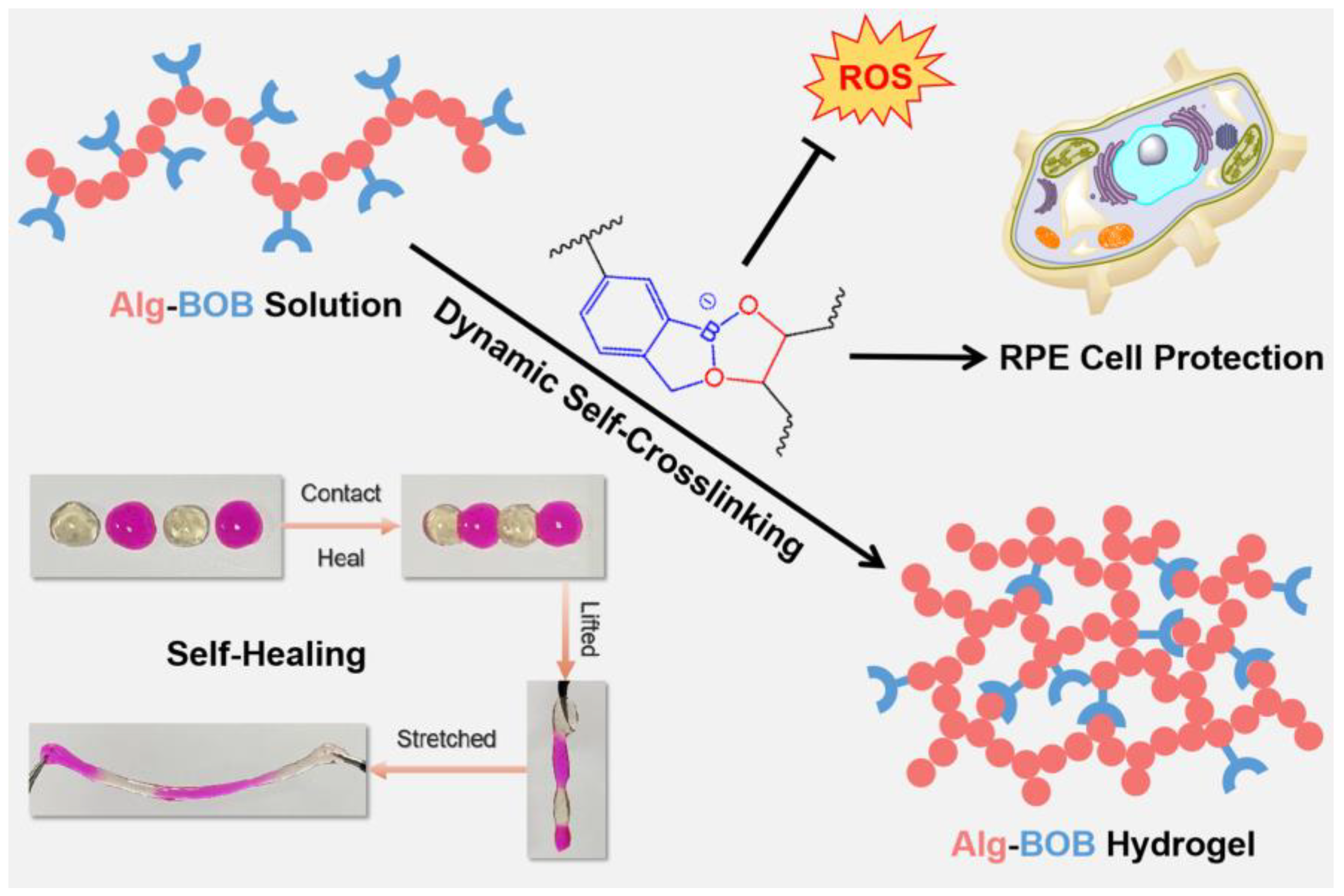
Self-Healing Alginate Hydrogel Formed by Dynamic Benzoxaborolate Chemistry Protects Retinal Pigment Epithelium Cells against Oxidative Damage
Abstract
1. Introduction
2. Results and Discussion
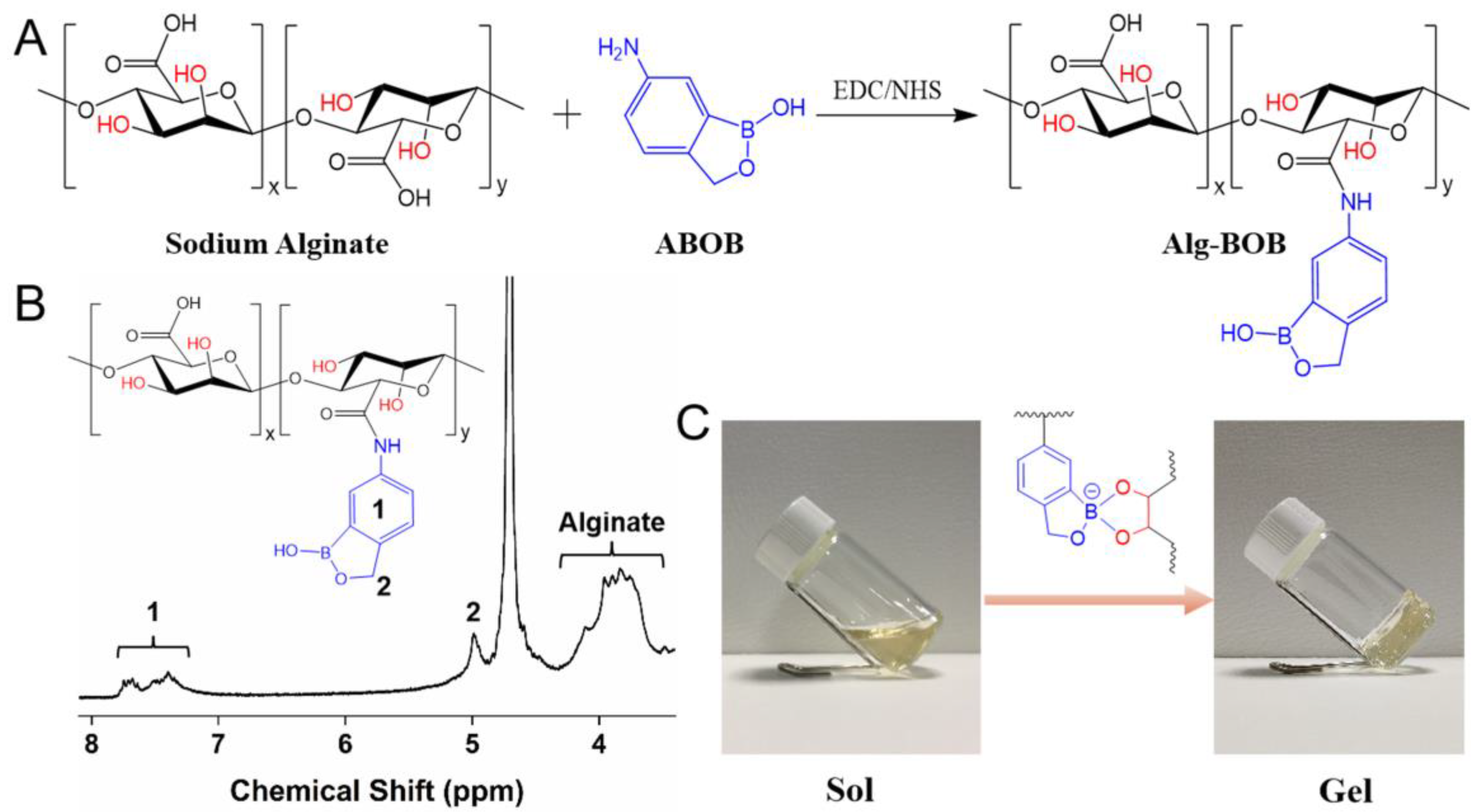
2.1. Synthesis of Alg-BOB Polymer and Hydrogel
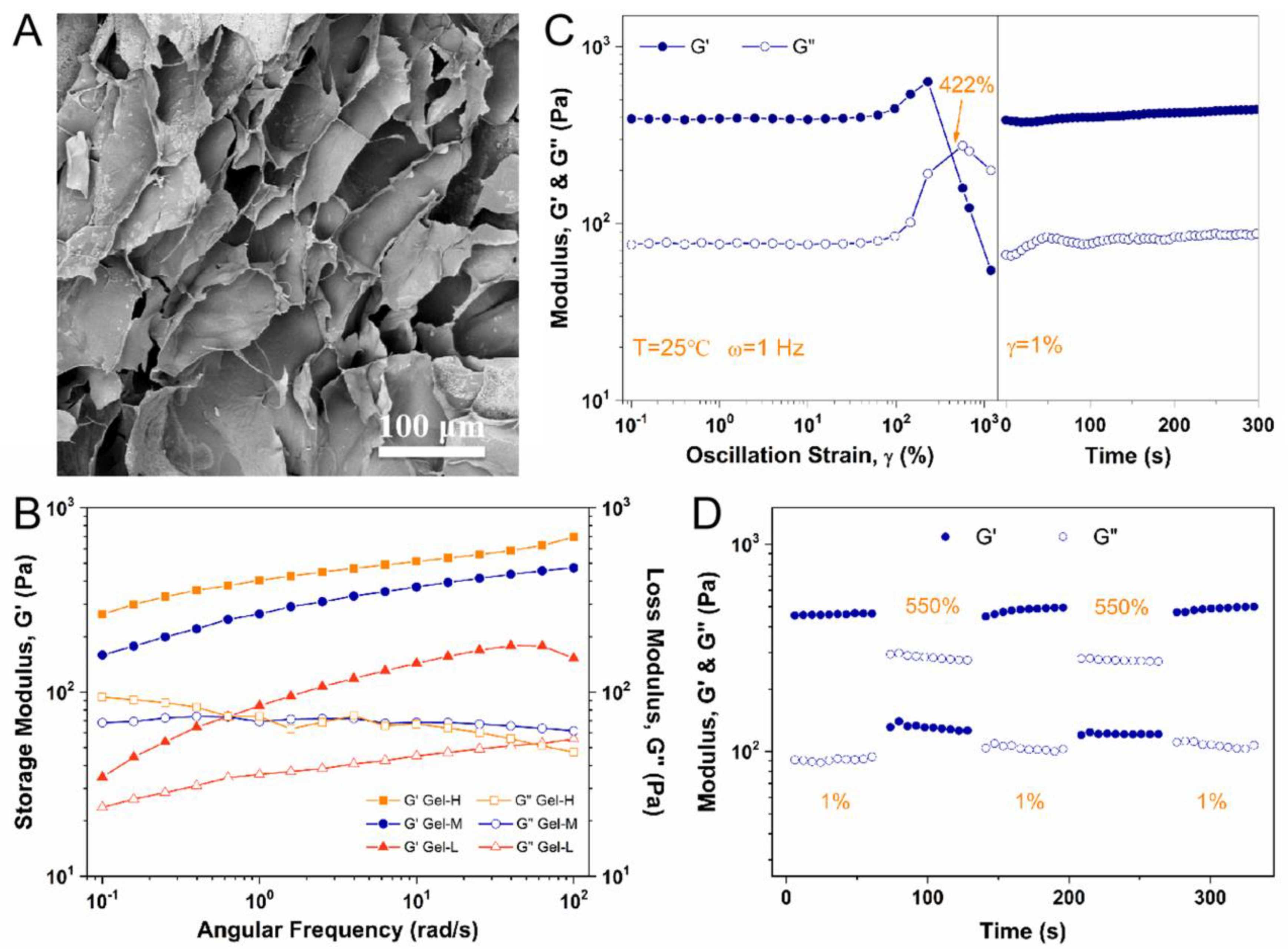
2.2. Characterization and Rheological Analysis of Alg-BOB Hydrogel
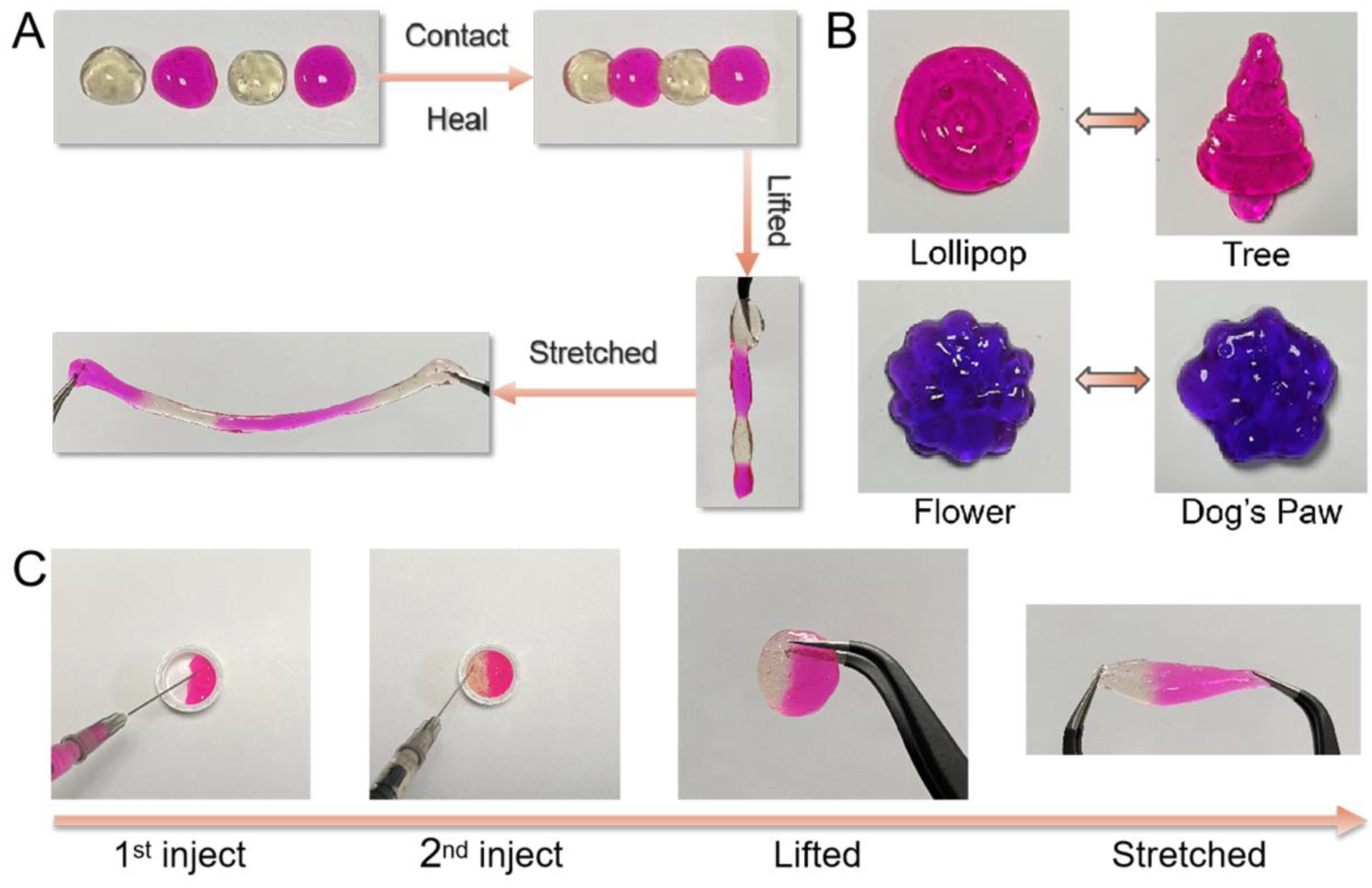
2.3. Self-Healing, Remolding, and Injectable Properties of Alg-BOB Hydrogel
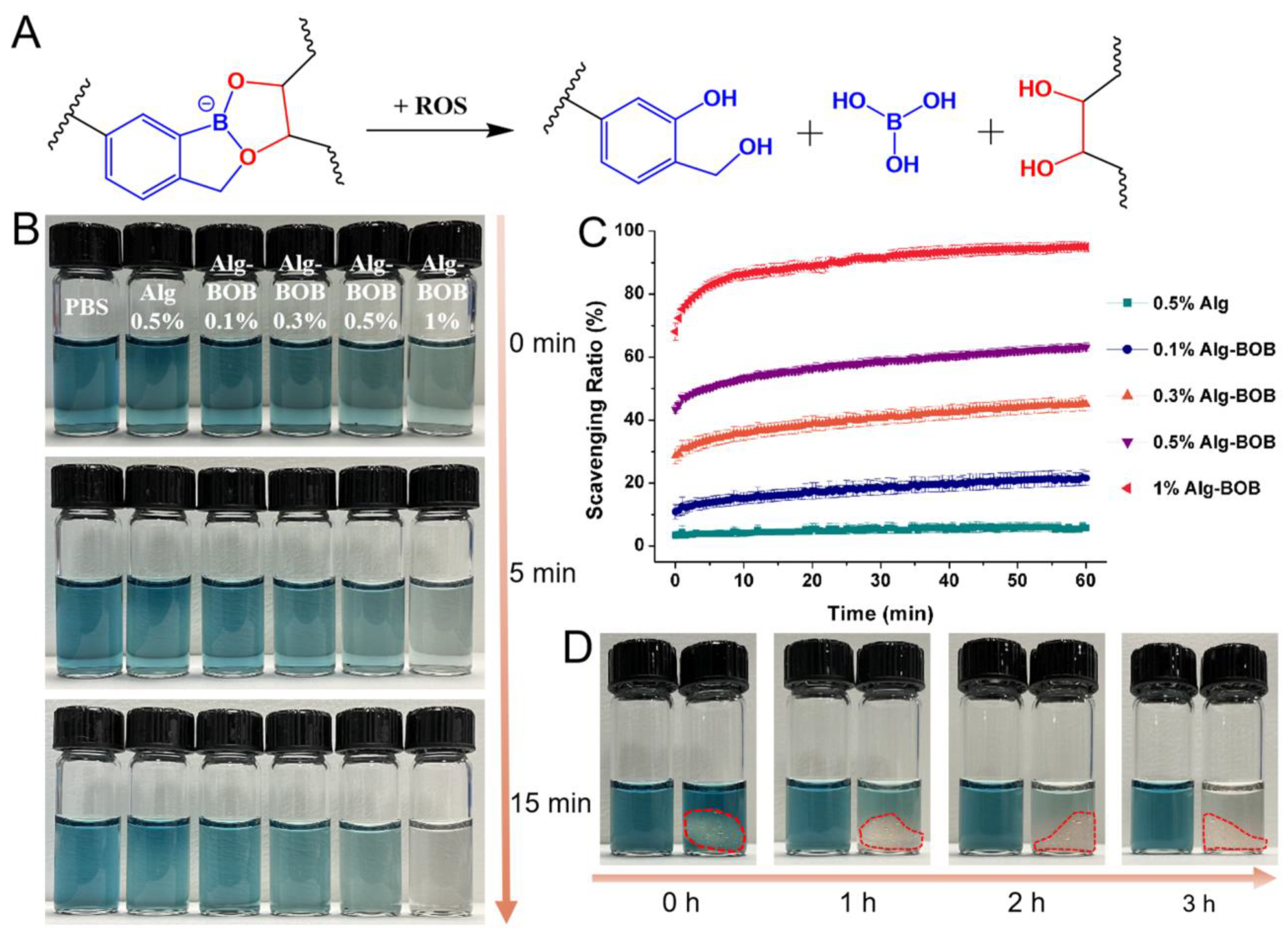
2.4. ROS Scavenging Ability
2.5. In Vitro Biocompatibility
2.6. In Vitro ROS Scavenging and RPE Protection
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Fabrication and Characterization of Alg-BOB Hydrogel
4.2.1. Synthesis and Characterization of Alg-BOB Polymer
4.2.2. Preparation of Alg-BOB Hydrogel
4.2.3. Morphology of Freeze-Dried Alg-BOB Hydrogel
4.2.4. Rheological Studies
4.2.5. Injectable, Moldable, and Self-Healing Properties
4.3. Antioxidant Property
4.4. In Vitro Cytocompatibility
4.4.1. Cell Culture
4.4.2. Cell Cytotoxicity via Cell Counting Kit-8 (CCK-8)
4.4.3. Live/Dead Staining
4.5. In Vitro ROS Scavenging and RPE Cell Protection by Hydrogel
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fleckenstein, M.; Keenan, T.; Guymer, R.; Chakravarthy, U.; Schmitz-Valckenberg, S.; Klaver, C.; Wong, W.; Chew, E. Age-related macular degeneration. Nat. Rev. Dis. Prim. 2021, 7, 31. [Google Scholar] [CrossRef]
- Sreekumar, P.G.; Kannan, R. Mechanisms of protection of retinal pigment epithelial cells from oxidant injury by humanin and other mitochondrial-derived peptides: Implications for age-related macular degeneration. Redox Biol. 2020, 37, 101663. [Google Scholar] [CrossRef] [PubMed]
