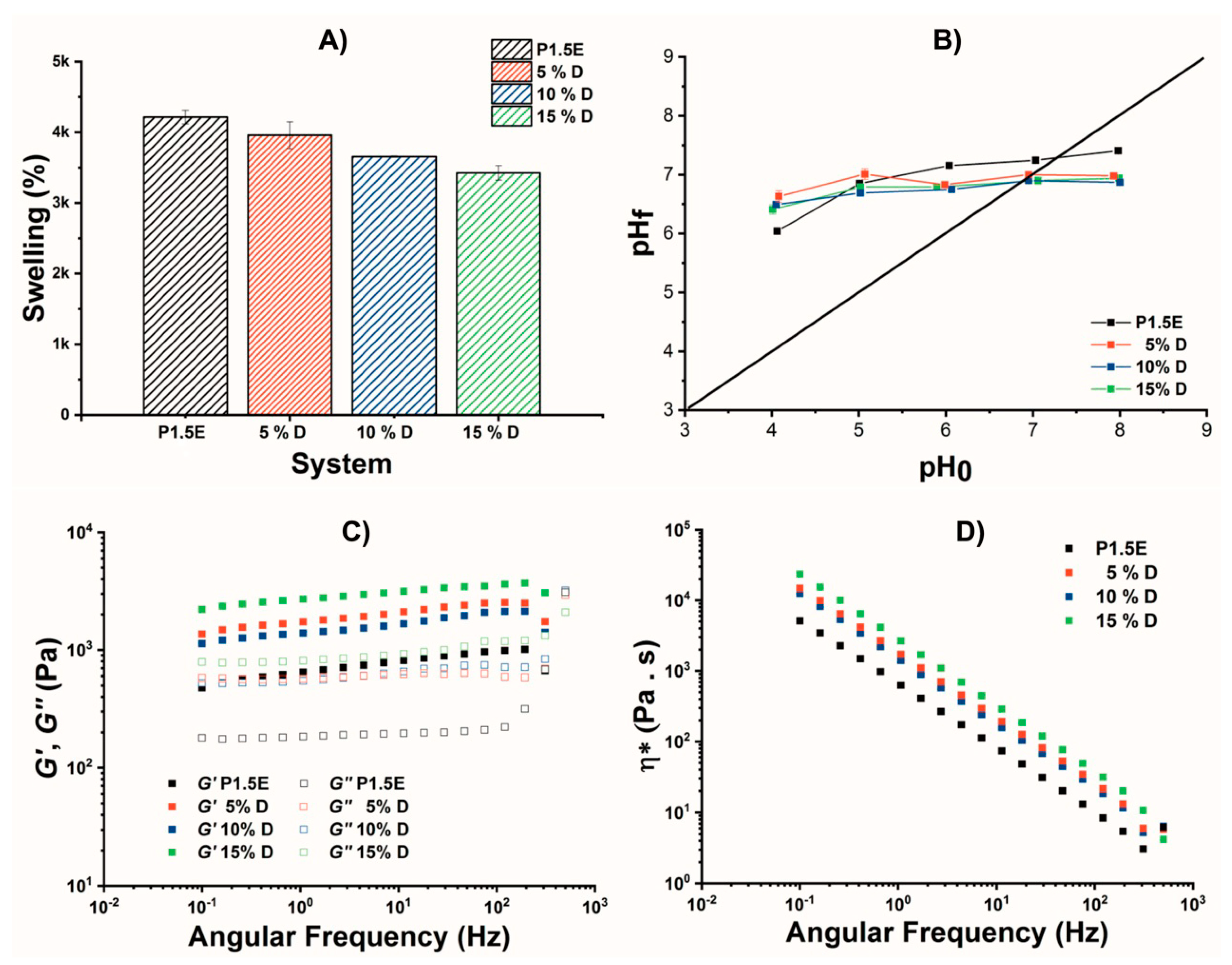
2.3. Thermal, Swelling, and Mechanical Properties of L-PEI·HCl@DUT-67 Hydrogels
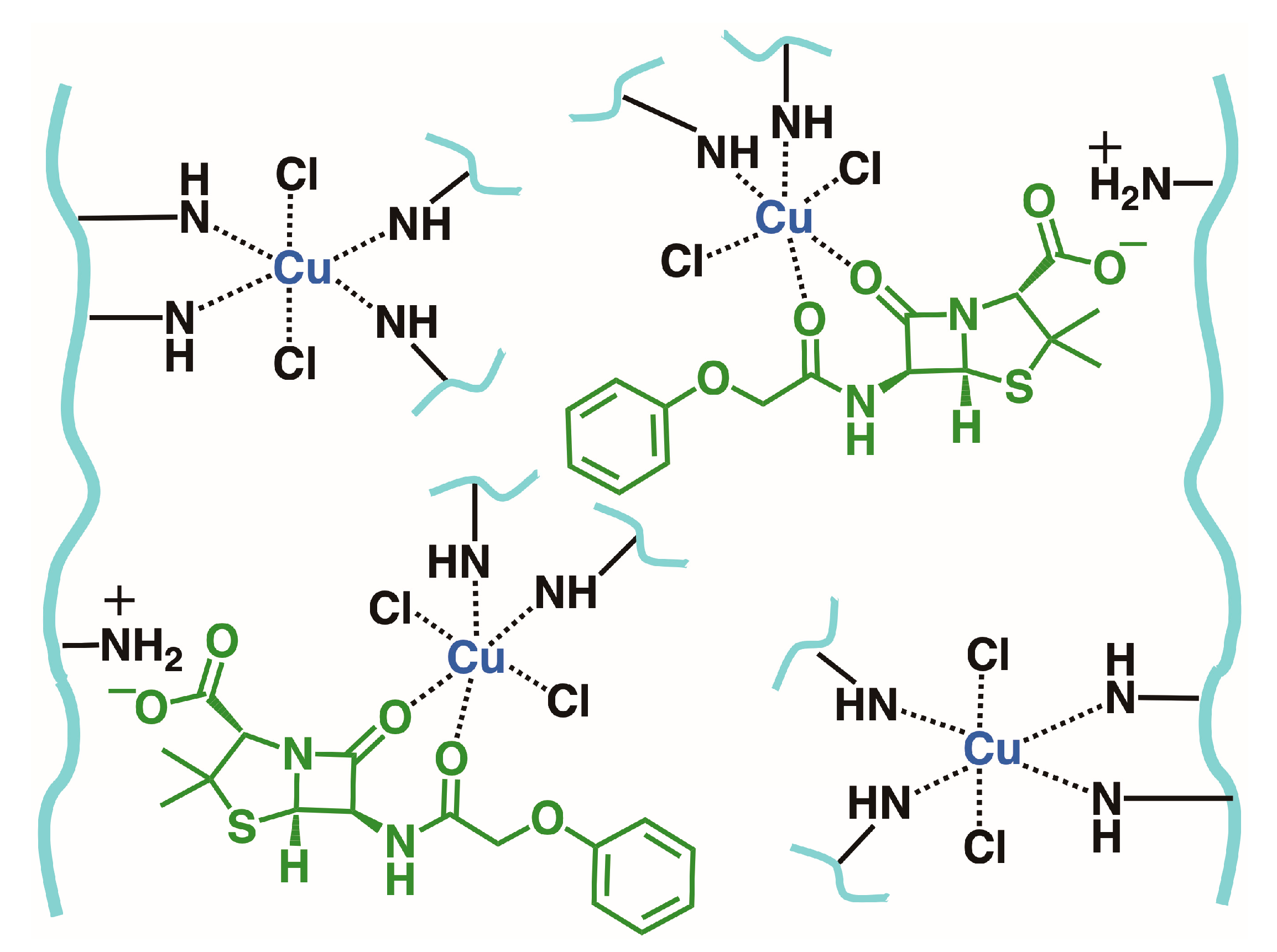
2.4. Functional Assessment of PEI@DUT-67 Hydrogels as Sorbents for Water Remediation
2.5. Effect of the pH on the Adsorption Capacity
2.6. Competitive Adsorption
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. DUT-67 Synthesis
4.3. MOF@L-PEI·HCl Composite Hydrogels Synthesis
4.4. Characterization Techniques
4.5. Kinetic and Adsorption Capacity Assessment
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foong, C.Y.; Wirzal, M.D.H.; Bustam, M.A. A Review on Nanofibers Membrane with Amino-Based Ionic Liquid for Heavy Metal Removal. J. Mol. Liq. 2020, 297, 111793. [Google Scholar] [CrossRef]
- Alzain, H.; Kalimugogo, V.; Hussein, K. A Review of Environmental Impact of Azo Dyes. Int. J. Res. Rev. 2023, 10, 64–689. [Google Scholar] [CrossRef]
- Pal, A.; Jayamani, J.; Prasad, R. An Urgent Need to Reassess the Safe Levels of Copper in the Drinking Water: Lessons from Studies on Healthy Animals Harboring No Genetic Deficits. Neurotoxicology 2014, 44, 58–60. [Google Scholar] [CrossRef]
- Peña-Guzmán, C.; Ulloa-Sánchez, S.; Mora, K.; Helena-Bustos, R.; Lopez-Barrera, E.; Alvarez, J.; Rodriguez-Pinzón, M. Emerging Pollutants in the Urban Water Cycle in Latin America: A Review of the Current Literature. J. Environ. Manag. 2019, 237, 408–423. [Google Scholar] [CrossRef] [PubMed]
- Sellaoui, L.; Gómez-Avilés, A.; Dhaouadi, F.; Bedia, J.; Bonilla-Petriciolet, A.; Rtimi, S.; Belver, C. Adsorption of Emerging Pollutants on Lignin-Based Activated Carbon: Analysis of Adsorption Mechanism via Characterization, Kinetics and Equilibrium Studies. Chem. Eng. J. 2023, 452, 139399. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, S.; Duan, H.; He, J.; Luo, Y. Removal of Anionic and Cationic Dyes Using Porous Chitosan/Carboxymethyl Cellulose-PEG Hydrogels: Optimization, Adsorption Kinetics, Isotherm and Thermodynamics Studies. Int. J. Biol. Macromol. 2023, 231, 123213. [Google Scholar] [CrossRef]
- Araque, L.M.; Pérez, C.J.; Infantes-Molina, A.; Rodríguez-Castellón, E.; Copello, G.J.; Lázaro-Martínez, J.M. Linear PEI-based Responsive Hydrogels: Synthesis and Characterization. J. Appl. Polym. Sci. 2023, 140, e54047. [Google Scholar] [CrossRef]
