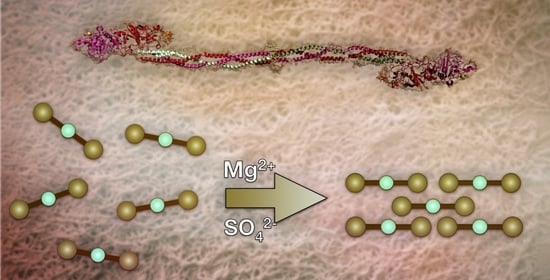
Thrombin-Free Fibrillogenesis and Gelation of Fibrinogen Triggered by Magnesium Sulfate
Abstract
1. Introduction
2. Results and Discussion
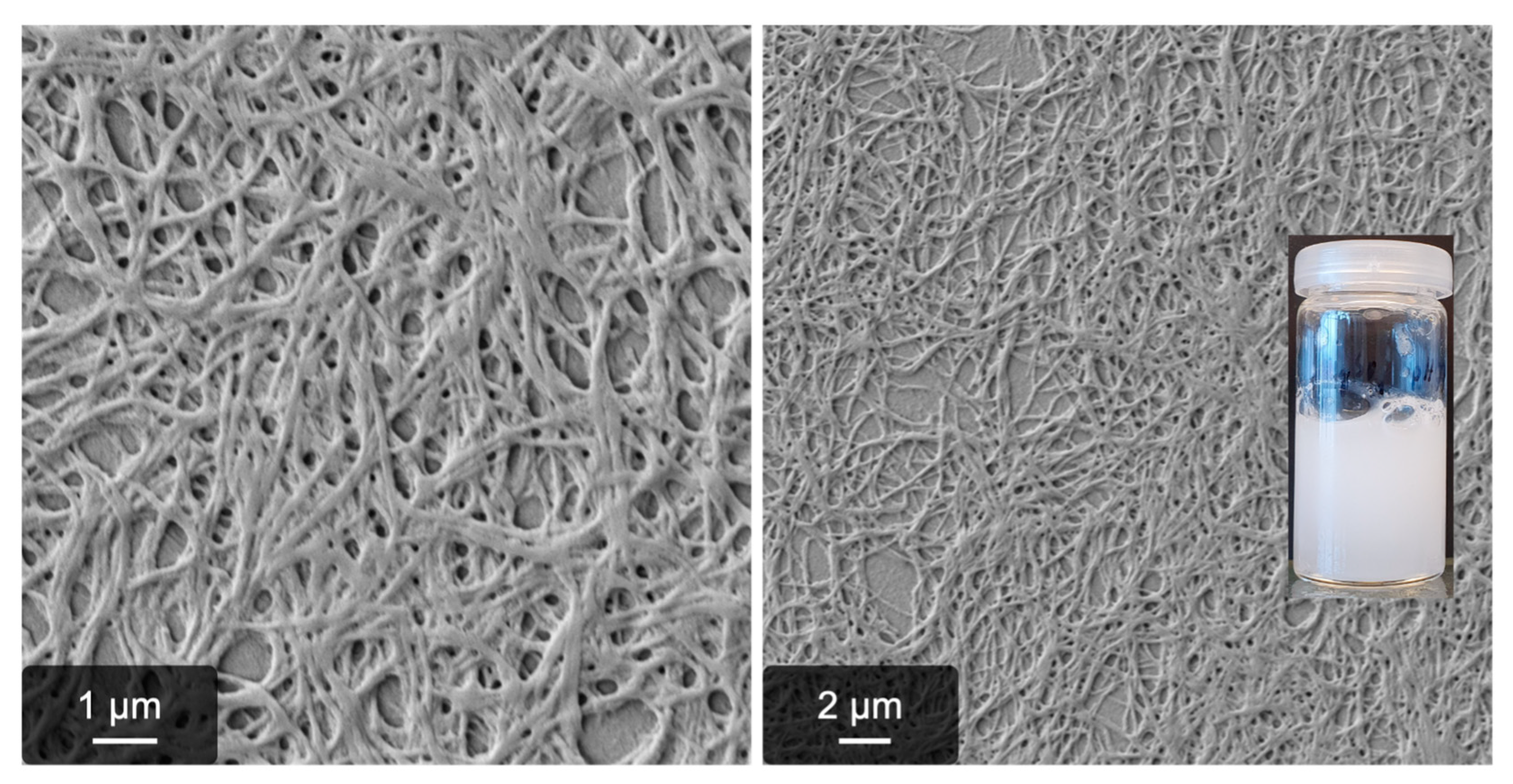
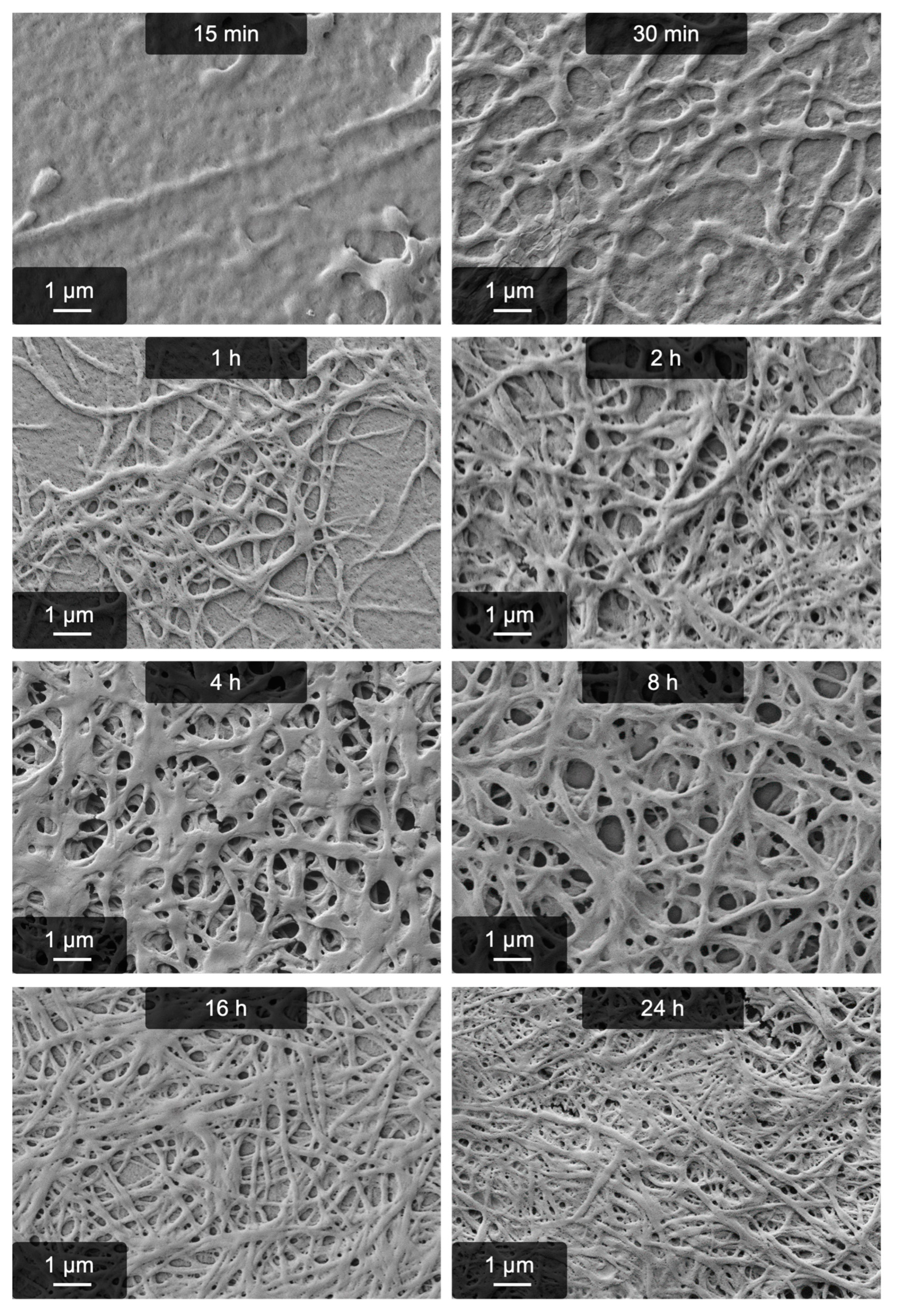
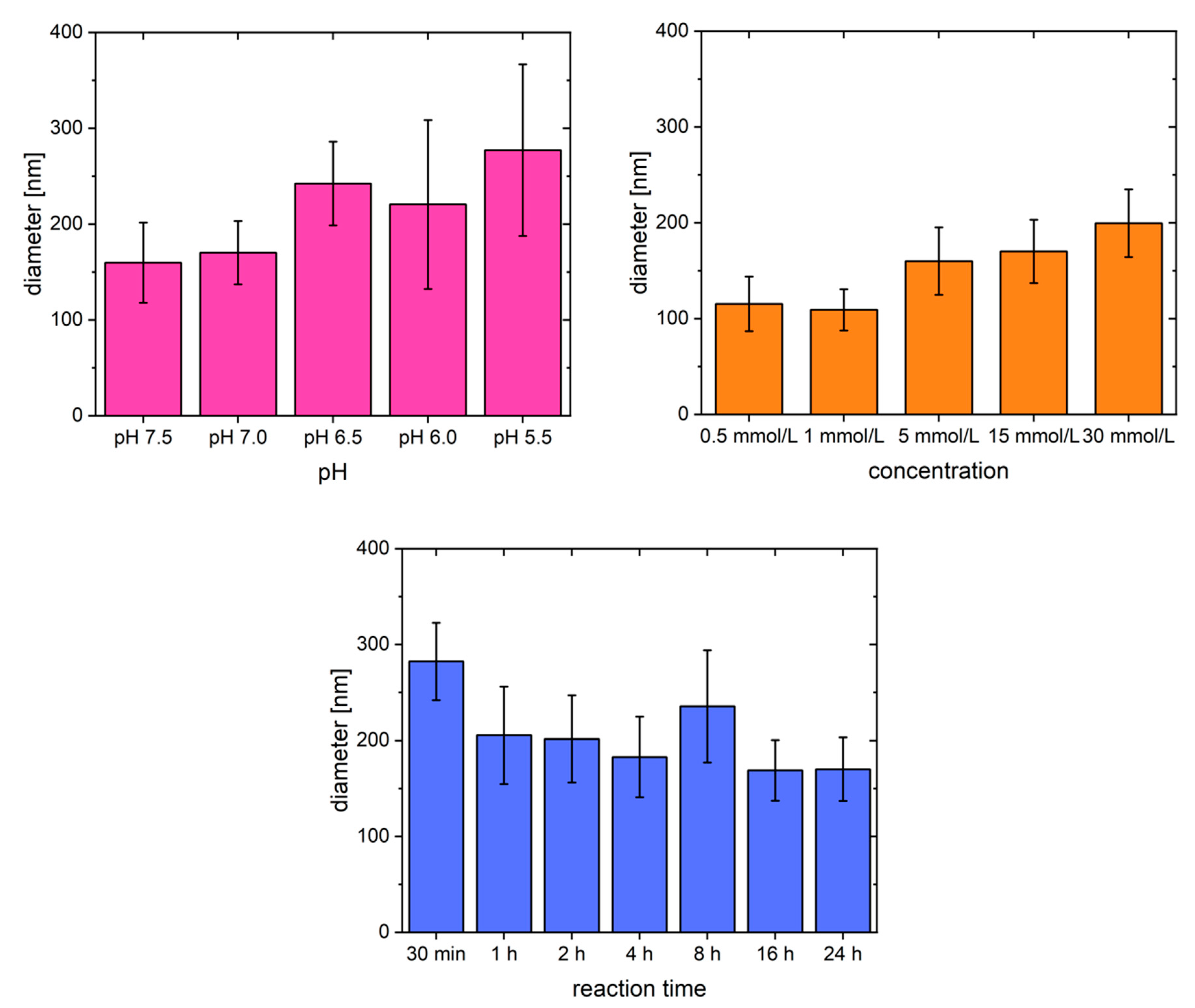
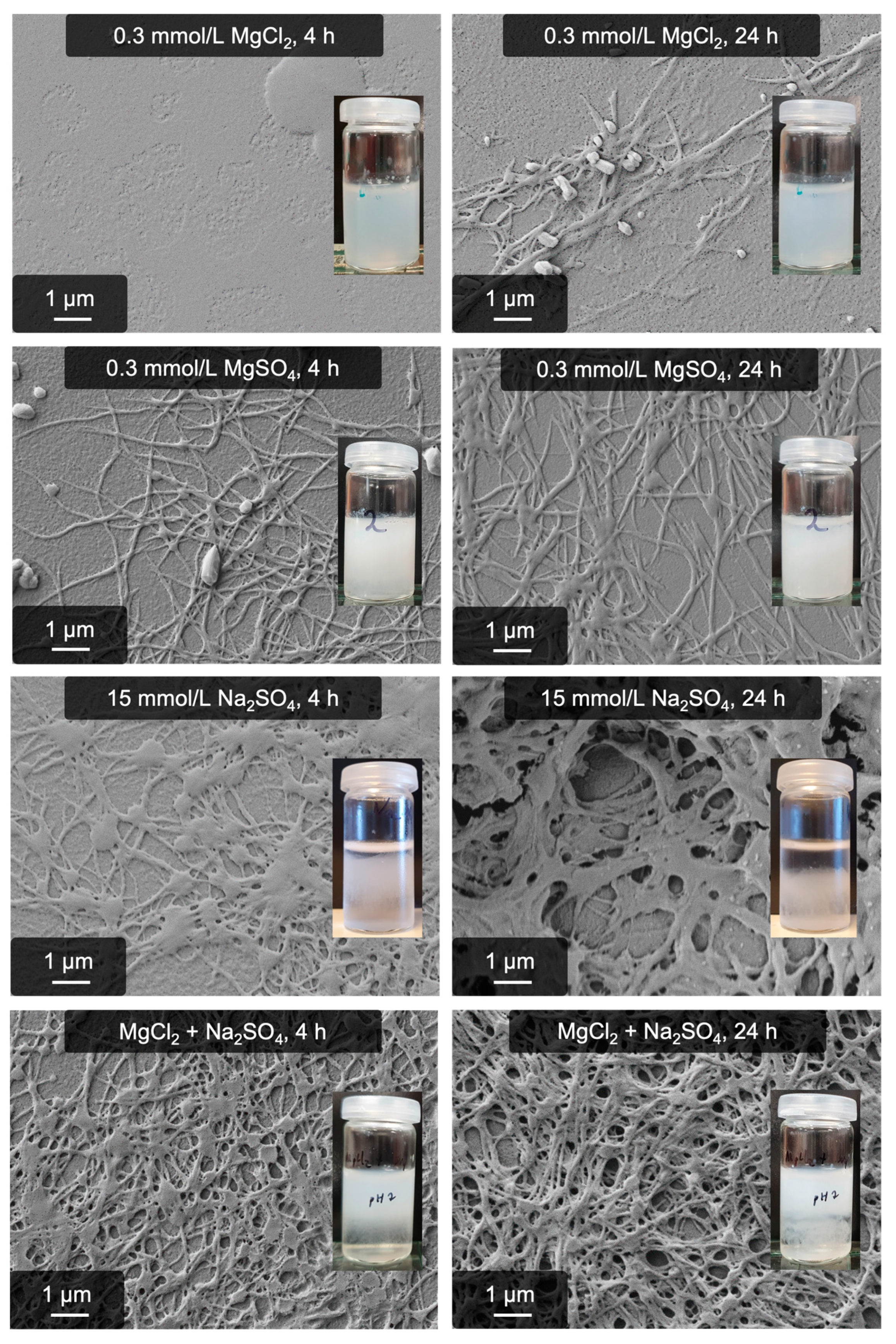
2.1. Fibrillogenesis Triggered by MgSO4 and Comparison to Our Previously Introduced Processes
2.2. Rheological Characterization
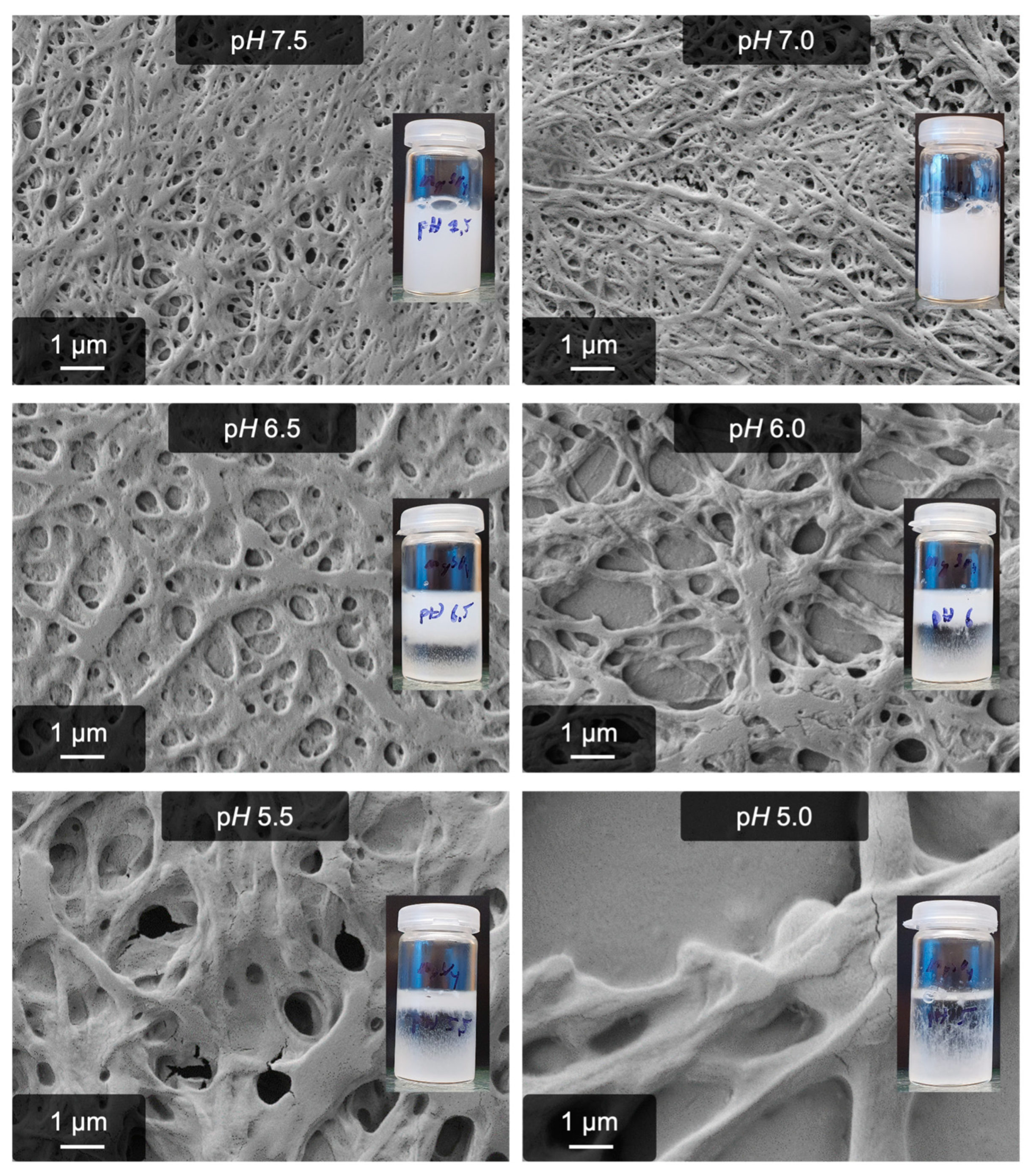
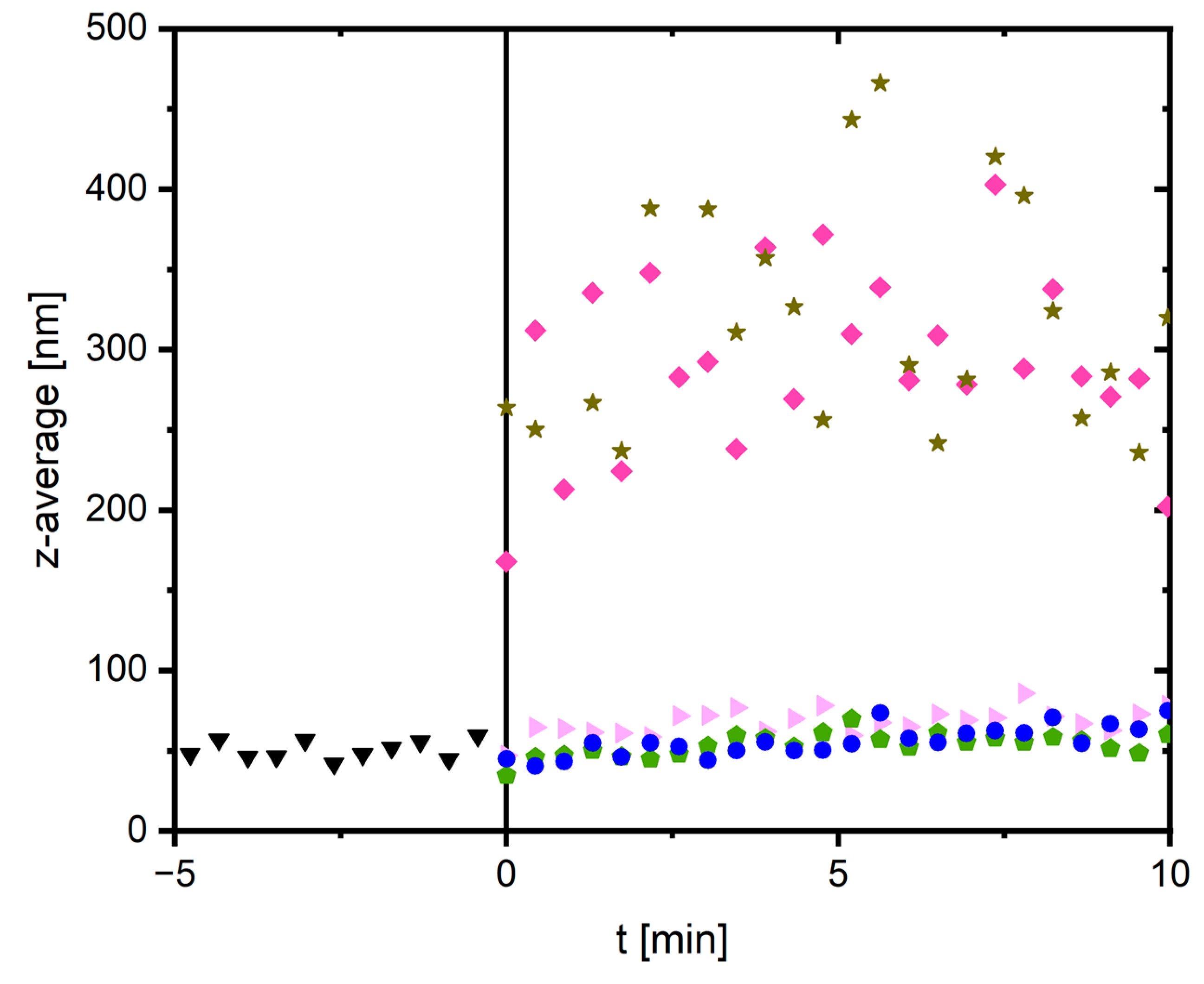
2.3. The Role of Binding Sites for Divalent Cations: Insights into the Mechanism
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Production of Hydrogels
4.3. Enzyme Inhibition
4.4. Occupancy of Binding Sites with 0.3 mmol/L Mg2+
4.5. Scanning Electron Microscopy (SEM) and Image Analysis
4.6. Rheology
4.7. Dynamic Light Scattering to Study Binding Site Occupancy
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rothemund, P.W.K. Folding DNA to Create Nanoscale Shapes and Patterns. Nature 2006, 440, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Aderibigbe, B.A.; Buyana, B. Alginate in Wound Dressings. Pharmaceutics 2018, 10, 1–19. [Google Scholar] [CrossRef]
- Rüdiger, A.A.; Bremser, W.; Strube, O.I. Nanoscaled Biocoatings via Enzyme Mediated Autodeposition of Casein. Macromol. Mater. Eng. 2016, 301, 1181–1190. [Google Scholar] [CrossRef]
- Rüdiger, A.A.; Brassat, K.; Lindner, J.K.N.; Bremser, W.; Strube, O.I. Easily Accessible Protein Nanostructures via Enzyme Mediated Addressing. Langmuir 2018, 34, 4264–4270. [Google Scholar] [CrossRef] [PubMed]
- Strube, O.I.; Büngeler, A.; Bremser, W. Enzyme-Mediated In Situ Synthesis and Deposition of Nonaggregated Melanin Pro-toparticles. Macromol. Mater. Eng. 2016, 301, 801–804. [Google Scholar] [CrossRef]
