Ex Vivo Functional Benchmarking of Hyaluronan-Based Osteoarthritis Viscosupplement Products: Comprehensive Assessment of Rheological, Lubricative, Adhesive, and Stability Attributes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Technical Benchmarking of Viscosupplementation Product Parameters and Specificities
- Durolane (Bioventus, Durham, NC, USA), with a specified polymer concentration of 20 mg/mL, which uses the NASHA technology, and which was approved by the FDA in 2017;
- Synolis VA 80/160 (Aptissen, Plan-Les-Ouates, Switzerland), with a specified concentration of 20 mg/mL of high MW HA and 40 mg/mL sorbitol, which received a CE-mark in 2019;
- Gel One (Zimmer Biomet, Warsaw, IN, USA), which is crosslinked and has a specified HA concentration of 10 mg/mL.
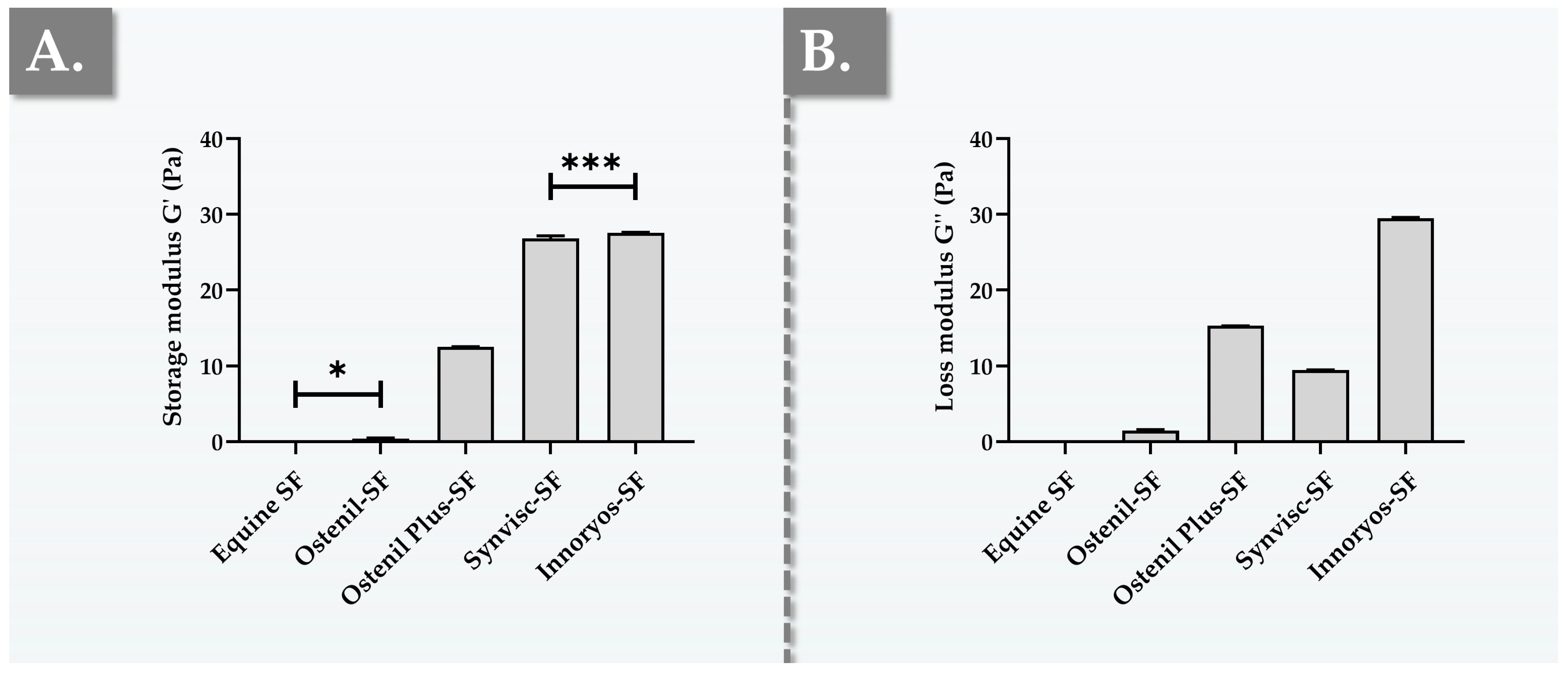
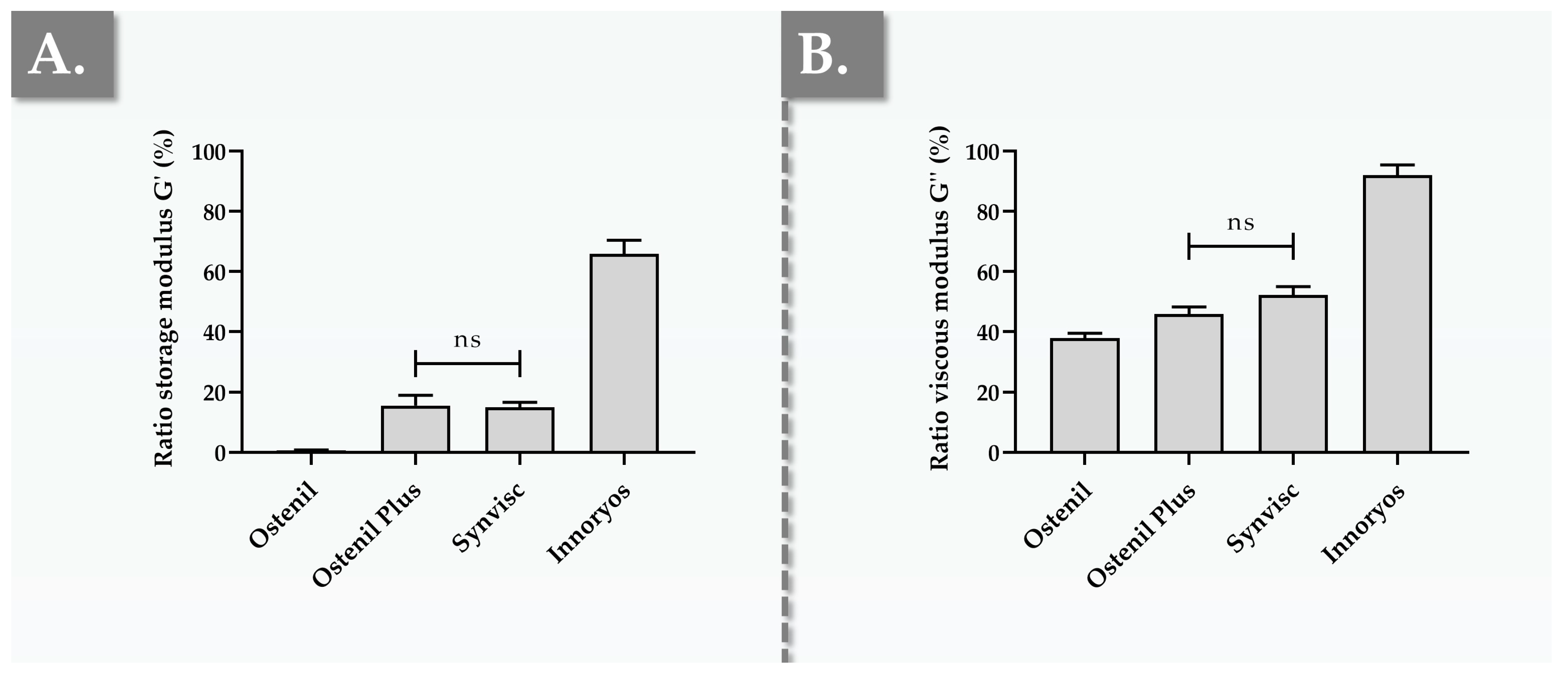
2.2. Ex Vivo Characterization Results of Hydrogel Rheological Behavior
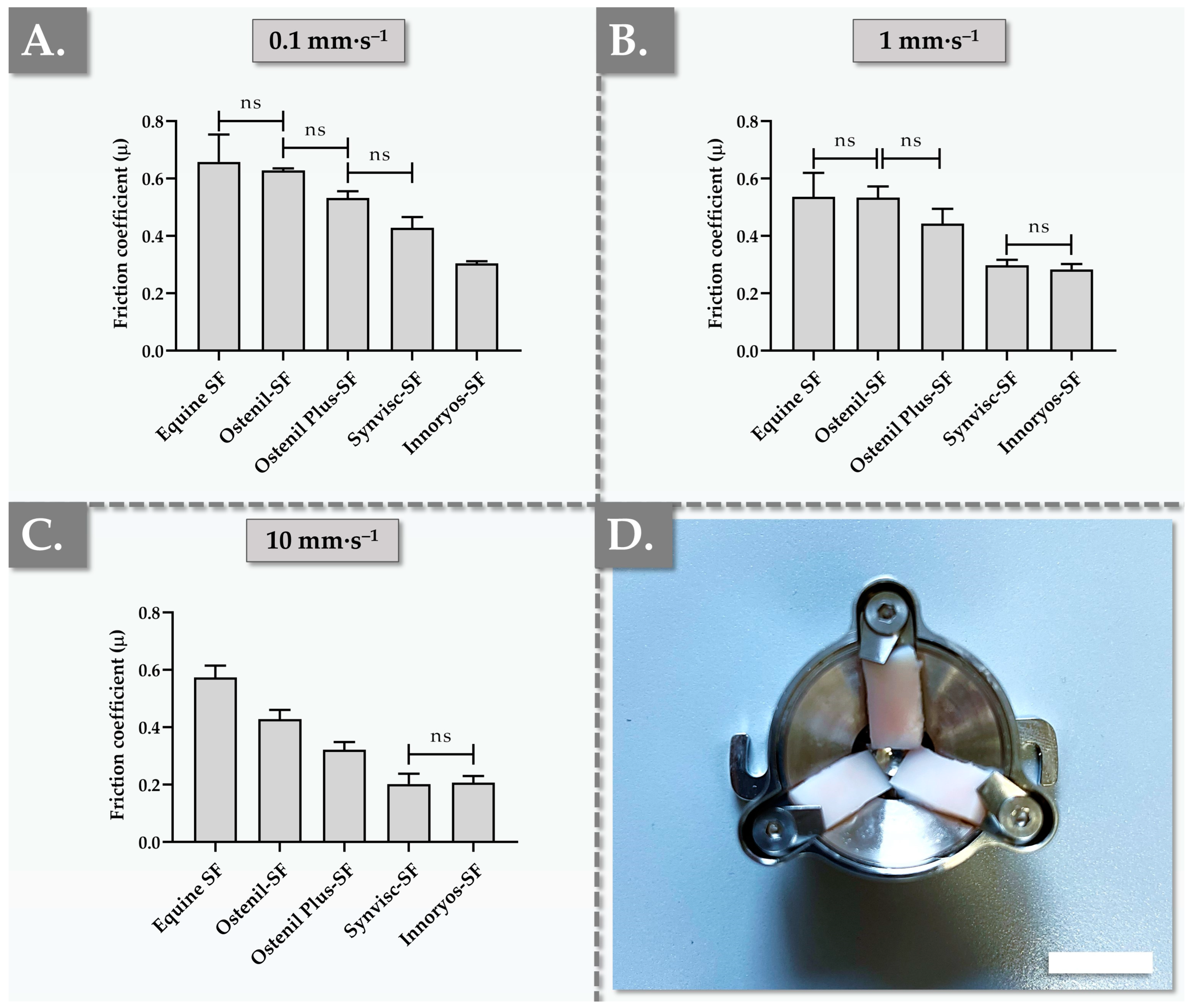
2.3. Ex Vivo Characterization Results of Hydrogel Lubrication Capacity
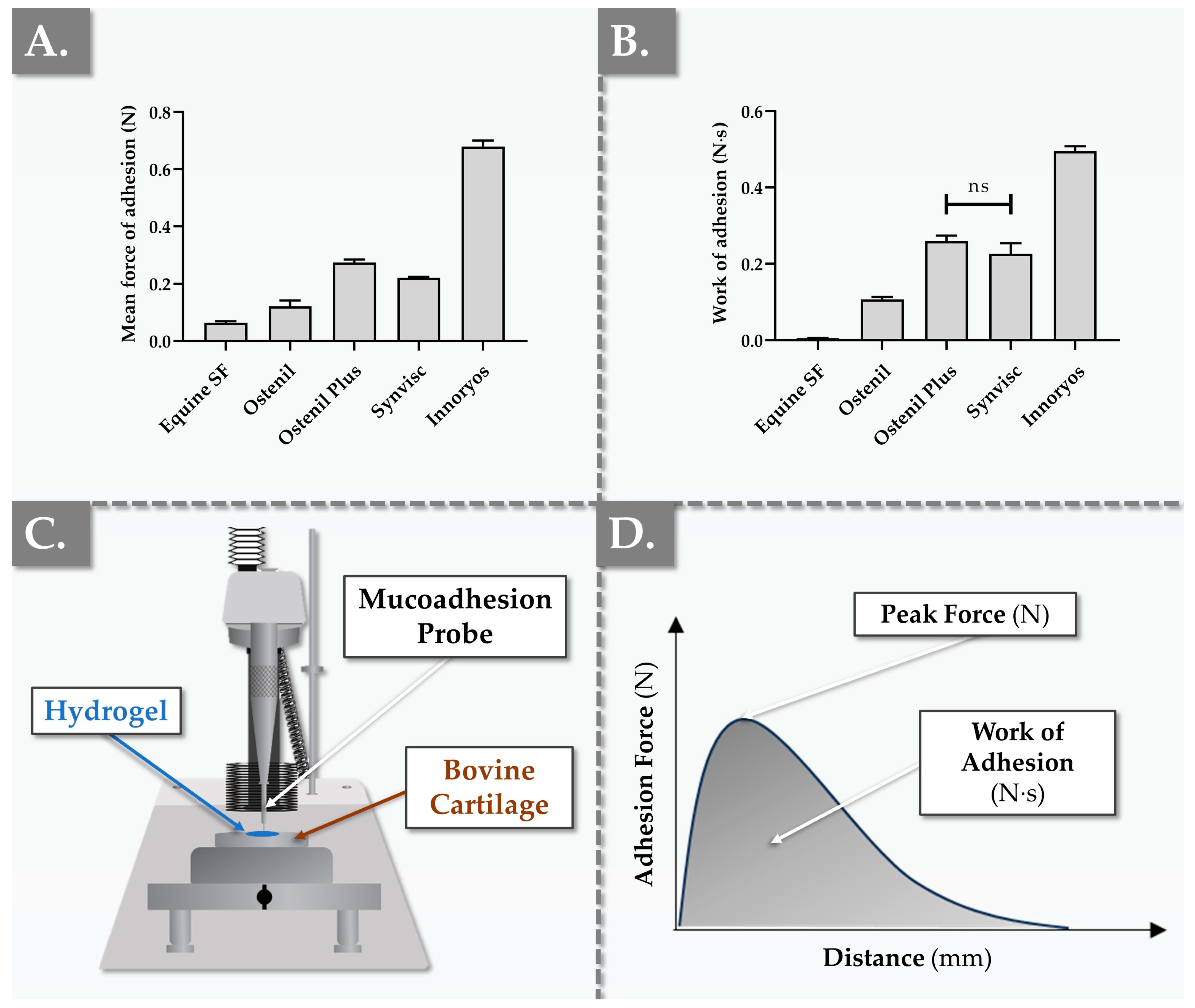
2.4. Ex Vivo Characterization Results of Hydrogel Bio-Adhesion Capacity
2.5. In Vitro Results of Hydrogel Resistance to Oxidative Degradation
2.6. Commercial Viscosupplementation Product Functional Benchmark Summary and Overall Comparative Assessment
2.7. Study Limitations and Future Perspectives
3. Conclusions
4. Materials and Methods
4.1. Materials and Consumables Used for the Study
4.2. Ex Vivo Rheological Behavior Setup
4.3. Ex Vivo Rotational Tribology Setup
4.4. Ex Vivo Bio-Adhesivity Evaluation Setup
4.5. Accelerated Hydrogel Degradation Assay
4.6. Benchmarking of Antioxidant Attributes for HA-Based Hydrogel Additives
4.7. Statistical Analysis and Data Presentation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASTM | American Society for Testing and Materials |
| CE | European mark of conformity |
| Da | Daltons |
| η* | complex viscosity |
| EP | European Pharmacopoeia |
| FDA | US Food and Drug Administration |
| FRAP | ferric reducing antioxidant power |
| G′ | storage modulus |
| G″ | loss modulus |
| GAG | glycosaminoglycan |
| GPC | gel permeation chromatography |
| H2O2 | hydrogen peroxide |
| HA | hyaluronic acid |
| HAS | hyaluronan synthases |
| HYAL | hyaluronidases |
| ISO | International Standards Organization |
| LVE | linear viscoelastic region |
| MD | medical device |
| MDa | megadalton |
| min | minute |
| MW | molecular weight |
| N | Newtons |
| NA | non-applicable |
