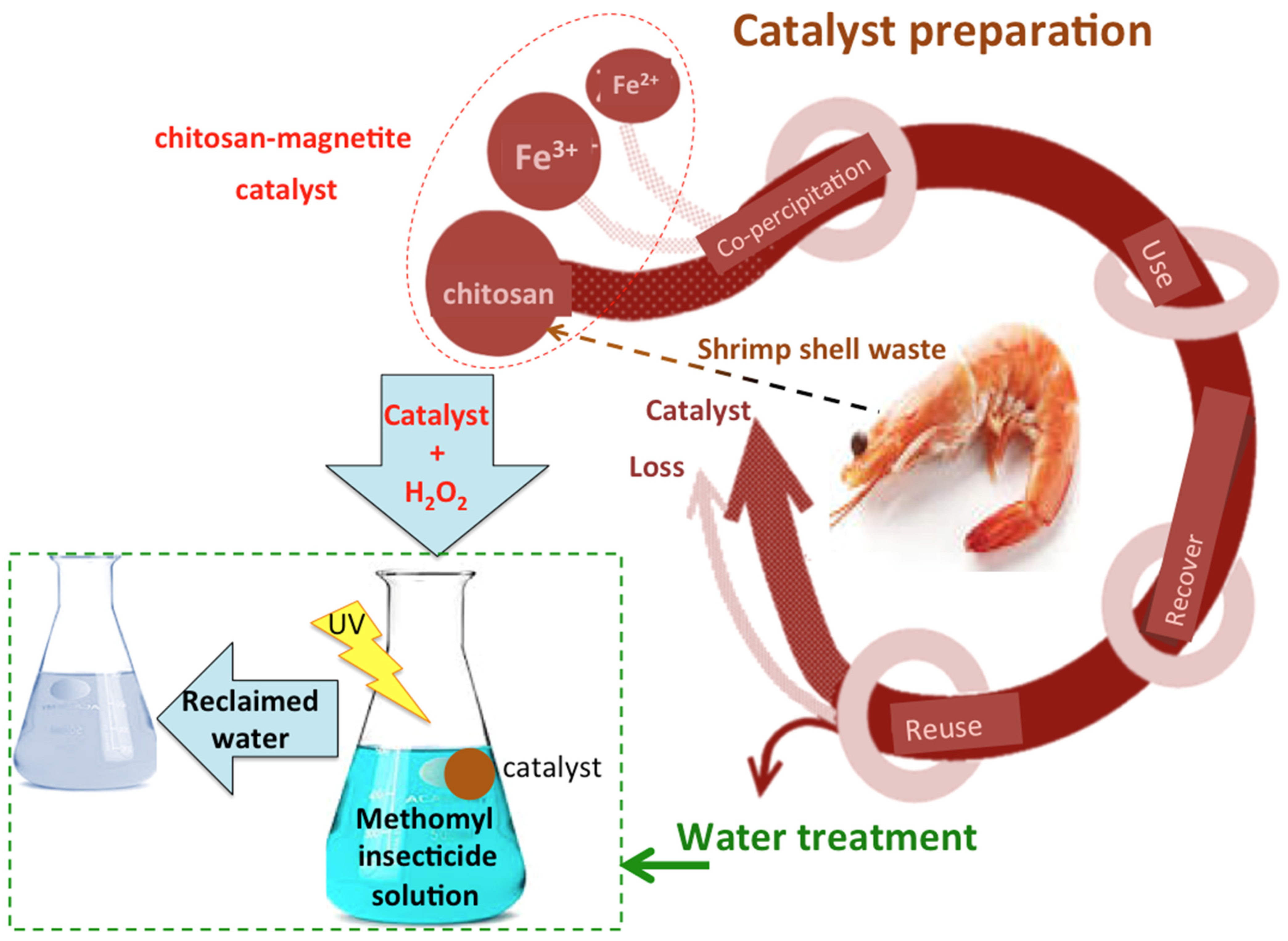
Nexus Advances Using Marine Biopolymeric Gel Material as a Photocatalyst for the Oxidation of Agricultural Wastewater Containing Insecticides
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Composite Material
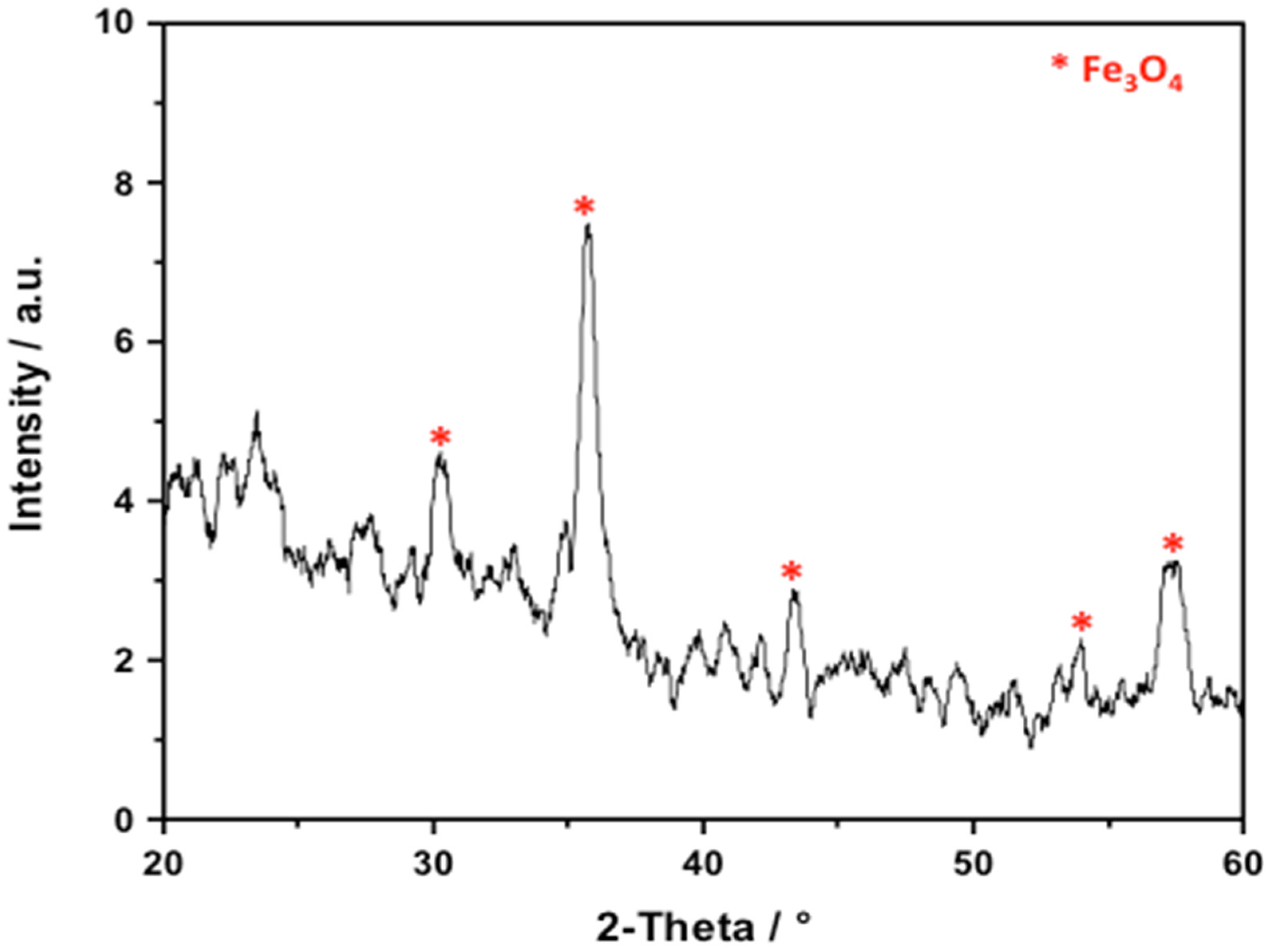
2.1.1. XRD Analysis
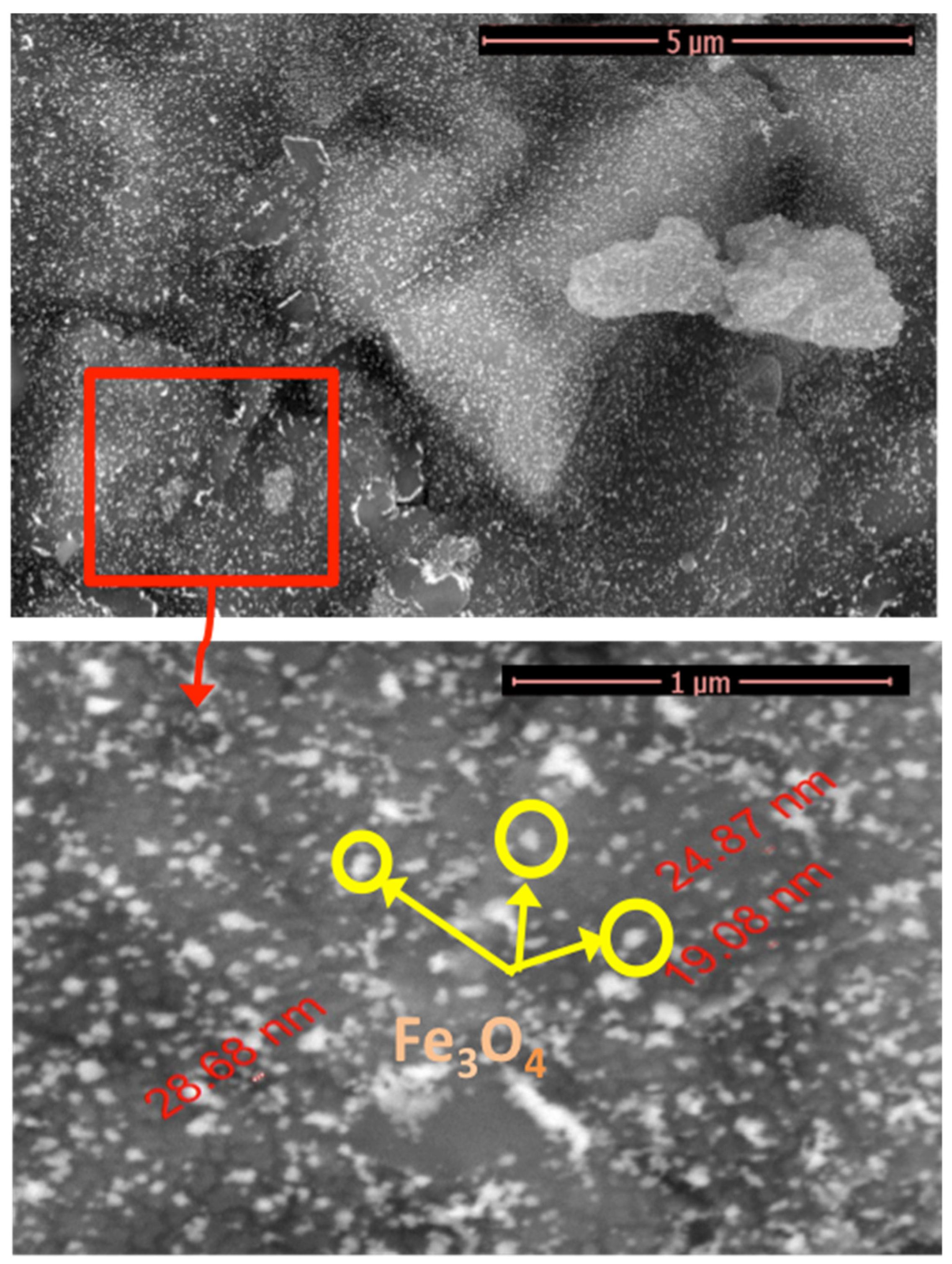
2.1.2. SEM Micrographs
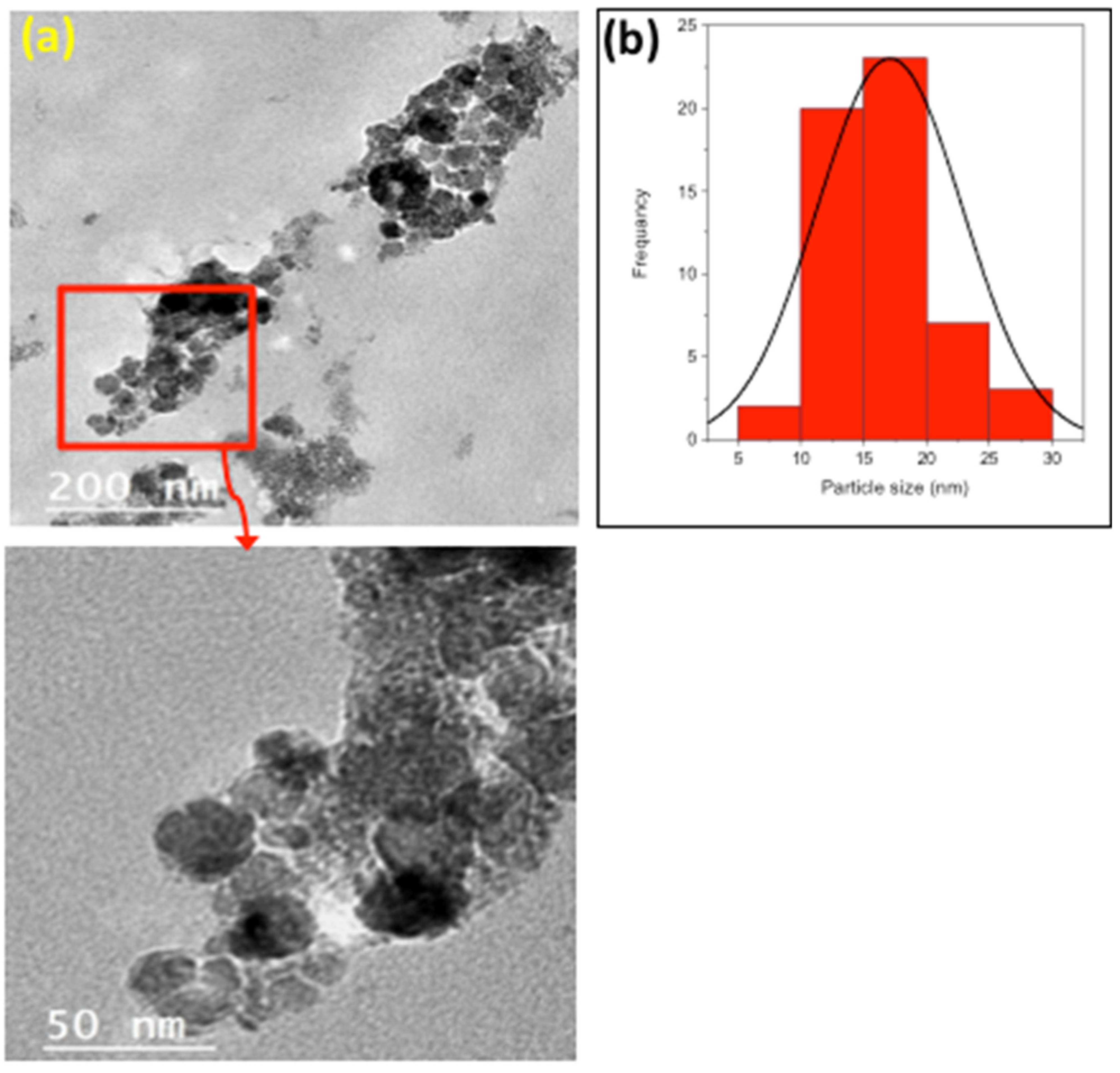
2.1.3. TEM Images
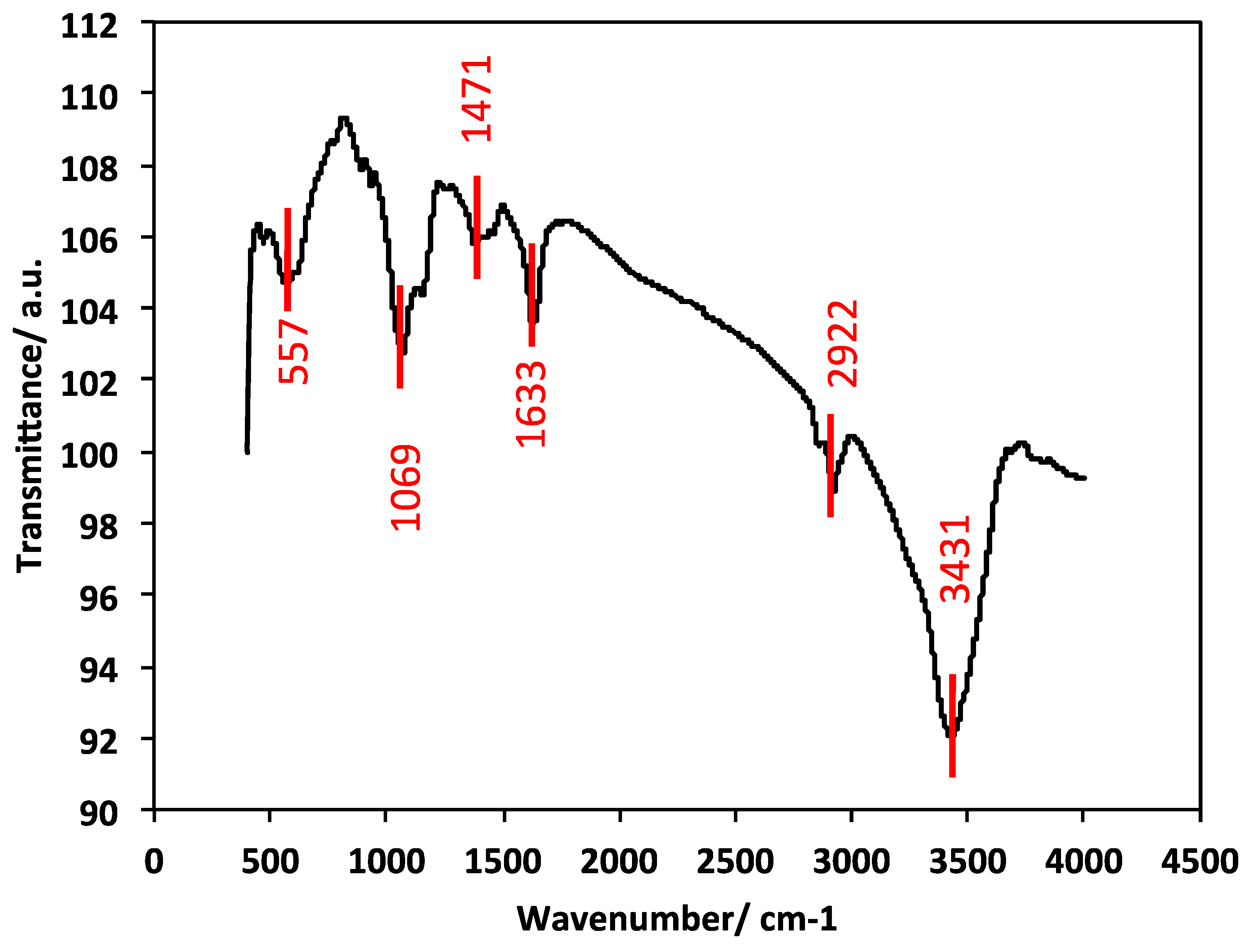
2.1.4. FTIR Analysis
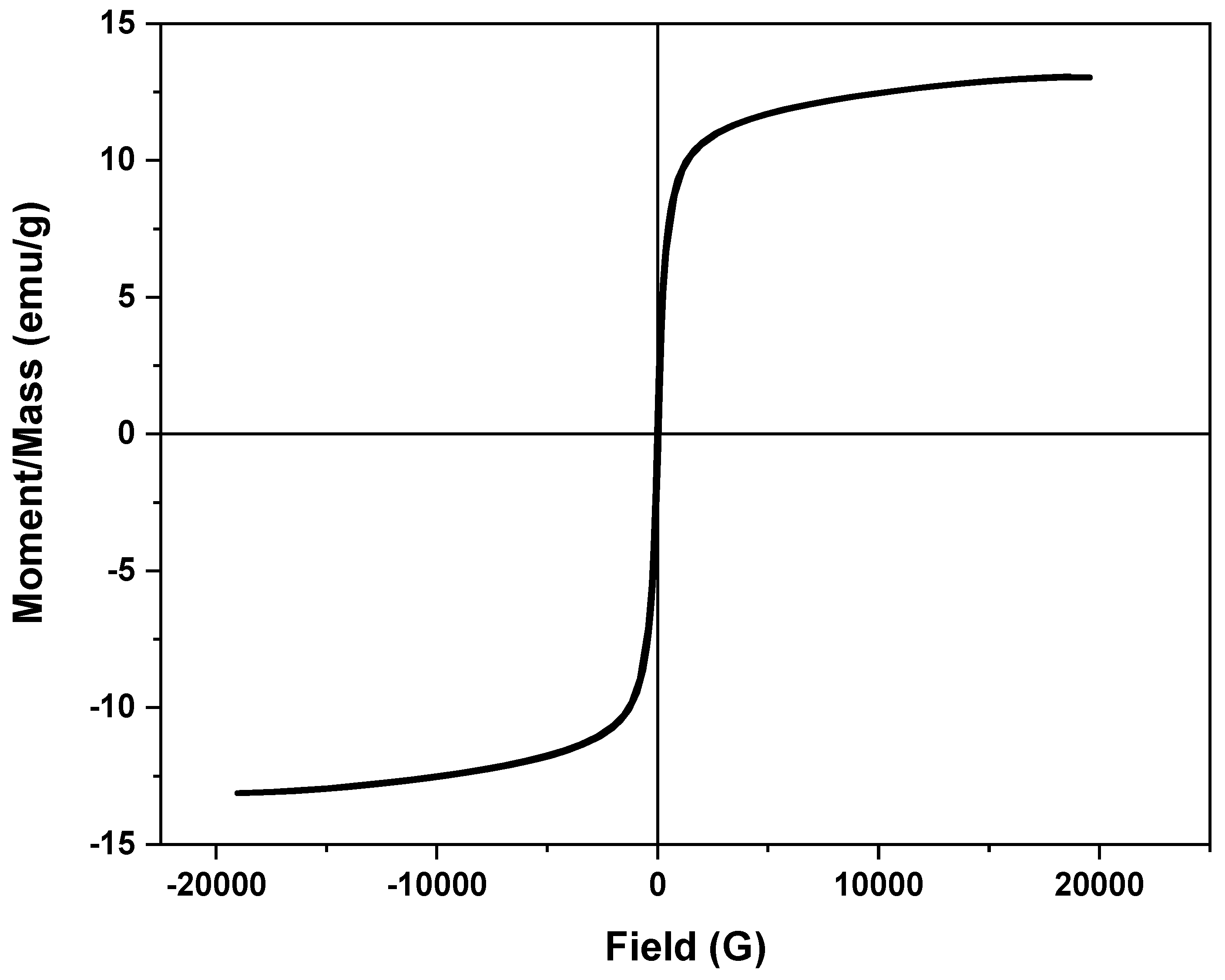
2.1.5. VSM Analysis
2.2. Studies on Methomyl Oxidation
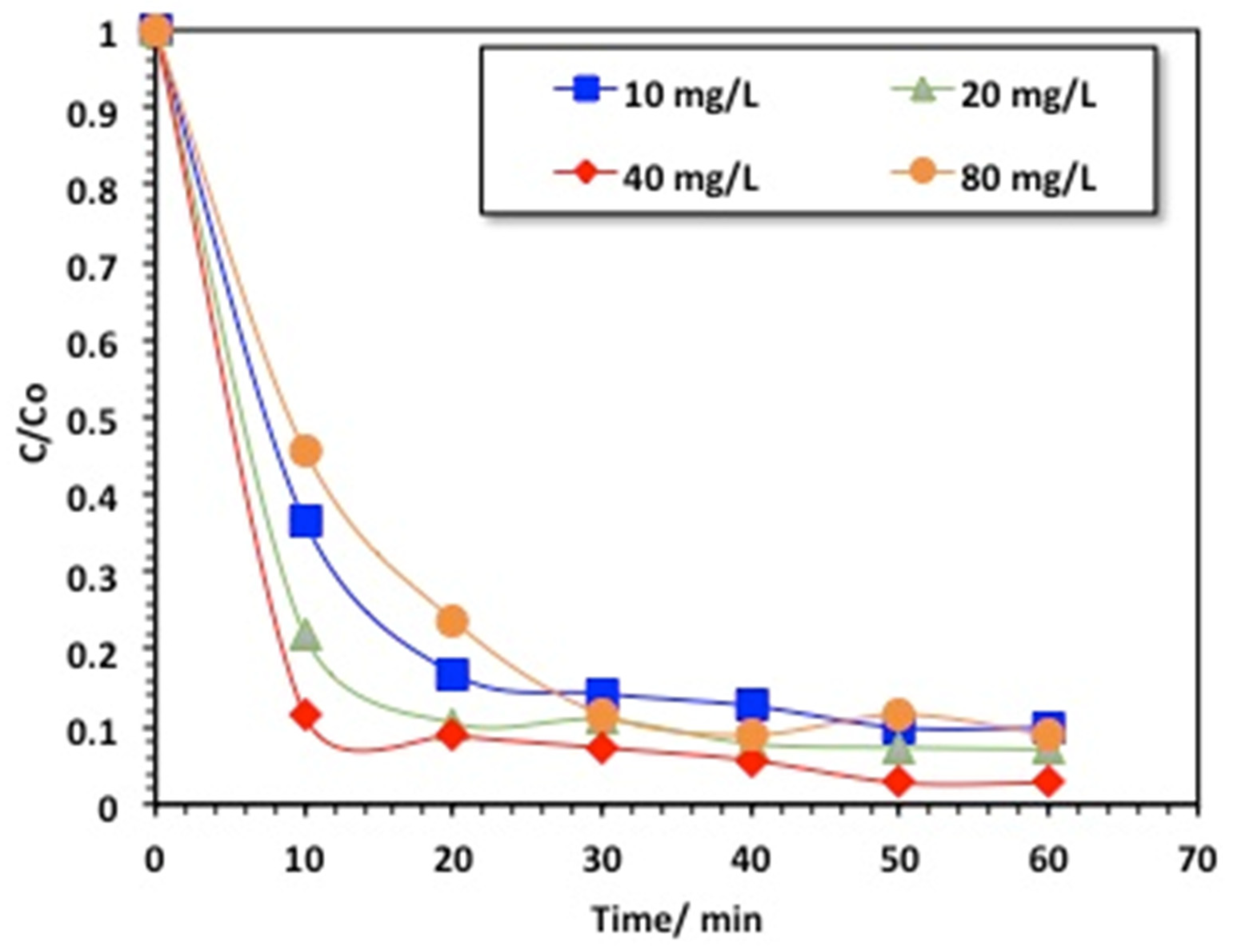
2.2.1. Effects of Reaction Time and Methomyl Loading
2.2.2. Effect of Hydrogen Peroxide
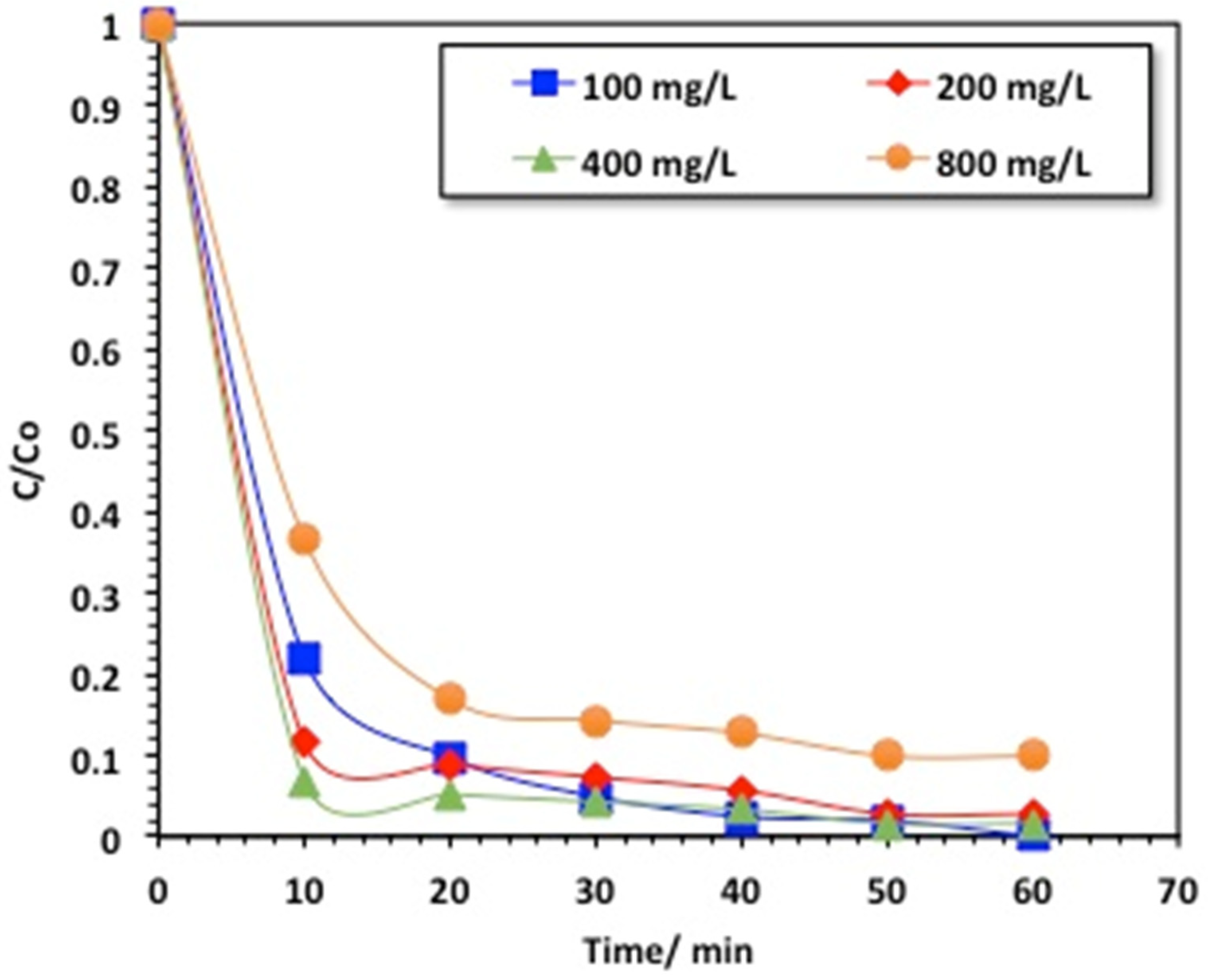
2.2.3. Effect of Chitosan–Magnetite Nanocomposite Loading
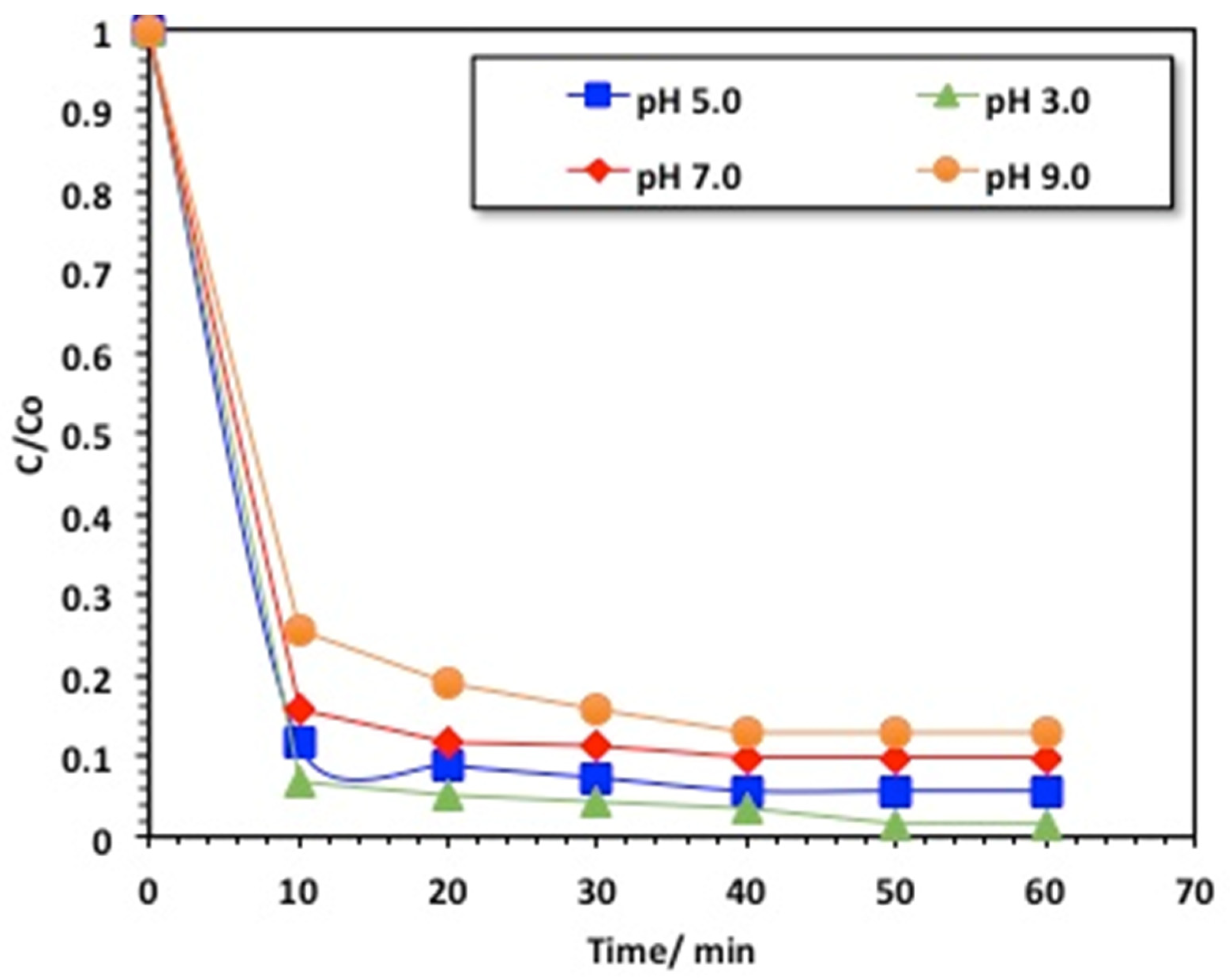
2.2.4. Effect of pH on the Composite Performance
2.2.5. Temperature Effect, Oxidation Kinetics, and Thermodynamic Determination
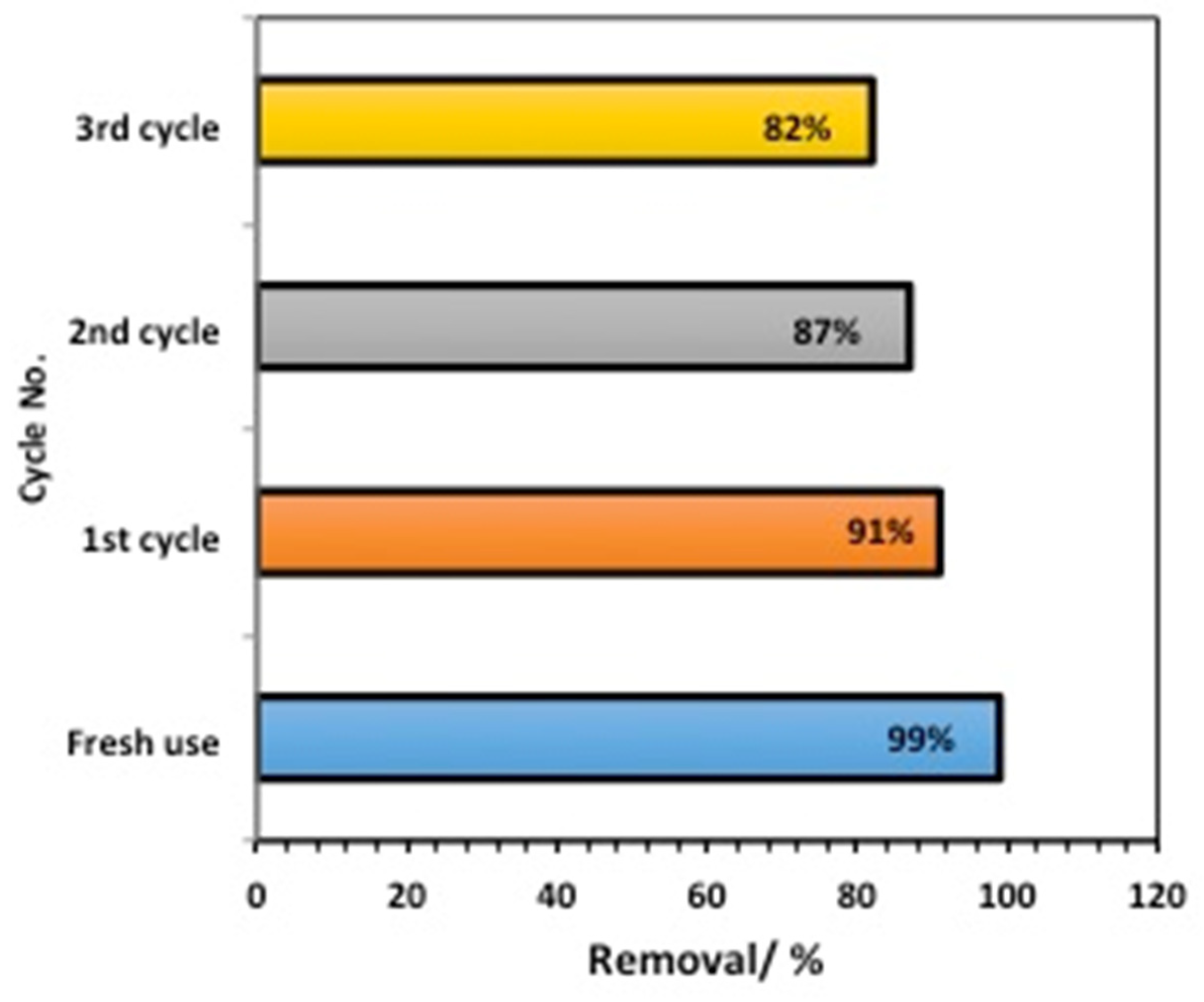
2.2.6. Catalyst Stability Assays
2.2.7. Comparative Investigation
| Composite Type | Induction Source of Fenton System | Pollutant | Pollutant Load | Catalyst Dose | pH | Oxidation (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Chitosan–magnetite | Ultraviolet | Methomyl insecticide | 50 ppm | 3.0 g/L | 3.0 | Complete oxidation | Current work |
| Chitosan–magnetite | Ultraviolet | Basic blue dye | 10 ppm | 2.4 mg/L | 7.0 | Complete oxidation | [8] |
| LaFeO3/BiOBr | Ultraviolet | Rhodamine B dye | 5 ppm | 0.1 mg | Not available | 98.2% | [14] |
| Mixed copper oxides | Microwave irradiance | Methomyl insecticide | 50 ppm | 3.0 g/L | 6.5 | 91% | [43] |
| Silica-supported iron | Solar radiation | Methomyl insecticide | 100 ppm | 103 mg/L | 2.8 | 98% | [44] |
| Magnetite–CeO2-g-C3N4 | Visible light | Tetracycline hydrochloride | 50 mg/L | 50 mg/L | 2.7 | 96.63% | [46] |