- Büngeler, A.; Hämisch, B.; Huber, K.; Bremser, W.; Strube, O.I. Insight into the Final Step of the Supramolecular Buildup of Eumelanin. Langmuir 2017, 33, 6895–6901. [Google Scholar] [CrossRef] [PubMed]
- Weisel, J.W. Fibrinogen and Fibrin. Adv. Protein. Chem. 2005, 70, 247–299. [Google Scholar] [CrossRef]
- Brennan, M. Fibrin Glue. Blood Rev. 1991, 5, 240–244. [Google Scholar] [CrossRef]
- Marchac, D.; Sàndor, G. Face Lifts and Sprayed Fibrin Glue: An Outcome Analysis of 200 Patients. Br. J. Plast. Surg 1994, 47, 306–309. [Google Scholar] [CrossRef]
- Mooney, E.; Loh, C.; Pu, L.L.Q. The Use of Fibrin Glue in Plastic Surgery. Plast. Reconstr. Surg 2009, 124, 989–992. [Google Scholar] [CrossRef]
- Silver, F.H.; Wang, M.-C.; Pins, G.D. Preparation and Use of Fibrin Glue in Surgery. Biomaterials 1995, 16, 891–903. [Google Scholar] [CrossRef] [PubMed]
- Moretz, W.H.; Shea, J.J.; Emmett, J.R. A Simple Autologous Fibrinogen Glue for Otologic Surgery. Otolaryngol.–Head Neck Surg. 1986, 95, 122–124. [Google Scholar] [CrossRef]
- Dye, J.F.; Sharma, V.; García, G.E.; Blackwood, K.A. Extracellular Matrix—Synthetic Skin Scaffold. U.S. Patent 10,357,592, 23 July 2019. [Google Scholar]
- Mol, A.; Van Lieshout, M.I.; Dam-De Veen, C.G.; Neuenschwander, S.; Hoerstrup, S.P.; Baaijens, F.P.T.; Bouten, C.V.C. Fibrin as a Cell Carrier in Cardiovascular Tissue Engineering Applications. Biomaterials 2005, 26, 3113–3121. [Google Scholar] [CrossRef] [PubMed]
- Kayal, T.A.; Buscemi, M.; Cavallo, A.; Foffa, I.; Soldani, G.; Losi, P. Plasminogen-Loaded Fibrin Scaffold as Drug Delivery System for Wound Healing Applications. Pharmaceutics 2022, 14, 251. [Google Scholar] [CrossRef] [PubMed]
