Calcium-Polyphosphate Submicroparticles (CaPP) Improvement Effect of the Experimental Bleaching Gels’ Chemical and Cellular-Viability Properties
Abstract
1. Introduction
2. Results
2.1. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-ray Analysis (EDX)
2.2. Transmission Electron Microscopy (TEM) and Dynamic Light Scattering (DLS)
2.3. X-ray Diffraction (XRD)
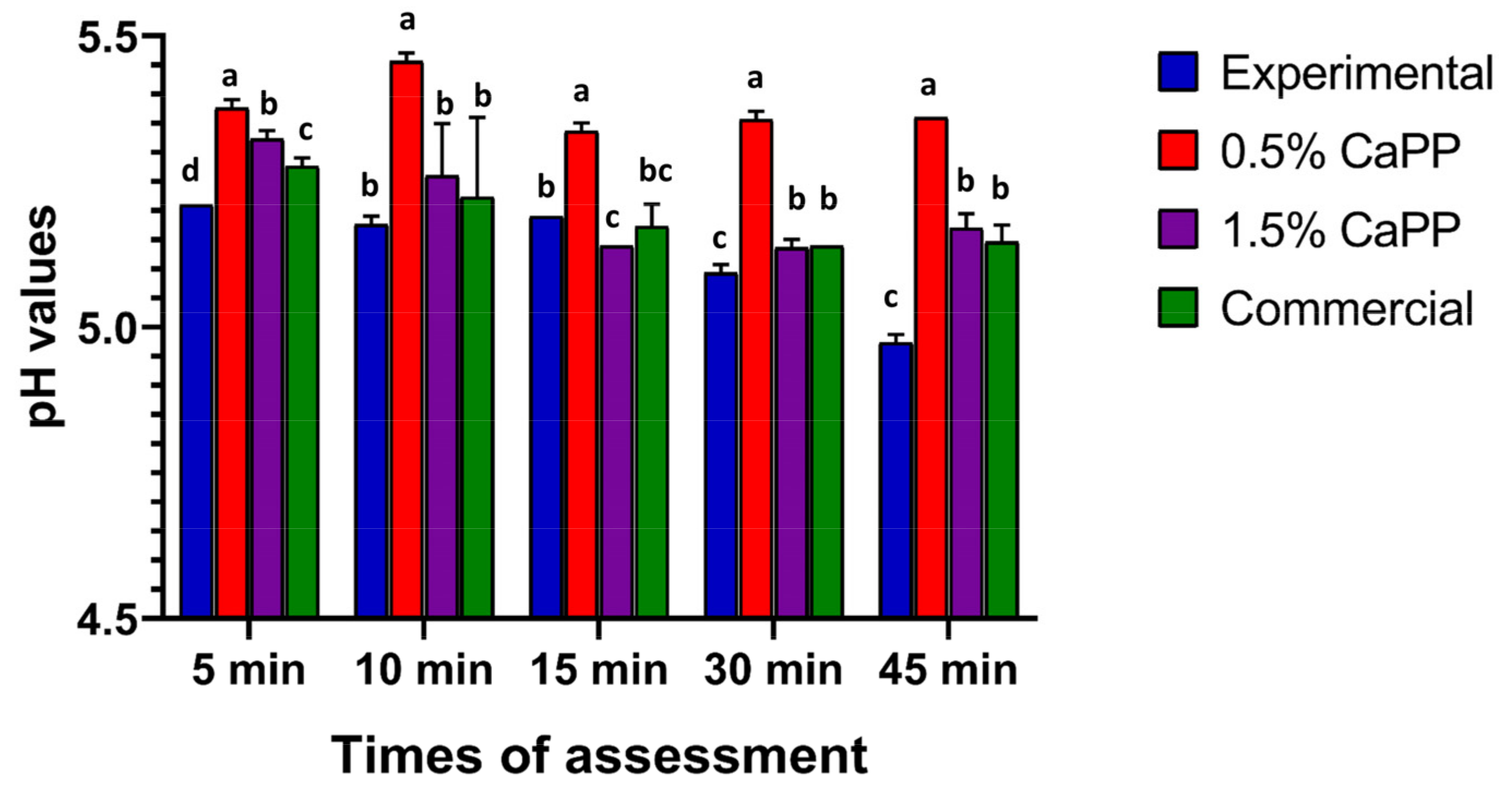
2.4. Visual and pH Evaluation Results
2.5. Hydrogen Peroxide Concentration of the HP-CaPP—Titrimetric Analysis (Iodometric)
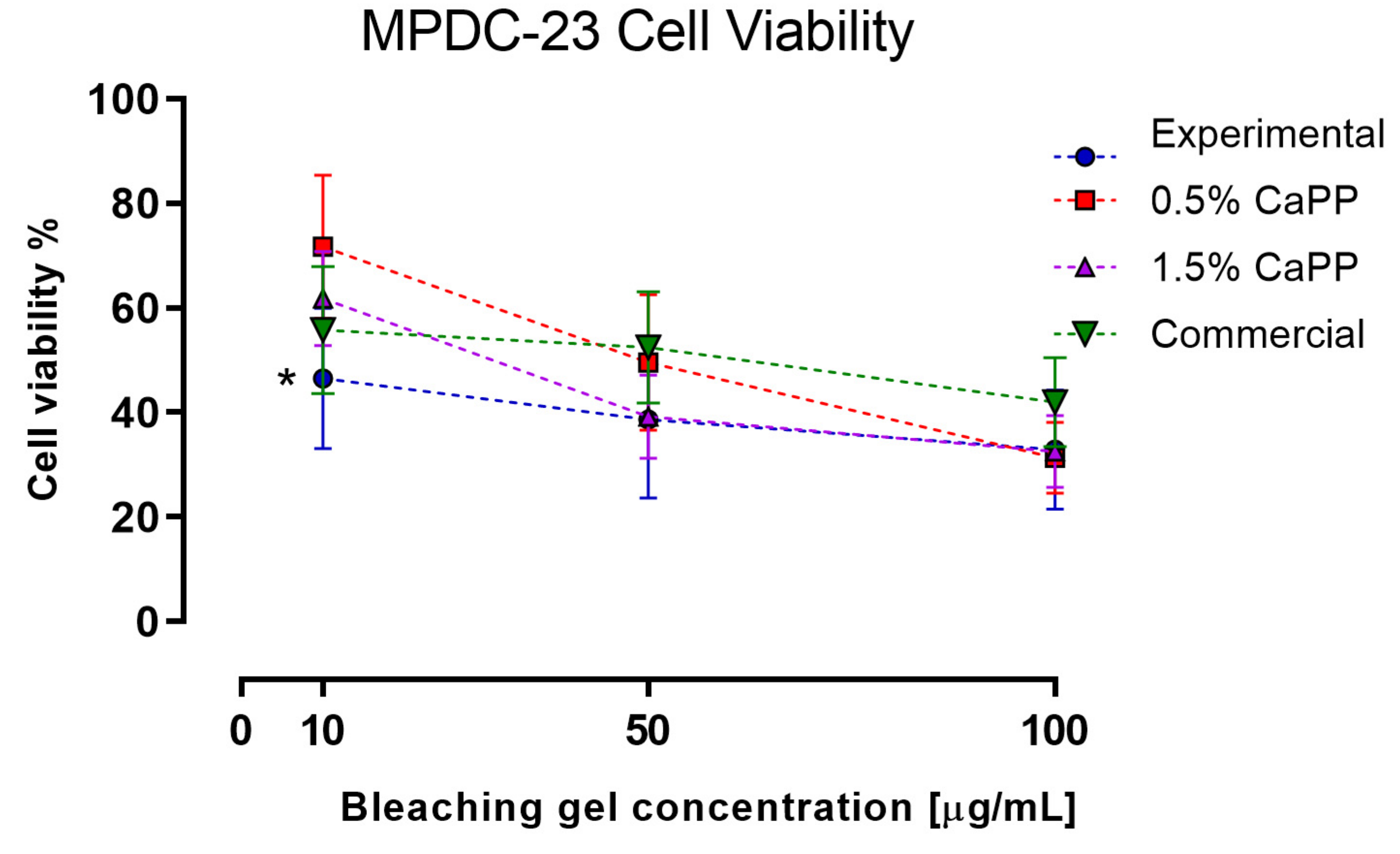
2.6. Odontoblasts-like Cells (MDPC-23) Viability after Exposure to the HP-CaPP Bleaching Gel
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Synthesis of Calcium-Polyphosphate Particles (CaPP) and Characterization
5.1.1. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-ray Analysis (EDX)
5.1.2. Transmission Electron Microscopy (TEM) and Dynamic Light Scattering (DLS)
5.1.3. X-ray Diffraction (XRD)
5.2. Experimental Bleaching Gel Containing Calcium-Polyphosphate Particles (HP-CaPP)
5.2.1. Evaluation of pH
5.2.2. Hydrogen Peroxide Concentration of the HP-CaPP—Titrimetric Analysis (Iodometric)
5.2.3. Odontoblasts-like Cells (MDPC-23) Viability in the HP-CaPP Bleaching Gel
5.3. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barbosa, J.G.; Benetti, F.; Gallinari, M.D.O.; Carminatti, M.; da Silva, A.B.D.; Lopes, I.N.I.; Briso, A.L.F.; Cintra, L.T.A. Bleaching gel mixed with MI Paste Plus reduces penetration of H2O2 and damage to pulp tissue and maintains bleaching effectiveness. Clin. Oral Investig. 2019, 24, 1299–1309. [Google Scholar] [CrossRef]