| Silver/bismuth/iron oxides | Visible light | Methyl orange | 40 ppm | 0.6 g/L | Not available | 97% | [11] |
| Cellulose–magnetite | Ultraviolet | Red K-HL dye | 50 ppm | 1 g/L | 3.0 | 99% | [45] |
| Alum sludge waste–magnetite | Ultraviolet | Methomyl insecticide | 50 ppm | 50 mg/L | 6.0 | Complete oxidation | [22] |
| Titanium/iron oxides | Ultraviolet light | Methyl orange | 80 ppm | 200 mg/L | 4.5 | 97% | [34] |
| Aluminum-based waste–magnetite | Ultraviolet | Levafix blue dye | 50 ppm | 2 g/L | 2.0 | Complete oxidation | [23] |
| TiO2@NH2-MIL-88B(Fe) | Visible light | Methylene blue | 100 ppm | 200 mg/L | 7.0 | Complete oxidation | [34] |
3. Conclusions
4. Materials and Methods
4.1. Synthesis of Chitosan–Magnetite Nanocomposite
4.2. Methomyl Oxidation Test
4.3. Characterization Techniques
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdou, K.A.; Mohammed, A.N.; Moselhy, W.; Farghali, A.A. Assessment of modified rice husk and sawdust as bio-adsorbent for heavy metals removal using nano particles in fish farm. Asian J. Anim. Vet. Adv. 2018, 13, 180–188. [Google Scholar] [CrossRef]
- Wulandari, I.O.; Mardila, V.T.; Santjojo, D.J.D.H.; Sabarudin, A. Preparation and Characterization of Chitosan-Coated Fe3O4 Nanoparticles Using Ex-Situ co-Precipitation Method and Tripolyphosphate/Sulphate as Dual Crosslinkers; IOP Publishing: Bristol, UK, 2018; Volume 299, p. 012064. [Google Scholar]
- Adesina, O.A.; Abdulkareem, F.; Yusuff, A.S.; Lala, M.; Okewale, A. Response surface methodology approach to optimization of process parameter for coagulation process of surface water using Moringa oleifera seed. S. Afr. J. Chem. Eng. 2019, 28, 46–51. [Google Scholar] [CrossRef]
- Rezgui, S.; Díez, A.M.; Monser, L.; Adhoum, N.; Pazos, M.; Sanromán, M.A. ZnFe2O4-chitosan magnetic beads for the removal of chlordimeform by photo-Fenton process under UVC irradiation. J. Environ. Manag. 2021, 283, 111987. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Behin, J.; Mahnam, A.R. Kinetics and thermodynamics of peroxydisulfate oxidation of Reactive Yellow 84. J. Saudi Chem. Soc. 2016, 20, 644–650. [Google Scholar] [CrossRef]