- Rubalskii, E.; Ruemke, S.; Salmoukas, C.; Aleshkin, A.; Bochkareva, S.; Modin, E.; Mashaqi, B.; Boyle, E.C.; Boethig, D.; Rubalsky, M.; et al. Fibrin Glue as a Local Drug-Delivery System for Bacteriophage PA5. Sci. Rep. 2019, 9, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Cortez-Jugo, C.; Li, J.; Lin, Z.; Richardson, R.T.; Han, Y.; Zhou, J.; Björnmalm, M.; Feeney, O.M.; Zhong, Q.Z.; et al. Engineering Biocoatings to Prolong Drug Release from Supraparticles. Biomacromolecules 2019, 20, 3425–3434. [Google Scholar] [CrossRef]
- Gorodetsky, R.; Clark, R.A.F.; An, J.; Gailit, J.; Levdansky, L.; Vexler, A.; Berman, E.; Marx, G. Fibrin Microbeads (FMB) as Biodegradable Carriers for Culturing Cells and for Accelerating Wound Healing. J. Investig. Dermatol. 1999, 112, 866–872. [Google Scholar] [CrossRef]
- de Melo, B.A.G.; Jodat, Y.A.; Cruz, E.M.; Benincasa, J.C.; Shin, S.R.; Porcionatto, M.A. Strategies to Use Fibrinogen as Bioink for 3D Bioprinting Fibrin-Based Soft and Hard Tissues. Acta Biomater. 2020, 117, 60–76. [Google Scholar] [CrossRef]
- Xu, T.; Gregory, C.A.; Molnar, P.; Cui, X.; Jalota, S.; Bhaduri, S.B.; Boland, T. Viability and Electrophysiology of Neural Cell Structures Generated by the Inkjet Printing Method. Biomaterials 2006, 27, 3580–3588. [Google Scholar] [CrossRef]
- Rejinold, N.S.; Muthunarayanan, M.; Deepa, N.; Chennazhi, K.P.; Nair, S.V.; Jayakumar, R. Development of Novel Fibrinogen Nanoparticles by Two-Step Co-Acervation Method. Int. J. Biol. Macromol. 2010, 47, 37–43. [Google Scholar] [CrossRef]