- Crespi, A.F.; Zomero, P.N.; Pérez, A.L.; Brondino, C.D.; Molina, A.I.; Linck, Y.G.; Monti, G.A.; Fernández, M.A.; Rodríguez-Castellón, E.; Lázaro-Martínez, J.M. Montmorillonite Materials with Paramagnetic Metal Complexes: Structural Studies and Catalytic Degradation of Emerging Pollutants. J. Environ. Chem. Eng. 2023, 11, 111420. [Google Scholar] [CrossRef]
- Sugawara, A.; Asoh, T.A.; Takashima, Y.; Harada, A.; Uyama, H. Composite Hydrogels Reinforced by Cellulose-Based Supramolecular Filler. Polym. Degrad. Stab. 2020, 177, 109157. [Google Scholar] [CrossRef]
- Rao, Z.; Dong, Y.; Liu, J.; Zheng, X.; Pei, Y.; Tang, K. Genipin-Crosslinked Gelatin-Based Composite Hydrogels Reinforced with Amino-Functionalized Microfibrillated Cellulose. Int. J. Biol. Macromol. 2022, 222, 3155–3167. [Google Scholar] [CrossRef]
- Liu, C.; Dai, T.; Wu, X.; Ma, J.; Liu, J.; Wu, S.; Yang, L.; Zhao, H. 3D Bioprinting of Cell-Laden Nano-Attapulgite/Gelatin Methacrylate Composite Hydrogel Scaffolds for Bone Tissue Repair. J. Mater. Sci. Technol. 2023, 135, 111–125. [Google Scholar] [CrossRef]
- Chen, L.; Wu, Y.; Xing, W.; Su, Q.; Tang, L.; Xue, H.; Gao, J. Mechanically Robust Composite Hydrogels for High Performance Solar Driven Interface Evaporation. Chem. Eng. Sci. 2023, 267, 118330. [Google Scholar] [CrossRef]
- Rehman Shah, H.U.; Ahmad, K.; Naseem, H.A.; Parveen, S.; Ashfaq, M.; Rauf, A.; Aziz, T. Water Stable Graphene Oxide Metal-Organic Frameworks Composite (ZIF-67@GO) for Efficient Removal of Malachite Green from Water. Food Chem. Toxicol. 2021, 154, 112312. [Google Scholar] [CrossRef]
- Ahmad, K.; Shah, H.U.R.; Parveen, S.; Aziz, T.; Naseem, H.A.; Ashfaq, M.; Rauf, A. Metal Organic Framework (KIUB-MOF-1) as Efficient Adsorbent for Cationic and Anionic Dyes from Brackish Water. J. Mol. Struct. 2021, 1242, 130898. [Google Scholar] [CrossRef]
- Ahmad, K.; Nazir, M.A.; Qureshi, A.K.; Hussain, E.; Najam, T.; Javed, M.S.; Shah, S.S.A.; Tufail, M.K.; Hussain, S.; Khan, N.A. Engineering of Zirconium Based Metal-Organic Frameworks (Zr-MOFs) as Efficient Adsorbents. Mater. Sci. Eng. B 2020, 262, 114766. [Google Scholar] [CrossRef]
- Ahmad, K.; Naseem, K.; Shah, H.-R.; Riaz, N.N.; Alhadhrami, A.; Majeed, H.; Ahmad, M.M.; Afzal Awan, M.M.; Ahmad, S.; Ashfaq, M. Towards Sustainable Water Purification: MOFs as a Promising Solution to Eliminate Toxic Water Pollutant Resorcinol. Z. Phys. Chem. 2023, 237, 1669–1689. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Z.-B.; Zeng, J.; Zhang, Z.-J.; Ma, S.; Tang, C.-M.; Xu, J.-Q. Preparation of Polyethyleneimine-Modified Chitosan/Ce-UIO-66 Composite Hydrogel for the Adsorption of Methyl Orange. Carbohydr. Polym. 2023, 299, 120079. [Google Scholar] [CrossRef]
