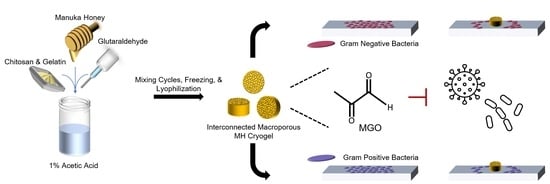
Antibacterial Efficacy of Manuka Honey-Doped Chitosan-Gelatin Cryogel and Hydrogel Scaffolds in Reducing Infection
Abstract
1. Introduction
2. Results and Discussion
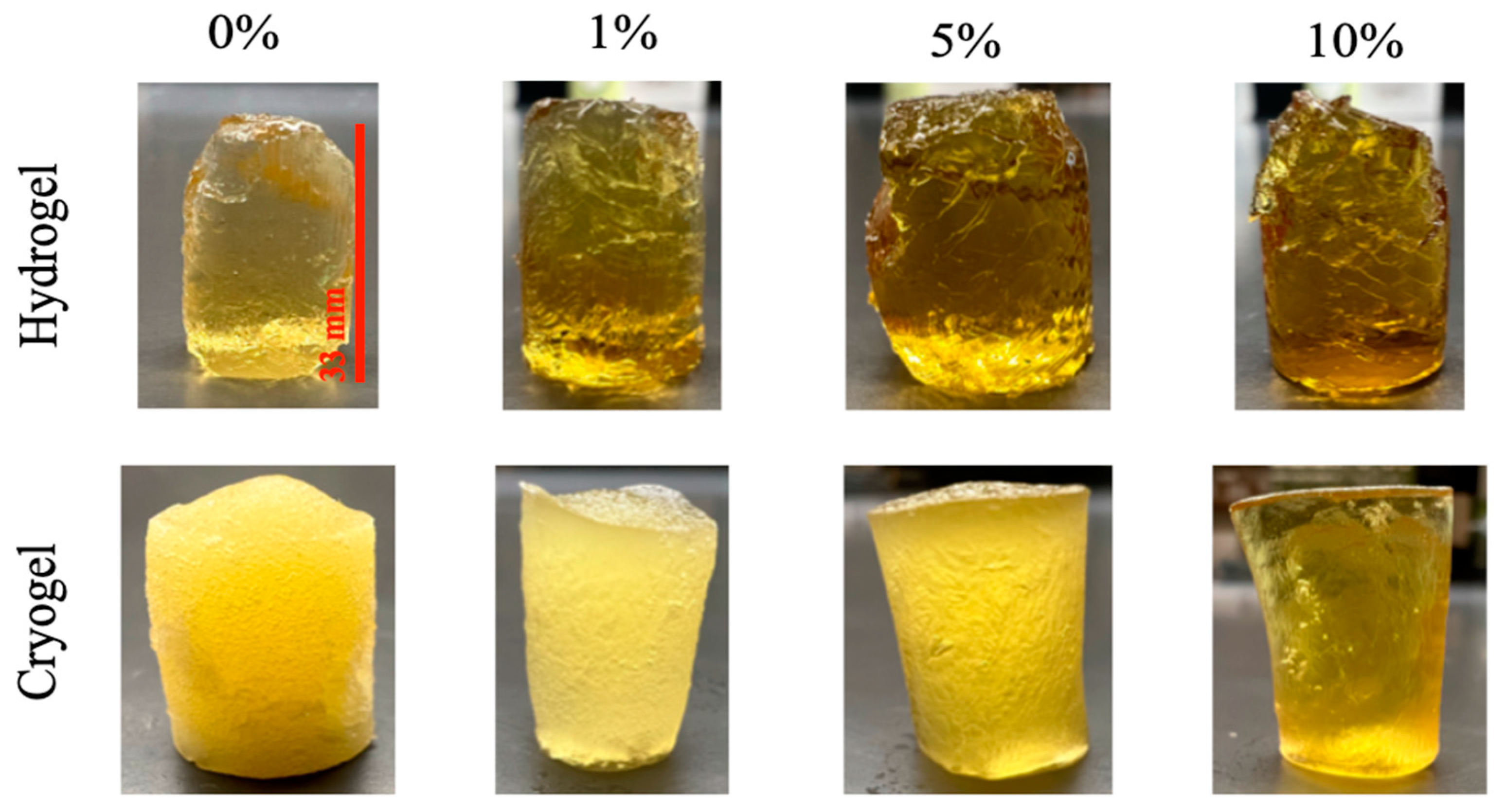
2.1. Scaffold Fabrication
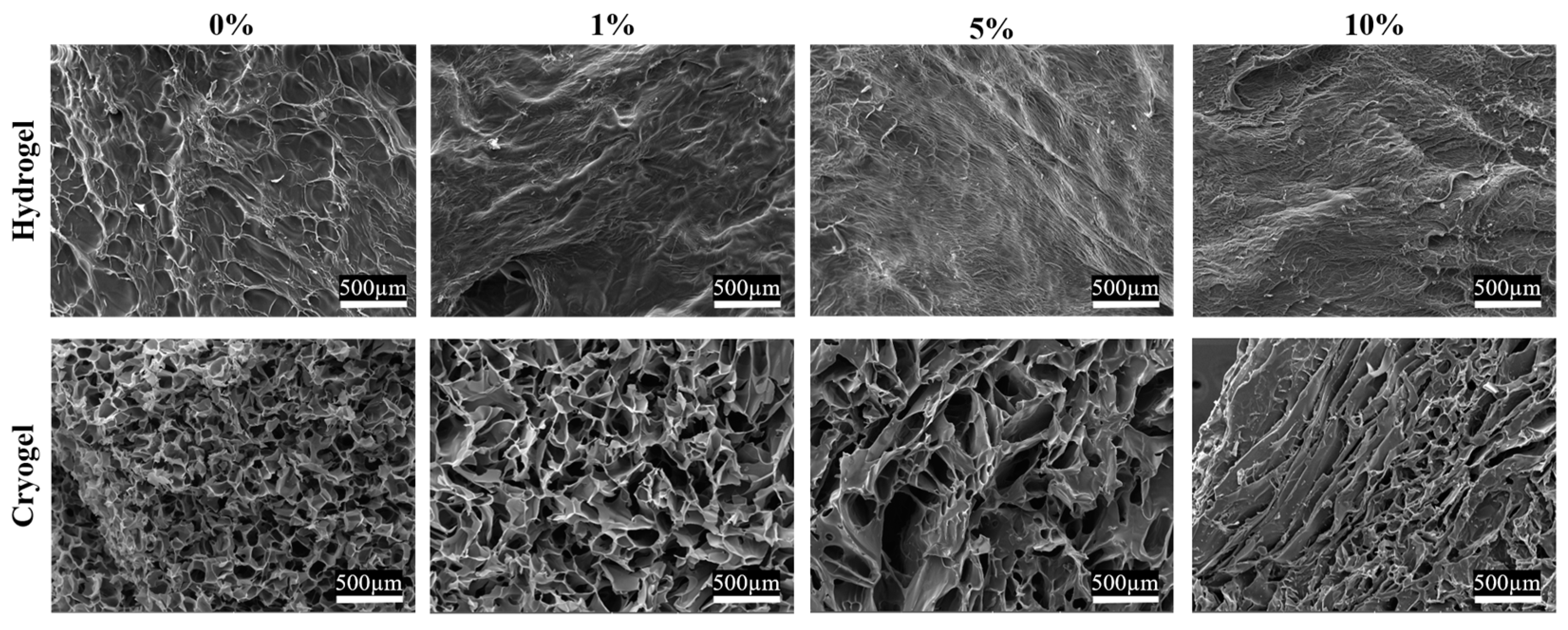
2.2. Scaffold Pore Analysis
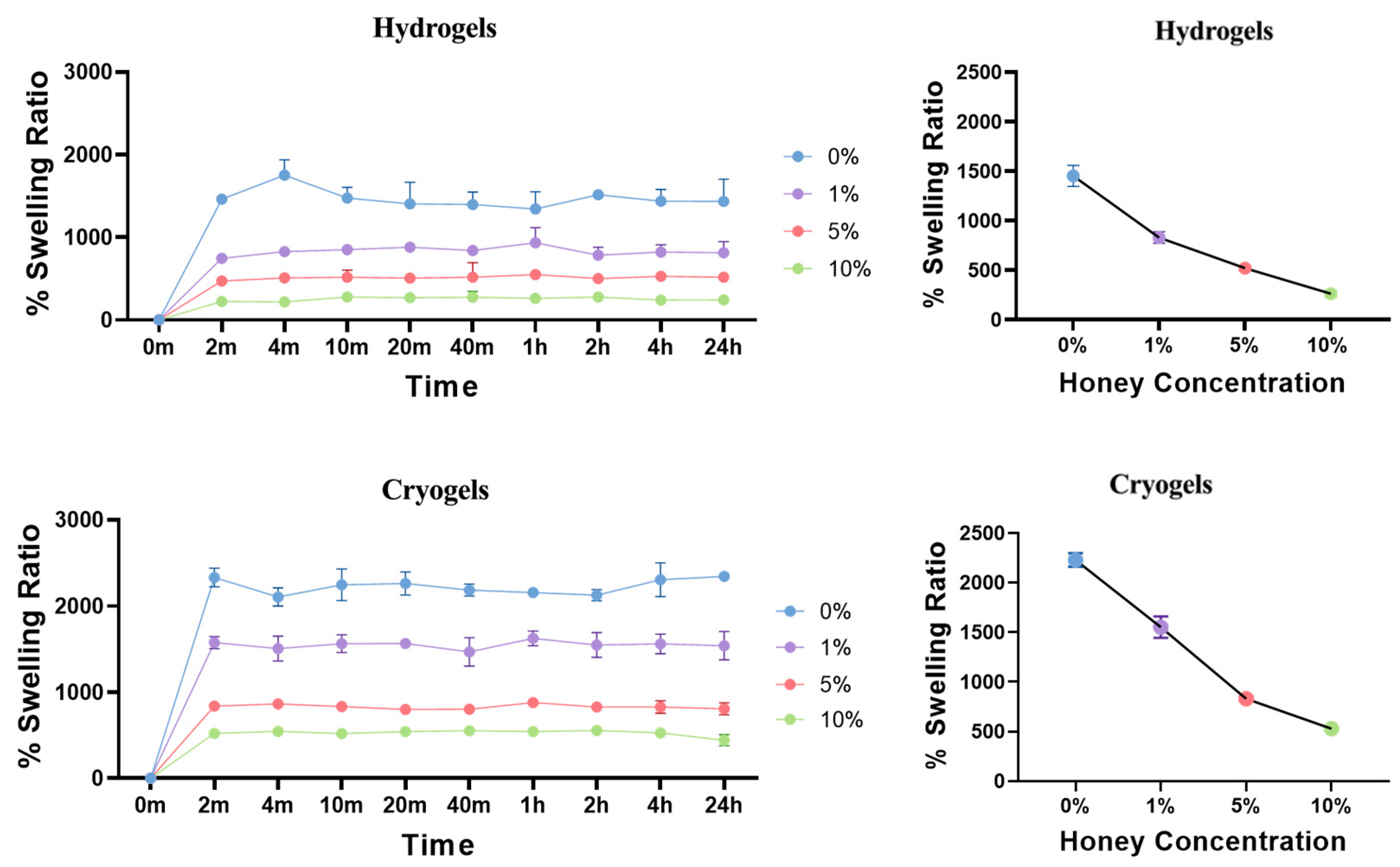