- Rejinold, N.S.; Muthunarayanan, M.; Chennazhi, K.P.; Nair, S.V.; Jayakumar, R. 5-Fluorouracil Loaded Fibrinogen Nanoparticles for Cancer Drug Delivery Applications. Int. J. Biol. Macromol. 2011, 48, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Rajangam, T.; Paik, H.-j.; An, S.S.A. Development of Fibrinogen Microspheres as a Biodegradable Carrier for Tissue Engineering. Biochip. J 2011, 5, 175–183. [Google Scholar] [CrossRef]
- Vasconcelos, D.M.; Gonçalves, R.M.; Almeida, C.R.; Pereira, I.O.; Oliveira, M.I.; Neves, N.; Silva, A.M.; Ribeiro, A.C.; Cunha, C.; Almeida, A.R.; et al. Fibrinogen Scaffolds with Immunomodulatory Properties Promote in Vivo Bone Regeneration. Biomaterials 2016, 111, 163–178. [Google Scholar] [CrossRef]
- Foegeding, E.A.; Allen, C.E.; Dayton, W.R. Effect of Heating Rate on Thermally Formed Myosin, Fibrinogen and Albumin Gels. J. Food Sci 1986, 51, 104–108. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Shum, P.; Yates, M.; Messersmith, P.B.; Thompson, D.H. Formation of Fibrinogen-Based Hydrogels Using Phototriggerable Diplasmalogen Liposomes. Bioconjug. Chem. 2002, 13, 640–646. [Google Scholar] [CrossRef]
- Blombäck, B.; Procyk, R.; Adamson, L.; Hessel, B. FXIII Induced Gelation of Human Fibrinogen - an Alternative Thiol Enhanced Thrombin Independent Pathway. Thromb. Res. 1985, 37, 613–628. [Google Scholar] [CrossRef]
- Siebenlist, K.R.; Meh, D.A.; Mosesson, M.W. Protransglutaminase (Factor XIII) Mediated Crosslinking of Fibrinogen and Fibrin. Thromb. Haemost. 2001, 86, 1221–1228. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lorand, L. Factor XIII: Structure, Activation, and Interactions with Fibrinogen and Fibrin. Ann. N. Y. Acad. Sci. 2001, 936, 291–311. [Google Scholar] [CrossRef]
- Steven, F.S.; Griffin, M.M.; Brown, B.S.; Hulley, T.P. Aggregation of Fibrinogen Molecules by Metal Ions. Int. J. Biol. Macromol. 1982, 4, 367–369. [Google Scholar] [CrossRef]