| N·s | Newton seconds |
| ns | non-significant |
| OA | osteoarthritis |
| Pa | Pascals |
| Pa·s | Pascal seconds |
| PBS | phosphate-buffered saline |
| PDMS | polydimethylsiloxane |
| ROS | reactive oxygen species |
| SEC | size-exclusion chromatography |
| SF | synovial fluid |
| TEAC | Trolox equivalent antioxidant capacity |
| USA | United States of America |
References
- Dou, H.; Wang, S.; Hu, J.; Song, J.; Zhang, C.; Wang, J.; Xiao, L. Osteoarthritis models: From animals to tissue engineering. J. Tissue Eng. 2023, 14, 20417314231172584. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Yu, H.; Huang, X.; Shen, J.; Xiao, G.; Chen, L.; Wang, H.; Xing, L.; Chen, D. Current understanding of osteoarthritis pathogenesis and relevant new approaches. Bone Res. 2022, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Primorac, D.; Molnar, V.; Rod, E.; Jeleč, Ž.; Čukelj, F.; Matišić, V.; Vrdoljak, T.; Hudetz, D.; Hajsok, H.; Borić, I. Knee osteoarthritis: A review of pathogenesis and state-of-the-art non-operative therapeutic considerations. Genes 2020, 11, 854. [Google Scholar] [CrossRef] [PubMed]
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M.; Conaghan, P.G.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.P. Osteoarthritis. Nat. Rev. Dis. Prim. 2016, 2, 16072. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheumatol. 2012, 64, 1697–1707. [Google Scholar] [CrossRef]
- Malekipour, F.; Lee, P.V. Shock absorbing ability in healthy and damaged cartilage-bone under high-rate compression. J. Mech. Behav. Biomed. Mat. 2019, 90, 388–394. [Google Scholar] [CrossRef]
- Tamer, T.M. Hyaluronan and synovial joint: Function, distribution, and healing. Interdiscip. Toxicol. 2013, 6, 111–125. [Google Scholar] [CrossRef]
- Kuiper, N.J.; Sharma, A. A detailed quantitative outcome measure of glycosaminoglycans in human articular cartilage for cell therapy and tissue engineering strategies. Osteoarthr. Cartil. 2015, 23, 2233–2241. [Google Scholar] [CrossRef]
- Stern, R.; Kogan, G.; Jedrzejas, M.J.; Soltés, L. The many ways to cleave hyaluronan. Biotechnol. Adv. 2007, 25, 537–557. [Google Scholar] [CrossRef]
- Žádníková, P.; Šínová, R.; Pavlík, V.; Šimek, M.; Šafránková, B.; Hermannová, M.; Nešporová, K.; Velebný, V. The degradation of hyaluronan in the skin. Biomolecules 2022, 12, 251. [Google Scholar] [CrossRef]
- Schanté, C.; Zubera, G.; Herlinb, C.; Vandamme, T.F. Synthesis of N-alanyl-hyaluronamide with high degree of substitution for enhanced resistance to hyaluronidase-mediated digestion. Carbohydr. Polym. 2011, 86, 747–752. [Google Scholar] [CrossRef]