- Jin, Y.; Li, Y.; Du, Q.; Chen, B.; Chen, K.; Zhang, Y.; Wang, M.; Sun, Y.; Zhao, S.; Jing, Z.; et al. Efficient Adsorption of Congo Red by MIL-53(Fe)/Chitosan Composite Hydrogel Spheres. Microporous Mesoporous Mater. 2023, 348, 112404. [Google Scholar] [CrossRef]
- Drache, F.; Bon, V.; Senkovska, I.; Marschelke, C.; Synytska, A.; Kaskel, S. Postsynthetic Inner-Surface Functionalization of the Highly Stable Zirconium-Based Metal-Organic Framework DUT-67. Inorg. Chem. 2016, 55, 7206–7213. [Google Scholar] [CrossRef]
- Zhuang, G.; Bai, J.; Tan, L.; Huang, H.; Gao, Y.; Zhong, X.; Zhong, C.; Wang, J. Preparation and Catalytic Properties of Pd Nanoparticles Supported on Micro-Crystal DUT-67 MOFs. RSC Adv. 2015, 5, 32714–32719. [Google Scholar] [CrossRef]
- Liu, D.; Qiu, J.; Xu, R.; Liu, J.; Feng, J.; Ouyang, L.; Qian, S.; Qiao, Y.; Liu, X. β-CD/PEI/PVA Composite Hydrogels with Superior Self-Healing Ability and Antibacterial Activity for Wound Healing. Compos. B Eng. 2022, 238, 109921. [Google Scholar] [CrossRef]
- Godiya, C.B.; Revadekar, C.; Kim, J.; Park, B.J. Amine-Bilayer-Functionalized Cellulose-Chitosan Composite Hydrogel for the Efficient Uptake of Hazardous Metal Cations and Catalysis in Polluted Water. J. Hazard. Mater. 2022, 436, 129112. [Google Scholar] [CrossRef] [PubMed]
- Mguni, L.L.; Yao, Y.; Ren, J.; Liu, X.; Hildebrandt, D. Modulated Synthesized Ni-Based MOF with Improved Adsorptive Desulfurization Activity. J. Clean. Prod. 2021, 323, 129196. [Google Scholar] [CrossRef]
- Usman, K.A.S.; Maina, J.W.; Seyedin, S.; Conato, M.T.; Payawan Jr, L.M.; Dumée, L.F.; Razal, J.M. Downsizing Metal–Organic Frameworks by Bottom-up and Top-down Methods. NPG Asia Mater. 2020, 12, 58. [Google Scholar] [CrossRef]
- Bigdeli, F.; Fetzer, M.N.A.; Nis, B.; Morsali, A.; Janiak, C. Coordination Modulation: A Way to Improve the Properties of Metal–Organic Frameworks. J. Mater. Chem. A Mater. 2023, 11, 22105–22131. [Google Scholar] [CrossRef]
