Dynamic Covalent Hydrogels: Strong yet Dynamic
Abstract
:1. Introduction
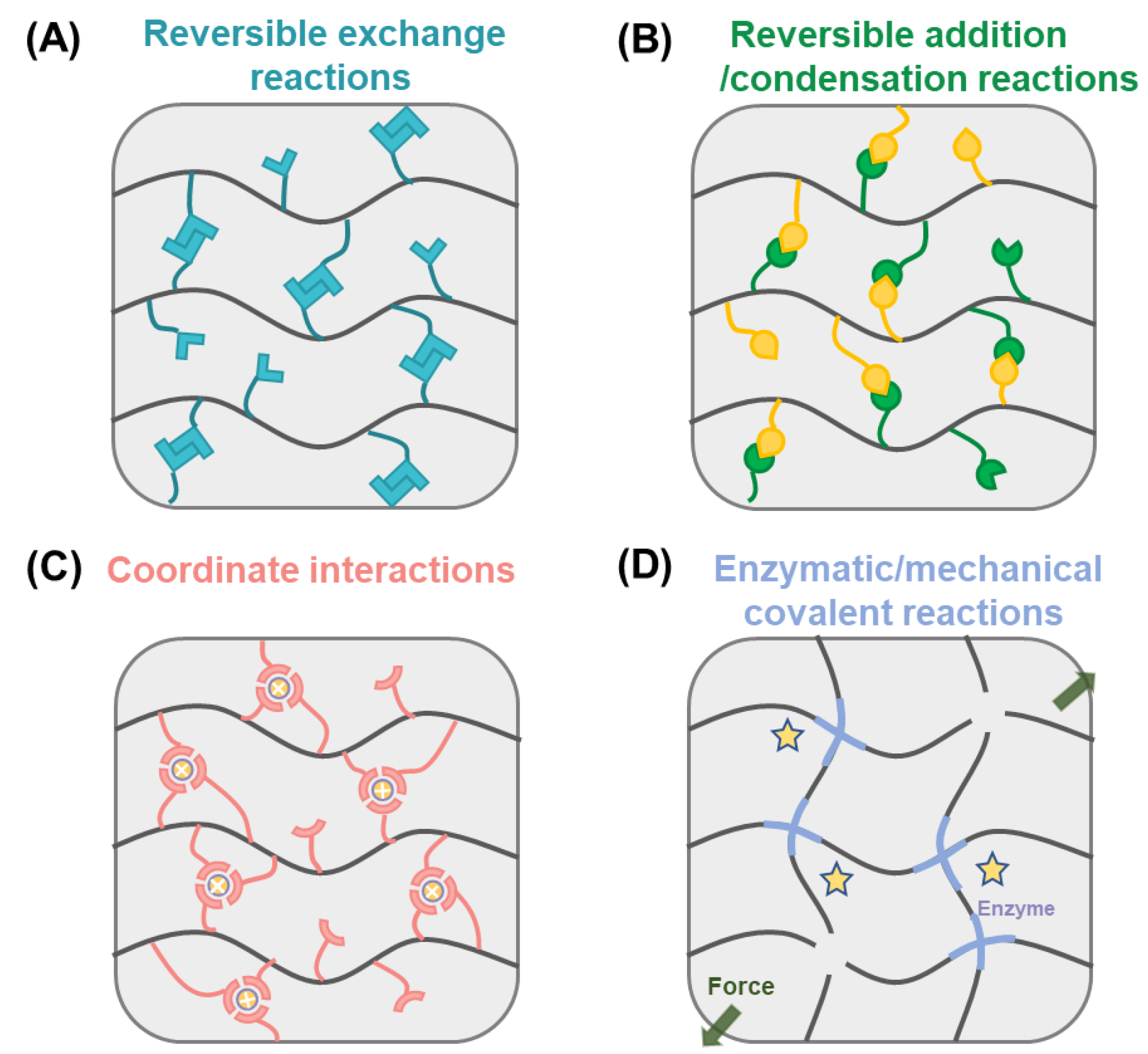
2. Dynamic Covalent Chemistry
2.1. Reversible Exchange
2.2. Reversible Addition/Condensation
2.3. Coordinate Interaction
2.4. Enzymatic/Mechanical Dynamic Covalent Reactions
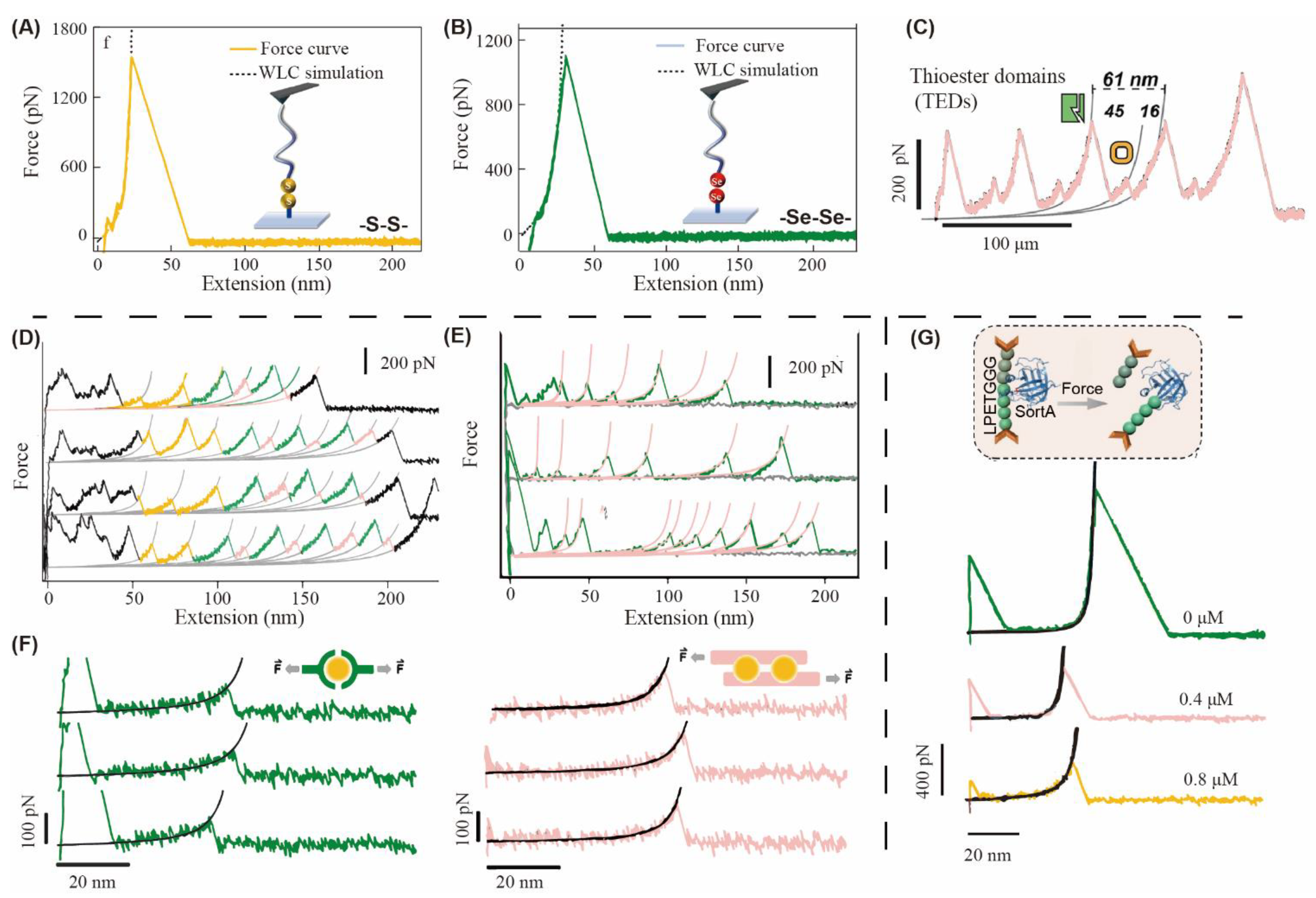
2.5. Properties of Dynamic Covalent Polymers
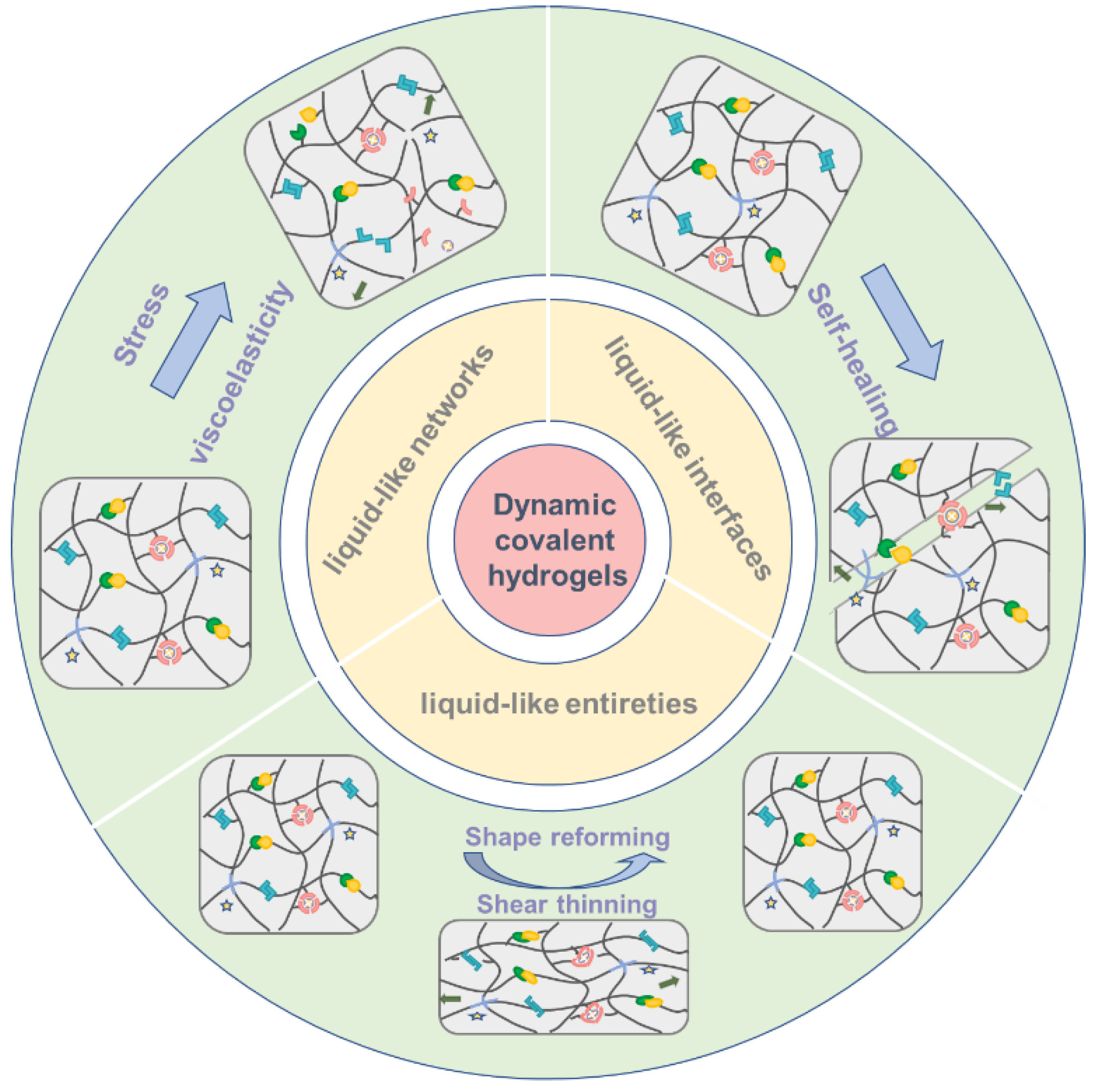
3. Dynamic Covalent Chemistry Endows Novel Properties of Hydrogels
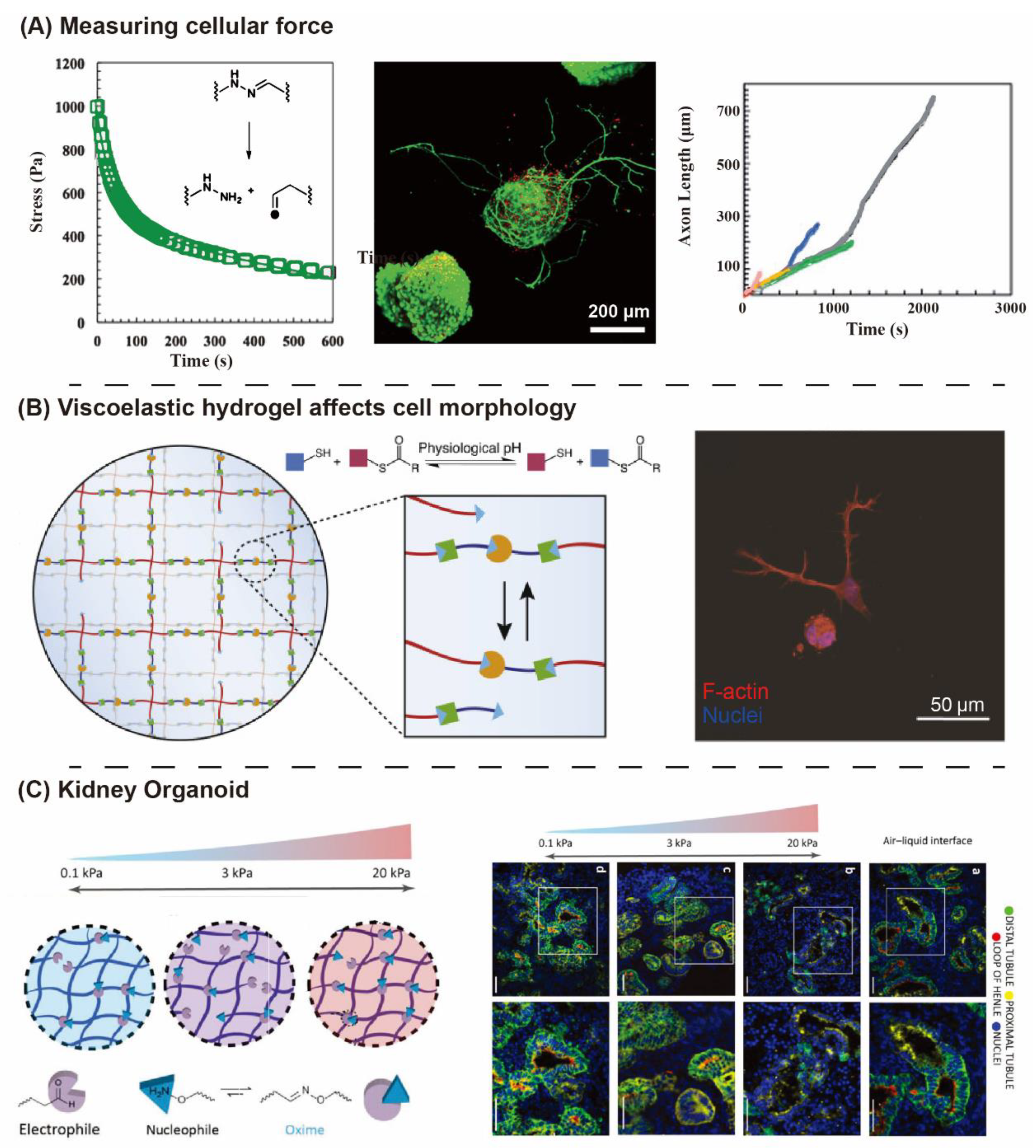
3.1. “Liquid-Like Networks” Change Dynamic Mechanical Properties
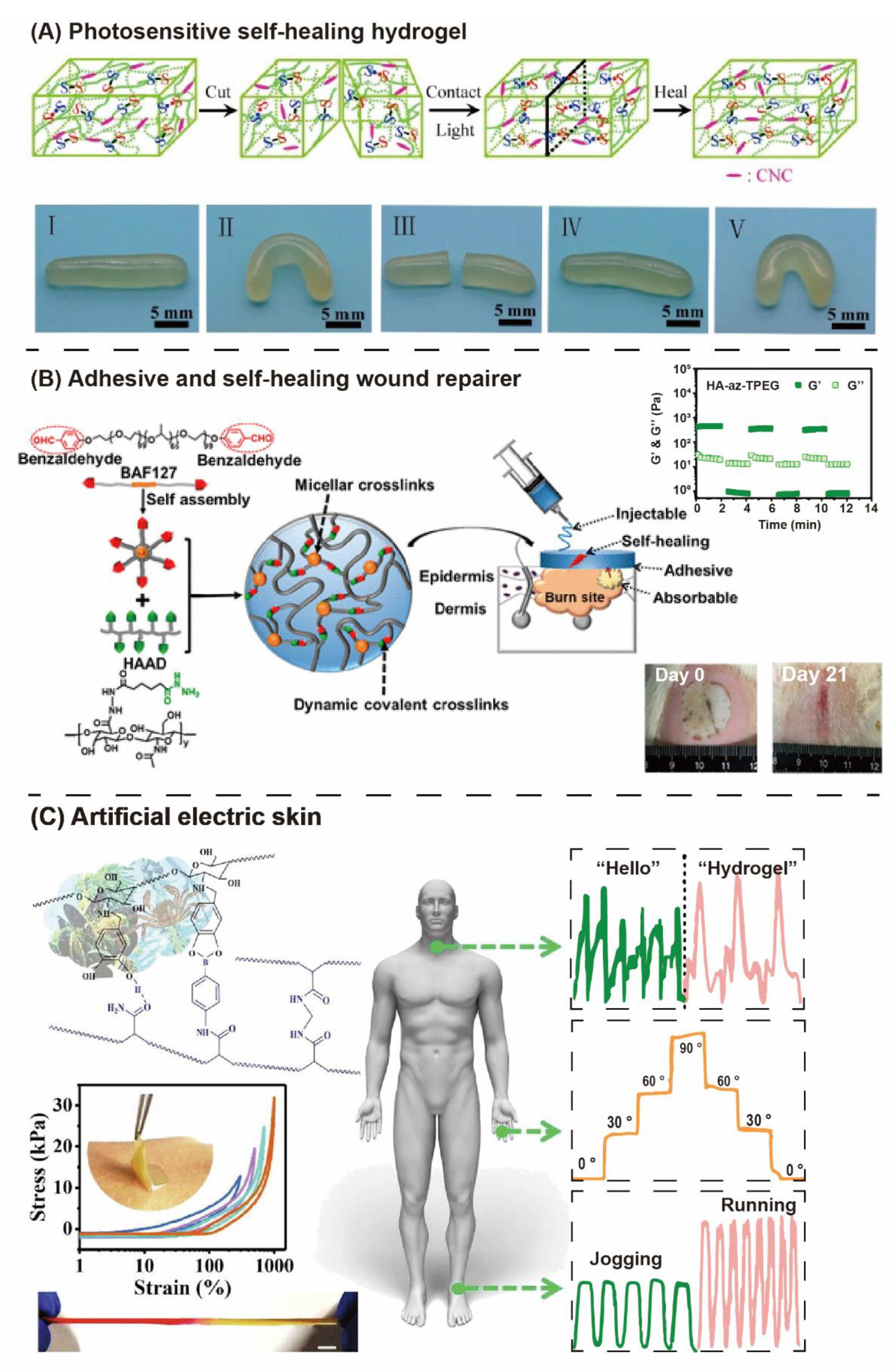
3.2. “Liquid-Like Interfaces” Outcome Self-Healing Properties
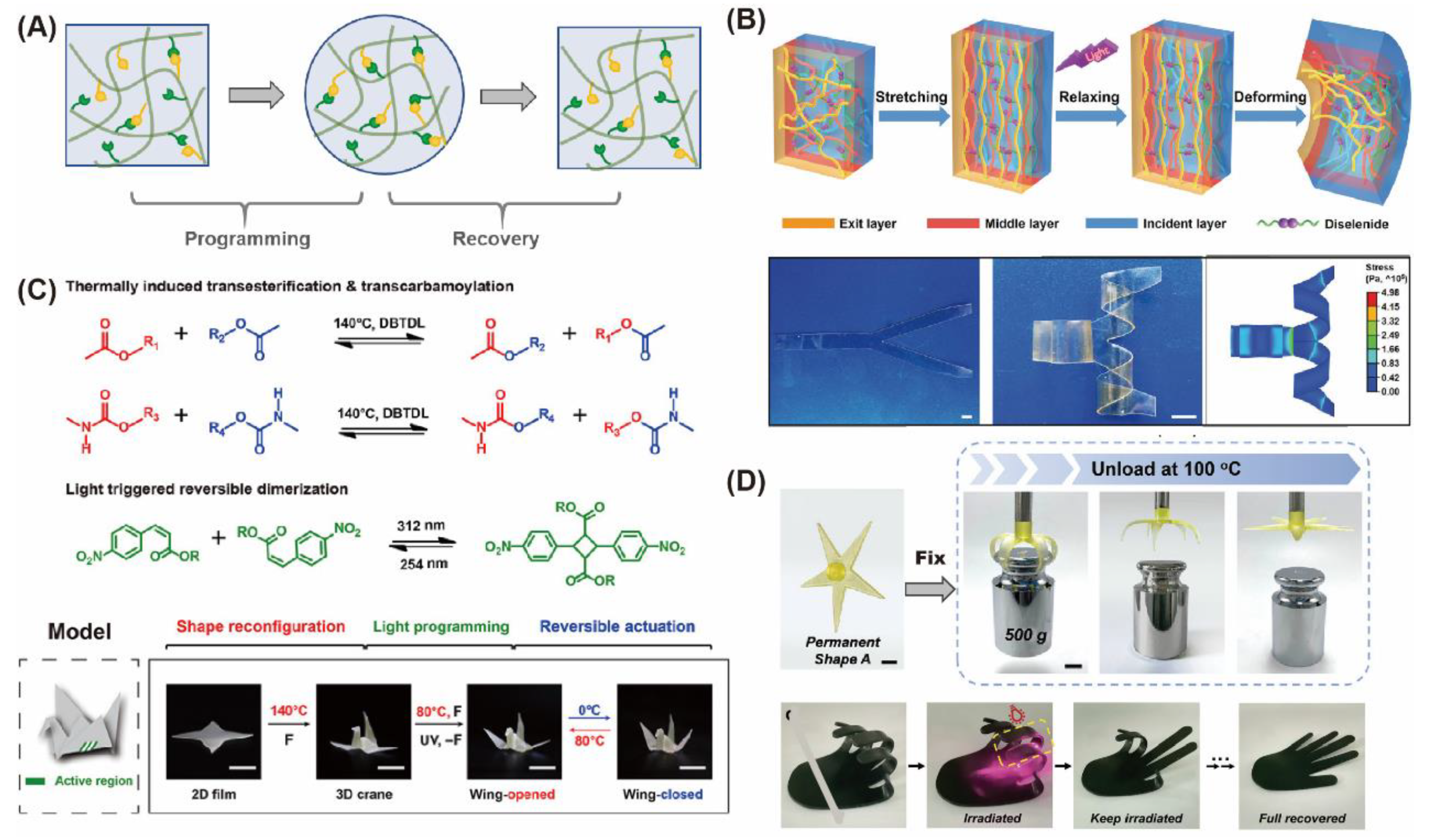
3.3. “Liquid-Like Entireties” Turn out Injectable and Shape-Shifting/Memory Materials
4. Conclusions and Perspectives
Funding
Conflicts of Interest
References
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Mahoney, M.J.; Anseth, K.S. Three-dimensional growth and function of neural tissue in degradable polyethylene glycol hydrogels. Biomaterials 2006, 27, 2265–2274. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Gu, J.; Li, L.; Yu, W.; Yin, S.; Qin, M.; Jiang, Q.; Wang, W.; Cao, Y. Hydrogel tapes for fault-tolerant strong wet adhesion. Nat. Commun. 2021, 12, 7156. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Xie, R.; Zhu, J.; Wu, J.; Hui, J.; Zheng, X.; Huo, F.; Fan, D. A temperature responsive adhesive hydrogel for fabrication of flexible electronic sensors. Npj Flex. Electron. 2022, 6, 68. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Lee, Y.; Song, W.J.; Sun, J.Y. Hydrogel soft robotics. Mater. Today Phys. 2020, 15, 100258. [Google Scholar] [CrossRef]
- Varghese, S.A.; Rangappa, S.M.; Siengchin, S.; Parameswaranpillai, J. Chapter 2—Natural polymers and the hydrogels prepared from them. In Hydrogels Based on Natural Polymers; Chen, Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 17–47. [Google Scholar]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef]
- Hodgson, S.M.; Bakaic, E.; Stewart, S.A.; Hoare, T.; Adronov, A. Properties of Poly(ethylene glycol) Hydrogels Cross-Linked via Strain-Promoted Alkyne–Azide Cycloaddition (SPAAC). Biomacromolecules 2016, 17, 1093–1100. [Google Scholar] [CrossRef]
- Park, E.J.; Gevrek, T.N.; Sanyal, R.; Sanyal, A. Indispensable Platforms for Bioimmobilization: Maleimide-Based Thiol Reactive Hydrogels. Bioconjugate Chem. 2014, 25, 2004–2011. [Google Scholar] [CrossRef]
- Song, J.; Lee, M.; Kim, T.; Na, J.; Jung, Y.; Jung, G.Y.; Kim, S.; Park, N. A RNA producing DNA hydrogel as a platform for a high performance RNA interference system. Nat. Commun. 2018, 9, 4331. [Google Scholar] [CrossRef] [Green Version]
- Mo, F.; Jiang, K.; Zhao, D.; Wang, Y.; Song, J.; Tan, W. DNA hydrogel-based gene editing and drug delivery systems. Adv. Drug Deliv. Rev. 2021, 168, 79–98. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, J.; Nie, J.; Du, B. Poly(N-isopropylacrylamide)-based ionic hydrogels: Synthesis, swelling properties, interfacial adsorption and release of dyes. Polym. J. 2016, 48, 431–438. [Google Scholar] [CrossRef]
- Lehn, J.-M. Supramolecular materials: Dynamic, responsive, adaptive. Supramol. Chem. 2022, 1, 100007. [Google Scholar] [CrossRef]
- Jiang, H.; Duan, L.; Ren, X.; Gao, G. Hydrophobic association hydrogels with excellent mechanical and self-healing properties. Eur. Polym. J. 2019, 112, 660–669. [Google Scholar] [CrossRef]
- Li, F.; Zhu, Y.; You, B.; Zhao, D.; Ruan, Q.; Zeng, Y.; Ding, C. Smart Hydrogels Co-switched by Hydrogen Bonds and π–π Stacking for Continuously Regulated Controlled-Release System. Adv. Funct. Mater. 2010, 20, 669–676. [Google Scholar] [CrossRef]
- Liu, L.; Han, Y.; Lv, S. Design of Self-Healing and Electrically Conductive Silk Fibroin-Based Hydrogels. ACS Appl. Mater. Interfaces 2019, 11, 20394–20403. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Fu, S.; Zhou, S.; Li, M.; Li, K.; Sun, W.; Zhai, Y. Advances in hydrogels based on dynamic covalent bonding and prospects for its biomedical application. Eur. Polym. J. 2020, 139, 110024. [Google Scholar] [CrossRef]