- Kaarniranta, K.; Uusitalo, H.; Blasiak, J.; Felszeghy, S.; Kannan, R.; Kauppinen, A.; Salminen, A.; Sinha, D.; Ferrington, D. Mechanisms of mitochondrial dysfunction and their impact on age-related macular degeneration. Prog. Retin. Eye Res. 2020, 79, 100858. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Junnuthula, V.; Dyawanapelly, S. Ocular Therapeutics and Molecular Delivery Strategies for Neovascular Age-Related Macular Degeneration (nAMD). Int. J. Mol. Sci. 2021, 22, 10594. [Google Scholar] [CrossRef] [PubMed]
- Nair, D.R.; Seiler, M.; Patel, K.; Thomas, V.; Camarillo, J.M.; Humayun, M.; Thomas, B. Tissue Engineering Strategies for Retina Regeneration. Appl. Sci. 2021, 11, 2154. [Google Scholar] [CrossRef]
- Zadeh, M.A.; Khoder, M.; Al-Kinani, A.A.; Younes, H.M.; Alany, R.G. Retinal cell regeneration using tissue engineered polymeric scaffolds. Drug Discov. Today 2019, 24, 1669–1678. [Google Scholar] [CrossRef]
- Song, W.K.; Park, K.-M.; Kim, H.-J.; Lee, J.H.; Choi, J.; Chong, S.Y.; Shim, S.H.; Del Priore, L.V.; Lanza, R. Treatment of Macular Degeneration Using Embryonic Stem Cell-Derived Retinal Pigment Epithelium: Preliminary Results in Asian Patients. Stem Cell Rep. 2015, 4, 860–872. [Google Scholar] [CrossRef]
- Mandai, M.; Watanabe, A.; Kurimoto, Y.; Hirami, Y.; Morinaga, C.; Daimon, T.; Fujihara, M.; Akimaru, H.; Sakai, N.; Shibata, Y.; et al. Autologous Induced Stem-Cell–Derived Retinal Cells for Macular Degeneration. N. Engl. J. Med. 2017, 376, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, Z.; Yazdian, F.; Tabandeh, F.; Sheikhpour, M. Regenerative medicine as a novel strategy for AMD treatment: A review. Biomed. Phys. Eng. Express 2019, 6, 012001. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, Y.; Ouyang, X.; Yang, Y.; Chen, Y.; Luo, Q.; Zhang, Y.; Zhu, D.; Yu, X.; Li, L. Biomimetic hybrid hydrogel for hemostasis, adhesion prevention and promoting regeneration after partial liver resection. Bioact. Mater. 2021, 11, 41–51. [Google Scholar] [CrossRef]
- Liu, M.-H.; Nan, K.-H.; Chen, Y.-J. The Progress in Thermogels Based on Synthetic Polymers for Treating Ophthalmic Diseases. Acta Polym. Sin. 2021, 52, 47–60. [Google Scholar]
