Degradation Kinetics of Disulfide Cross-Linked Microgels: Real-Time Monitoring by Confocal Microscopy
Abstract
:1. Introduction
2. Results and Discussion
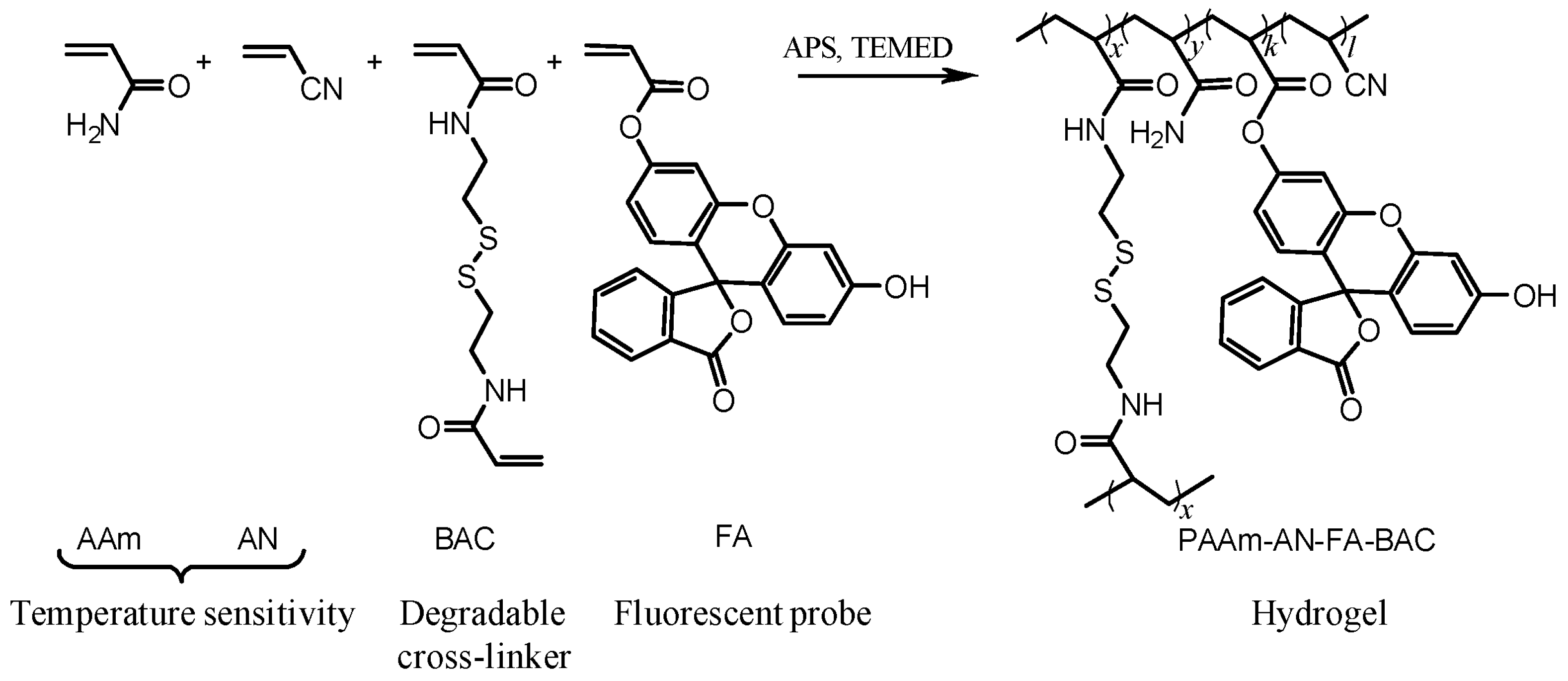
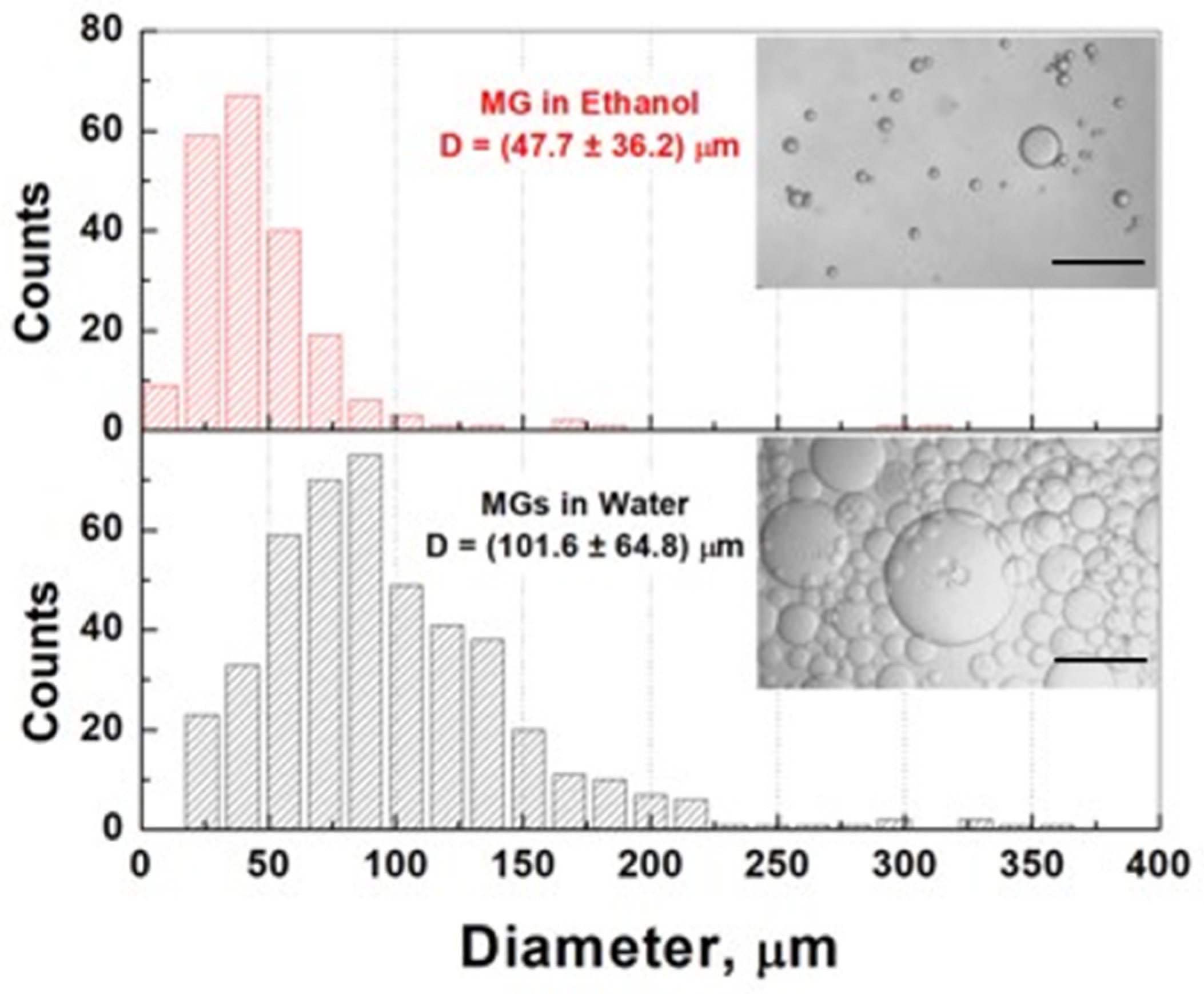
2.1. Properties of Microgels
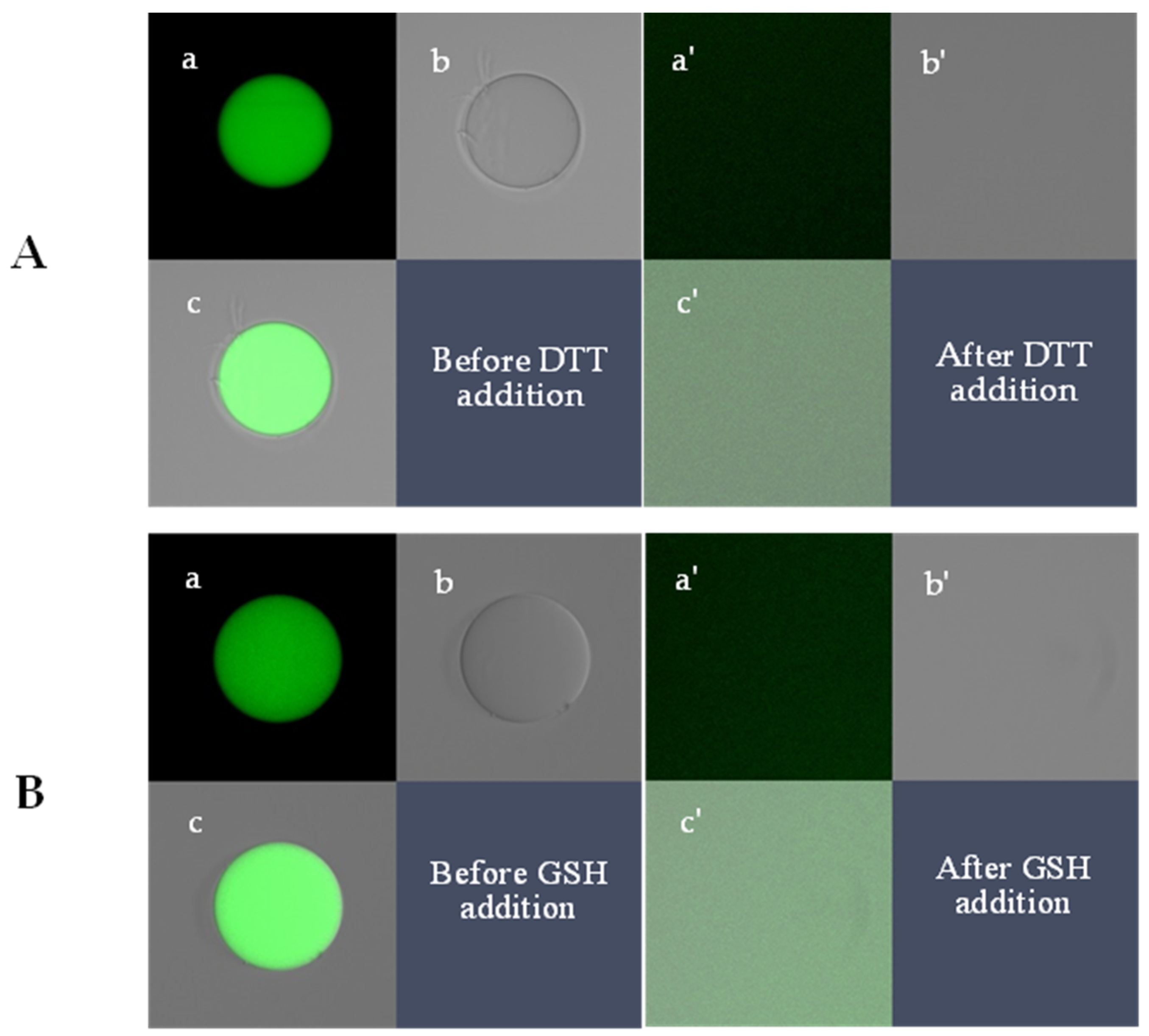
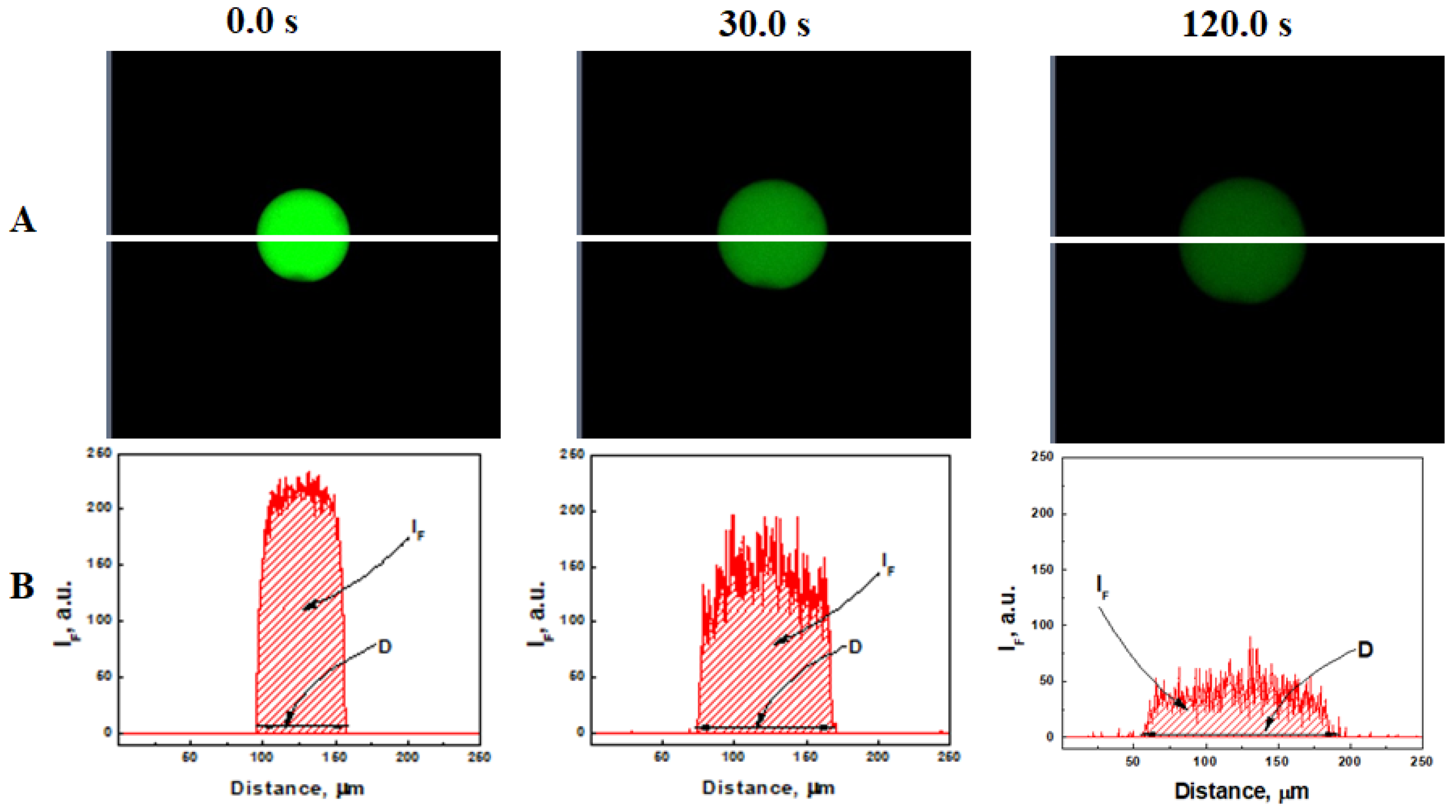
2.2. Confocal Imaging and Quantification of Microgel Size (D) and Fluorescence Intensity (IF)
2.2.1. Quantitative Analysis of Microgel Size Change in 2.3 mM GSH
2.2.2. Quantitative Analysis of Fluorescence Intensity in 2.3 mM GSH
2.3. Swelling Kinetic Parameters (Volume Change) of the Microgel at Different Concentrations of Glutathione
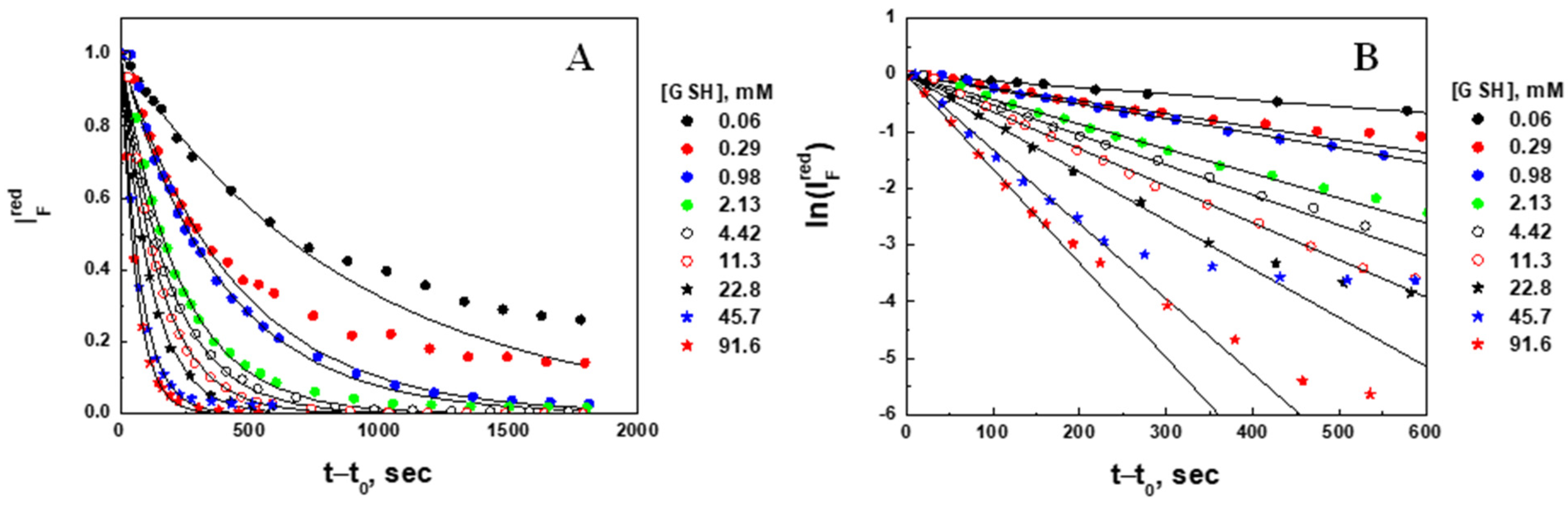
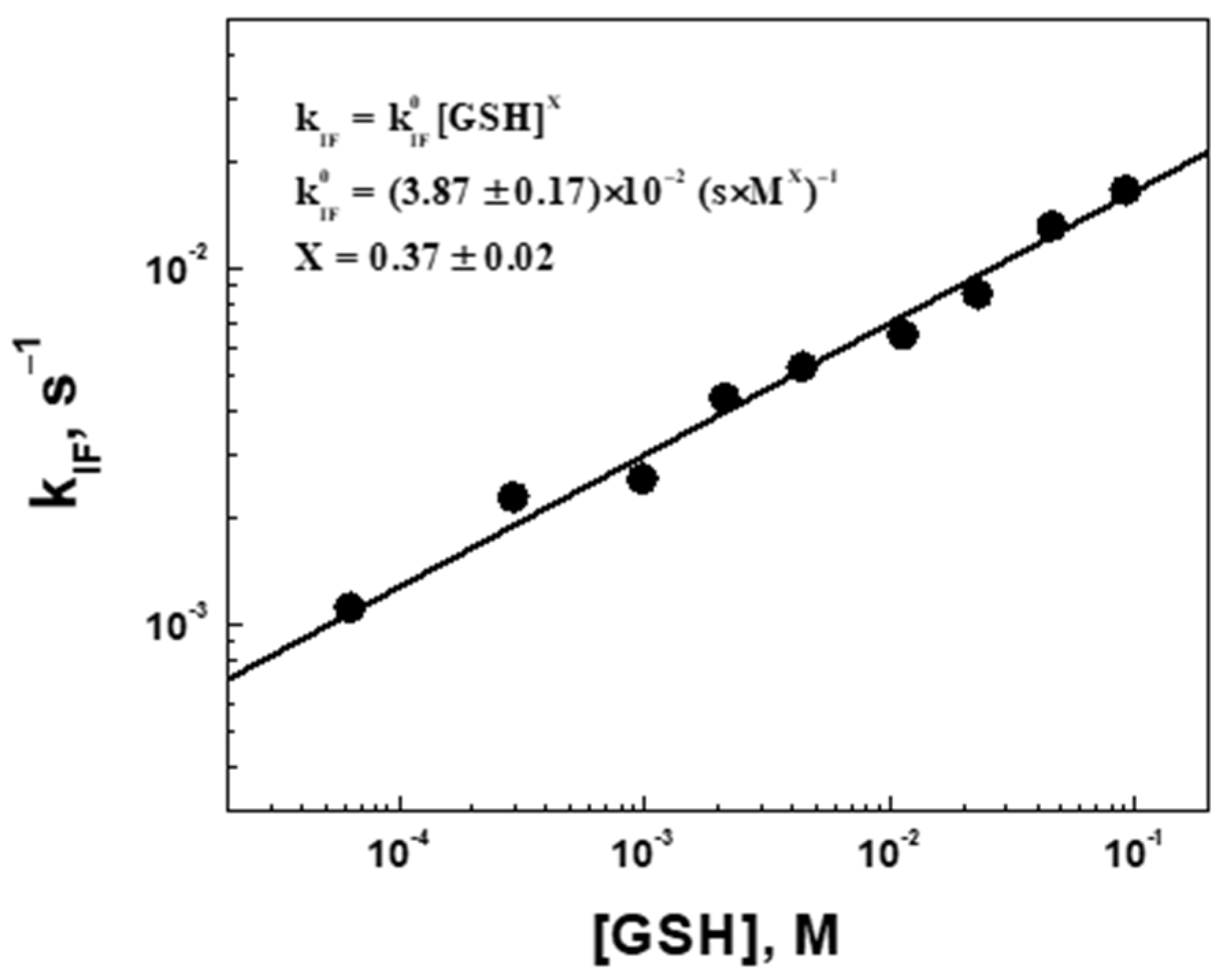
2.4. Degradation Kinetic Parameters (Fluorescence Intensity Decrease) of Microgel at Different Concentrations of Glutathione
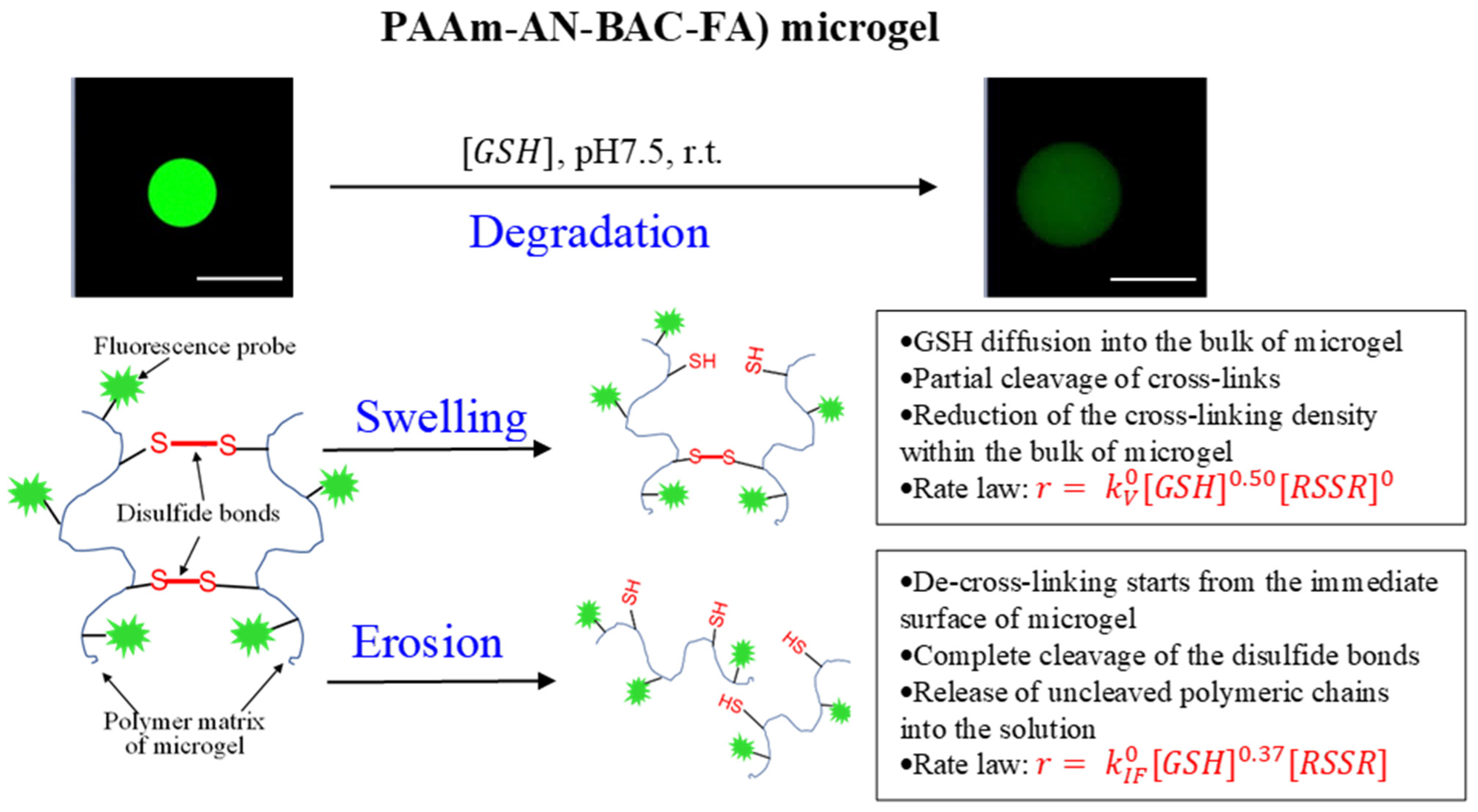
2.5. Mechanism of Degradation (Swelling and Erosion) of PAAm-AN-BAC-FA Microgel in PBS Buffer (pH 7.5) in the Presense of Glutathione as a Reducing Agent
3. Conclusions
4. Materials and Methods
4.1. Chemicals and Materials
4.2. Microgel Synthesis
4.3. Instrumentation
4.3.1. Optical Microscopy (OM) Imaging of Microgels
4.3.2. Confocal Laser Scanning Microscopy (CLSM) Imaging of Microgels