- Zhang, J.; Lei, H.; Qin, M.; Wang, W.; Cao, Y. Quantifying cation-π interactions in marine adhesive proteins using single-molecule force spectroscopy. Supramol. Chem. 2022, 1, 100005. [Google Scholar] [CrossRef]
- Rizwan, M.; Baker, A.E.G.; Shoichet, M.S. Designing Hydrogels for 3D Cell Culture Using Dynamic Covalent Crosslinking. Adv. Healthc. Mater. 2021, 10, e2100234. [Google Scholar] [CrossRef]
- Lehn, J.-M. Dynamic Combinatorial Chemistry and Virtual Combinatorial Libraries. Chem.—A Eur. J. 1999, 5, 2455–2463. [Google Scholar] [CrossRef]
- Rowan, S.J.; Cantrill, S.J.; Cousins, G.R.L.; Sanders, J.K.M.; Stoddart, J.F. Dynamic Covalent Chemistry. Angew. Chem. Int. Ed. 2002, 41, 898–952. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, Y.; Wang, S.; Zhao, C.; Gong, X. The dissociation of physical interaction clusters under tensile deformation of hybrid double network gels. Polymer 2020, 210, 122995. [Google Scholar] [CrossRef]
- Huang, S.; Kong, X.; Xiong, Y.; Zhang, X.; Chen, H.; Jiang, W.; Niu, Y.; Xu, W.; Ren, C. An overview of dynamic covalent bonds in polymer material and their applications. Eur. Polym. J. 2020, 141, 110094. [Google Scholar] [CrossRef]
- Gharakhloo, M.; Karbarz, M. Autonomous self-healing hydrogels: Recent development in fabrication strategies. Eur. Polym. J. 2022, 165, 111004. [Google Scholar] [CrossRef]
- Perera, M.M.; Ayres, N. Dynamic covalent bonds in self-healing, shape memory, and controllable stiffness hydrogels. Polym. Chem. 2020, 11, 1410–1423. [Google Scholar] [CrossRef]
- Ni, C.; Chen, D.; Zhang, Y.; Xie, T.; Zhao, Q. Autonomous Shapeshifting Hydrogels via Temporal Programming of Photoswitchable Dynamic Network. Chem. Mater. 2021, 33, 2046–2053. [Google Scholar] [CrossRef]
- Chen, X.; Han, Y.; Gao, P.; Yang, M.; Xiao, L.; Xiong, X.; Zhao, H.; Tang, C.; Chen, G.; Zhu, X.; et al. Disulfide-bond A oxidoreductase-like protein protects against ectopic fat deposition and lipid-related kidney damage in diabetic nephropathy. Kidney Int. 2019, 95, 880–895. [Google Scholar] [CrossRef]
- Lukesh, J.C.; Palte, M.J.; Raines, R.T. A Potent, Versatile Disulfide-Reducing Agent from Aspartic Acid. J. Am. Chem. Soc. 2012, 134, 4057–4059. [Google Scholar] [CrossRef]
- Bechtel, T.J.; Weerapana, E. From structure to redox: The diverse functional roles of disulfides and implications in disease. Proteomics 2017, 17, 1600391. [Google Scholar] [CrossRef]
- Lee, S.F.; Davey, L. Disulfide Bonds: A Key Modification in Bacterial Extracytoplasmic Proteins. J. Dent. Res. 2017, 96, 1465–1473. [Google Scholar] [CrossRef]
- Xia, J.; Li, H.; Xu, H. Measuring the Strength of S/Se Based Dynamic Covalent Bonds. Acta Polym. Sin. 2020, 51, 205–213. [Google Scholar]
- Lei, H.; Ma, Q.; Li, W.; Wen, J.; Ma, H.; Qin, M.; Wang, W.; Cao, Y. An ester bond underlies the mechanical strength of a pathogen surface protein. Nat. Commun. 2021, 12, 5082. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, H.F. Thiol/disulfide exchange equilibria and disulfide bond stability. Methods Enzym. 1995, 251, 8–28. [Google Scholar]
- Gulden, M.; Jess, A.; Kammann, J.; Maser, E.; Seibert, H. Cytotoxic potency of H2O2 in cell cultures: Impact of cell concentration and exposure time. Free Radic. Biol. Med. 2010, 49, 1298–1305. [Google Scholar] [CrossRef]
- Han, Y.; Liu, C.; Xu, H.; Cao, Y. Engineering Reversible Hydrogels for 3D Cell Culture and Release Using Diselenide Catalyzed Fast Disulfide Formation. Chin. J. Chem. 2022, 40, 1578–1584. [Google Scholar] [CrossRef]
- Canal-Martin, A.; Perez-Fernandez, R. Biomimetic selenocystine based dynamic combinatorial chemistry for thiol-disulfide exchange. Nat. Commun. 2021, 12, 163. [Google Scholar] [CrossRef]
- Echelman, D.J.; Lee, A.Q.; Fernandez, J.M. Mechanical forces regulate the reactivity of a thioester bond in a bacterial adhesin. J. Biol. Chem. 2017, 292, 8988–8997. [Google Scholar] [CrossRef]
- Lei, H.; Guo, Y.; Hu, X.; Hu, C.; Hu, X.; Li, H. Reversible Unfolding and Folding of the Metalloprotein Ferredoxin Revealed by Single-Molecule Atomic Force Microscopy. J. Am. Chem. Soc. 2017, 139, 1538–1544. [Google Scholar] [CrossRef]