- Al, M.F.; Mo’ayyad, S.; Ahmad, S.; Mohammad, A.-S. Impact of Fenton and ozone on oxidation of wastewater containing nitroaromatic compounds. J. Environ. Sci. 2008, 20, 675–682. [Google Scholar]
- Argun, M.E.; Karatas, M. Application of Fenton process for decolorization of reactive black 5 from synthetic wastewater: Kinetics and thermodynamics. Environ. Prog. Sustain. Energy 2011, 30, 540–548. [Google Scholar] [CrossRef]
- Guan, X.H.; Chen, G.H.; Shang, C. Re-use of water treatment works sludge to enhance particulate pollutant removal from sewage. Water Res. 2015, 39, 3433–3440. [Google Scholar] [CrossRef]
- Bounab, L.; Iglesias, O.; González-Romero, E.; Pazos, M.; Sanromán, M.Á. Effective heterogeneous electro-Fenton process of m-cresol with iron loaded actived carbon. RSC Adv. 2015, 5, 31049–31056. [Google Scholar] [CrossRef]
- Clark, J.H.; Farmer, T.J.; Herrero-Davila, L.; Sherwood, J. Circular economy design considerations for research and process development in the chemical sciences. Green Chem. 2016, 18, 3914–3934. [Google Scholar] [CrossRef]
- Di, L.; Yang, H.; Xian, T.; Liu, X.; Chen, X. Photocatalytic and photo-Fenton catalytic degradation activities of Z-scheme Ag2S/BiFeO3 heterojunction composites under visible-light irradiation. Nanomaterials 2019, 9, 399. [Google Scholar] [CrossRef]
- El-Desoky, H.S.; Ghoneim, M.M.; El-Sheikh, R.; Zidan, N.M. Oxidation of Levafix CA reactive azo-dyes in industrial wastewater of textile dyeing by electro-generated Fenton’s reagent. J. Hazard. Mater. 2010, 175, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Hosseinzadeh, S.; Wardenier, N.; Verheust, Y.; Chys, M.; Van Hulle, S. Combining ozone with UV and H2O2 for the degradation of micropollutants from different origins: Lab-scale analysis and optimization. Environ. Technol. 2019, 40, 3773–3782. [Google Scholar] [CrossRef] [PubMed]