- Correa, S.; Grosskopf, A.K.; Hernandez, H.L.; Chan, D.; Yu, A.C.; Stapleton, L.M.; Appel, E.A. Translational Applications of Hydrogels. Chem. Rev. 2021, 121, 11385–11457. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.R.; Kondiah, P.P.D.; Choonara, Y.E.; Du Toit, L.C.; Ally, N.; Pillay, V. Hydrogel Biomaterials for Application in Ocular Drug Delivery. Front. Bioeng. Biotechnol. 2020, 8, 228. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M.; Liu, Z.Q.; Feng, Z.H.; Xu, F.; Liu, J.K. Adhesive protein-free synthetic hydrogels for retinal pigment epithelium cell culture with low ROS level. J. Biomed. Mater. Res. Part A 2013, 102, 2258–2267. [Google Scholar] [CrossRef]
- Park, J.H.; Shin, E.Y.; Shin, M.E.; Choi, M.J.; Carlomagno, C.; Song, J.E.; Khang, G. Enhanced retinal pigment epithelium (RPE) regeneration using curcumin/alginate hydrogels: In vitro evaluation. Int. J. Biol. Macromol. 2018, 117, 546–552. [Google Scholar] [CrossRef]
- Shin, E.Y.; Park, J.H.; Shin, M.E.; Song, J.E.; Thangavelu, M.; Carlomagno, C.; Motta, A.; Migliaresi, C.; Khang, G. Injectable taurine-loaded alginate hydrogels for retinal pigment epithelium (RPE) regeneration. Mater. Sci. Eng. C 2019, 103, 109787. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, D.; Jeong, Y.W.; Choi, M.J.; Lee, G.W.; Thangavelu, M.; Song, J.E.; Khang, G. Engineering retinal pigment epithelial cells regeneration for transplantation in regenerative medicine using PEG/Gellan gum hydrogels. Int. J. Biol. Macromol. 2019, 130, 220–228. [Google Scholar] [CrossRef]
- Jeong, Y.W.; Kim, H.S.; Thangavelu, M.; Choi, M.J.; Lee, G.W.; Song, C.U.; Song, J.E.; Khang, G. Progress in Silk Fibroin Based Composite Scaffold/Hydrogel: Silk Fibroin/PEG Hydrogel for the RPE Regeneration a Promising Biomaterial for Clinical Application. Front. Mater. 2020, 7, 504642. [Google Scholar] [CrossRef]