- Drache, F.; Bon, V.; Senkovska, I.; Getzschmann, J.; Kaskel, S. The Modulator Driven Polymorphism of Zr(IV) Based Metal–Organic Frameworks. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2017, 375, 20160027. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Y.; Shi, Y.; Wang, Z.; Guo, W.; Bi, J.; Wu, L. Au Nanoparticles-Anchored Defective Metal–Organic Frameworks for Photocatalytic Transformation of Amines to Imines under Visible Light. J. Colloid Interface Sci. 2023, 631, 154–163. [Google Scholar] [CrossRef]
- Liu, H.-X.; Liu, T.-T.; Huang, T.; Fang, Z.-B.; Li, L.; Yin, Q.; Cao, R.; Gong, X.-Q.; Liu, T.-F. Trace of Molecular Doping in Metal–Organic Frameworks: Drastic Change in the Electronic Band Structure with a Preserved Topology and Porosity. J. Mater. Chem. A Mater. 2020, 8, 12370–12377. [Google Scholar] [CrossRef]
- Liu, Y.; Zou, J.; Guo, B.; Ren, Y.; Wang, Z.; Song, Y.; Yu, Y.; Wu, L. Selective Photocatalytic Oxidation of Thioanisole on DUT-67(Zr) Mediated by Surface Coordination. Langmuir 2020, 36, 2199–2208. [Google Scholar] [CrossRef]
- Xu, M.; Zhao, X.; Jiang, H.; Song, X.; Zhou, W.; Liu, X.; Liu, Z.; Wang, H.; Huo, P. Constructing Schottky Junctions via Pd Nanosheets on DUT-67 Surfaces to Accelerate Charge Transfer. J. Colloid Interface Sci. 2022, 608, 3022–3029. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, M.; Song, X.; Zhou, W.; Liu, X.; Wang, H.; Huo, P. Integration of 3D Macroscopic Reduced Graphene Oxide Aerogel with DUT-67 for Selective CO2 Photoreduction to CO in Gas-Solid Reaction. Chem. Eng. J. 2022, 446, 137034. [Google Scholar] [CrossRef]
- Drache, F.; Cirujano, F.G.; Nguyen, K.D.; Bon, V.; Senkovska, I.; Llabrés i Xamena, F.X.; Kaskel, S. Anion Exchange and Catalytic Functionalization of the Zirconium-Based Metal–Organic Framework DUT-67. Cryst. Growth Des. 2018, 18, 5492–5500. [Google Scholar] [CrossRef]
- Sangeetha Margreat, S.; Ramalingam, S.; Sebastian, S.; Xavier, S.; Periandy, S.; Daniel, J.C.; Maria Julie, M. DFT, Spectroscopic, DSC/TGA, Electronic, Biological and Molecular Docking Investigation of 2,5-Thiophenedicarboxylic Acid: A Promising Anticancer Agent. J. Mol. Struct. 2020, 1200, 127099. [Google Scholar] [CrossRef]
- Borges, M.M.C.; Pires, B.C.; Vieira, S.S.; Borges, K.B.; de Lima Guimarães, L.G. Magnetic and PH Responsive Composite Hydrogel-Based on Poly(2-(Diethylamino)Ethyl Methacrylate)/Chitosan for Fipronil Removal from Aqueous Medium. React. Funct. Polym. 2021, 168, 105050. [Google Scholar] [CrossRef]