2.3. Swelling Kinetics
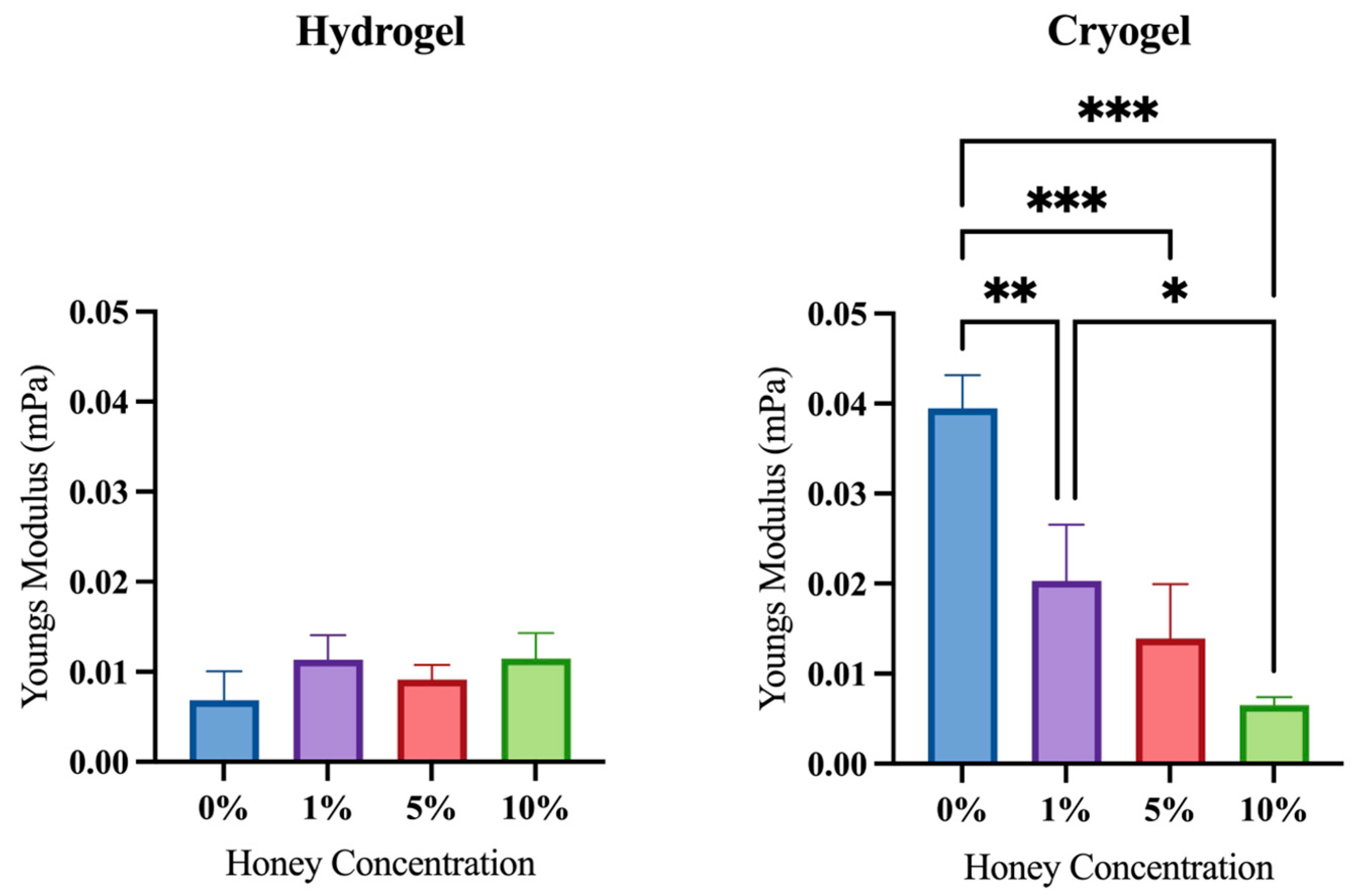
2.4. Mechanical Testing
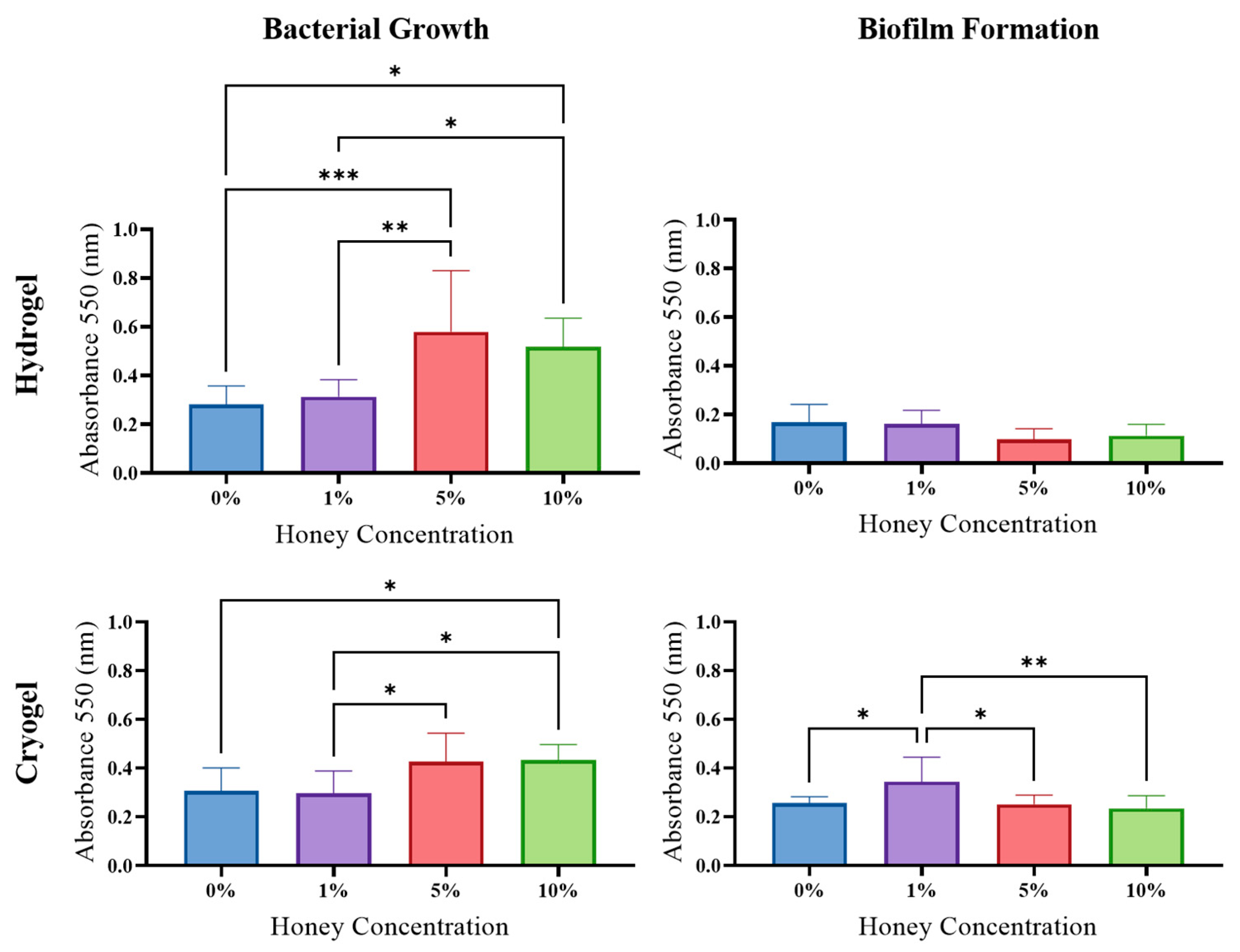
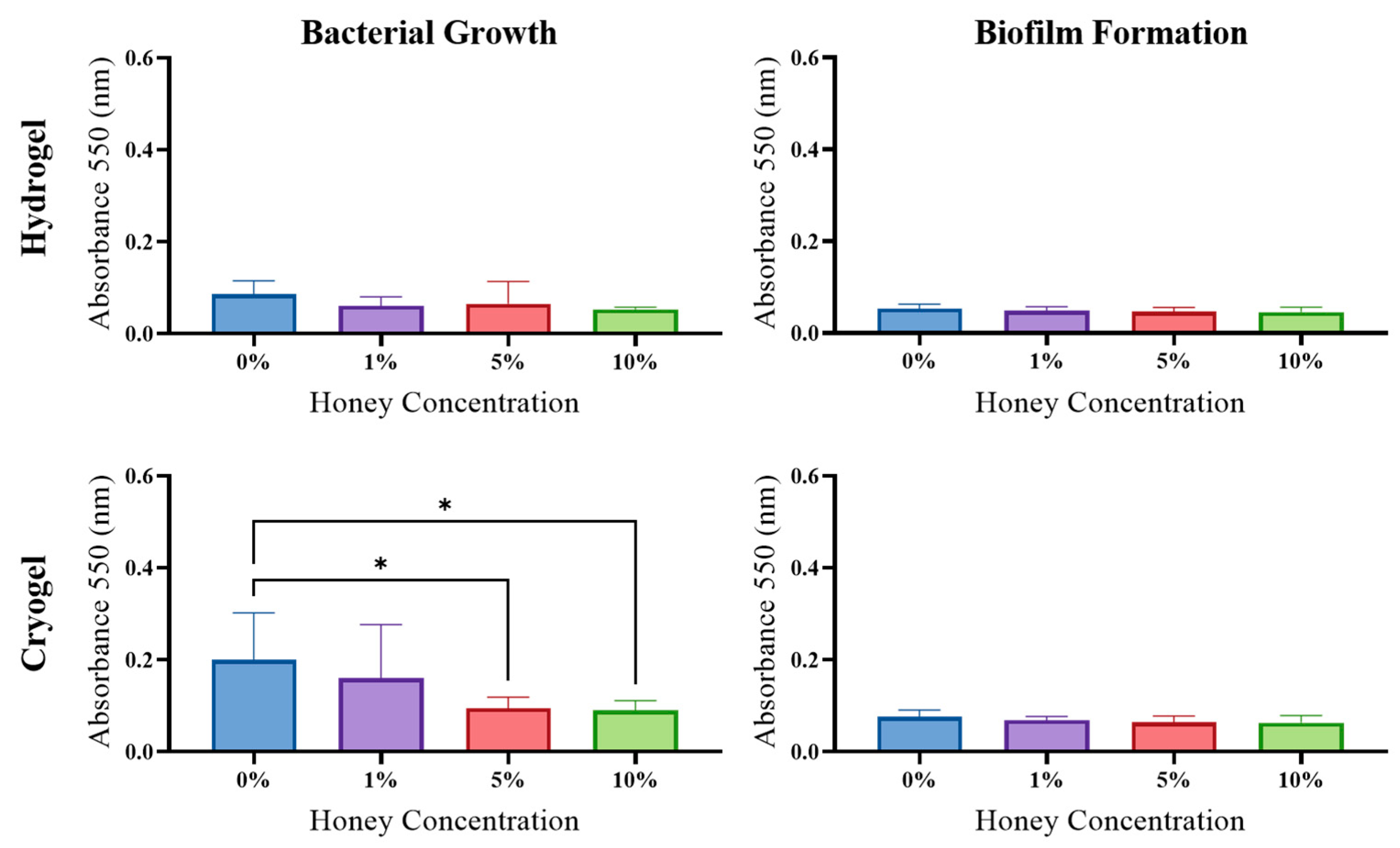
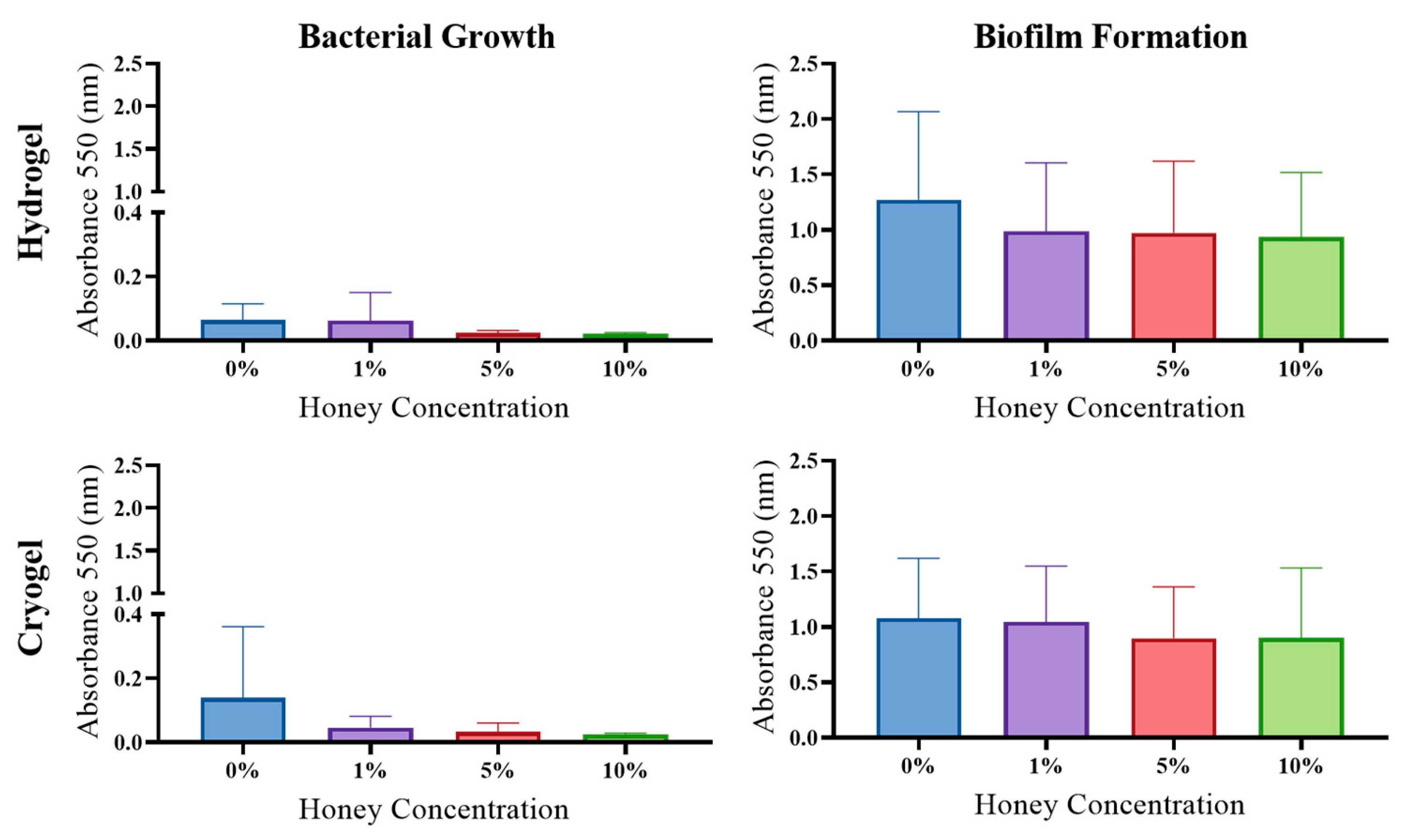
2.5. Antibacterial Activity
2.6. Biofilm Formation
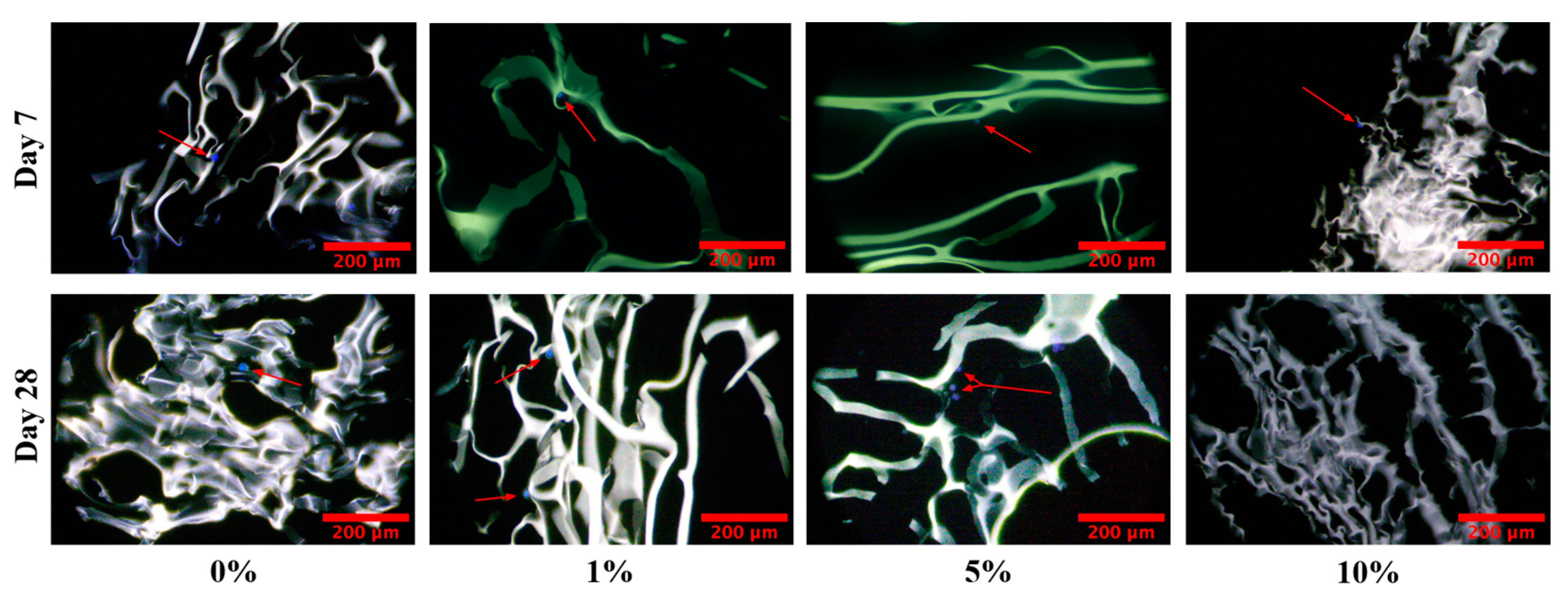
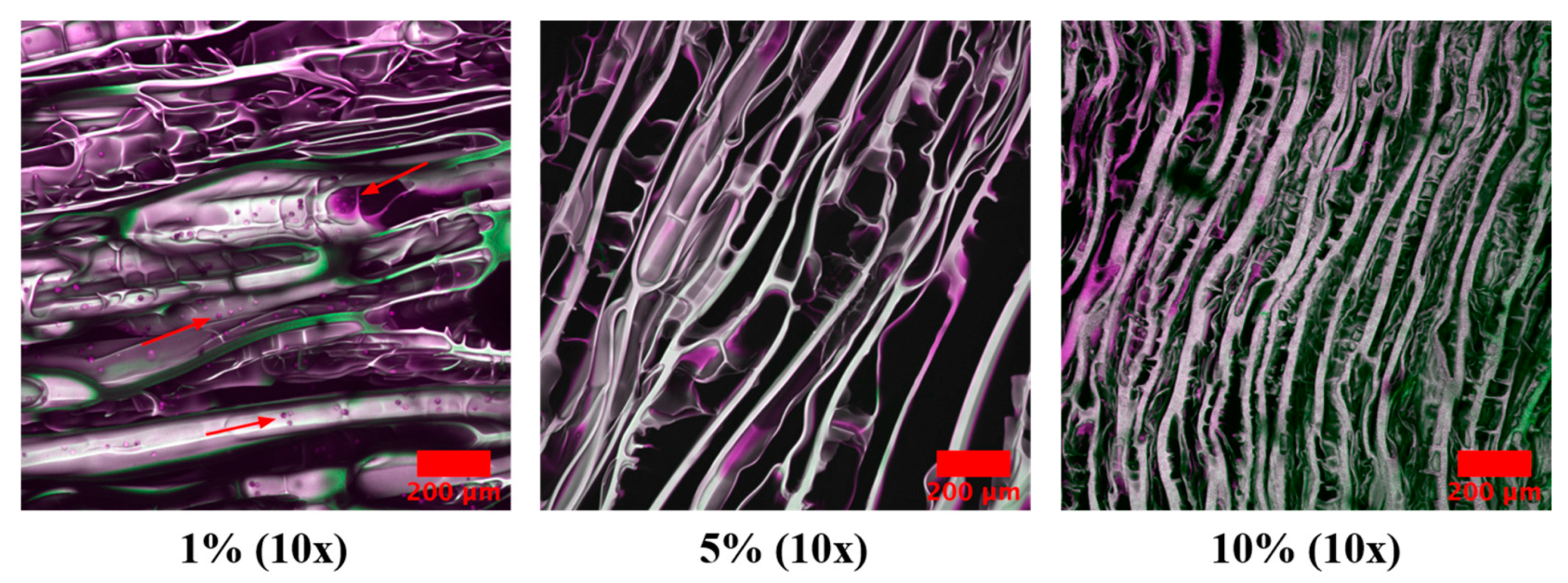
2.7. Cell Infiltration