- Mitrousis, N.; Hacibekiroglu, S.; Ho, M.T.; Sauvé, Y.; Nagy, A.; van der Kooy, D.; Shoichet, M.S. Hydrogel-mediated co-transplantation of retinal pigmented epithelium and photoreceptors restores vision in an animal model of advanced retinal degeneration. Biomaterials 2020, 257, 120233. [Google Scholar] [CrossRef]
- Nita, M.; Grzybowski, A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxidative Med. Cell. Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef]
- Wang, K.; Zheng, M.; Lester, K.; Han, Z. Light-induced Nrf2(−/−) mice as atrophic age-related macular degeneration model and treatment with nanoceria laden injectable hydrogel. Sci. Rep. 2019, 9, 14573. [Google Scholar] [CrossRef]
- Ruan, Y.; Jiang, S.; Musayeva, A.; Gericke, A. Oxidative Stress and Vascular Dysfunction in the Retina: Therapeutic Strategies. Antioxidants 2020, 9, 761. [Google Scholar] [CrossRef]
- YGe, Y.; Zhang, A.; Sun, R.; Xu, J.; Yin, T.; He, H.; Gou, J.; Kong, J.; Zhang, Y.; Tang, X. Penetratin-modified lutein nanoemulsion in situ gel for the treatment of age-related macular degeneration. Expert Opin. Drug Deliv. 2020, 17, 603–619. [Google Scholar]
- Tisi, A.; Feligioni, M.; Passacantando, M.; Ciancaglini, M.; Maccarone, R. The Impact of Oxidative Stress on Blood-Retinal Barrier Physiology in Age-Related Macular Degeneration. Cells 2021, 10, 64. [Google Scholar] [CrossRef]
- Wang, K.; Mitra, R.N.; Zheng, M.; Han, Z. Nanoceria-loaded injectable hydrogels for potential age-related macular degeneration treatment. J. Biomed. Mater. Res. Part A 2018, 106, 2795–2804. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Zang, J.; Abdullah, A.A.I.; Li, Y.; Dong, H. Design Strategies and Applications of ROS-Responsive Phenylborate Ester-Based Nanomedicine. ACS Biomater. Sci. Eng. 2020, 6, 6510–6527. [Google Scholar] [CrossRef]
- Shi, W.; Fang, F.; Kong, Y.; Greer, S.E.; Kuss, M.; Liu, B.; Xue, W.; Jiang, X.; Lovell, P.; Mohs, A.M.; et al. Dynamic hyaluronic acid hydrogel with covalent linked gelatin as an anti-oxidative bioink for cartilage tissue engineering. Biofabrication 2022, 14, 014107. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Zhang, Y. Boronic acid-containing hydrogels: Synthesis and their applications. Chem. Soc. Rev. 2013, 42, 8106–8121. [Google Scholar] [CrossRef]