- Tan, L.; Zhang, P.; Zhao, X.; Fan, C.; Zhang, C.; Yan, Y.; Von Gadow, K. Analysing species abundance distribution patterns across sampling scales in three natural forests in Northeastern China. Iforest Biogeosci. For. 2020, 13, 482. [Google Scholar] [CrossRef]
- Gomes, M.N.; Rodrigues, F.P.; Silikas, N.; Francci, C.E. Micro-CT and FE-SEM enamel analyses of calcium-based agent application after bleaching. Clin. Oral Investig. 2017, 22, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Vieira-Junior, W.; Ferraz, L.; Pini, N.; Ambrosano, G.; Aguiar, F.; Tabchoury, C.; Lima, D. Effect of Toothpaste Use Against Mineral Loss Promoted by Dental Bleaching. Oper. Dent. 2018, 43, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.; Zanatta, R.; Silva, T.; Borges, A. Effect of Calcium and Fluoride Addition to Hydrogen Peroxide Bleaching Gel On Tooth Diffusion, Color, and Microhardness. Oper. Dent. 2019, 44, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Vieira-Junior, W.; Ferraz, L.; Giorgi, M.; Ambrosano, G.; Aguiar, F.; Lima, D. Effect of Mouth Rinse Treatments on Bleached Enamel Properties, Surface Morphology, and Tooth Color. Oper. Dent. 2019, 44, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Wijetunga, C.L.; Otsuki, M.; Hiraishi, N.; Luong, M.N.; Tagami, J. Effect of pH of bleaching agent on