- Sun, W.; Xue, B.; Fan, Q.; Tao, R.; Wang, C.; Wang, X.; Li, Y.; Qin, M.; Wang, W.; Chen, B.; et al. Molecular engineering of metal coordination interactions for strong, tough, and fast-recovery hydrogels. Sci. Adv. 2020, 6, eaaz9531. [Google Scholar] [CrossRef]
- He, G.; Lei, H.; Sun, W.; Gu, J.; Yu, W.; Zhang, D.; Chen, H.; Li, Y.; Qin, M.; Xue, B.; et al. Strong and Reversible Covalent Double Network Hydrogel Based on Force-Coupled Enzymatic Reactions. Angew. Chem. Int. Ed. Engl. 2022, 61, e202201765. [Google Scholar] [CrossRef]
- Kildahl, N.K. Bond Energy Data Summarized. J. Chem. Educ. 1995, 72, 423–424. [Google Scholar] [CrossRef]
- Ji, S.; Cao, W.; Yu, Y.; Xu, H. Dynamic Diselenide Bonds: Exchange Reaction Induced by Visible Light without Catalysis. Angew. Chem. Int. Ed. 2014, 53, 6781–6785. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.Q.; Ji, S.B.; Sun, C.X.; Liu, C.; Yu, Y.; Fu, Y.; Xu, H.P. Wavelength-Controlled Dynamic Metathesis: A Light-Driven Exchange Reaction between Disulfide and Diselenide Bonds. Angew Chem. Int. Ed. 2018, 57, 16426–16430. [Google Scholar] [CrossRef]
- Lin, W.; Xue, Z.; Wen, L.; Li, Y.; Liang, Z.; Xu, J.; Yang, C.; Gu, Y.; Zhang, J.; Zu, X.; et al. Mesoscopic simulations of drug-loaded diselenide crosslinked micelles: Stability, drug loading and release properties. Colloids Surf B Biointerfaces 2019, 182, 110313. [Google Scholar] [CrossRef]
- Sun, C.; Tan, Y.; Xu, H. From Selenite to Diselenide-Containing Drug Delivery Systems. ACS Mater. Lett. 2020, 2, 1173–1177. [Google Scholar] [CrossRef]
- Pan, S.; Yang, J.; Ji, S.; Li, T.; Xu, H. Cancer Therapy by Targeting Thioredoxin Reductase Based on Selenium-Containing Dynamic Covalent Bond. CCS Chem. 2020, 2, 225–235. [Google Scholar] [CrossRef]
- Ji, S.; Fan, F.; Sun, C.; Yu, Y.; Xu, H. Visible Light-Induced Plasticity of Shape Memory Polymers. ACS Appl. Mater. Interfaces 2017, 9, 33169–33175. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Yuan, Y.; Chen, F.; Sun, C.; Xu, H.; Chen, Y. Diselenide-Linked Polymers under Sonication. ACS Macro Lett. 2020, 9, 1547–1551. [Google Scholar] [CrossRef]
- Xia, J.; Zhao, P.; Pan, S.; Xu, H. Diselenide-Containing Polymeric Vesicles with Osmotic Pressure Response. ACS Macro Lett. 2019, 8, 629–633. [Google Scholar] [CrossRef]
- Liu, C.; Xia, J.H.; Ji, S.B.; Fan, Z.Y.; Xu, H.P. Visible-light-induced metathesis reaction between diselenide and ditelluride. Chem. Commun. 2019, 55, 2813–2816. [Google Scholar] [CrossRef]
- Yi, Y.; Xu, H.; Wang, L.; Cao, W.; Zhang, X. A new dynamic covalent bond of Se-N: Towards controlled self-assembly and disassembly. Chemistry 2013, 19, 9506–9510. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Fang, R.; Wang, D.; Wang, J.; Xu, H.; Wang, Y.; Zhang, X. Tuning polymeric amphiphilicity via Se-N interactions: Towards one-step double emulsion for highly selective enzyme mimics. Small 2015, 11, 1537–1541. [Google Scholar] [CrossRef] [PubMed]
- Franke, J.; Hertweck, C. Biomimetic Thioesters as Probes for Enzymatic Assembly Lines: Synthesis, Applications, and Challenges. Cell Chem. Biol. 2016, 23, 1179–1192. [Google Scholar] [CrossRef] [PubMed]
- Folikumah, M.Y.; Behl, M.; Lendlein, A. Thiol–Thioester Exchange Reactions in Precursors Enable pH-Triggered Hydrogel Formation. Biomacromolecules 2021, 22, 1875–1884. [Google Scholar] [CrossRef]
- Hupe, D.J.; Jencks, W.P. Nonlinear structure-reactivity correlations. Acyl transfer between sulfur and oxygen nucleophiles. J. Am. Chem. Soc. 1977, 99, 451–464. [Google Scholar] [CrossRef]
- Brown, T.E.; Carberry, B.J.; Worrell, B.T.; Dudaryeva, O.Y.; McBride, M.K.; Bowman, C.N.; Anseth, K.S. Photopolymerized dynamic hydrogels with tunable viscoelastic properties through thioester exchange. Biomaterials 2018, 178, 496–503. [Google Scholar] [CrossRef]
- Liu, F.; Lagares, D.; Choi, K.M.; Stopfer, L.; Marinkovic, A.; Vrbanac, V.; Probst, C.K.; Hiemer, S.E.; Sisson, T.H.; Horowitz, J.C.; et al. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am. J. Physiol.-Lung Cell Mol. Physiol. 2015, 308, L344–L357. [Google Scholar] [CrossRef]