- Marco-Dufort, B.; Tibbitt, M. Design of moldable hydrogels for biomedical applications using dynamic covalent boronic esters. Mater. Today Chem. 2019, 12, 16–33. [Google Scholar] [CrossRef]
- Aeridou, E.; Díaz, D.D.; Alemán, C.; Pérez-Madrigal, M.M. Advanced Functional Hydrogel Biomaterials Based on Dynamic B–O Bonds and Polysaccharide Building Blocks. Biomacromolecules 2020, 21, 3984–3996. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zeng, Y.; Wu, H.; Zhou, C.; Tao, L. An antioxidant self-healing hydrogel for 3D cell cultures. J. Mater. Chem. B 2020, 8, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Li, D.; Li, Q.; Li, S.; Huang, H.; Li, H.; Dong, H.; Cao, X. Dynamic Nanocomposite Microgel Assembly with Microporosity, Injectability, Tissue-Adhesion, and Sustained Drug Release Promotes Articular Cartilage Repair and Regeneration. Adv. Health Mater. 2021, 11, e2102395. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, S.; Yoshizaki, Y.; Konno, T. Phospholipid polymer hydrogels with rapid dissociation for reversible cell immobilization. J. Mater. Chem. B 2022, 10, 2628–2636. [Google Scholar] [CrossRef]
- Pettignano, A.; Grijalvo, S.; Häring, M.; Eritja, R.; Tanchoux, N.; Quignard, F.; Díaz, D.D. Boronic acid-modified alginate enables direct formation of injectable, self-healing and multistimuli-responsive hydrogels. Chem. Commun. 2017, 53, 3350–3353. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Long, L.; Yang, L.; Fu, D.; Hu, C.; Kong, Q.; Wang, Y. Inflammation-Responsive Drug-Loaded Hydrogels with Sequential Hemostasis, Antibacterial, and Anti-Inflammatory Behavior for Chronically Infected Diabetic Wound Treatment. ACS Appl. Mater. Interfaces 2021, 13, 33584–33599. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk-Woźniak, A.; Borys, K.M.; Sporzyński, A. Recent Developments in the Chemistry and Biological Applications of Benzoxaboroles. Chem. Rev. 2015, 115, 5224–5247. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Diaz-Dussan, D.; Wu, D.; Wang, W.; Peng, Y.-Y.; Asha, A.B.; Hall, D.G.; Ishihara, K.; Narain, R. Bioinspired Self-Healing Hydrogel Based on Benzoxaborole-Catechol Dynamic Covalent Chemistry for 3D Cell Encapsulation. ACS Macro Lett. 2018, 7, 904–908. [Google Scholar] [CrossRef]
- Chen, Y.; Tan, Z.; Wang, W.; Peng, Y.-Y.; Narain, R. Injectable, Self-Healing, and Multi-Responsive Hydrogels via Dynamic Covalent Bond Formation between Benzoxaborole and Hydroxyl Groups. Biomacromolecules 2018, 20, 1028–1035. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, W.; Wu, D.; Zeng, H.; Hall, D.G.; Narain, R. Multiresponsive and Self-Healing Hydrogel via Formation of Polymer–Nanogel Interfacial Dynamic Benzoxaborole Esters at Physiological pH. ACS Appl. Mater. Interfaces 2019, 11, 44742–44750. [Google Scholar] [CrossRef]
- Wu, D.; Wang, W.; Diaz-Dussan, D.; Peng, Y.Y.; Chen, Y.; Narain, R.; Hall, D.G. In Situ Forming, Dual-Crosslink Network, Self-Healing Hydrogel Enabled by a Bioorthogonal Nopoldiol–Benzoxaborolate Click Reaction with a Wide pH Range. Chem. Mater. 2019, 31, 4092–4102. [Google Scholar] [CrossRef]
- Sun, P.; Huang, T.; Wang, X.; Wang, G.; Liu, Z.; Chen, G.; Fan, Q. Dynamic-Covalent Hydrogel with NIR-Triggered Drug Delivery for Localized Chemo-Photothermal Combination Therapy. Biomacromolecules 2019, 21, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, T.; Cosenza, V.; Ogawa, Y.; Jeacomine, I.; Vallet, A.; Ortega, S.; Michel, R.; Olsson, J.D.M.; Gerfaud, T.; Boiteau, J.-G.; et al. Boronic acid and diol-containing polymers: How to choose the correct couple to form “strong” hydrogels at physiological pH. Soft Matter 2020, 16, 3628–3641. [Google Scholar] [CrossRef]