tooth bleaching action in vitro. Dent. Mater. J. 2021, 40, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Guo, Y.; Feng, X.; Sa, Y.; Yang, X.; Wang, M.; Li, P.; Wang, Y. Hydrogen Peroxide Might Bleach Natural Dentin by Oxidizing Phosphoprotein. J. Dent. Res. 2018, 97, 1339–1345. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Li, Q.; Wang, Y. Effects of pH Values of Hydrogen Peroxide Bleaching Agents on Enamel Surface Properties. Oper. Dent. 2011, 36, 554–562. [Google Scholar] [CrossRef]
- Elfallah, H.M.; Bertassoni, L.E.; Charadram, N.; Rathsam, C.; Swain, M.V. Effect of tooth bleaching agents on protein content and mechanical properties of dental enamel. Acta Biomater. 2015, 20, 120–128. [Google Scholar] [CrossRef]
- Vilhena, K.F.B.; Nogueira, B.C.L.; Fagundes, N.C.F.; Loretto, S.C.; Angelica, R.S.; Lima, R.R.; E Souza, M.H.S. Dental enamel bleached for a prolonged and excessive time: Morphological changes. PLoS ONE 2019, 14, e0214948. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Pallavi, F.; Shi, Y.; Oyoyo, U.; Mohraz, A.; Li, Y. Effect of Bleaching Gel Viscosity on Tooth Whitening Efficacy and Pulp Chamber Penetration: An In Vitro Study. Oper. Dent. 2018, 43, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Pfefferkorn, F.; Ilie, N.; Blunck, U. Northern Lights 2018: Konsensus. 2018. Available online: https://www.zmk-aktuell.de/fachgebiete/allgemeine-zahnheilkunde/story/northern-lights-2018-konsensus__7162.html (accessed on 1 December 2022).
