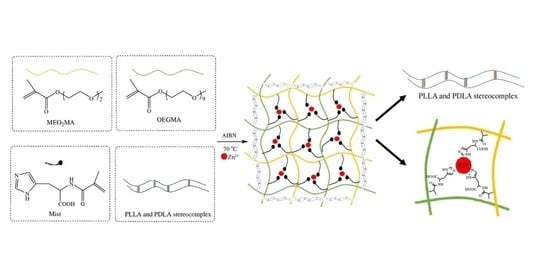
A Temperature/pH Double-Responsive and Physical Double-Crosslinked Hydrogel Based on PLA and Histidine
Abstract
:1. Introduction
2. Results and Discussion
2.1. HEMA-PLLA30/PDLA30 and Mist Monomers
2.1.1. HEMA-PLLA30/PDLA30 Macromonomers
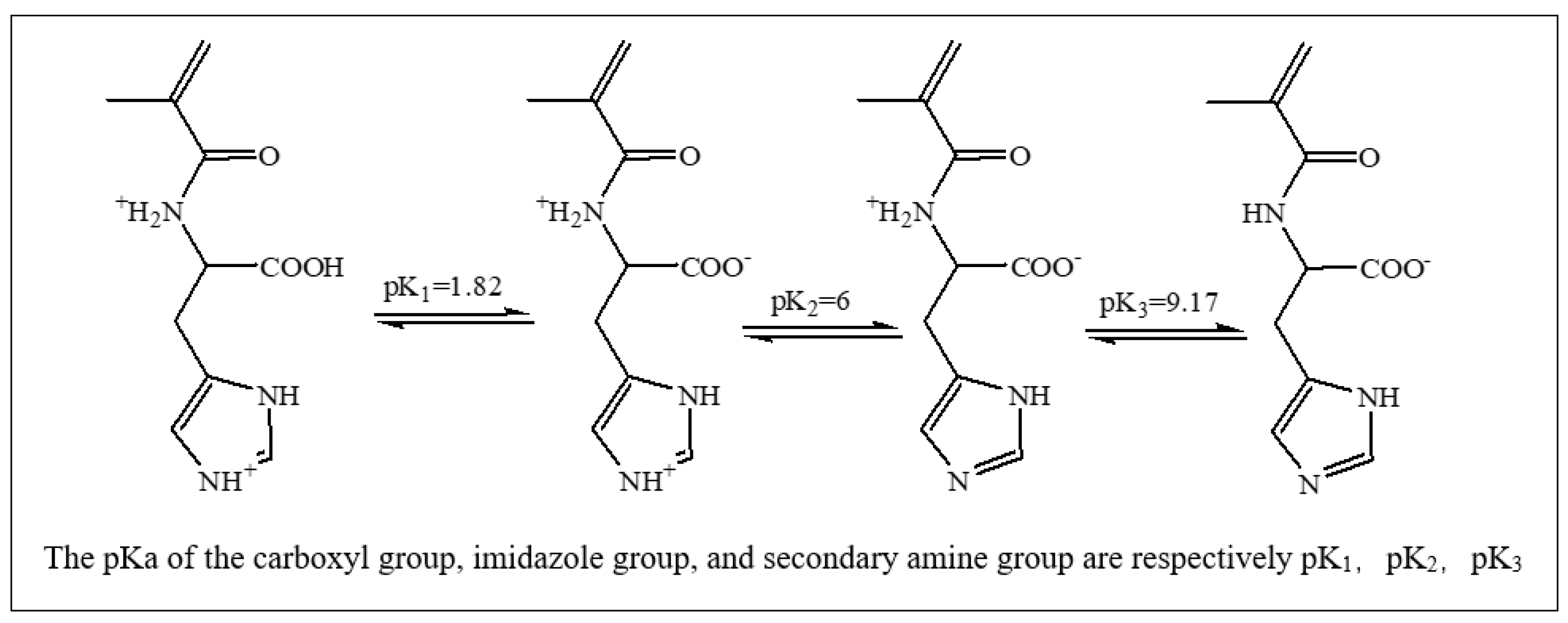