- Ghobril, C.; Charoen, K.; Rodriguez, E.K.; Nazarian, A.; Grinstaff, M.W. A Dendritic Thioester Hydrogel Based on Thiol-Thioester Exchange as a Dissolvable Sealant System for Wound Closure. Angew. Chem.-Int. Ed. 2013, 52, 14070–14074. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. Engl. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Chen, X.; Dam, M.A.; Ono, K.; Mal, A.K.; Shen, H.; Nutt, S.R.; Sheran, K.; Wudl, F. A Thermally Re-mendable Cross-Linked Polymeric Material. Science 2002, 295, 1698–1702. [Google Scholar] [CrossRef]
- Da Cunha, L.; Garrigues, B. A new study on the Diels-Alder reaction between anthracene and maleic anhydride under ultrasound. Bull. Des Soc. Chim. Belg. 1997, 106, 817–823. [Google Scholar]
- Duan, H.-Y.; Wang, Y.-X.; Wang, L.-J.; Min, Y.-Q.; Zhang, X.-H.; Du, B.-Y. An Investigation of the Selective Chain Scission at Centered Diels–Alder Mechanophore under Ultrasonication. Macromolecules 2017, 50, 1353–1361. [Google Scholar] [CrossRef]
- Bailey, S.J.; Barney, C.W.; Sinha, N.J.; Pangali, S.V.; Hawker, C.J.; Helgeson, M.E.; Valentine, M.T.; Read de Alaniz, J. Rational mechanochemical design of Diels–Alder crosslinked biocompatible hydrogels with enhanced properties. Mater. Horiz. 2022, 9, 1947–1953. [Google Scholar] [CrossRef] [PubMed]
- Sander, E.G.; Jencks, W.P. Equilibria for additions to the carbonyl group. J. Am. Chem. Soc. 1968, 90, 6154–6162. [Google Scholar] [CrossRef]
- Kool, E.T.; Crisalli, P.; Chan, K.M. Fast alpha nucleophiles: Structures that undergo rapid hydrazone/oxime formation at neutral pH. Org. Lett. 2014, 16, 1454–1457. [Google Scholar] [CrossRef]
- Kolmel, D.K.; Kool, E.T. Oximes and Hydrazones in Bioconjugation: Mechanism and Catalysis. Chem. Rev. 2017, 117, 10358–10376. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Y.; Hsu, S.-H. Hydrogels Based on Schiff Base Linkages for Biomedical Applications. Molecules 2019, 24, 3005. [Google Scholar] [CrossRef]
- Zhang, Z.; He, C.; Chen, X. Hydrogels based on pH-responsive reversible carbon–nitrogen double-bond linkages for biomedical applications. Mater. Chem. Front. 2018, 2, 1765–1778. [Google Scholar] [CrossRef]
- Kalia, J.; Raines, R.T. Hydrolytic stability of hydrazones and oximes. Angew. Chem. Int. Ed. Engl. 2008, 47, 7523–7526. [Google Scholar] [CrossRef]
- Tang, S.; Richardson, B.M.; Anseth, K.S. Dynamic covalent hydrogels as biomaterials to mimic the viscoelasticity of soft tissues. Prog. Mater. Sci. 2021, 120, 100738. [Google Scholar] [CrossRef]
- Jencks, W.P. Studies on the Mechanism of Oxime and Semicarbazone Formation1. J. Am. Chem. Soc. 1959, 81, 475–481. [Google Scholar] [CrossRef]
- Grover, G.N.; Lam, J.; Nguyen, T.H.; Segura, T.; Maynard, H.D. Biocompatible hydrogels by oxime Click chemistry. Biomacromolecules 2012, 13, 3013–3017. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Zhang, Y.J. Boronic acid-containing hydrogels: Synthesis and their applications. Chem. Soc. Rev. 2013, 42, 8106–8121. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Fullenkamp, D.E.; Rivera, J.G.; Messersmith, P.B. pH responsive self-healing hydrogels formed by boronate–catechol complexation. Chem. Commun. 2011, 47, 7497–7499. [Google Scholar] [CrossRef]
- Wang, R.; Bian, Z.; Zhan, D.; Wu, Z.; Yao, Q.; Zhang, G. Boronic acid-based sensors for small-molecule reactive species: A review. Dye. Pigment. 2021, 185, 108885. [Google Scholar] [CrossRef]
- Williams, G.T.; Sedgwick, A.C.; Sen, S.; Gwynne, L.; Gardiner, J.E.; Brewster, J.T.; Hiscock, J.R.; James, T.D.; Jenkins, A.T.A.; Sessler, J.L. Boronate ester cross-linked PVA hydrogels for the capture and H2O2-mediated release of active fluorophores. Chem. Commun. 2020, 56, 5516–5519. [Google Scholar] [CrossRef]
- Springsteen, G.; Wang, B. A detailed examination of boronic acid–diol complexation. Tetrahedron 2002, 58, 5291–5300. [Google Scholar] [CrossRef]
- Tseng, T.-C.; Hsieh, F.-Y.; Theato, P.; Wei, Y.; Hsu, S.-H. Glucose-sensitive self-healing hydrogel as sacrificial materials to fabricate vascularized constructs. Biomaterials 2017, 133, 20–28. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, W.; Wu, D.; Nagao, M.; Hall, D.G.; Thundat, T.; Narain, R. Injectable Self-Healing Zwitterionic Hydrogels Based on Dynamic Benzoxaborole-Sugar Interactions with Tunable Mechanical Properties. Biomacromolecules 2018, 19, 596–605. [Google Scholar] [CrossRef]