- Yang, S.-Y.; Choi, J.-W.; Kim, K.-M.; Kwon, J.-S. Effects of 35% hydrogen peroxide solution containing hydrated calcium silicate on enamel surface. Clin. Oral Investig. 2021, 26, 2133–2142. [Google Scholar] [CrossRef]
- Pinto, A.; Bridi, E.; Amaral, F.; França, F.; Turssi, C.; Pérez, C.; Martinez, E.; Flório, F.; Basting, R. Enamel Mineral Content Changes After Bleaching With High and Low Hydrogen Peroxide Concentrations: Colorimetric Spectrophotometry and Total Reflection X-ray Fluorescence Analyses. Oper. Dent. 2017, 42, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Pini, N.I.P.; Piccelli, M.R.; Vieira-Junior, W.F.; Ferraz, L.N.; Aguiar, F.H.B.; Lima, D.A.N.L. In-office tooth bleaching with chitosan-enriched hydrogen peroxide gels: In vitro results. Clin. Oral Investig. 2021, 26, 471–479. [Google Scholar] [CrossRef]
- Yang, S.-Y.; Han, A.R.; Kim, K.-M.; Kwon, J.-S. Effects of incorporating 45S5 bioactive glass into 30% hydrogen peroxide solution on whitening efficacy and enamel surface properties. Clin. Oral Investig. 2022, 26, 5301–5312. [Google Scholar] [CrossRef]
- Orilisi, G.; Tosco, V.; Monterubbianesi, R.; Notarstefano, V.; Özcan, M.; Putignano, A.; Orsini, G. ATR-FTIR, EDS and SEM evaluations of enamel structure after treatment with hydrogen peroxide bleaching agents loaded with nano-hydroxyapatite particles. Peerj 2021, 9, e10606. [Google Scholar] [CrossRef]
- Müller, W.E.; Neufurth, M.; Tolba, E.; Wang, S.; Geurtsen, W.; Feng, Q.; Schröder, H.C.; Wang, X. A biomimetic approach to ameliorate dental hypersensitivity by amorphous polyphosphate microparticles. Dent. Mater. 2016, 32, 775–783. [Google Scholar] [CrossRef]
- Tolba, E.; Wang, X.; Wang, S.; Neufurth, M.; Ackermann, M.; Schröder, H.C.; Müller, W.E.G. Amorphous Polyphosphate and Ca-Carbonate Nanoparticles Improve the Self-Healing Properties of both Technical and Medical Cements. Biotechnol. J. 2020, 15, e2000101. [Google Scholar] [CrossRef]
- Müller, W.E.; Tolba, E.; Schröder, H.C.; Wang, S.; Glaßer, G.; Muñoz-Espí, R.; Link, T.; Wang, X. A new polyphosphate calcium material with morphogenetic activity. Mater. Lett. 2015, 148, 163–166. [Google Scholar] [CrossRef]
- Müller, W.E.; Neufurth, M.; Ackermann, M.; Tolba, E.; Korzhev, M.; Wang, S.; Feng, Q.; Schröder, H.C.; Wang, X. Bifunctional dentifrice: Amorphous polyphosphate a regeneratively active sealant with potent anti- Streptococcus mutans activity. Dent. Mater. 2017, 33, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, M.; Tolba, E.; Neufurth, M.; Wang, S.; Schröder, H.C.; Wang, X.; Müller, W.E. Biomimetic transformation of polyphosphate microparticles during restoration of damaged teeth. Dent. Mater. 2019, 35, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Wang, S.; Tolba, E.; Neufurth, M.; Ackermann, M.; Muñoz-Espí, R.; Lieberwirth, I.; Glasser, G.; Schröder, H.C.; Wang, X. Transformation of Amorphous Polyphosphate Nanoparticles into Coacervate Complexes: An Approach for the Encapsulation of Mesenchymal Stem Cells. Small 2018, 14, e1801170. [Google Scholar] [CrossRef] [PubMed]