- Curtis, K.A.; Miller, D.; Millard, P.; Basu, S.; Horkay, F.; Chandran, P.L. Unusual Salt and PH Induced Changes in Polyethylenimine Solutions. PLoS ONE 2016, 11, e0158147. [Google Scholar] [CrossRef]
- Shu, X.; Wei, Y.; Luo, X.; Liu, J.; Mao, L.; Yuan, F.; Gao, Y. κ-Carrageenan/Konjac Glucomannan Composite Hydrogel Filled with Rhamnolipid-Stabilized Nanostructured Lipid Carrier: Improvement of Structure and Properties. Food Hydrocoll. 2023, 134, 108088. [Google Scholar] [CrossRef]
- Zhang, Z.; Lucia, L. Toward Synergistic Reinforced Graphene Nanoplatelets Composite Hydrogels with Self-Healing and Multi-Stimuli Responses. Polymer 2021, 234, 124228. [Google Scholar] [CrossRef]
- Bhat, M.A.; Rather, R.A.; Shalla, A.H. Texture and Rheological Features of Strain and PH Sensitive Chitosan-Imine Graphene-Oxide Composite Hydrogel with Fast Self-Healing Nature. Int. J. Biol. Macromol. 2022, 222, 3129–3141. [Google Scholar] [CrossRef]
- Nnaji, N.J.N.; Sonde, C.U.; Nwanji, O.L.; Ezeh, G.C.; Onuigbo, A.U.; Ojukwu, A.M.; Mbah, P.C.; Adewumi, A.O.; Unoka, E.C.; Otedo, J.O.; et al. Dacryodes Edulis Leaf Derived Biochar for Methylene Blue Biosorption. J. Environ. Chem. Eng. 2023, 11, 109638. [Google Scholar] [CrossRef]
- Aldahash, S.A.; Siddiqui, S.; Uddin, M.K. Eco-Friendly Synthesis of Copper Nanoparticles from Fiber of Trapa Natans L. Shells and Their Impregnation Onto Polyamide-12 for Environmental Applications. J. Nat. Fibers 2023, 20, 2224976. [Google Scholar] [CrossRef]
- Yang, J.; Huang, Y.; Cheng, Y.; Wu, X.; Lu, J.; Wan, Q.; Feng, J.; Zeng, Q.; Zhao, S.; Yu, L.; et al. Long-Acting Removal of High-Toxic p-Nitrophenol in Wastewater via Peroxymonosulfate Activation by Cyclic Membrane Catalysis. J. Clean. Prod. 2023, 401, 136739. [Google Scholar] [CrossRef]
- Asgari, P.; Mousavi, S.H.; Aghayan, H.; Ghasemi, H.; Yousefi, T. Nd-BTC Metal-Organic Framework (MOF); Synthesis, Characterization and Investigation on Its Adsorption Behavior toward Cesium and Strontium Ions. Microchem. J. 2019, 150, 104188. [Google Scholar] [CrossRef]
- Rao, R.A.K.; Kashifuddin, M. Pottery Glaze-An Excellent Adsorbent for the Removal of Cu(II) from Aqueous Solution. Chin. J. Geochem. 2012, 31, 136–146. [Google Scholar] [CrossRef]
- Semenova, A.; Giles, L.W.; Vidallon, M.L.P.; Follink, B.; Brown, P.L.; Tabor, R.F. Copper-Binding Properties of Polyethylenimine–Silica Nanocomposite Particles. Langmuir 2022, 38, 10585–10600. [Google Scholar] [CrossRef] [PubMed]