2.8. Discussion
3. Conclusions
4. Materials and Methods
4.1. Scaffold Fabrication Optimization
4.2. Cryogel Pore Assessment
4.3. Swell Testing
4.4. Mechanical Testing
4.5. Antibacterial Assay
4.6. Biofilm Formation Assay
4.7. Cell Infiltration
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnston, M.; Mcbride, M.; Dahiya, D.; Owusu-Apenten, R.; Nigam, P.S. Antibacterial activity of Manuka honey and its components: An overview. AIMS Microbiol. 2018, 4, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Henriques, A.F.; Jenkins, R.E.; Burton, N.F.; Cooper, R.A. The intracellular effects of manuka honey on Staphylococcus aureus. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 45–50. [Google Scholar] [CrossRef]
- Cooper, R.A.; Molan, P.C.; Harding, K.G. The sensitivity to honey of Gram-positive cocci of clinical significance isolated from wounds. J. Appl. Microbiol. 2002, 93, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Maddocks, S.E.; Lopez, M.S.; Rowlands, R.S.; Cooper, R.A. Manuka honey inhibits the development of Streptococcus pyogenes biofilms and causes reduced expression of two fibronectin binding proteins. Microbiology 2012, 158, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.; Reygaert, W.C. Gram Negative Bacteria. In Infection Management for Geriatrics in Long-Term Care Facilities, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2022; pp. 427–443. Available online: https://www.ncbi.nlm.nih.gov/books/NBK538213/ (accessed on 28 April 2023).