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kazakov, S. Liposome-nanogel structures for future pharmaceutical applications: An updated review. Curr. Pharm. Des. 2016, 10, 1391–1413. [Google Scholar] [CrossRef] [PubMed]
- Kazakov, S. Lipobeads. In Liposomes; Catala, A., Ed.; InTech: Rijeka, Croatia, 2017; Chapter 3; pp. 49–93. ISBN 978-953-51-5467-9. [Google Scholar]
- Rahni, S.; Kazakov, S. Hydrogel micro-/nanosphere coated by a lipid bilayer: Preparation and microscopic probing. Gels 2017, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Aldosari, B.; Alfagih, I.; Almurshedi, A. Lipid nanoparticles as delivery systems for RNA-based vaccines. Pharmaceutics 2021, 13, 206. [Google Scholar] [CrossRef] [PubMed]
- Granot-Matok, Y.; Ezra, A.; Ramishetti, S.; Sharma, P.; Somu Naidu, G.; Benhar, I.; Peer, D. Lipid nanoparticles-loaded with toxin mRNA represents a new strategy for the treatment of solid tumors. Theranostics 2023, 13, 3497–3508. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Korsmeyer, R.W. Dynamically swelling hydrogels in controlled release applications. In Hydrogels in Medicine and Pharmacy; Peppas, N.A., Ed.; CRC Press: Boca Raton, FL, USA, 1987. [Google Scholar]
- Harland, R.S.; Prud’homme, R.K. Polyelectrolyte Gels: Properties, Preparation, and Applications; American Chemical Society: Washington, DC, USA, 1992. [Google Scholar]
- Shamay, Y.; Adar, L.; Ashkenasy, G.; Davis, A. Light induced drug delivery into cancer cells. Biomaterials 2011, 32, 1377–1386. [Google Scholar] [CrossRef]