- Valverde, A.; Tovar, G.I.; Rio-López, N.A.; Torres, D.; Rosales, M.; Wuttke, S.; Fidalgo-Marijuan, A.; Porro, J.M.; Jiménez-Ruiz, M.; García Sakai, V.; et al. Designing Metal-Chelator-like Traps by Encoding Amino Acids in Zirconium-Based Metal–Organic Frameworks. Chem. Mater. 2022, 34, 9666–9684. [Google Scholar] [CrossRef]
- Tovar, G.I.; Valverde, A.; Mendes-Felipe, C.; Wuttke, S.; Fidalgo-Marijuan, A.; Larrea, E.S.; Lezama, L.; Zheng, F.; Reguera, J.; Lanceros-Méndez, S. Chitin/Metal-Organic Framework Composites as Wide-Range Adsorbent. ChemSusChem 2021, 14, 2892–2901. [Google Scholar] [CrossRef]
- Valverde, A.; de Fernandez-de Luis, R.; Salazar, H.; Gonçalves, B.F.; King, S.; Almásy, L.; Kriechbaum, M.; Laza, J.M.; Vilas-Vilela, J.L.; Martins, P.M. On The Multiscale Structure and Morphology of Pvdf-Hfp@ Mof Membranes in The Scope of Water Remediation Applications. Adv. Mater. Interfaces 2023, 10, 2300424. [Google Scholar] [CrossRef]
- Zhong, J.; Wang, B.; Sun, K.; Duan, J. Hyperbranched Polyethylenimine–Based Polymeric Nanoparticles: Synthesis, Properties, and an Application in Selective Response to Copper Ion. Colloid Polym. Sci. 2021, 299, 1577–1586. [Google Scholar] [CrossRef]
- Sher, A.; Veber, M.; Marolt-Gomišček, M. Spectroscopic and Polarographic Investigations: Copper(II)-Penicillin Derivatives. Int. J. Pharm. 1997, 148, 191–199. [Google Scholar] [CrossRef]
- van Krimpen, P.C.; van Bennekom, W.P.; Bult, A. A Study of the Metal Complexation Behaviour of Some Penicillins, Cephalosporins and Their Derivatives. Pharm. Weekbl. 1988, 10, 259–266. [Google Scholar] [CrossRef]
- Lázaro-Martínez, J.M.; Rodríguez-Castellón, E.; Vega, D.; Monti, G.A.; Chattah, A.K. Solid-State Studies of the Crystalline/Amorphous Character in Linear Poly (Ethylenimine Hydrochloride) (PEI·HCl) Polymers and Their Copper Complexes. Macromolecules 2015, 48, 1115–1125. [Google Scholar] [CrossRef]
- Reinsch, H.; Waitschat, S.; Chavan, S.M.; Lillerud, K.P.; Stock, N. A Facile “Green” Route for Scalable Batch Production and Continuous Synthesis of Zirconium MOFs. Eur. J. Inorg. Chem. 2016, 2016, 4490–4498. [Google Scholar] [CrossRef]
- Kim, K.C.; Yoon, T.-U.; Bae, Y.-S. Applicability of Using CO2 Adsorption Isotherms to Determine BET Surface Areas of Microporous Materials. Microporous Mesoporous Mater. 2016, 224, 294–301. [Google Scholar] [CrossRef]