- Čadež, V.; Erceg, I.; Selmani, A.; Jurašin, D.D.; Šegota, S.; Lyons, D.M.; Kralj, D.; Sikirić, M.D. Amorphous Calcium Phosphate Formation and Aggregation Process Revealed by Light Scattering Techniques. Crystals 2018, 8, 254. [Google Scholar] [CrossRef]
- Mellgren, T.; Qin, T.; Öhman-Mägi, C.; Zhang, Y.; Wu, B.; Xia, W.; Engqvist, H. Calcium Phosphate Microspheres as a Delivery Vehicle for Tooth-Bleaching Agents. J. Dent. Res. 2017, 97, 283–288. [Google Scholar] [CrossRef]
- Ionescu, A.C.; Degli Esposti, L.; Iafisco, M.; Brambilla, E. Dental tissue remineralization by bioactive calcium phosphate nanoparticles formulations. Sci. Rep. 2022, 12, 5994. [Google Scholar] [CrossRef]
- Borges, A.; Zanatta, R.; Barros, A.; Silva, L.; Pucci, C.; Torres, C. Effect of Hydrogen Peroxide Concentration on Enamel Color and Microhardness. Oper. Dent. 2015, 40, 96–101. [Google Scholar] [CrossRef]
- Wang, K.; Chen, F.; Liu, C.; Rüssel, C. The effect of polymeric chain-like structure on the degradation and cellular biocompatibility of calcium polyphosphate. Mater. Sci. Eng. C 2008, 28, 1572–1578. [Google Scholar] [CrossRef]
- Balladares, L.; Alegría-Acevedo, L.; Montenegro-Arana, A.; Arana-Gordillo, L.; Pulido, C.; Salazar-Gracez, M.; Reis, A.; Loguercio, A. Effects of pH and Application Technique of In-office Bleaching Gels on Hydrogen Peroxide Penetration into the Pulp Chamber. Oper. Dent. 2019, 44, 659–667. [Google Scholar] [CrossRef]
- Qin, T.; Mellgren, T.; Jefferies, S.; Xia, W.; Engqvist, H. A Study for Tooth Bleaching via Carbamide Peroxide-Loaded Hollow Calcium Phosphate Spheres. Dent. J. 2016, 5, 3. [Google Scholar] [CrossRef]
- Jones, P.; Tobe, M.L.; Wynne-Jones, W.F.K. Hydrogen peroxide + water mixtures. Part 5.—Stabilization against the iron perchlorate catalyzed decomposition of hydrogen peroxide. Trans. Faraday Soc. 1959, 55, 91–97. [Google Scholar] [CrossRef]
- Vandersmissen, K.; De Smedt, F.; Vinckier, C. The Impact of Traces of Hydrogen Peroxide and Phosphate on the Ozone Decomposition Rate in “Pure Water”. Ozone Sci. Eng. 2008, 30, 300–309. [Google Scholar] [CrossRef]
- Jang, D.; Kwon, S.; Jo, S. Effect of Phosphate Stabilizers in Hydrogen Peroxide Decomposition on Manganese-Based Catalysts. J. Propuls. Power 2015, 31, 904–911. [Google Scholar] [CrossRef]