- Gallo-Cordova, A.; Castro, J.J.; Winkler, E.L.; Lima, E., Jr.; Zysler, R.D.; del Puerto Morales, M.; Ovejero, J.G.; Streitwieser, D.A. Improving degradation of real wastewaters with self-heating magnetic nanocatalysts. J. Clean. Prod. 2021, 308, 127385. [Google Scholar] [CrossRef]
- Guedes, A.M.F.M.; Madeira, L.M.P.; Boaventura, R.A.R.; Costa, C.A.V. Fenton oxidation of cork cooking wastewater—Overall kinetic analysis. Water Res. 2003, 37, 3061–3069. [Google Scholar] [CrossRef]
- Hilder, M.; Winther-Jensen, O.; Winther-Jensen, B.; MacFarlane, D.R. Graphene/zinc nano-composites by electrochemical co-deposition. Phys. Chem. Chem. Phys. 2012, 14, 14034–14040. [Google Scholar] [CrossRef]
- Ioannou, L.A.; Fatta-Kassinos, D. Solar photo-Fenton oxidation against the bioresistant fractions of winery wastewater. J. Environ. Chem. Eng. 2013, 1, 703–712. [Google Scholar] [CrossRef]
- Laib, S.; Rezzaz-Yazid, H.; Yatmaz, H.C.; Sadaoui, Z. Low cost effective heterogeneous photo-Fenton catalyst from drinking water treatment residuals for reactive blue 19 degradation: Preparation and characterization. Water Environ. Res. 2021, 93, 1097–1106. [Google Scholar] [CrossRef]
- Li, X.; Cui, J.; Pei, Y. Granulation of drinking water treatment residuals as applicable media for phosphorus removal. J. Environ. Manag. 2018, 213, 36–46. [Google Scholar] [CrossRef]
- Qu, R.; Zhang, W.; Liu, N.; Zhang, Q.; Liu, Y.; Li, X.; Feng, L. Antioil Ag3PO4 nanoparticle/polydopamine/Al2O3 sandwich structure for complex wastewater treatment: Dynamic catalysis under natural light. ACS Sustain. Chem. Eng. 2018, 6, 8019–8028. [Google Scholar] [CrossRef]
- Muthukannan, V.; Praveen, K.; Natesan, B. Fabrication and characterization of magnetite/reduced graphene oxide composite incurred from iron ore tailings for high performance application. Mater. Chem. Phys. 2015, 162, 400–407. [Google Scholar] [CrossRef]