- Cheng, K.L.; Bray, R.H. 1-(2-Pyridylazo)-2-Naphthol as Possible Analytical Reagent. Anal. Chem. 1955, 27, 782–785. [Google Scholar] [CrossRef]
- Hartmann, S.R.; Hahn, E.L. Nuclear Double Resonance in the Rotating Frame. Phys. Rev. 1962, 128, 2042–2053. [Google Scholar] [CrossRef]
- Fung, B.M.; Khitrin, A.K.; Ermolaev, K. An Improved Broadband Decoupling Sequence for Liquid Crystals and Solids. J. Magn. Reson. 2000, 142, 97–101. [Google Scholar] [CrossRef]
- van Rossum, B.-J.; Förster, H.; de Groot, H.J.M. High-Field and High-Speed CP-MAS 13C NMR Heteronuclear Dipolar-Correlation Spectroscopy of Solids with Frequency-Switched Lee–Goldburg Homonuclear Decoupling. J. Magn. Reson. 1997, 124, 516–519. [Google Scholar] [CrossRef]
- Feike, M.; Demco, D.E.; Graf, R.; Gottwald, J.; Hafner, S.; Spiess, H.W. Broadband Multiple-Quantum NMR Spectroscopy. J. Magn. Reson. A 1996, 122, 214–221. [Google Scholar] [CrossRef]
- Sun, H.; Zhan, J.; Chen, L.; Zhao, Y. Preparation of CTS/PAMAM/SA/Ca2+ Hydrogel and Its Adsorption Performance for Heavy Metal Ions. Appl. Surf. Sci. 2023, 607. [Google Scholar] [CrossRef]
- Lopez-Ramon, M.V.; Stoeckli, F.; Moreno-Castilla, C.; Carrasco-Marin, F. On the Characterization of Acidic and Basic Surface Sites on Carbons by Various Techniques. Carbon 1999, 37, 1215–1221. [Google Scholar] [CrossRef]
- Ho, Y. The Kinetics of Sorption of Divalent Metal Ions onto Sphagnum Moss Peat. Water Res. 2000, 34, 735–742. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Rejeena, S.R.; Tharun, A.R. Preparation, Characterization and Adsorption Behavior of Tannin-Modified Poly(Glycidylmethacrylate)-Grafted Zirconium Oxide-Densified Cellulose for the Selective Separation of Bovine Serum Albumin. Colloids Surf. B Biointerfaces 2012, 93, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.W.; Porter, J.F.; Mckay, G. Sorption Kinetic Analysis for the Removal of Cadmium Ions from Effluents Using Bone Char. Water Res. 2001, 35, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; Hsieh, C.-T. Activation Energy for Oxygen Chemisorption on Carbon at Low Temperatures. Ind. Eng. Chem. Res. 1999, 38, 292–297. [Google Scholar] [CrossRef]
- Kuo, S.; Lotse, E.G. Kinetics of Phosphate Adsorption and Desorption by Hematite and Gibbsite. Soil. Sci. 1973, 116, 400–406. [Google Scholar] [CrossRef]