- Conrozier, T.; Mathieu, P.; Rinaudo, M. Mannitol preserves the viscoelastic properties of hyaluronic acid in an in vitro model of oxidative stress. Rheumatol. Ther. 2014, 1, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Waller, K.A.; Zhang, L.X.; Elsaid, K.A.; Fleming, B.C.; Warman, M.L.; Jay, G.D. Role of lubricin and boundary lubrication in the prevention of chondrocyte apoptosis. Proc. Natl. Acad. Sci. USA 2013, 110, 5852–5857. [Google Scholar] [CrossRef] [PubMed]
- Jay, G.D.; Torres, J.R.; Warman, M.L.; Breuer, K.S. The role of lubricin in the mechanical behavior of synovial fluid. Proc. Natl. Acad. Sci. USA 2007, 104, 6194–6199. [Google Scholar] [CrossRef] [PubMed]
- Temple-Wong, M.M.; Ren, S.; Quach, P.; Hansen, B.C.; Chen, A.C.; Hasegawa, A.; D’Lima, D.D.; Koziol, J.; Masuda, K.; Lotz, M.K.; et al. Hyaluronan concentration and size distribution in human knee synovial fluid: Variations with age and cartilage degeneration. Arthritis Res. Ther. 2016, 18, 18. [Google Scholar] [CrossRef]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef]
- Yao, Q.; Wu, X.; Tao, C.; Gong, W.; Chen, M.; Qu, M.; Zhong, Y.; He, T.; Chen, S.; Xiao, G. Osteoarthritis: Pathogenic signaling pathways and therapeutic targets. Sig. Transduct. Target Ther. 2023, 8, 56. [Google Scholar] [CrossRef]
- Bowman, S.; Awad, M.E.; Hamrick, M.W.; Hunter, M.; Fulzele, S. Recent advances in hyaluronic acid based therapy for osteoarthritis. Clin. Transl. Med. 2018, 7, 6. [Google Scholar] [CrossRef]
- Arrich, J.; Piribauer, F.; Mad, P.; Schmid, D.; Klaushofer, K.; Müllner, M. Intra-articular hyaluronic acid for the treatment of osteoarthritis of the knee: Systematic review and meta-analysis. Can. Med. Assoc. J. 2005, 172, 1039–1043. [Google Scholar] [CrossRef]
- Pereira, T.V.; Jüni, P.; Saadat, P.; Xing, D.; Yao, L.; Bobos, P.; Agarwal, A.; Hincapié, C.A.; da Costa, B.R. Viscosupplementation for knee osteoarthritis: Systematic review and meta-analysis. BMJ 2022, 378, e069722. [Google Scholar] [CrossRef]
- Testa, G.; Giardina, S.M.C.; Culmone, A.; Vescio, A.; Turchetta, M.; Cannavò, S.; Pavone, V. Intra-articular injections in knee osteoarthritis: A review of literature. J. Funct. Morphol. Kinesiol. 