- Hribar, K.C.; Lee, M.H.; Lee, D.; Burdick, J.A. Enhanced release of small molecules from near-infrared light responsive polymer-nanorod composites. ACS Nano 2011, 5, 2948–2956. [Google Scholar] [CrossRef]
- Mamada, A.; Tanaka, T.; Kungwatchakun, D.; Irie, M. Photoinduced phase transition of gels. Macromolecules 1990, 23, 1517–1519. [Google Scholar] [CrossRef]
- Suzuki, A.; Tanaka, T. Phase transitions in polymer gels induced by visible light. Nature 1990, 346, 345–347. [Google Scholar] [CrossRef]
- Tanaka, T.; Nishio, I.; Sun, S.T.; Nishio, S.U. Collapse of gels in an electric field. Science 1982, 218, 467–469. [Google Scholar] [CrossRef]
- Plunkett, K.; Kraft, M.; Yu, Q.; Moore, J. Swelling kinetics of disulfide cross-linked microgels. Macromolecules 2003, 36, 3960–3966. [Google Scholar] [CrossRef]
- Bromberg, L.; Temchenko, M.; Alakhov, V.; Hatton, T. Kinetics of swelling of polyether-modified poly(acrylic acid) microgels with permanent and degradable cross-links. Langmuir 2005, 21, 1590–1598. [Google Scholar] [CrossRef] [PubMed]
- Engineer, C.; Parikh, J.; Raval, A. Review on hydrolytic degradation behavior of biodegradable polymers from controlled drug delivery systems. Trends Biomater. Artif. Organs 2011, 25, 79–85. [Google Scholar]