- Kim, S.Y.; Kang, S.S. Anti-Biofilm Activities of Manuka Honey against Escherichia coli O157:H7. Food Sci. Anim. Resour. 2020, 40, 668. [Google Scholar] [CrossRef] [PubMed]
- Clements, A.; Young, J.C.; Constantinou, N.; Frankel, G. Infection strategies of enteric pathogenic Escherichia coli. Gut Microbes 2012, 3, 71–87. [Google Scholar] [CrossRef]
- Cooper, R.A.; Halas, E.; Molan, P.C. The efficacy of honey in inhibiting strains of Pseudomonas aeruginosa from infected burns. J. Burn. Care Rehabil. 2002, 23, 366–370. [Google Scholar] [CrossRef]
- Henriques, A.F.; Jenkins, R.E.; Burton, N.F.; Cooper, R.A. The effect of manuka honey on the structure of Pseudomonas aeruginosa. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 167–171. [Google Scholar] [CrossRef]
- Malhotra, S.; Hayes, D.; Wozniak, D.J. Cystic fibrosis and pseudomonas aeruginosa: The host-microbe interface. Clin. Microbiol. Rev. 2019, 32, e00138-18. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, C.K.; Upadhyay, N.; Jain, A.; Verma, A.; Narayana Charyulu, R.; Jain, S. Hydrogels-Promising Candidates for Tissue Engineering. In Nanotechnology Applications for Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2015; pp. 77–94. [Google Scholar]
- Hixon, K.R.; Lu, T.; Sell, S.A. A comprehensive review of cryogels and their roles in tissue engineering applications. Acta Biomater. 2017, 62, 29–41. [Google Scholar] [CrossRef]
- Sasikala, L.; Durai, B.; Rathinamoorthy, R. Manuka honey loaded chitosan hydrogel films for wound dressing applications. Int. J. PharmTech Res. 2013, 5, 1774–1785. [Google Scholar]
- Bonifacio, M.A.; Cometa, S.; Cochis, A.; Gentile, P.; Ferreira, A.M.; Azzimonti, B.; Procino, G.; Ceci, E.; Rimondini, L.; De Giglio, E. Antibacterial effectiveness meets improved mechanical properties: Manuka honey/gellan gum composite hydrogels for cartilage repair. Carbohydr. Polym. 2018, 198, 462–472. [Google Scholar] [CrossRef]
- Hixon, K.R.; Bogner, S.J.; Ronning-Arnesen, G.; Janowiak, B.E.; Sell, S.A. Investigating Manuka Honey Antibacterial Properties When Incorporated into Cryogel, Hydrogel, and Electrospun Tissue Engineering Scaffolds. Gels 2019, 5, 21. [Google Scholar] [CrossRef]
- Abd El-Malek, F.F.; Yousef, A.S.; El-Assar, S.A. Hydrogel film loaded with new formula from manuka honey for treatment of chronic wound infections. J. Glob. Antimicrob. Resist. 2017, 11, 171–176. [Google Scholar] [CrossRef]
- Hixon, K.R.; Lu, T.; McBride-Gagyi, S.H.; Janowiak, B.E.; Sell, S.A. A comparison of tissue engineering scaffolds incorporated with Manuka honey of varying UMF. BioMed Res. Int. 2017, 2017, 4843065. [Google Scholar] [CrossRef] [PubMed]
- Hixon, K.R.; Lu, T.; Carletta, M.N.; McBride-Gagyi, S.H.; Janowiak, B.E.; Sell, S.A. A preliminary in vitro evaluation of the bioactive potential of cryogel scaffolds incorporated with Manuka honey for the treatment of chronic bone infections. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 1918–1933. [Google Scholar] [CrossRef]
- Bonifacio, M.A.; Cometa, S.; Cochis, A.; Gentile, P.; Ferreira, A.M.; Azzimonti, B.; Procino, G.; Ceci, E.; Rimondini, L.; De Giglio, E. Data on Manuka Honey/Gellan Gum composite hydrogels for cartilage repair. Data Brief 2018, 20, 831–839. [Google Scholar] [CrossRef]
- Ullah, A.; Ullah, S.; Khan, M.Q.; Hashmi, M.; Nam, P.D.; Kato, Y.; Tamada, Y.; Kim, I.S. Manuka honey incorporated cellulose acetate nanofibrous mats: Fabrication and in vitro evaluation as a potential wound dressing. Int. J. Biol. Macromol. 2020, 155, 479–489. [Google Scholar] [CrossRef]
- Yilmaz Atay, H. Antibacterial activity of chitosan-based systems. Funct. Chitosan 2020, 2020, 457–489. [Google Scholar]
- Yan, D.; Li, Y.; Liu, Y.; Li, N.; Zhang, X.; Yan, C. Antimicrobial properties of chitosan and chitosan derivatives in the treatment of enteric infections. Molecules 2021, 26, 7136. [Google Scholar] [CrossRef]
- Ke, C.L.; Deng, F.S.; Chuang, C.Y.; Lin, C.H. Antimicrobial actions and applications of Chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef]
- Kathuria, N.; Tripathi, A.; Kar, K.K.; Kumar, A. Synthesis and characterization of elastic and macroporous chitosan-gelatin cryogels for tissue engineering. Acta Biomater. 2009, 5, 406–418. [Google Scholar] [CrossRef]
- Shirbin, S.J.; Karimi, F.; Chan, N.J.A.; Heath, D.E.; Qiao, G.G. Macroporous Hydrogels Composed Entirely of Synthetic Polypeptides: Biocompatible and Enzyme Biodegradable 3D Cellular Scaffolds. Biomacromolecules 2016, 17, 2981–2991. [Google Scholar] [CrossRef]
- Hixon, K.R.; Eberlin, C.T.; Kadakia, P.U.; McBride-Gagyi, S.H.; Jain, E.; Sell, S.A. A comparison of cryogel scaffolds to identify an appropriate structure for promoting bone regeneration. Biomed. Phys. Eng. Express 2016, 2, 035014. [Google Scholar] [CrossRef]
- Rogers, Z.J.; Bencherif, S.A. Cryogelation and cryogels. Gels 2019, 5, 46. [Google Scholar] [CrossRef]
- Mandal, M.D.; Mandal, S. Honey: Its medicinal property and antibacterial activity. Asian Pac. J. Trop. Biomed. 2011, 1, 154–160. [Google Scholar] [CrossRef]