2021, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- McGrory, B.; Weber, K.; Lynott, J.A.; Richmond, J.C.; Davis, C.M., III; Yates, A., Jr.; Kamath, A.F.; Dasa, V.; Brown, G.A.; Gerlinger, T.L.; et al. The American Academy of Orthopaedic Surgeons evidence-based clinical practice guideline on surgical management of osteoarthritis of the knee. J. Bone Jt. Surg. Am. 2016, 98, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Peck, J.; Slovek, A.; Miro, P.; Vij, N.; Traube, B.; Lee, C.; Berger, A.A.; Kassem, H.; Kaye, A.D.; Sherman, W.F.; et al. A comprehensive review of viscosupplementation in osteoarthritis of the knee. Orthop. Rev. 2021, 13, 25549. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, C.L.; Davies, M.J. Direct detection and identification of radicals generated during the hydroxyl radical-induced degradation of hyaluronic acid and related materials. Free Radic. Biol. Med. 1996, 21, 275–290. [Google Scholar] [CrossRef]
- Zahan, O.M.; Serban, O.; Gherman, C.; Fodor, D. The evaluation of oxidative stress in osteoarthritis. Med. Pharm. Rep. 2020, 93, 12–22. [Google Scholar] [CrossRef]
- La Gatta, A.; Stellavato, A.; Vassallo, V.; Di Meo, C.; Toro, G.; Iolascon, G.; Schiraldi, C. Hyaluronan and derivatives: An in vitro multilevel assessment of their potential in viscosupplementation. Polymers 2021, 13, 3208. [Google Scholar] [CrossRef]
- Zhang, F.X.; Liu, P.; Ding, W.; Meng, Q.B.; Su, D.H.; Zhang, Q.C.; Lian, R.X.; Yu, B.Q.; Zhao, M.D.; Dong, J.; et al. Injectable mussel-inspired highly adhesive hydrogel with exosomes for endogenous cell recruitment and cartilage defect regeneration. Biomaterials 2021, 278, 121169. [Google Scholar] [CrossRef]
- Porcello, A.; Gonzalez-Fernandez, P.; Jeannerat, A.; Peneveyre, C.; Abdel-Sayed, P.; Scaletta, C.; Raffoul, W.; Hirt-Burri, N.; Applegate, L.A.; Allémann, E.; et al. Thermo-responsive hyaluronan-based hydrogels combined with allogeneic cytotherapeutics for the treatment of osteoarthritis. Pharmaceutics 2023, 15, 1528. [Google Scholar] [CrossRef]
- Hintze, V.; Schnabelrauch, M.; Rother, S. Chemical modification of hyaluronan and their biomedical applications. Front. Chem. 2022, 10, 830671. [Google Scholar] [CrossRef]
- Lin, Y.W.; Fang, C.H.; Meng, F.Q.; Ke, C.J.; Lin, F.H. Hyaluronic acid loaded with cerium oxide nanoparticles as antioxidant in hydrogen peroxide induced chondrocytes injury: An in vitro osteoarthritis model. Molecules 2020, 25, 4407. [Google Scholar] [CrossRef]
- Zhuang, C.; Ni, S.; Yang, Z.-C.; Liu, R.-P. Oxidative stress induces chondrocyte apoptosis through caspase-dependent and caspase-independent mitochondrial pathways and the antioxidant mechanism of Angelica Sinensis polysaccharide. Oxid. Med. Cell Longev. 2020, 2020, 3240820. [Google Scholar] [CrossRef] [PubMed]