- Artzi, N.; Oliva, N.; Puron, C.; Shitreet, S.; Artzi, S.; bon Ramos, A.; Groothuis, A.; Sahagian, G.; Edelman, E.R. In vivo and in vitro tracking of erosion in biodegradable materials using non-invasive fluorescence imaging. Nat. Mater. 2012, 10, 704–709. [Google Scholar]
- Gyarmati, B.; Nemethy, A.; Szilagyi, A. Reversible disulphide formation in polymer networks: A versatile functional group from synthesis to applications. Eur. Polym. J. 2013, 49, 1268–1286. [Google Scholar] [CrossRef]
- Pedre, B.; Barayeu, U.; Ezeriņa, D.; Dick, T. The mechanism of action of N-acetylcysteine (NAC): The emerging role of H2S and sulfane sulfur species. Pharmacol. Ther. 2021, 228, 107916. [Google Scholar] [CrossRef] [PubMed]
- Zinatullina, K.; Orekhova, A.; Kasaikina, O.; Khrameeva, K.; Berezin, M.; Rusina, I. N-Acetylcysteine is an effective analog of glutathione in reactions with reactive oxygen species. Russ. Chem. Bull. 2021, 70, 1934–1938. [Google Scholar] [CrossRef]
- Yi, M.; Khosla, C. Thiol-disufide exchange reactions in the mammalian extracellular environment. Annu. Rev. Chem. Biomol. Eng. 2016, 7, 197–222. [Google Scholar] [CrossRef]
- Mittler, R. ROS Are Good. Cell Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D. Activation of apoptosis signalling pathways by reactive oxygen species. BBA-Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Schumacker, P.T. Reactive oxygen species in cancer cells: Live by the sword, die by the sword. Cancer Cell 2006, 10, 241–252. [Google Scholar] [CrossRef]