- Wei, W.; Sun, Y.; Zhu, M.; Liu, X.; Sun, P.; Wang, F.; Gui, Q.; Meng, W.; Cao, Y.; Zhao, J. Structural Insights and the Surprisingly Low Mechanical Stability of the Au-S Bond in the Gold-Specific Protein GolB. J. Am. Chem. Soc. 2015, 137, 15358–15361. [Google Scholar] [CrossRef]
- Xiang, W.; Li, Z.; Xu, C.-Q.; Li, J.; Zhang, W.; Xu, H. Quantifying the Bonding Strength of Gold-Chalcogen Bonds in Block Copolymer Systems. Chem.–Asian J. 2019, 14, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Muddassir, M. Blue light-induced low mechanical stability of ruthenium-based coordination bonds: An AFM-based single-molecule force spectroscopy study. RSC Adv. 2020, 10, 40543–40551. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wen, J.; Qin, M.; Cao, Y.; Ma, H.; Wang, W. Single-Molecule Mechanics of Catechol-Iron Coordination Bonds. ACS Biomater. Sci. Eng. 2017, 3, 979–989. [Google Scholar] [CrossRef]
- Xue, X.; Hu, Y.; Wang, S.; Chen, X.; Jiang, Y.; Su, J. Fabrication of physical and chemical crosslinked hydrogels for bone tissue engineering. Bioact. Mater. 2022, 12, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cheng, R.; Zhao, X.; Zhang, Y.; Tam, A.; Yan, Y.; Shen, H.; Zhang, Y.S.; Qi, J.; Feng, Y.; et al. An injectable self-healing coordinative hydrogel with antibacterial and angiogenic properties for diabetic skin wound repair. NPG Asia Mater. 2019, 11, 3. [Google Scholar] [CrossRef]
- García, F.; Pelss, J.; Zuilhof, H.; Smulders, M.M.J. Multi-responsive coordination polymers utilising metal-stabilised, dynamic covalent imine bonds. Chem. Commun. 2016, 52, 9059–9062. [Google Scholar] [CrossRef]
- Yang, R.; Li, G.; Zhuang, C.; Yu, P.; Ye, T.; Zhang, Y.; Shang, P.; Huang, J.; Cai, M.; Wang, L.; et al. Gradient bimetallic ion-based hydrogels for tissue microstructure reconstruction of tendon-to-bone insertion. Sci. Adv. 2021, 7, 3816. [Google Scholar] [CrossRef]
- Li, X.; Egervari, G.; Wang, Y.; Berger, S.L.; Lu, Z. Regulation of chromatin and gene expression by metabolic enzymes and metabolites. Nat. Rev. Mol. Cell Biol. 2018, 19, 563–578. [Google Scholar] [CrossRef]
- Alvarenga, E.C.; Fonseca, M.C.; Carvalho, C.C.; Florentino, R.M.; Franca, A.; Matias, E.; Guimaraes, P.B.; Batista, C.; Freire, V.; Carmona, A.K.; et al. Angiotensin Converting Enzyme Regulates Cell Proliferation and Migration. PLoS ONE 2016, 11, e0165371. [Google Scholar]
- Wang, L.; Wang, C.C. Oxidative protein folding fidelity and redoxtasis in the endoplasmic reticulum. Trends Biochem. Sci. 2022. Available online: https://pubmed.ncbi.nlm.nih.gov/35871147/ (accessed on 20 July 2022). [CrossRef]
- Wei, Q.; Xu, W.; Zhang, Q.; Zhang, S.; Cheng, L.; Wang, Q. Dynamic hydrogels produced via monoamine oxidase B-catalyzed deamination and aldimine crosslinking for 3D printing. J. Mater. Chem. B 2017, 5, 5092–5095. [Google Scholar] [CrossRef] [PubMed]
- Hyoudou, K.; Nishikawa, M.; Ikemura, M.; Kobayashi, Y.; Mendelsohn, A.; Miyazaki, N.; Tabata, Y.; Yamashita, F.; Hashida, M. Cationized catalase-loaded hydrogel for growth inhibition of peritoneally disseminated tumor cells. J. Control Release 2007, 122, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Gong, Z.; Wang, J.; Wang, C. Enzymatic hydrogelation of self-assembling peptide I4K2 and its antibacterial and drug sustained-release activities. RSC Adv. 2017, 7, 48631–48638. [Google Scholar] [CrossRef]
- Mai, A.Q.; Bánsági, T.; Taylor, A.F.; Pojman, J.A. Reaction-diffusion hydrogels from urease enzyme particles for patterned coatings. Commun. Chem. 2021, 4, 101. [Google Scholar] [CrossRef]
- Kutcherlapati, S.N.; Yeole, N.; Jana, T. Urease immobilized polymer hydrogel: Long-term stability and enhancement of enzymatic activity. J. Colloid Interface Sci. 2016, 463, 164–172. [Google Scholar] [CrossRef]
- Han, Y.; Yu, S.; Liu, L.; Zhao, S.; Yang, T.; Yang, Y.; Fang, Y.; Lv, S. Silk fibroin-based hydrogels as a protective matrix for stabilization of enzymes against pH denaturation. Mol. Catal. 2018, 457, 24–32. [Google Scholar] [CrossRef]