- Asha, A.; Peng, Y.; Cheng, Q.; Ishihara, K.; Liu, Y.; Narain, R. Dopamine Assisted Self-Cleaning, Antifouling, and Antibacterial Coating via Dynamic Covalent Interactions. ACS Appl. Mater. Interfaces 2022, 14, 9557–9569. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, X. Alginate hydrogel dressings for advanced wound management. Int. J. Biol. Macromol. 2020, 162, 1414–1428. [Google Scholar] [CrossRef] [PubMed]
- Raus, R.A.; Nawawi, W.M.F.W.; Nasaruddin, R.R. Alginate and alginate composites for biomedical applications. Asian J. Pharm. Sci. 2021, 16, 280–306. [Google Scholar] [CrossRef]
- Teng, K.; An, Q.; Chen, Y.; Zhang, Y.; Zhao, Y. Recent Development of Alginate-Based Materials and Their Versatile Functions in Biomedicine, Flexible Electronics, and Environmental Uses. ACS Biomater. Sci. Eng. 2021, 7, 1302–13377. [Google Scholar] [CrossRef]
- Kong, X.; Chen, L.; Li, B.; Quan, C.; Wu, J. Applications of oxidized alginate in regenerative medicine. J. Mater. Chem. B 2021, 9, 2785–2801. [Google Scholar] [CrossRef]
- Hong, S.; Shin, M.; Park, E.; Ryu, J.; Burdick, J.; Lee, H. Alginate-Boronic Acid: pH-Triggered Bioinspired Glue for Hydrogel Assembly. Adv. Funct. Mater. 2019, 30, 1908497. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, W.; Wu, D.; Nagao, M.; Hall, D.G.; Thundat, T.; Narain, R. Injectable Self-Healing Zwitterionic Hydrogels Based on Dynamic Benzoxaborole–Sugar Interactions with Tunable Mechanical Properties. Biomacromolecules 2018, 19, 596–605. [Google Scholar] [CrossRef]
- Hakuto, N.; Saito, K.; Kirihara, M.; Kotsuchibashi, Y. Preparation of cross-linked poly(vinyl alcohol) films from copolymers with benzoxaborole and carboxylic acid groups, and their degradability in an oxidizing environment. Polym. Chem. 2020, 11, 2469–2474. [Google Scholar] [CrossRef]