- Rota, F.; Cavassi, M.; Niego, D.; Gorlani, R.; Vianelli, L.; Tatti, L.; Muntau, H. Mathematical modelling of photomineralization of phenols in aqueous solution, by photocatalytic membranes immobilizing titanium dioxide. Chemosphere 1996, 33, 2159–2173. [Google Scholar] [CrossRef]
- Oyewo, O.A.; Adeniyi, A.; Sithole, B.B.; Onyango, M.S. Sawdust-based cellulose nanocrystals incorporated with ZnO nanoparticles as efficient adsorption media in the removal of methylene blue dye. ACS Omega 2020, 5, 18798–18807. [Google Scholar] [CrossRef] [PubMed]
- Pourali, P.; Behzad, M.; Arfaeinia, H.; Ahmadfazeli, A.; Afshin, S.; Poureshgh, Y.; Rashtbari, Y. Removal of acid blue 113 from aqueous solutions using low-cost adsorbent: Adsorption isotherms, thermodynamics, kinetics and regeneration studies. Sep. Sci. Technol. 2021, 56, 3079–3091. [Google Scholar] [CrossRef]
- Rezgui, S.; Ghazouani, M.; Bousselmi, L.; Akrout, H. Efficient treatment for tannery wastewater through sequential electro-Fenton and electrocoagulation processes. J. Environ. Chem. Eng. 2022, 10, 107424. [Google Scholar] [CrossRef]
- Thabet, R.H.; Fouad, M.K.; Ali, I.A.; El Sherbiney, S.A.; Tony, M.A. Magnetite-based nanoparticles as an efficient hybrid heterogeneous adsorption/oxidation process for reactive textile dye removal from wastewater matrix. Int. J. Environ. Anal. Chem. 2021, 103, 2636–2658. [Google Scholar] [CrossRef]
- Saad, R.A.; Younes, G.; El-Dakdouki, M.H.; Al-Oweini, R. Vanadium-substituted polyoxomolybdates for methylene blue adsorption from aqueous solutions. J. Clust. Sci. 2021, 33, 2077–2083. [Google Scholar] [CrossRef]
- Setyono, D.; Valiyaveettil, S. Chemically modified sawdust as renewable adsorbent for arsenic removal from water. ACS Sustain. Chem. Eng. 2014, 2, 2722–2729. [Google Scholar] [CrossRef]
- Shaheen, T.I.; Emam, H.E. Sono-chemical synthesis of cellulose nanocrystals from wood sawdust using acid hydrolysis. Int. J. Biol. Macromol. 2018, 107, 1599–1606. [Google Scholar] [CrossRef]