- Yao, R.; Li, X.; Xiao, N.; Weng, W.; Zhang, W. Single-molecule observation of mechanical isomerization of spirothiopyran and subsequent Click addition. Nano Res. 2020, 14, 2654–2658. [Google Scholar] [CrossRef]
- Belowich, M.E.; Stoddart, J.F. Dynamic imine chemistry. Chem. Soc. Rev. 2012, 41, 2003–2024. [Google Scholar] [CrossRef]
- Chakma, P.; Konkolewicz, D. Dynamic Covalent Bonds in Polymeric Materials. Angew. Chem. Int. Ed. Engl. 2019, 58, 9682–9695. [Google Scholar] [CrossRef]
- Roy, N.; Bruchmann, B.; Lehn, J.M. DYNAMERS: Dynamic polymers as self-healing materials. Chem. Soc. Rev. 2015, 44, 3786–3807. [Google Scholar] [CrossRef]
- Lu, L.; Pan, J.; Li, G. Recyclable high-performance epoxy based on transesterification reaction. J. Mater. Chem. A 2017, 5, 21505–21513. [Google Scholar] [CrossRef]
- Zheng, N.; Xu, Y.; Zhao, Q.; Xie, T. Dynamic Covalent Polymer Networks: A Molecular Platform for Designing Functions beyond Chemical Recycling and Self-Healing. Chem. Rev. 2021, 121, 1716–1745. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Dong, J.; Luo, Y.; Zhao, Q.; Xie, T. Dynamic Covalent Polymer Networks: From Old Chemistry to Modern Day Innovations. Adv. Mater. 2017, 29, 1606100. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Coste, M.; Diaconu, A.; Barboiu, M.; Ulrich, S. Cationic dynamic covalent polymers for gene transfection. J. Mater. Chem. B 2020, 8, 9385–9403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qi, Y.; Ulrich, S.; Barboiu, M.; Ramström, O. Dynamic Covalent Polymers for Biomedical Applications. Mater. Chem. Front. 2020, 4, 489–506. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, J.; Xiao, F.; Zhao, D.; Luan, Y. Disulfide bond based polymeric drug carriers for cancer chemotherapy and relevant redox environments in mammals. Med. Res. Rev. 2018, 38, 1485–1510. [Google Scholar] [CrossRef]
- Cao, Y.; Yao, Y.; Li, Y.; Yang, X.; Cao, Z.; Yang, G. Tunable keratin hydrogel based on disulfide shuffling strategy for drug delivery and tissue engineering. J. Colloid Interface Sci. 2019, 544, 121–129. [Google Scholar] [CrossRef]
- Bansal, A.; Simon, M.C. Glutathione metabolism in cancer progression and treatment resistance. J. Cell Biol. 2018, 217, 2291–2298. [Google Scholar] [CrossRef]
- Sun, P.; Huang, T.; Wang, X.; Wang, G.; Liu, Z.; Chen, G.; Fan, Q. Dynamic-Covalent Hydrogel with NIR-Triggered Drug Delivery for Localized Chemo-Photothermal Combination Therapy. Biomacromolecules 2020, 21, 556–565. [Google Scholar] [CrossRef]
- Otsuka, H.; Nagano, S.; Kobashi, Y.; Maeda, T.; Takahara, A. A dynamic covalent polymer driven by disulfide metathesis under photoirradiation. Chem. Commun. 2010, 46, 1150–1152. [Google Scholar] [CrossRef]
- Winne, J.M.; Leibler, L.; Du Prez, F.E. Dynamic covalent chemistry in polymer networks: A mechanistic perspective. Polym. Chem. 2019, 10, 6091–6108. [Google Scholar] [CrossRef]
- Nakajima, T. Generalization of the sacrificial bond principle for gel and elastomer toughening. Polym. J. 2017, 49, 477–485. [Google Scholar] [CrossRef]
- Duval, K.; Grover, H.; Han, L.H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiol. Bethesda 2017, 32, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Mih, J.D.; Marinkovic, A.; Liu, F.; Sharif, A.S.; Tschumperlin, D.J. Matrix stiffness reverses the effect of actomyosin tension on cell proliferation. J. Cell Sci. 2012, 125 Pt 24, 5974–5983. [Google Scholar] [CrossRef] [Green Version]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef]
- Yang, C.; DelRio Frank, W.; Ma, H.; Killaars Anouk, R.; Basta Lena, P.; Kyburz Kyle, A.; Anseth Kristi, S. Spatially patterned matrix elasticity directs stem cell fate. Proc. Natl. Acad. Sci. 2016, 113, E4439–E4445. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A.; Mooney, D.J.; Shenoy, V.B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 2020, 584, 535–546. [Google Scholar] [CrossRef]
- Wu, D.; Lei, H.; Xie, X.; Zhou, L.; Zheng, P.; Cao, Y.; Zhang, Y. Self-sorting double network hydrogels with photo-definable biochemical cues as artificial synthetic extracellular matrix. Nano Res. 2020, 15, 4294–4301. [Google Scholar] [CrossRef]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef]