- Long, L.; Hu, C.; Liu, W.; Wu, C.; Lu, L.; Yang, L.; Wang, Y. Injectable multifunctional hyaluronic acid/methylcellulose hydrogels for chronic wounds repairing. Carbohydr. Polym. 2022, 289, 119456. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Deng, M.; Wu, Y.; Hu, C.; Zhang, B.; Guo, C.; Song, H.; Kong, Q.; Wang, Y. Sustained gene delivery from inflammation-responsive anti-inflammatory hydrogels promotes extracellular matrix metabolism balance in degenerative nucleus pulposus. Compos. Part B Eng. 2022, 236, 109806. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, S.; Ning, P.; Lu, K.; Ma, S.; Wang, Y.; Lü, S. Phosphate enhanced self-healing property of phenylborate-based hydrogel at neutral environment. Polymer 2021, 225, 123749. [Google Scholar] [CrossRef]
- Azim, E.A.A.; Elkheshen, S.A.; Hathout, R.M.; Fouly, M.A.; El Hoffy, N.M. Augmented in vitro and in vivo Profiles of Brimonidine Tartrate Using Gelatinized-Core Liposomes. Int. J. Nanomed. 2022, 17, 2753–2776. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Huang, Y.; Tao, C.; Yang, W.; Chen, J.; Zhu, L.; Pan, T.; Narain, R.; Nan, K.; Chen, Y. Self-Healing Alginate Hydrogel Formed by Dynamic Benzoxaborolate Chemistry Protects Retinal Pigment Epithelium Cells against Oxidative Damage. Gels 2023, 9, 24. https://doi.org/10.3390/gels9010024
Liu M, Huang Y, Tao C, Yang W, Chen J, Zhu L, Pan T, Narain R, Nan K, Chen Y. Self-Healing Alginate Hydrogel Formed by Dynamic Benzoxaborolate Chemistry Protects Retinal Pigment Epithelium Cells against Oxidative Damage. Gels. 2023; 9(1):24. https://doi.org/10.3390/gels9010024
Chicago/Turabian StyleLiu, Minhua, Yate Huang, Chunwen Tao, Weijia Yang, Junrong Chen, Li Zhu, Tonghe Pan, Ravin Narain, Kaihui Nan, and Yangjun Chen. 2023. "Self-Healing Alginate Hydrogel Formed by Dynamic Benzoxaborolate Chemistry Protects Retinal Pigment Epithelium Cells against Oxidative Damage" Gels 9, no. 1: 24. https://doi.org/10.3390/gels9010024
APA StyleLiu, M., Huang, Y., Tao, C., Yang, W., Chen, J., Zhu, L., Pan, T., Narain, R., Nan, K., & Chen, Y. (2023). Self-Healing Alginate Hydrogel Formed by Dynamic Benzoxaborolate Chemistry Protects Retinal Pigment Epithelium Cells against Oxidative Damage. Gels, 9(1), 24. https://doi.org/10.3390/gels9010024