- Wang, X.; Ackermann, M.; Wang, S.; Tolba, E.; Neufurth, M.; Feng, Q.; Schröder, H.C.; Müller, W.E.G. Amorphous polyphosphate/amorphous calcium carbonate implant material with enhanced bone healing efficacy in a critical-size defect in rats. Biomed. Mater. 2016, 11, 035005. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Schröder, H.C.; Müller, W.E.G. Amorphous polyphosphate, a smart bioinspired nano-/bio-material for bone and cartilage regeneration: Towards a new paradigm in tissue engineering. J. Mater. Chem. B 2018, 6, 2385–2412. [Google Scholar] [CrossRef] [PubMed]
- Dhivya, S.; Narayan, A.K.; Kumar, R.L.; Chandran, S.V.; Vairamani, M.; Selvamurugan, N. Proliferation and differentiation of mesenchymal stem cells on scaffolds containing chitosan, calcium polyphosphate and pigeonite for bone tissue engineering. Cell Prolif. 2017, 51, e12408. [Google Scholar] [CrossRef]
- Darvishnia, F.; Rabiee, S.M.; Sabour, D. Bioactivity evaluation of printable calcium polyphosphate/alginoplast cement for bone tissue engineering; In vitro study. Bioprinting 2022, 27, e00210. [Google Scholar] [CrossRef]
- Públio, J.D.C.; Zeczkowski, M.; Burga-Sánchez, J.; Ambrosano, G.M.B.; Groppo, F.C.; Aguiar, F.H.B.; Lima, D.A.N.L. Influence of different thickeners in at-home tooth bleaching: A randomized clinical trial study. Clin. Oral Investig. 2018, 23, 2187–2198. [Google Scholar] [CrossRef]
- Acuña, E.D.; Parreiras, S.O.; Dds, M.W.F.; Cruz, G.P.; Gomes, A.; Dds, C.P.F.B.; Loguercio, A.D.; Reis, A. In-office bleaching with a commercial 40% hydrogen peroxide gel modified to have different pHs: Color change, surface morphology, and penetration of hydrogen peroxide into the pulp chamber. J. Esthet. Restor. Dent. 2019, 34, 322–327. [Google Scholar] [CrossRef]
- Soares, D.G.; Marcomini, N.; Duque, C.C.D.O.; Bordini, E.A.F.; Zuta, U.O.; Basso, F.G.; Hebling, J.; Costa, C.A.D.S. Increased whitening efficacy and reduced cytotoxicity are achieved by the chemical activation of a highly concentrated hydrogen peroxide bleaching gel. J. Appl. Oral Sci. 2019, 27, e20180453. [Google Scholar] [CrossRef]
- Desbord, M.; Soulié, J.; Rey, C.; Combes, C. Tunable Behavior in Solution of Amorphous Calcium Ortho/Pyrophosphate Materials: An Acellular In Vitro Study. ACS Biomater. Sci. Eng. 2022, 8, 2363–2374. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, V.; Da Rosa, D.A.; Da Silva, D.P.; Kury, M.; Liporoni, P.C.S.; Soares, L.E.S.; Martins, A.A. Effects of experimental bleaching agents on the mineral content of sound and demineralized enamels. J. Appl. Oral Sci. 2018, 26, e20170589. [Google Scholar] [CrossRef] [PubMed]
- Marson, F.; Gonçalves, R.; Silva, C.; Cintra, L.; Pascotto, R.; dos Santos, P.; Briso, A. Penetration of Hydrogen Peroxide and Degradation Rate of Different Bleaching Products. Oper. Dent. 2015, 40, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Baccan, N.; de Andrade, J.C.; Godinho, O.E.; Barone, J.S. Química Analítica Quantitativa Elementar, 3rd ed.; Edgard Blucher Ltda: Sao Paulo, Brazil, 2001. [Google Scholar]