- Lappas, M.; Permezel, M. The anti-inflammatory and antioxidative effects of nicotinamide, a vitamin B(3) derivative, are elicited by FoxO3 in human gestational tissues: Implications for preterm birth. J. Nutr. Biochem. 2011, 22, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C. Mechanistic basis and clinical evidence for the applications of nicotinamide (niacinamide) to control skin aging and pigmentation. Antioxidants 2021, 10, 1315. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.; Bhandari, M.; Grant, J.; Bedi, A.; Trojian, T.; Johnson, A.; Schemitsch, E. A systematic review of current clinical practice guidelines on intra-articular hyaluronic acid, corticosteroid, and platelet-rich plasma injection for knee osteoarthritis: An international perspective. Orthop. J. Sport. Med. 2021, 9, 23259671211030272. [Google Scholar] [CrossRef] [PubMed]
- Richardson, C.; Plaas, A.; Block, J.A. Intra-articular hyaluronan therapy for symptomatic knee osteoarthritis. Rheum. Dis. Clin. N. Am. 2019, 45, 439–451. [Google Scholar] [CrossRef]
- Singh, H.; Knapik, D.M.; Polce, E.M.; Eikani, C.K.; Bjornstad, A.H.; Gursoy, S.; Perry, A.K.; Westrick, J.C.; Yanke, A.B.; Verma, N.N.; et al. Relative efficacy of intra-articular injections in the treatment of knee osteoarthritis: A systematic review and network meta-analysis. Am. J. Sport. Med. 2022, 50, 3140–3148. [Google Scholar] [CrossRef]
- Jang, C.W.; Bang, M.; Park, J.H.; Cho, H.E. Impact of changes in clinical practice guidelines for intra-articular injection treatments for knee osteoarthritis on public interest and social media. Osteoarthr. Cart. 2023, 31, 793–801. [Google Scholar] [CrossRef]
- Webner, D.; Huang, Y.; Hummer, C.D. Intraarticular hyaluronic acid preparations for knee osteoarthritis: Are some better than others? Cartilage 2021, 13, 1619S–1636S. [Google Scholar] [CrossRef]
- More, S.; Kotiya, A.; Kotia, A.; Ghosh, S.K.; Spyrou, L.A.; Sarris, I.E. Rheological properties of synovial fluid due to viscosupplements: A review for osteoarthritis remedy. Comput. Methods Programs Biomed. 2020, 196, 105644. [Google Scholar] [CrossRef]
- Bhuanantanondh, P. Rheology of Synovial Fluid with and without Viscosupplements in Patients with Osteoarthritis: A Pilot Study. University of British Columbia. 2009. Available online: https://open.library.ubc.ca/collections/ubctheses/24/items/1.0069367 (accessed on 11 August 2023).