- Soliman, E.M.; Ahmed, S.A.; Fadl, A.A. Adsorptive removal of oil spill from sea water surface using magnetic wood sawdust as a novel nano-composite synthesized via microwave approach. J. Environ. Health Sci. Eng. 2020, 18, 79–90. [Google Scholar] [CrossRef]
- Sun, J.-H.; Sun, S.-P.; Fan, M.-H.; Guo, H.-Q.; Qiao, L.-P.; Sun, R.-X. A kinetic study on the degradation of p-nitroaniline by Fenton oxidation process. J. Hazard. Mater. 2007, 148, 172–177. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, H. Solar driven photocatalysis-an efficient method for removal of pesticides from water and wastewater. Biointerface Res. Appl. Chem. 2021, 11, 9071–9084. [Google Scholar]
- Thabet, R.H.; Fouad, M.K.; Sherbiny, S.A.E.; Tony, M.A. Zero-Waste Approach: Assessment of Aluminum-Based Waste as a Photocatalyst for Industrial Wastewater Treatment Ecology. Int. J. Environ. Res. 2022, 16, 36. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, M.; Wu, J.; Liang, J.; Zhou, L.; Lǚ, B. Visible light-degradation of azo dye methyl orange using TiO2/β-FeOOH as a heterogeneous photo-Fenton-like catalyst. Water Sci. Technol. 2013, 68, 2178–2185. [Google Scholar] [CrossRef] [PubMed]
- Tony, M.A.; Lin, L.-S. Iron Coated-Sand from Acid Mine Drainage Waste for Being a Catalytic Oxidant Towards Municipal Wastewater Remediation. Int. J. Environ. Res. 2021, 15, 191–201. [Google Scholar] [CrossRef]
- Ahamad, T.; Naushad, M.; Mousa, R.H.; Alshehri, S.M. Fabrication of starch-salicylaldehyde based polymer nanocomposite (PNC) for the removal of pollutants from contaminated water. Int. J. Biol. Macromol. 2020, 165, 2731–2738. [Google Scholar] [CrossRef]
- Zhang, Y.; Klamerth, N.; Messele, S.A.; Chelme-Ayala, P.; El-Din, M.G. Kinetics study on the degradation of a model naphthenic acid by ethylenediamine-N, N’-disuccinic acid-modified Fenton process. J. Hazard. Mater. 2016, 318, 371–378. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, J.; Fang, Y.; Cao, Z.; Chen, D.; Li, N.; Xu, Q.; Lu, J. TiO2 nanoparticles anchored onto the metal–organic framework NH2-MIL-88B (Fe) as an adsorptive photocatalyst with enhanced fenton-like degradation of organic pollutants under visible light irradiation. ACS Sustain. Chem. Eng. 2018, 6, 16186–16197. [Google Scholar] [CrossRef]
- Kim, I.T.; Magasinski, A.; Jacob, K.; Yushin, G.; Tannenbaum, R. Synthesis and electrochemical performance of reduced graphene oxide/maghemite composite anode for lithium ion batteries. Carbon 2013, 52, 56–64. [Google Scholar] [CrossRef]
- Najjar, W.; Chirchi, L.; Santos, E.; Ghorhel, A. Kinetic study of 2-nitrophenol photodegradation on Al-pillared montmorillonite doped with copper. J. Environ. Monit. 2001, 3, 697–701. [Google Scholar] [CrossRef]
- Jusin, J.W.; Aziz, M.; Sean, G.P.; Jaafar, J. Preparation and characterization of graphene-based magnetic hybrid nanocomposite. Malays. J. Anal. Sci. 2016, 20, 149–156. [Google Scholar] [CrossRef]
- Tony, M.A.; Eltabey, M.M. End-of-life waste criteria: Synthesis and utilization of Mn–Zn ferrite nanoparticles as a superparamagnetic photocatalyst for synergistic wastewater remediation. Appl. Water Sci. 2022, 12, 21. [Google Scholar] [CrossRef]
- Pan, W.; Zhang, G.; Zheng, T.; Wang, P. Degradation of p-nitrophenol using CuO/Al 2 O 3 as a Fenton-like catalyst under microwave irradiation. RSC Adv. 2015, 34, 27043–27051. [Google Scholar] [CrossRef]
- Tony, M.A. Pattern, Forms and Bibliometric Analysis for Systematic Study of Silica-Supported Heterogeneous Solar Photocatalyst for Lannate Insecticide Abatement from Aqueous Stream. Arab. J. Sci. Eng. 2022, 48, 8417–8430. [Google Scholar] [CrossRef]
- Gherghel, A.; Teodosiu, C.; De Gisi, S. A review on wastewater sludge valorisation and its challenges in the context of circular economy. J. Clean. Prod. 2019, 228, 244–263. [Google Scholar] [CrossRef]