- Gollwitzer, R.; Bode, W.; Karges, H.E. On the Aggregation of Fibrinogen Molecules. Thromb. Res. 1983, 29, 41–53. [Google Scholar] [CrossRef]
- Wei, G.; Reichert, J.; Bossert, J.; Jandt, K.D. Novel Biopolymeric Template for the Nucleation and Growth of Hydroxyapatite Crystals Based on Self-Assembled Fibrinogen Fibrils. Biomacromolecules 2008, 9, 3258–3267. [Google Scholar] [CrossRef]
- Wei, G.; Reichert, J.; Jandt, K.D. Controlled Self-Assembly and Templated Metallization of Fibrinogen Nanofibrils. Chem. Commun. 2008, 3903–3905. [Google Scholar] [CrossRef]
- Stapelfeldt, K.; Stamboroski, S.; Mednikova, P.; Brüggemann, D. Fabrication of 3D-Nanofibrous Fibrinogen Scaffolds Using Salt-Induced Self Assembly. Biofabrication 2019, 11, 025010. [Google Scholar] [CrossRef] [PubMed]
- Stapelfeldt, K.; Stamboroski, S.; Walter, I.; Suter, N.; Kowalik, T.; Michaelis, M.; Brüggemann, D. Controlling the Multiscale Structure of Nanofibrous Fibrinogen Scaffolds for Wound Healing. Nano Lett. 2019, 19, 6554–6563. [Google Scholar] [CrossRef]
- Stamboroski, S.; Boateng, K.; Lierath, J.; Kowalik, T.; Thiel, K.; Köppen, S.; Noeske, P.-L.M.; Brüggemann, D. Influence of Divalent Metal Ions on the Precipitation of the Plasma Protein Fibrinogen. Biomacromolecules 2021, 22, 4642–4658. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Casey, B.; Galanakis, D.K.; Marmorat, C.; Skoog, S.; Vorvolakos, K.; Simon, M.; Rafailovich, M.H. The Influence of Surface Chemistry on Adsorbed Fibrinogen Conformation, Orientation, Fiber Formation and Platelet Adhesion. Acta Biomater. 2017, 54, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.; Rafailovich, M.H.; Medved, L.; Tsurupa, G.; Kudryk, B.J.; Liu, Y.; Galanakis, D.K. Evaluation of Fibrinogen Self-Assembly: Role of Its AC Region. J. Thromb. Haemost. 2010, 8, 2727–2735. [Google Scholar] [CrossRef]