- Traverso, N.; Ricciarelli, R.; Nitti, M.; Pronzato, M.A.; Marinari, U.M.; Domenicotti, C. Role of glutathione in cancer progression and chemoresistance. Oxidative Med. Cell. Longev. 2013, 2013, 972913. [Google Scholar] [CrossRef] [PubMed]
- Ortega, A.; Mena, S.; Estela, J. Glutathione in cancer cell death. Cancers 2011, 3, 1285–1310. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative stress in cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef] [PubMed]
- Gamcsik, M.; Ksibhatla, M.; Teeter, S.; Colvin, O. Glutathione levels in human tumors. Biomarkers 2012, 17, 671–691. [Google Scholar] [CrossRef]
- Cook, J.; Pass, H.; Iype, S.; Friedman, N.; DeGraff, W.; Russo, A.; Mitchell, J. Cellular glutathione and thiol measurements from surgically resected human lung tumor and normal lung tissue. Cancer Res. 1991, 51, 4287–4294. [Google Scholar]
- Kudo, H.; Mio, T.; Kokunai, T.; Tamaki, N.; Sumino, K.; Matsumoto, S. Quantitative analysis of glutathione in human brain tumors. J. Neurosurg. 1990, 72, 610–615. [Google Scholar] [CrossRef]
- Opstad, K.; Provencher, S.; Bell, B.; Griffiths, J.; Howe, F. Detection of elevated glutathione in meningiomas by quantitative in vivo 1H MRS. Magn. Reson. Med. 2003, 49, 632–637. [Google Scholar] [CrossRef]