- Von Lospichl, B.; Hemmati-Sadeghi, S.; Dey, P.; Dehne, T.; Haag, R.; Sittinger, M.; Ringe, J.; Gradzielski, M. Injectable hydrogels for treatment of osteoarthritis—A rheological study. Colloids Surf. B. Interfac. 2017, 159, 477–483. [Google Scholar] [CrossRef]
- Fam, H.; Bryant, J.T.; Kontopoulou, M. Rheological properties of synovial fluids. Biorheology 2007, 44, 59–74. [Google Scholar] [PubMed]
- Falcone, S.J.; Palmeri, D.M.; Berg, R.A. Rheological and cohesive properties of hyaluronic acid. J. Biomed. Mat. Res. 2006, 76, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, M.; Manjoo, A.; Shaw, P.; Niazi, F.; Rosen, J. Rheological properties of commercially available hyaluronic acid products in the United States for the treatment of osteoarthritis knee pain. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2018, 11, 1179544117751622. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, M.; Manjoo, A.; Shaw, P.; Niazi, F.; Rosen, J. A comparison between rheological properties of intra-articular hyaluronic acid preparations and reported human synovial fluid. Adv. Ther. 2018, 35, 523–530. [Google Scholar] [CrossRef]
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic acid: Molecular mechanisms and therapeutic trajectory. Front. Vet. Sci. 2019, 6, 192. [Google Scholar] [CrossRef]
- Wu, Y.Z.; Huang, H.T.; Ho, C.J.; Shih, C.L.; Chen, C.H.; Cheng, T.L.; Wang, Y.C.; Lin, S.Y. Molecular weight of hyaluronic acid has major influence on its efficacy and safety for viscosupplementation in hip osteoarthritis: A systematic review and meta-analysis. Cartilage 2021, 13, 169S–184S. [Google Scholar] [CrossRef]
- Hummer, C.D.; Angst, F.; Ngai, W.; Whittington, C.; Yoon, S.S.; Duarte, L.; Manitt, C.; Schemitsch, E. High molecular weight intraarticular hyaluronic acid for the treatment of knee osteoarthritis: A network meta-analysis. BMC Musculoskelet. Disord. 2020, 21, 702. [Google Scholar] [CrossRef]
- Bonnevie, E.D.; Galesso, D.; Secchieri, C.; Bonassar, L.J. Frictional characterization of injectable hyaluronic acids is more predictive of clinical outcomes than traditional rheological or viscoelastic characterization. PLoS ONE 2019, 14, e0216702. [Google Scholar] [CrossRef]
- Rebenda, D.; Vrbka, M.; Čípek, P.; Toropitsyn, E.; Nečas, D.; Pravda, M.; Hartl, M. On the dependence of rheology of hyaluronic acid solutions and frictional behavior of articular cartilage. Materials 2020, 13, 2659. [Google Scholar] [CrossRef]
- Zhu, L.; Seror, J.; Day, A.J.; Kampf, N.; Klein, J. Ultra-low friction between boundary layers of hyaluronan-phosphatidylcholine complexes. Acta Biomater. 2017, 59, 283–292. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, W.; Fan, Y.; Kampf, N.; Wang, Y.; Klein, J. Effects of hyaluronan molecular weight on the lubrication of cartilage-emulating boundary layers. Biomacromolecules 2020, 21, 4345–4354. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Mears, L.L.E.; Wang, Y.; Su, R.; Qi, W.; He, Z.; Valtiner, M. Lubricants for osteoarthritis treatment: From natural to bioinspired and alternative strategies. Adv. Colloid Interface Sci. 2023, 311, 102814. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Banquy, X.; Greene, G.W.; Lowrey, D.D.; Israelachvili, J.N. The boundary lubrication of chemically grafted and cross-linked hyaluronic acid in phosphate buffered saline and lipid solutions measured by the surface forces apparatus. Langmuir 2012, 28, 2244–2250. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Banquy, X.; Zappone, B.; Greene, G.W.; Jay, G.D.; Israelachvili, J.N. Synergistic interactions between grafted hyaluronic acid and lubricin provide enhanced wear protection and lubrication. Biomacromolecules 2013, 14, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- Seror, J.; Merkher, Y.; Kampf, N.; Collinson, L.; Day, A.J.; Maroudas, A.; Klein, J. Articular cartilage proteoglycans as boundary lubricants: Structure and frictional interaction of surface-attached hyaluronan and hyaluronan—Aggrecan complexes. Biomacromolecules 2011, 12, 3432–3443. [Google Scholar] [CrossRef]
- Mederake, M.; Trappe, D.; Jacob, C.; Hofmann, U.K.; Schüll, D.; Dalheimer, P.; Exner, L.; Walter, C. Influence of hyaluronic acid on intra-articular friction—A biomechanical study in whole animal joints. BMC Musculoskel. Disord. 2022, 23, 927. [Google Scholar] [CrossRef]
- Simou, K.; Jones, S.W.; Davis, E.T.; Preece, J.; Zhang, Z.J. Rheological and interface adhesive properties of osteoarthritic synovial fluids. Biotribology 2022, 32, 100227. [Google Scholar] [CrossRef]
- Wang, S.; Qiu, Y.; Qu, L.; Wang, Q.; Zhou, Q. Hydrogels for treatment of different degrees of osteoarthritis. Front. Bioeng. Biotechnol. 2022, 10, 858656. [Google Scholar] [CrossRef]