- Marx, G. Protofibrin Clots Induced by Calcium and Zinc. Biopolymers 1987, 26, 911–920. [Google Scholar] [CrossRef]
- Marx, G. Divalent Cations Induce Protofibril Gelation. Am. J. Hematol 1988, 27, 104–109. [Google Scholar] [CrossRef]
- Marx, G. Mechanism of Fibrin Coagulation Based on Selective, Cation-driven, Protofibril Association. Biopolymers 1988, 27, 763–774. [Google Scholar] [CrossRef]
- Hense, D.; Büngeler, A.; Kollmann, F.; Hanke, M.; Orive, A.; Keller, A.; Grundmeier, G.; Huber, K.; Strube, O.I. Self-Assembled Fibrinogen Hydro- And Aerogels with Fibrin-like 3D Structures. Biomacromolecules 2021, 22, 4084–4094. [Google Scholar] [CrossRef] [PubMed]
- Hämisch, B.; Büngeler, A.; Kielar, C.; Keller, A.; Strube, O.I.; Huber, K. Self-Assembly of Fibrinogen in Aqueous, Thrombin-Free Solutions of Variable Ionic Strengths. Langmuir 2019, 35, 12113–12122. [Google Scholar] [CrossRef] [PubMed]
- Hense, D.; Strube, O.I. Fibrillogenesis and Hydrogel Formation from Fibrinogen Induced by Calcium Salts. Gels 2023, 9, 175. [Google Scholar] [CrossRef]
- Credo, R.B.; Curtis, C.G.; Lorand, L. Ca2+-Related Regulatory Function of Fibrinogen. Proc. Natl. Acad. Sci. USA 1978, 75, 4234–4237. [Google Scholar] [CrossRef]
- Marguerie, G.; Chagniel, G.; Suscillon, M. The Binding of Calcium to Bovine Fibrinogen. Biochim. Biophys. Acta 1977, 490, 94–103. [Google Scholar] [CrossRef]
- Marguerie, G. The Binding of Calcium to Fibrinogen: Some Structural Features. Biochim. Biophys. Acta 1977, 494, 172–181. [Google Scholar] [CrossRef]
- Stojkov, G.; Niyazov, Z.; Picchioni, F.; Bose, R.K. Relationship between Structure and Rheology of Hydrogels for Various Applications. Gels 2021, 7, 255. [Google Scholar] [CrossRef]