- Monteiro, P.F.; Travanut, A.; Conte, C.; Alexander, C. Reduction-responsive polymers for drug delivery in cancer therapy-Is there anything new to discover? Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1678. [Google Scholar] [CrossRef]
- Rosen, H.; Abribat, T. The rise and rise of drug delivery. Nat. Rev. Drug Discov. 2005, 4, 381–385. [Google Scholar] [CrossRef]
- Seuring, J.; Agarwal, S. First example of a universal and cost-effective approach: Polymers with tunable upper critical solution temperature in water and electrolyte solution. Macromolecules 2012, 45, 3910–3918. [Google Scholar] [CrossRef]
- Alpaslan, D.; Aktas, N.; Yilmaz, S.; Sahiner, N. The preparation of p(acrylonitrile-co-acrylamide) hydrogels for uranyl ion recovery from aqueous environments. Hacet. J. Biol. Chem. 2014, 42, 89–97. [Google Scholar] [CrossRef]
- Scarpa, M.; Momo, F.; Viglino, P.; Vianello, F.; Rigo, A. Activated oxygen species in the oxidation of glutathione A kinetic study. Biophys. Chem. 1996, 60, 53–61. [Google Scholar] [CrossRef]
- Abedinzadehm, Z.; Gardes-Albert, M.; Ferradini, C. Kinetic study of the oxidation mechanism of glutathione by hydrogen peroxide in neutral aqueous medium. J. Chem. 1989, 67, 1247–1255. [Google Scholar]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef] [PubMed]
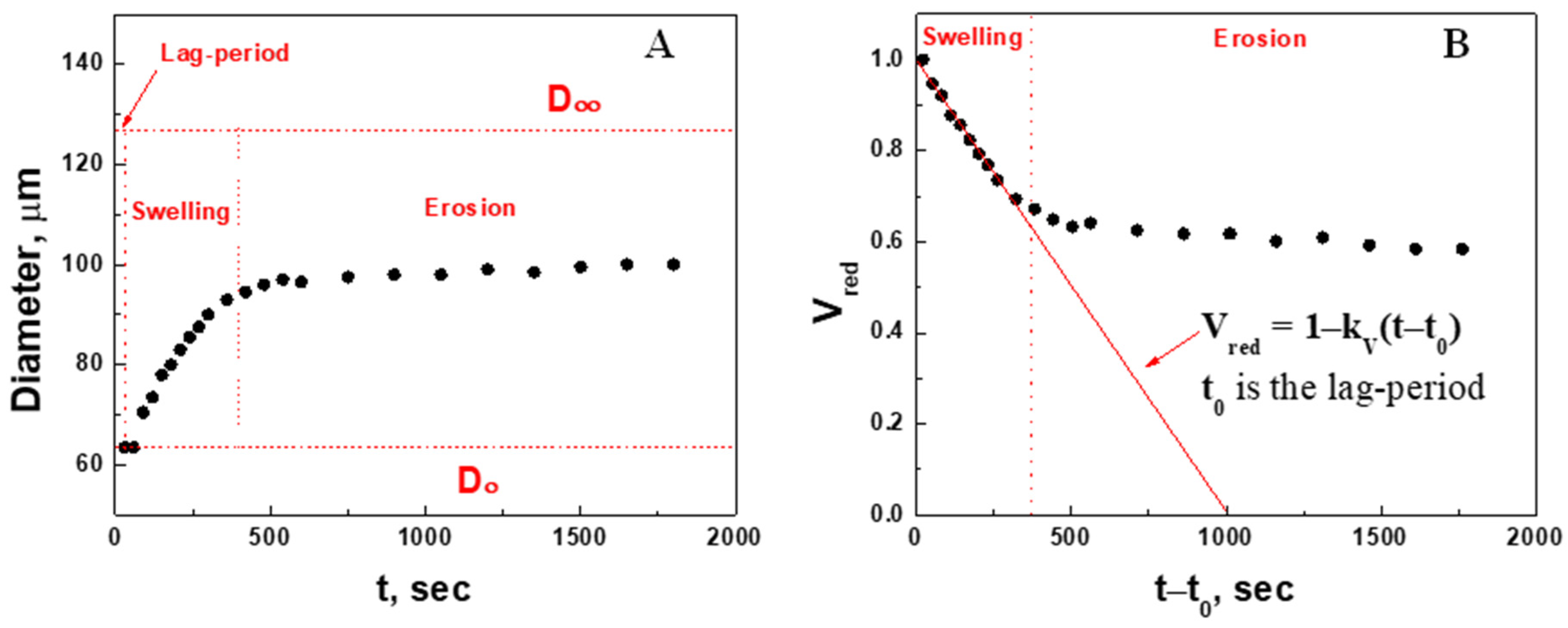
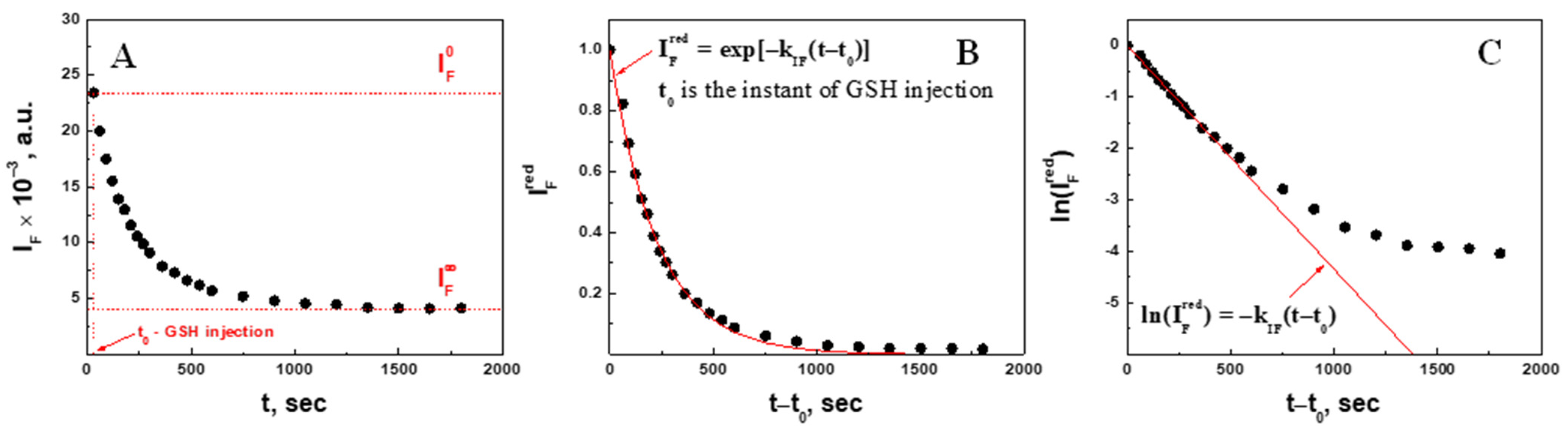
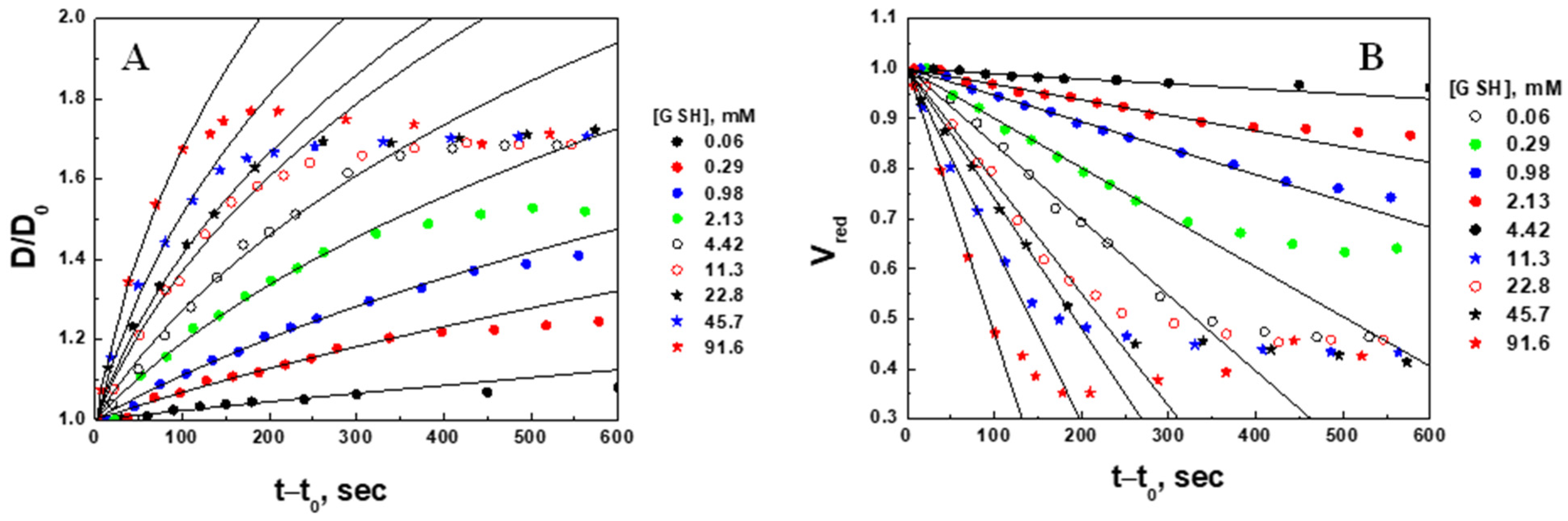
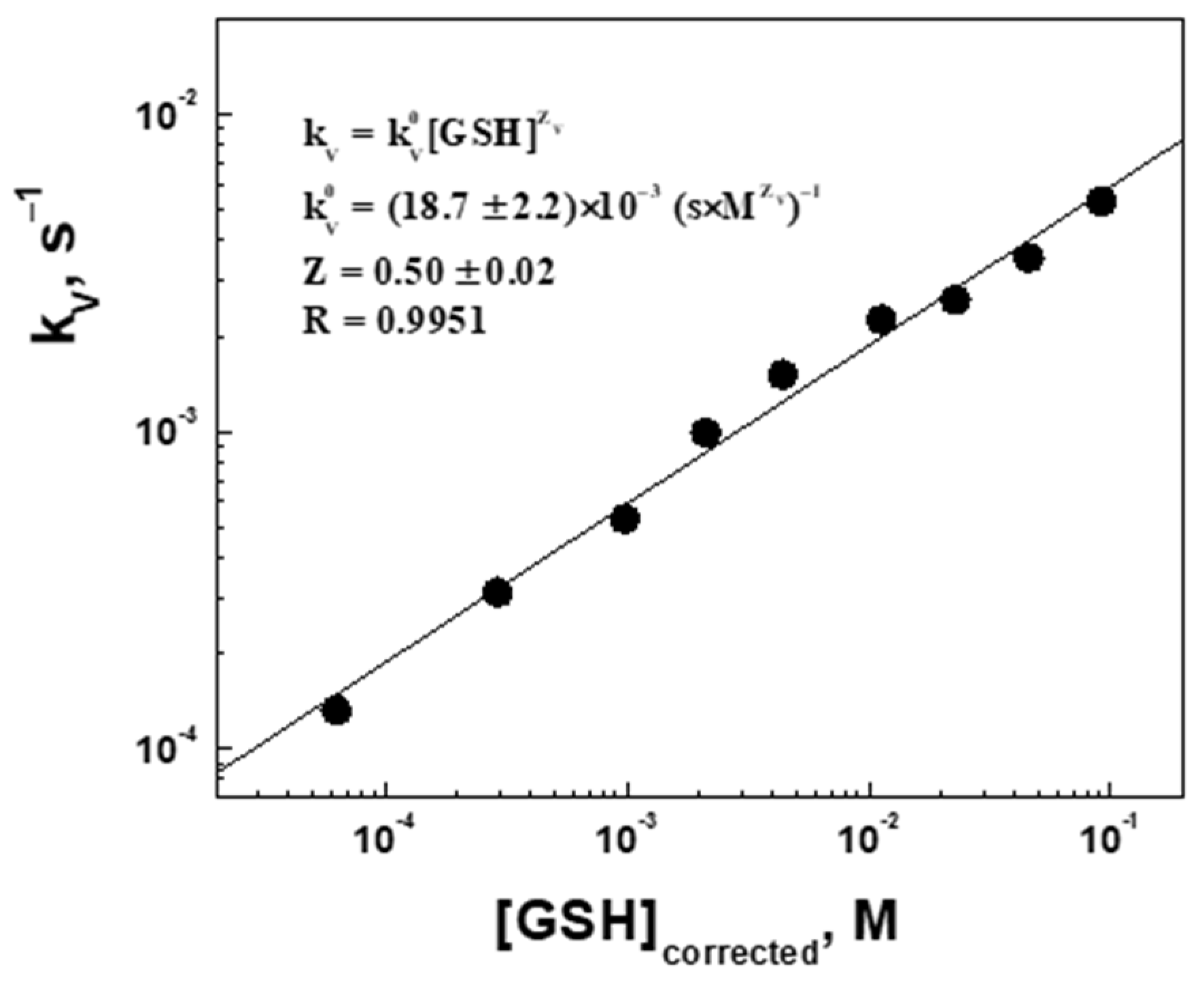