- Amariei, S.; Norocel, L.; Scripcă, L.A. An innovative method for preventing honey crystallization. Innov. Food Sci. Emerg. Technol. 2020, 66, 102481. [Google Scholar] [CrossRef]
- Robin Lim, A.H.; Sam, L.M.; Gobilik, J.; Ador, K.; Choon, J.L.N.; Majampan, J.; Benedick, S. Physicochemical Properties of Honey from Contract Beekeepers, Street Vendors and Branded Honey in Sabah, Malaysia. Asian Pac. J. Trop. Biomed. 2022, 33, 61. [Google Scholar]
- Lee, K.Y.; Mooney, D.J. Hydrogels for tissue engineering. Chem. Rev. 2001, 101, 1869–1879. [Google Scholar] [CrossRef]
- Sudarshan, N.R.; Hoover, D.G.; Knorr, D. Antibacterial action of chitosan. Food Biotechnol. 2009, 6, 257–272. [Google Scholar] [CrossRef]
- Yabes, J.M.; White, B.K.; Murray, C.K.; Sanchez, C.J.; Mende, K.; Beckius, M.L.; Zera, W.C.; Wenke, J.C.; Akers, K.S. In Vitro activity of Manuka Honey and polyhexamethylene biguanide on filamentous fungi and toxicity to human cell lines. Med. Mycol. 2017, 55, 334–343. [Google Scholar] [CrossRef][Green Version]
- Main, E.N.; Bowlin, G.L. Potential for Manuka honey-inspired therapeutics to improve the host–biomaterial response. MedComm Biomater. Appl. 2022, 1, e18. [Google Scholar] [CrossRef]
- Minden-Birkenmaier, B.A.; Cherukuri, K.; Smith, R.A.; Radic, M.Z.; Bowlin, G.L. Manuka Honey Modulates the Inflammatory Behavior of a dHL-60 Neutrophil Model under the Cytotoxic Limit. Int. J. Biomater. 2019, 2019, 6132581. [Google Scholar] [CrossRef]
- Hixon, K.R.; Carletta, M.N.; Neal, S.M.; Talovic, M.; Dunn, A.J.; Garg, K.; Sell, S.A. Mineralization and antibacterial potential of bioactive cryogel scaffolds in vitro. Int. J. Polym. Mater. Polym. Biomater. 2018, 68, 901–914. [Google Scholar] [CrossRef]
- Dewey, M.J.; Collins, A.J.; Tiffany, A.; Barnhouse, V.R.; Lu, C.; Kolliopoulos, V.; Mutreja, I.; Hickok, N.J.; Harley, B.A.C. Evaluation of bacterial attachment on mineralized collagen scaffolds and addition of manuka honey to increase mesenchymal stem cell osteogenesis. Biomaterials 2023, 294, 122015. [Google Scholar] [CrossRef]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef]
- Wilson, C.; Lukowicz, R.; Merchant, S.; Valquier-Flynn, H.; Caballero, J.; Sandoval, J.; Okuom, M.; Huber, C.; Brooks, T.D.; Wilson, E.; et al. Quantitative and Qualitative Assessment Methods for Biofilm Growth: A Mini-review. Res. Rev. J. Eng. Technol. 2017, 6. Available online: http://www.rroij.com/open-access/quantitative-and-qualitative-assessment-methods-for-biofilm-growth-a-minireview-.pdf (accessed on 9 February 2023).
- O’Toole, G.A.; Kolter, R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 1998, 30, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Kanematsu, H.; Nakagawa, R.; Sano, K.; Barry, D.M.; Ogawa, A.; Hirai, N.; Kogo, T.; Kuroda, D.; Wada, N.; Lee, S.; et al. Graphene-dispersed silane compound used as a coating to sense immunity from biofilm formation. Med. Devices Sens. 2019, 2, e10043. [Google Scholar] [CrossRef]
- Tanaka, N.; Kogo, T.; Hirai, N.; Ogawa, A.; Kanematsu, H.; Takahara, J.; Awazu, A.; Fujita, N.; Haruzono, Y.; Ichida, S.; et al. In-situ detection based on the biofilm hydrophilicity for environmental biofilm formation. Sci. Rep. 2019, 9, 8070. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, R.; Kanematsu, H.; Ogawa, A.; Kogo, T.; Miura, H.; Kawai, R.; Hirai, N.; Kato, T.; Yoshitake, M.; Barry, D.M. Quantitative Analyses of Biofilm by Using Crystal Violet Staining and Optical Reflection. Materials 2022, 15, 6727. [Google Scholar] [CrossRef]
- Kanematsu, H.; Ikigai, H.; Yoshitake, M. Evaluation of various metallic coatings on steel to mitigate biofilm formation. Int. J. Mol. Sci. 2009, 10, 559–571. [Google Scholar] [CrossRef]
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.T. Porous scaffolds for bone regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Murphy, C.M.; O’Brien, F.J. Understanding the effect of mean pore size on cell activity in collagen-glycosaminoglycan scaffolds. Cell Adhes. Migr. 2010, 4, 377–381. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitchell, K.; Panicker, S.S.; Adler, C.L.; O’Toole, G.A.; Hixon, K.R. Antibacterial Efficacy of Manuka Honey-Doped Chitosan-Gelatin Cryogel and Hydrogel Scaffolds in Reducing Infection. Gels 2023, 9, 877. https://doi.org/10.3390/gels9110877
Mitchell K, Panicker SS, Adler CL, O’Toole GA, Hixon KR. Antibacterial Efficacy of Manuka Honey-Doped Chitosan-Gelatin Cryogel and Hydrogel Scaffolds in Reducing Infection. Gels. 2023; 9(11):877. https://doi.org/10.3390/gels9110877
Chicago/Turabian StyleMitchell, Karina, Sreejith S. Panicker, Calista L. Adler, George A. O’Toole, and Katherine R. Hixon. 2023. "Antibacterial Efficacy of Manuka Honey-Doped Chitosan-Gelatin Cryogel and Hydrogel Scaffolds in Reducing Infection" Gels 9, no. 11: 877. https://doi.org/10.3390/gels9110877
APA StyleMitchell, K., Panicker, S. S., Adler, C. L., O’Toole, G. A., & Hixon, K. R. (2023). Antibacterial Efficacy of Manuka Honey-Doped Chitosan-Gelatin Cryogel and Hydrogel Scaffolds in Reducing Infection. Gels, 9(11), 877. https://doi.org/10.3390/gels9110877