- Nam, S.; Hu, K.H.; Butte, M.J.; Chaudhuri, O. Strain-enhanced stress relaxation impacts nonlinear elasticity in collagen gels. Proc. Natl. Acad. Sci. USA 2016, 113, 5492–5497. [Google Scholar] [CrossRef]
- Munster, S.; Jawerth, L.M.; Leslie, B.A.; Weitz, J.I.; Fabry, B.; Weitz, D.A. Strain history dependence of the nonlinear stress response of fibrin and collagen networks. Proc. Natl. Acad. Sci. USA 2013, 110, 12197–12202. [Google Scholar] [CrossRef]
- Lou, J.; Friedowitz, S.; Will, K.; Qin, J.; Xia, Y. Predictably Engineering the Viscoelastic Behavior of Dynamic Hydrogels via Correlation with Molecular Parameters. Adv. Mater. 2021, 33, e2104460. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Morán, H.; Ahmadi, A.; Vogler, B.; Roh, K.-H. Oxime Cross-Linked Alginate Hydrogels with Tunable Stress Relaxation. Biomacromolecules 2019, 20, 4419–4429. [Google Scholar] [CrossRef]
- Mihajlovic, M.; Rikkers, M.; Mihajlovic, M.; Viola, M.; Schuiringa, G.; Ilochonwu, B.C.; Masereeuw, R.; Vonk, L.; Malda, J.; Ito, K.; et al. Viscoelastic Chondroitin Sulfate and Hyaluronic Acid Double-Network Hydrogels with Reversible Cross-Links. Biomacromolecules 2022, 23, 1350–1365. [Google Scholar] [CrossRef]
- Ruiter, F.A.A.; Morgan, F.L.C.; Roumans, N.; Schumacher, A.; Slaats, G.; Moroni, L.; LaPointe, V.L.S.; Baker, M. Soft, dynamic hydrogel confinement improves kidney organoid lumen morphology and reduces epithelial—Mesenchymal transition in culture. bioRxiv 2021. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, D.D.; Domaille, D.W.; Brown, T.E.; Kyburz, K.A.; Kiyotake, E.; Cha, J.N.; Anseth, K.S. Measuring cellular forces using bis-aliphatic hydrazone crosslinked stress-relaxing hydrogels. Soft Matter. 2014, 10, 9230–9236. [Google Scholar] [CrossRef]
- Baker, A.E.G.; Tam, R.Y.; Shoichet, M.S. Independently Tuning the Biochemical and Mechanical Properties of 3D Hyaluronan-Based Hydrogels with Oxime and Diels–Alder Chemistry to Culture Breast Cancer Spheroids. Biomacromolecules 2017, 18, 4373–4384. [Google Scholar] [CrossRef] [PubMed]
- Devi, V.K.A.; Shyam, R.A.-O.; Palaniappan, A.A.-O.; Jaiswal, A.K.; Oh, T.H.; Nathanael, A.A.-O. Self-Healing Hydrogels: Preparation, Mechanism and Advancement in Biomedical Applications. Polymers 2021, 13, 3782. [Google Scholar] [CrossRef]
- Hai, M.; Zhang, Q.; Li, Z.; Cheng, M.; Kuehne, A.J.C.; Shi, F. Visualizing polymer diffusion in hydrogel self-healing. Supramol. Chem. 2022, 1, 100009. [Google Scholar] [CrossRef]
- Li, W.; Lu, S.; Zhao, M.; Lin, X.; Zhang, M.; Xiao, H.; Liu, K.; Huang, L.; Chen, L.; Ouyang, X.; et al. Self-Healing Cellulose Nanocrystals-Containing Gels via Reshuffling of Thiuram Disulfide Bonds. Polymers 2018, 12, 1392. [Google Scholar] [CrossRef]
- Chen, M.; Tian, J.; Liu, Y.; Cao, H.; Li, R.; Wang, J.; Wu, J.; Zhang, Q. Dynamic covalent constructed self-healing hydrogel for sequential delivery of antibacterial agent and growth factor in wound healing. Chem. Eng. J. 2019, 373, 413–424. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, F.; Li, Z.; Lin, S.; Chen, L.; Liu, L.; Chen, Y. Hydrogel Cross-Linked with Dynamic Covalent Bonding and Micellization for Promoting Burn Wound Healing. ACS Appl. Mater. Interfaces 2018, 10, 25194–25202. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Han, L.; Huang, H.; Xuan, X.; Pan, G.; Wan, L.; Lu, T.; Xu, M.; Pan, L. A direction-aware and ultrafast self-healing dual network hydrogel for a flexible electronic skin strain sensor. J. Mater. Chem. A 2020, 8, 26109–26118. [Google Scholar] [CrossRef]
- Zou, Z.; Zhu, C.; Li, Y.; Lei, X.; Zhang, W.; Xiao, J. Rehealable, fully recyclable, and malleable electronic skin enabled by dynamic covalent thermoset nanocomposite. Sci. Adv. 2018, 4, eaaq0508. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Ren, Z.J.; Liu, F.F.; Zhao, L.; Ling, Q.J.; Gu, H.B. Multifunctional Self-Healing Dual Network Hydrogels Constructed via Host-Guest Interaction and Dynamic Covalent Bond as Wearable Strain Sensors for Monitoring Human and Organ Motions. Acs Appl. Mater. Interfaces 2021, 13, 14625–14635. [Google Scholar] [CrossRef]