| , mM Injected | , mM Corrected | , µm | , µm | Swelling Ratio, | Apparent Swelling Rate Constant, , × 10−4 s−1 | Swelling Characteristic Time, , min | Regression Correlation Coefficient, R |
|---|---|---|---|---|---|---|---|
| 0.230 | 0.063 | 83.2 | 166 | 7.9 | 1.32 ± 0.11 | 126 ± 11 | 0.9868 |
| 0.459 | 0.293 | 98.1 | 197 | 8.1 | 3.11 ± 0.12 | 53.6 ± 2.1 | 0.9923 |
| 1.15 | 0.984 | 91.5 | 183 | 8.0 | 5.29 ± 0.14 | 31.5 ± 0.8 | 0.9965 |
| 2.30 | 2.13 | 63.5 | 126 | 7.8 | 9.91 ± 0.31 | 16.8 ± 0.5 | 0.9957 |
| 4.59 | 4.42 | 115.8 | 232 | 8.0 | 15.1 ± 0.5 | 11.0 ± 0.4 | 0.9958 |
| 11.5 | 11.3 | 111.7 | 224 | 8.1 | 22.5 ± 1.2 | 7.41 ± 0.40 | 0.9914 |
| 23.0 | 22.8 | 100.1 | 199 | 7.9 | 26.0 ± 0.6 | 6.41 ± 0.15 | 0.9977 |
| 45.9 | 45.7 | 74.6 | 148 | 7.8 | 35.3 ± 0.9 | 4.73 ± 0.12 | 0.9954 |
| 91.8 | 91.6 | 80.0 | 160 | 8.0 | 53.0 ± 1.0 | 3.14 ± 0.06 | 0.9996 |
| , mM Injected | , mM Corrected | , × 10−3 a.u. | , × 10−3 a.u. | Degradation Ratio, | Apparent Degradation Rate Constant, × 10−4 s−1 | Characteristic Time, , min | Regression Correlation Coefficient, R |
|---|---|---|---|---|---|---|---|
| 0.230 | 0.063 | 31.3 | 16.0 | 0.49 | 11.2 ± 0.3 | 14.9 ± 0.4 | 0.9878 |
| 0.459 | 0.293 | 37.9 | 12.0 | 0.68 | 22.9 ± 0.7 | 7.28 ± 0.22 | 0.9968 |
| 1.15 | 0.984 | 34.3 | 7.3 | 0.79 | 25.8 ± 0.4 | 6.46 ± 0.10 | 0.9989 |
| 2.30 | 2.13 | 23.4 | 4.0 | 0.83 | 43.5 ± 1.1 | 3.83 ± 0.10 | 0.9977 |