- Wang, S.; Long, J.; Jiang, T.; Shao, L.; Li, D.; Xie, X.; Xu, F. Magnetic Fe3O4/CeO2/g-C3N4 composites with a visible-light response as a high efficiency Fenton photocatalyst to synergistically degrade tetracycline. Sep. Purif. Technol. 2021, 278, 119609. [Google Scholar] [CrossRef]


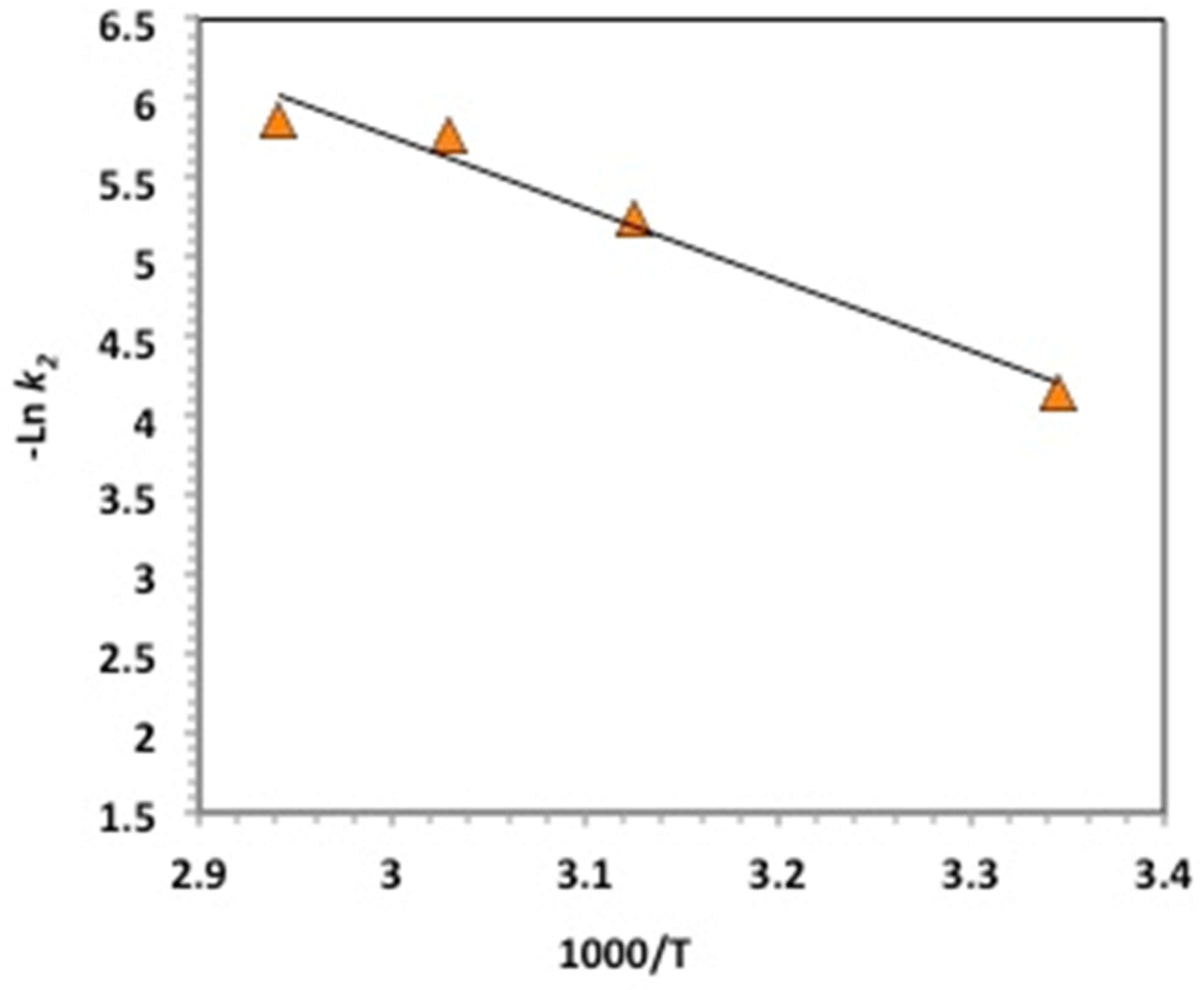
| Kinetics Model | Parameters | Values | |||
|---|---|---|---|---|---|
| T, °C | |||||
| 26 °C | 40 °C | 50 °C | 60 °C | ||
| Pseudo-first-order ) | k1 (min−1) | 0.129 | 0.090 | 0.101 | 0.072 |
| t1/2 (min) | 9.6 | 6.8 | 7.7 | 5.3 | |
| R2 | 0.6 | 0.59 | 0.54 | 0.59 | |
| Pseudo-second-order () | k2 (L·mg−1·min−1) × 10−2 | 1.57 | 0.53 | 0.31 | 0.28 |
| t1/2 (min) | 1.27 | 3.77 | 6.45 | 7.14 | |
| R2 | 0.86 | 0.94 | 0.92 | 0.90 | |
| Thermodynamic Parameters | T/°C | |||
|---|---|---|---|---|
| 26 °C | 40 °C | 50 °C | 60 °C | |
| ∆G′ (kJ/mol) | 83.56 | 92.50 | 96.94 | 100.25 |
| ∆H′ (kJ/mol) | 34.91 | 34.73 | 34.65 | 34.57 |
| ∆S′ (J/mol K) | −162.70 | −180.50 | −188.75 | −193.18 |
| Ea (kJ/mol) | 37.39 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, E.A.; Tony, M.A. Nexus Advances Using Marine Biopolymeric Gel Material as a Photocatalyst for the Oxidation of Agricultural Wastewater Containing Insecticides. Gels 2023, 9, 864. https://doi.org/10.3390/gels9110864
Hassan EA, Tony MA. Nexus Advances Using Marine Biopolymeric Gel Material as a Photocatalyst for the Oxidation of Agricultural Wastewater Containing Insecticides. Gels. 2023; 9(11):864. https://doi.org/10.3390/gels9110864
Chicago/Turabian StyleHassan, Ehssan Ahmed, and Maha A. Tony. 2023. "Nexus Advances Using Marine Biopolymeric Gel Material as a Photocatalyst for the Oxidation of Agricultural Wastewater Containing Insecticides" Gels 9, no. 11: 864. https://doi.org/10.3390/gels9110864
APA StyleHassan, E. A., & Tony, M. A. (2023). Nexus Advances Using Marine Biopolymeric Gel Material as a Photocatalyst for the Oxidation of Agricultural Wastewater Containing Insecticides. Gels, 9(11), 864. https://doi.org/10.3390/gels9110864