- Duan, W.L.; Zhang, L.N.; Bohara, R.; Martin-Saldaña, S.; Yang, F.; Zhao, Y.; Xie, Y.; Bu, Y.; Pandit, A. Adhesive hydrogels in osteoarthritis: From design to application. Mil. Med. Res. 2023, 10, 4. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Wang, L.; Zhang, X.; Heng, B.C.; Wang, D.A.; Ge, Z. Modified hyaluronic acid hydrogels with chemical groups that facilitate adhesion to host tissues enhance cartilage regeneration. Bioact. Mater. 2020, 6, 1689–1698. [Google Scholar] [CrossRef]
- Lin, W.; Liu, Z.; Kampf, N.; Klein, J. The role of hyaluronic acid in cartilage boundary lubrication. Cells 2020, 9, 1606. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, Z.; Yang, H.; Zhong, H.; Peng, W.; Xie, R. Recent advances in understanding the role of cartilage lubrication in osteoarthritis. Molecules 2021, 26, 6122. [Google Scholar] [CrossRef] [PubMed]
- Banquy, X.; Lee, D.W.; Das, S.; Hogan, J.; Israelachvili, J.N. Shear-induced aggregation of mammalian synovial fluid components under boundary lubrication conditions. Adv. Funct. Mater. 2014, 24, 3152–3161. [Google Scholar] [CrossRef]
- Bu, Y.; Pandit, A. Cohesion mechanisms for bioadhesives. Bioact. Mater. 2022, 13, 105–118. [Google Scholar] [CrossRef]
- Newton, M.D.; Osborne, J.; Gawronski, K.; Baker, K.C.; Maerz, T. Articular cartilage surface roughness as an imaging-based morphological indicator of osteoarthritis: A preliminary investigation of osteoarthritis initiative subjects. J. Orthop. Res. 2017, 35, 2755–2764. [Google Scholar] [CrossRef]
- Rinaudo, M.; Lardy, B.; Grange, L.; Conrozier, T. Effect of mannitol on hyaluronic acid stability in two in vitro models of oxidative stress. Polymers 2014, 6, 1948–1957. [Google Scholar] [CrossRef]
- Faivre, J.; Gallet, M.; Tremblais, E.; Trévidic, P.; Bourdon, F. Advanced concepts in rheology for the evaluation of hyaluronic acid-based soft tissue fillers. Dermatol. Surg. 2021, 47, e159–e167. [Google Scholar] [CrossRef]
- Flégeau, K.; Jing, J.; Brusini, R.; Gallet, M.; Moreno, C.; Walker, L.; Bourdon, F.; Faivre, J. Multidose hyaluronidase administration as an optimal procedure to degrade resilient hyaluronic acid soft tissue fillers. Molecules 2023, 28, 1003. [Google Scholar] [CrossRef]
- Porcello, A.; Gonzalez-Fernandez, P.; Jordan, O.; Allémann, E. Nanoforming hyaluronan-based thermoresponsive hydrogels: Optimized and tunable functionality in osteoarthritis management. Pharmaceutics 2022, 14, 659. [Google Scholar] [CrossRef]
- Levin, J.; Momin, S.B. How much do we really know about our favorite cosmeceutical ingredients? J. Clin. Aesthet. Dermatol. 2010, 3, 22–41. [Google Scholar]
- Zhen, A.X.; Piao, M.J.; Kang, K.A.; Madushan Fernando, P.D.S.; Kang, H.K.; Koh, Y.S.; Yi, J.M.; Hyun, J.W. Niacinamide protects skin cells from oxidative stress induced by particulate matter. Biomol. Ther. 2019, 27, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Kamat, J.P.; Devasagayam, T.P.A. Nicotinamide (vitamin B3) as an effective antioxidant against oxidative damage in rat brain mitochondria. Redox Rep. 1999, 4, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Huma, S.; Khan, H.M.S.; Ijaz, S.; Sarfraz, M.; Zaka, H.S.; Ahmad, A. Development of niacinamide/ferulic acid-loaded multiple emulsion and its in vitro/in vivo investigation as a cosmeceutical product. Biomed. Res. Int. 2022, 2022, 1725053. [Google Scholar] [CrossRef] [PubMed]
- Janaszewska, A.; Bartosz, G. Assay of total antioxidant capacity: Comparison of four methods as applied to human blood plasma. Scand. J. Clin. Lab. Investig. 2002, 62, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Philips, N.; Chalensouk-Khaosaat, J.; Gonzalez, S. Simulation of the elastin and fibrillin in non-irradiated or UVA radiated fibroblasts, and direct inhibition of elastase or matrix metalloptoteinases activity by nicotinamide or its derivatives. J. Cosmet. Sci. 2018, 69, 47–56. [Google Scholar]
- Laurent, A.; Porcello, A.; Jeannerat, A.; Peneveyre, C.; Coeur, A.; Abdel-Sayed, P.; Scaletta, C.; Michetti, M.; de Buys Roessingh, A.; Jordan, O.; et al. Lyophilized progenitor tenocyte extracts: Sterilizable cytotherapeutic derivatives with antioxidant properties and hyaluronan hydrogel functionalization effects. Antioxidants 2023, 12, 163. [Google Scholar] [CrossRef]
- Bovone, G.; Dudaryeva, O.Y.; Marco-Dufort, B.; Tibbitt, M.W. Engineering hydrogel adhesion for biomedical applications via chemical design of the junction. ACS Biomater. Sci. Eng. 2021, 7, 4048–4076. [Google Scholar] [CrossRef]
- Nejati, S.; Mongeau, L. Injectable, pore-forming, self-healing, and adhesive hyaluronan hydrogels for soft tissue engineering applications. Sci. Rep. 2023, 13, 14303. [Google Scholar] [CrossRef]
- Cook, S.G.; Bonassar, S.J. Interaction with cartilage increases the viscosity of hyaluronic acid solutions. ACS Biomater. Sci. Eng. 2020, 6, 2787–2795. [Google Scholar] [CrossRef]
- Kaderli, S.; Boulocher, C.; Pillet, E.; Watrelot-Virieux, D.; Rougemont, A.L.; Roger, T.; Viguier, E.; Gurny, R.; Scapozza, L.; Jordan, O. A novel biocompatible hyaluronic acid-chitosan hybrid hydrogel for osteoarthrosis therapy. Int. J. Pharm. 2015, 483, 158–168. [Google Scholar] [CrossRef]