| 4.59 | 4.42 | 44.5 | 6.9 | 0.84 | 53.0 ± 0.8 | 3.14 ± 0.05 | 0.9967 |
| 11.5 | 11.3 | 41.2 | 7.4 | 0.82 | 65.3 ± 0.9 | 2.55 ± 0.04 | 0.9990 |
| 23.0 | 22.8 | 13.5 | 1.7 | 0.87 | 85.1 ± 1.6 | 1.96 ± 0.04 | 0.9990 |
| 45.9 | 45.7 | 32.8 | 3.5 | 0.89 | 131.7 ± 4.8 | 1.27 ± 0.05 | 0.9960 |
| 91.8 | 91.6 | 28.7 | 2.0 | 0.93 | 166.2 ± 3.1 | 1.00 ± 0.02 | 0.9993 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mercer, I.G.; Italiano, A.N.; Gazaryan, I.G.; Steiner, A.B.; Kazakov, S.V. Degradation Kinetics of Disulfide Cross-Linked Microgels: Real-Time Monitoring by Confocal Microscopy. Gels 2023, 9, 782. https://doi.org/10.3390/gels9100782
Mercer IG, Italiano AN, Gazaryan IG, Steiner AB, Kazakov SV. Degradation Kinetics of Disulfide Cross-Linked Microgels: Real-Time Monitoring by Confocal Microscopy. Gels. 2023; 9(10):782. https://doi.org/10.3390/gels9100782
Chicago/Turabian StyleMercer, Iris G., Angelina N. Italiano, Irina G. Gazaryan, Aaron B. Steiner, and Sergey V. Kazakov. 2023. "Degradation Kinetics of Disulfide Cross-Linked Microgels: Real-Time Monitoring by Confocal Microscopy" Gels 9, no. 10: 782. https://doi.org/10.3390/gels9100782
APA StyleMercer, I. G., Italiano, A. N., Gazaryan, I. G., Steiner, A. B., & Kazakov, S. V. (2023). Degradation Kinetics of Disulfide Cross-Linked Microgels: Real-Time Monitoring by Confocal Microscopy. Gels, 9(10), 782. https://doi.org/10.3390/gels9100782