- Weisel, J.W. The Mechanical Properties of Fibrin for Basic Scientists and Clinicians. Biophys. Chem. 2004, 112, 267–276. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Cichocki, B.; Ekiel-Jezewska, M.L.; Słowicka, A.; Wajnryb, E.; Wasilewska, M. Fibrinogen Conformations and Charge in Electrolyte Solutions Derived from DLS and Dynamic Viscosity Measurements. J. Colloid Interface Sci. 2012, 385, 244–257. [Google Scholar] [CrossRef] [PubMed]
- Apap-Bologna, A.; Webster, A.; Raitt, F.; Kemp, G. The Influence of Calcium Ions on Fibrinogen Conformation. Biochim. Biophys. Acta 1989, 995, 70–74. [Google Scholar] [CrossRef]


| Anion-Induced Pseudo-Fibrin | Ca2+-Induced Pseudo-Fibrin | |
|---|---|---|
| Triggers | Kosmotropic anions (sodium phosphate, sodium sulfate, …) | Ca2+ salts independent of the anion |
| Fiber yield, gel stability | Medium fiber yield, low stability | High fiber yield, high stability |
| Temperatures | 5–8 °C (irreversible dissolution at higher temperatures) | 5–37 °C |
| Trigger concentrations | 5–20 mmol/L | 1–30 mmol/L |
| pH range | 6.5–7.5 | 7.0–9.5 |
| Reaction time | 4 h | 16 h |
| Pseudo-Fibrin | Fibrin | Fibrinogen | |
|---|---|---|---|
| G’ after 4 h [Pa] | 10 | 2 | 0.02 |
| G’ after 24 h [Pa] | 15 | 20 | No reliable information |
| Crossover after 4 h [Hz] | None observed | None observed | 0.5 |
| Crossover after 24 h [Hz] | None observed | None observed | No reliable information |
| pH | Number of High Affinity Binding Sites (Only for Ca2+) | Number of Low Affinity Binding Sites (for Ca2+ and Mg2+) |
|---|---|---|
| 5.5 | 2 | 0 |
| 6.0 | 2 | 0 |
| 7.5 | 3 | 14–16 |
| 8.5 | 3 | 10–12 |
| 9.0 | 3 | 10–12 |
| Nr. | Salt Addition | Purpose |