- Gou, S.; Porcello, A.; Allémann, E.; Salomon, D.; Micheels, P.; Jordan, O.; Kalia, Y.N. Injectable hyaluronan-based thermoresponsive hydrogels for dermatological applications. Pharmaceutics 2023, 15, 1708. [Google Scholar] [CrossRef]
- Prikhnenko, S. Polycomponent mesotherapy formulations for the treatment of skin aging and improvement of skin quality. Clin. Cosmet. Investig. Dermatol. 2015, 8, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Iranmanesh, B.; Khalili, M.; Mohammadi, S.; Amiri, R.; Aflatoonian, M. Employing hyaluronic acid-based mesotherapy for facial rejuvenation. J. Cos. Derm. 2022, 21, 6605–6618. [Google Scholar] [CrossRef] [PubMed]
- Zelenetskii, A.N.; Uspenskii, S.; Zaboronok, A.; Cherkaev, G.; Shchegolihin, A.; Mathis, B.J.; Selyanin, M.; Yamamoto, T.; Matsumura, A. Polycomplexes of hyaluronic acid and borates in a solid state and solution: Synthesis, characterization and perspectives of application in boron neutron capture therapy. Polymers 2018, 10, 181. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Investigated Commercial Hydrogel Products | |||
|---|---|---|---|---|
| Ostenil | Ostenil Plus | Synvisc | Innoryos | |
| Manufacturer | TRB Chemedica; Geneva, Switzerland | TRB Chemedica; Geneva, Switzerland | Sanofi Genzyme; Cambridge, MA, USA | Albomed; Schwarzenbruck, Germany |
| Market Launch Year | 1998 | 2009 | 1997 | 2022 |
| Regulatory Classification | Class III device | Class III device | Class III device | Class III device |
| Specified Indications | Pain and mobility reduction in degenerative and traumatic affections of the knee and other synovial articulations | Pain and mobility reduction in degenerative and traumatic affections of the knee and other synovial articulations | Pain in OA of the knee in patients who have failed to respond adequately to conservative nonpharmacologic therapy and simple analgesics | Pain and decreased articular mobility associated with degenerative lesions of the knee and other synovial joints, including OA |
| HA Concentration/Polymer Type | 10 mg/mL; Linear HA | 20 mg/mL; Linear HA | 8 mg/mL; Chemically cross-linked HA | 22 mg/mL; Linear HA |
| HA Molecular Weight (Molecular Weight Class) | 1.6 MDa (Intermediate) | 1.6 MDa (Intermediate) | 6 MDa (Hylan A; High) | 1.2–2.2 MDa (Intermediate) |
| HA Sourcing | Biotechnology | Biotechnology | Avian | Biotechnology |
| Composition | 1% HA; injectable buffer solution | 2% HA; 1% mannitol; injectable buffer solution | 0.8% Hylan G-F 20 1; injectable buffer solution | 2.2% HA; 1.5% niacinamide; injectable buffer solution |
| Quantities/Additives | NA | Mannitol | NA | Niacinamide 2 |
| Volume/Unit | 2.0 mL | 2.0 mL | 2.0 mL | 2.0 mL |
| Administration Regimen | 3–5 injections; 1 week interval | 1–3 injections; 1 week interval | 3 injections; 1 week interval | 3 injections; 1 week interval |
| Functional Parameter | Overall Assessment/Comparative Operator Gradings 1 | |||
|---|---|---|---|---|
| Ostenil | Ostenil Plus | Synvisc | Innoryos | |
| Viscoelasticity | + | ++ | +++ | +++ |
| Lubricity | + | + | +++ | +++ |
| Bio-Adhesion | + | ++ | ++ | +++ |
| Stability | – | ++ | ++ | +++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porcello, A.; Hadjab, F.; Ajouaou, M.; Philippe, V.; Martin, R.; Abdel-Sayed, P.; Hirt-Burri, N.; Scaletta, C.; Raffoul, W.; Applegate, L.A.; et al. Ex Vivo Functional Benchmarking of Hyaluronan-Based Osteoarthritis Viscosupplement Products: Comprehensive Assessment of Rheological, Lubricative, Adhesive, and Stability Attributes. Gels 2023, 9, 808. https://doi.org/10.3390/gels9100808
Porcello A, Hadjab F, Ajouaou M, Philippe V, Martin R, Abdel-Sayed P, Hirt-Burri N, Scaletta C, Raffoul W, Applegate LA, et al. Ex Vivo Functional Benchmarking of Hyaluronan-Based Osteoarthritis Viscosupplement Products: Comprehensive Assessment of Rheological, Lubricative, Adhesive, and Stability Attributes. Gels. 2023; 9(10):808. https://doi.org/10.3390/gels9100808
Chicago/Turabian StylePorcello, Alexandre, Farid Hadjab, Maryam Ajouaou, Virginie Philippe, Robin Martin, Philippe Abdel-Sayed, Nathalie Hirt-Burri, Corinne Scaletta, Wassim Raffoul, Lee Ann Applegate, and et al. 2023. "Ex Vivo Functional Benchmarking of Hyaluronan-Based Osteoarthritis Viscosupplement Products: Comprehensive Assessment of Rheological, Lubricative, Adhesive, and Stability Attributes" Gels 9, no. 10: 808. https://doi.org/10.3390/gels9100808
APA StylePorcello, A., Hadjab, F., Ajouaou, M., Philippe, V., Martin, R., Abdel-Sayed, P., Hirt-Burri, N., Scaletta, C., Raffoul, W., Applegate, L. A., Allémann, E., Jordan, O., & Laurent, A. (2023). Ex Vivo Functional Benchmarking of Hyaluronan-Based Osteoarthritis Viscosupplement Products: Comprehensive Assessment of Rheological, Lubricative, Adhesive, and Stability Attributes. Gels, 9(10), 808. https://doi.org/10.3390/gels9100808