- Peng, H.; Lv, Y.; Wei, G.; Zhou, J.; Gao, X.; Sun, K.; Ma, G.; Lei, Z. A flexible and self-healing hydrogel electrolyte for smart supercapacitor. J. Power Sources 2019, 431, 210–219. [Google Scholar] [CrossRef]
- Wang, P.; Pei, D.; Wang, Z.; Li, M.; Ma, X.; You, J.; Li, C. Biocompatible and self-healing ionic gel skin as shape-adaptable and skin-adhering sensor of human motions. Chem. Eng. J. 2020, 398, 125540. [Google Scholar] [CrossRef]
- Sun, Y.; Nan, D.; Jin, H.; Qu, X. Recent advances of injectable hydrogels for drug delivery and tissue engineering applications. Polym. Test. 2019, 81, 106283. [Google Scholar] [CrossRef]
- Béduer, A.; Bonini, F.; Verheyen, C.A.; Genta, M.; Martins, M.; Brefie-Guth, J.; Tratwal, J.; Filippova, A.; Burch, P.; Naveiras, O.; et al. An Injectable Meta-Biomaterial: From Design and Simulation to In Vivo Shaping and Tissue Induction. Adv. Mater. 2021, 33, 2102350. [Google Scholar] [CrossRef]
- Liu, L.; Xiang, Y.; Wang, Z.; Yang, X.; Yu, X.; Lu, Y.; Deng, L.; Cui, W. Adhesive liposomes loaded onto an injectable, self-healing and antibacterial hydrogel for promoting bone reconstruction. NPG Asia Mater. 2019, 11, 81. [Google Scholar] [CrossRef]
- Yu, Z.; Xu, Y.; Yao, H.; Si, X.; Ji, G.; Dong, S.; Zhao, J.; Tang, Z.; Fang, X.; Song, W.; et al. A simple and general strategy for postsurgical personalized cancer vaccine therapy based on an injectable dynamic covalent hydrogel. Biomater. Sci. 2021, 9, 6879–6888. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Xie, J.; Liu, Y.; Xiao, M.; Li, Y.; Yang, J.; Yang, J.; Liu, W.A.-O. Injectable Hyaluronic Acid Hydrogel Loaded with Functionalized Human Mesenchymal Stem Cell Aggregates for Repairing Infarcted Myocardium. ACS Biomater. Sci. Eng. 2020, 6, 6926–6937. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Zheng, J.; Liu, L.; Zhang, Q. Three-Dimensional Printing Self-Healing Dynamic/Photocrosslinking Gelatin-Hyaluronic Acid Double-Network Hydrogel for Tissue Engineering. ACS Omega 2022, 7, 12076–12088. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; He, Y.; Zhang, F.; Liu, Y.; Leng, J. A Review of Shape Memory Polymers and Composites: Mechanisms, Materials, and Applications. Adv. Mater. 2020, 33, e2000713. [Google Scholar] [CrossRef] [PubMed]
- Löwenberg, C.; Balk, M.; Wischke, C.; Behl, M.; Lendlein, A. Shape-Memory Hydrogels: Evolution of Structural Principles To Enable Shape Switching of Hydrophilic Polymer Networks. Acc. Chem. Res. 2017, 50, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Haleem, A.; Javaid, M.; Singh, R.P.; Suman, R. Significant roles of 4D printing using smart materials in the field of manufacturing. Adv. Ind. Eng. Polym. Res. 2021, 4, 301–311. [Google Scholar] [CrossRef]
- Kuang, X.; Qi, H.J. Modular 4D Printing Assisted by Dynamic Chemical Bonds. Matter 2020, 2, 1080–1082. [Google Scholar] [CrossRef]
- Liu, C.; Tan, Y.; He, C.; Ji, S.; Xu, H. Unconstrained 3D Shape Programming with Light-Induced Stress Gradient. Adv. Mater. 2021, 33, e2105194. [Google Scholar] [CrossRef]
- Jin, B.; Song, H.; Jiang, R.; Song, J.; Zhao, Q.; Xie, T. Programming a crystalline shape memory polymer network with thermo-and photo-reversible bonds toward a single-component soft robot. Sci. Adv. 2018, 4, eaao3865. [Google Scholar] [CrossRef]
- Cui, C.; An, L.; Zhang, Z.; Ji, M.; Chen, K.; Yang, Y.; Su, Q.; Wang, F.; Cheng, Y.; Zhang, Y. Reconfigurable 4D Printing of Reprocessable and Mechanically Strong Polythiourethane Covalent Adaptable Networks. Adv. Funct. Mater. 2022, 32, 2203720. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Cao, Y.; Lei, H. Dynamic Covalent Hydrogels: Strong yet Dynamic. Gels 2022, 8, 577. https://doi.org/10.3390/gels8090577
Han Y, Cao Y, Lei H. Dynamic Covalent Hydrogels: Strong yet Dynamic. Gels. 2022; 8(9):577. https://doi.org/10.3390/gels8090577
Chicago/Turabian StyleHan, Yueying, Yi Cao, and Hai Lei. 2022. "Dynamic Covalent Hydrogels: Strong yet Dynamic" Gels 8, no. 9: 577. https://doi.org/10.3390/gels8090577
APA StyleHan, Y., Cao, Y., & Lei, H. (2022). Dynamic Covalent Hydrogels: Strong yet Dynamic. Gels, 8(9), 577. https://doi.org/10.3390/gels8090577