- Mazilu, A.; Sarosi, C.; Moldovan, M.; Miuta, F.; Prodan, D.; Antoniac, A.; Prejmerean, C.; Dumitrescu, L.S.; Popescu, V.; Raiciu, A.D.; et al. Preparation and Characterization of Natural Bleaching Gels Used in Cosmetic Dentistry. Materials 2019, 12, 2106. [Google Scholar] [CrossRef]
- Soares, D.G.; Basso, F.G.; Hebling, J.; Costa, C.A.D.S. Concentrations of and application protocols for hydrogen peroxide bleaching gels: Effects on pulp cell viability and whitening efficacy. J. Dent. 2014, 42, 185–198. [Google Scholar] [CrossRef]




| Gel | 15 min | 30 min | 45 min | 90 min | 150 min |
|---|---|---|---|---|---|
| Hydrogen Peroxide Concentration Mean (wt%) * | |||||
| Experimental | 26.74 a | 25.28 a | 21.39 a | 17.01 b | 14.10 c |
| 0.5% CaPP | 25.16 a | 24.67 a | 23.19 a | 22.69 a | 23.68 a |
| 1.5% CaPP | 24.91 a | 24.91 a | 23.25 a | 23.50 a | 21.85 a |
| Commercial | 29.86 a | 23.08 a | 22.10 a | 22.10 a | 18.79 b |
| Reagent | Brand | Lot |
|---|---|---|
| CaCl2⋅2H2O (C3306-1000g) | Sigma | #SLBZ8395 |
| Na-PolyP (DP-PCB/O-2018-001) | Chemische Fabrik Budenheim | MV58/371 |
| NaOH (Pearls) | Labsynth | H2000.01.AH |
| Component A | Component B |
|---|---|
| H2O2 (50% sol) | H2O distilled |
| Carbopol 940 | CaPP (0.5 or 1.5 wt%) |
| Glycerol | Carbopol 940 |
| Propylene glycol | Glycerol |
| Citric acid | Propylene glycol |
| - | NaPP |
| NaOH to adjust the pH ≈ 1.8 | NaOH to adjust the pH ≈ 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guanipa Ortiz, M.I.; Santos, J.J.d.; Burga Sánchez, J.; Rodrigues-Filho, U.P.; Aguiar, F.H.B.; Rischka, K.; Lima, D.A.N.L. Calcium-Polyphosphate Submicroparticles (CaPP) Improvement Effect of the Experimental Bleaching Gels’ Chemical and Cellular-Viability Properties. Gels 2023, 9, 42. https://doi.org/10.3390/gels9010042
Guanipa Ortiz MI, Santos JJd, Burga Sánchez J, Rodrigues-Filho UP, Aguiar FHB, Rischka K, Lima DANL. Calcium-Polyphosphate Submicroparticles (CaPP) Improvement Effect of the Experimental Bleaching Gels’ Chemical and Cellular-Viability Properties. Gels. 2023; 9(1):42. https://doi.org/10.3390/gels9010042
Chicago/Turabian StyleGuanipa Ortiz, Mariángela Ivette, Juliana Jarussi dos Santos, Jonny Burga Sánchez, Ubirajara Pereira Rodrigues-Filho, Flávio Henrique Baggio Aguiar, Klaus Rischka, and Débora Alves Nunes Leite Lima. 2023. "Calcium-Polyphosphate Submicroparticles (CaPP) Improvement Effect of the Experimental Bleaching Gels’ Chemical and Cellular-Viability Properties" Gels 9, no. 1: 42. https://doi.org/10.3390/gels9010042
APA StyleGuanipa Ortiz, M. I., Santos, J. J. d., Burga Sánchez, J., Rodrigues-Filho, U. P., Aguiar, F. H. B., Rischka, K., & Lima, D. A. N. L. (2023). Calcium-Polyphosphate Submicroparticles (CaPP) Improvement Effect of the Experimental Bleaching Gels’ Chemical and Cellular-Viability Properties. Gels, 9(1), 42. https://doi.org/10.3390/gels9010042