- Suen, S.-Y. A Comparison of Isotherm and Kinetic Models for Binary-Solute Adsorption to Affinity Membranes. J. Chem. Technol. Biotechnol. 1996, 65, 249–257. [Google Scholar] [CrossRef]


| pH 4 | pH 7 | pH 10 | ||||
|---|---|---|---|---|---|---|
| System | MO (mg g−1) | PEN (mg g−1) | MO (mg g−1) | PEN (mg g−1) | MO (mg g−1) | PEN (mg g−1) |
| P1.5E | 696 ± 4 | 98 ± 9 | 462 ± 9 | 24 ± 3 | 78 ± 5 | 1.6 ± 1.0 |
| 15%D | 799 ± 32 | 115 ± 4 | 584 ± 20 | 29 ± 3 | 92 ± 11 | 2.3 ± 0.6 |
| System | Cu2+ (mmol g−1) | PEN (mmol g−1) | Cu2+ Mixed (mmol g−1) | PEN Mixed (mmol g−1) |
|---|---|---|---|---|
| P1.5E | 0.20 ± 0.01 | 0.32 ± 0.02 | 0.34 ± 0.06 | 0.22 ± 0.01 |
| 15%D | 0.38 ± 0.02 | 0.36 ± 0.01 | 0.59 ± 0.02 | 0.29 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araque, L.M.; Fernández de Luis, R.; Fidalgo-Marijuan, A.; Infantes-Molina, A.; Rodríguez-Castellón, E.; Pérez, C.J.; Copello, G.J.; Lázaro-Martínez, J.M. Linear Polyethyleneimine-Based and Metal Organic Frameworks (DUT-67) Composite Hydrogels as Efficient Sorbents for the Removal of Methyl Orange, Copper Ions, and Penicillin V. Gels 2023, 9, 909. https://doi.org/10.3390/gels9110909
Araque LM, Fernández de Luis R, Fidalgo-Marijuan A, Infantes-Molina A, Rodríguez-Castellón E, Pérez CJ, Copello GJ, Lázaro-Martínez JM. Linear Polyethyleneimine-Based and Metal Organic Frameworks (DUT-67) Composite Hydrogels as Efficient Sorbents for the Removal of Methyl Orange, Copper Ions, and Penicillin V. Gels. 2023; 9(11):909. https://doi.org/10.3390/gels9110909
Chicago/Turabian StyleAraque, Luis M., Roberto Fernández de Luis, Arkaitz Fidalgo-Marijuan, Antonia Infantes-Molina, Enrique Rodríguez-Castellón, Claudio J. Pérez, Guillermo J. Copello, and Juan M. Lázaro-Martínez. 2023. "Linear Polyethyleneimine-Based and Metal Organic Frameworks (DUT-67) Composite Hydrogels as Efficient Sorbents for the Removal of Methyl Orange, Copper Ions, and Penicillin V" Gels 9, no. 11: 909. https://doi.org/10.3390/gels9110909
APA StyleAraque, L. M., Fernández de Luis, R., Fidalgo-Marijuan, A., Infantes-Molina, A., Rodríguez-Castellón, E., Pérez, C. J., Copello, G. J., & Lázaro-Martínez, J. M. (2023). Linear Polyethyleneimine-Based and Metal Organic Frameworks (DUT-67) Composite Hydrogels as Efficient Sorbents for the Removal of Methyl Orange, Copper Ions, and Penicillin V. Gels, 9(11), 909. https://doi.org/10.3390/gels9110909