|---|---|---|
| 1 | 0.3 mmol/L MgCl2 | Saturating all binding sites with Mg2+ |
| 2 | 15 mmol/L Na2SO4 | Triggering the anion-induced pathway to create pseudo-fibrin |
| 3 | 0.3 mmol/L MgCl2 + 15 mmol/L Na2SO4 | Combining the anion process with binding site occupancy |
| 4 | 0.3 mmol/L MgSO4 | Reference, since #3 yields 0.3 mmol/L MgSO4 |
| Nr. | Sample | Addition of Stock Solution |
|---|---|---|
| 1 | Reference of pure fibrinogen | --- |
| 2 | Aggregation triggered by MgSO4 | 1 mL of a 30 mmol/L MgSO4 solution |
| 3 | Anion-induced pseudo-fibrin with 15 mmol/L Na2SO4 | 1 mL of a 30 mmol/L sodium sulfate solution |
| 4 | Binding site occupancy with 0.3 mmol/L MgSO4 | 1 mL of a 0.6 mmol/L MgSO4 solution |
| 5 | Binding site occupancy with 0.3 mmol/L MgCl2 | 1 mL of a 0.6 mmol/L MgCl2 solution |
| 6 | Anion-induced pseudo-fibrin with binding site occupancy (sodium sulfate was added to sample 5) | 61.9 µL of a Na2SO4 solution added to sample 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hense, D.; Strube, O.I. Thrombin-Free Fibrillogenesis and Gelation of Fibrinogen Triggered by Magnesium Sulfate. Gels 2023, 9, 892. https://doi.org/10.3390/gels9110892
Hense D, Strube OI. Thrombin-Free Fibrillogenesis and Gelation of Fibrinogen Triggered by Magnesium Sulfate. Gels. 2023; 9(11):892. https://doi.org/10.3390/gels9110892
Chicago/Turabian StyleHense, Dominik, and Oliver I. Strube. 2023. "Thrombin-Free Fibrillogenesis and Gelation of Fibrinogen Triggered by Magnesium Sulfate" Gels 9, no. 11: 892. https://doi.org/10.3390/gels9110892
APA StyleHense, D., & Strube, O. I. (2023). Thrombin-Free Fibrillogenesis and Gelation of Fibrinogen Triggered by Magnesium Sulfate. Gels, 9(11), 892. https://doi.org/10.3390/gels9110892