2.1.2. Mist Monomer
2.2. Synthesis and Characterization of Temperature/pH Double-Responsive and Physical Double-Crosslinked Hydrogel
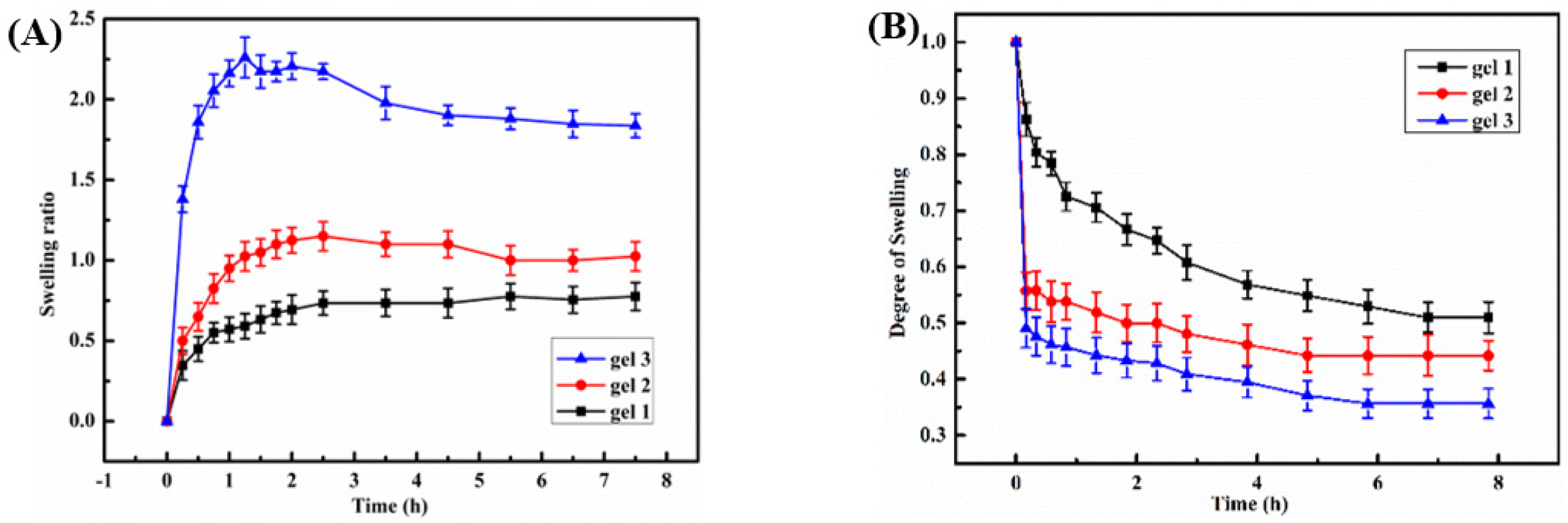
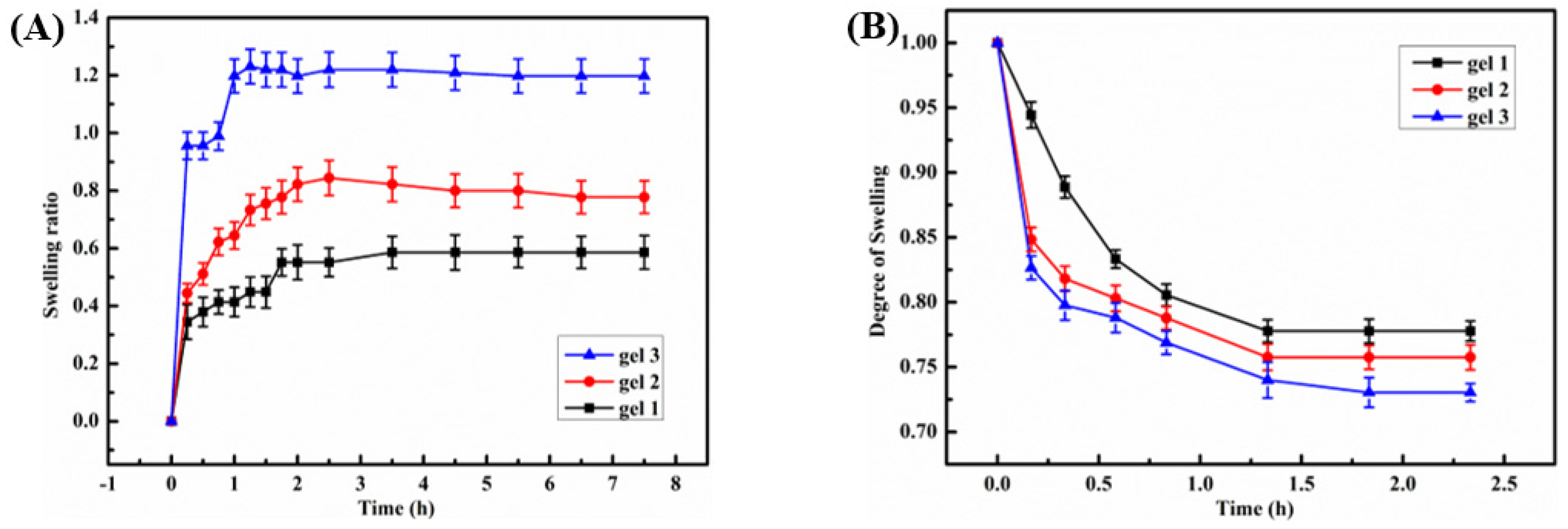
2.3. Temperature Sensitivity of the Hydrogels
2.4. The pH Sensitivity of the Hydrogel
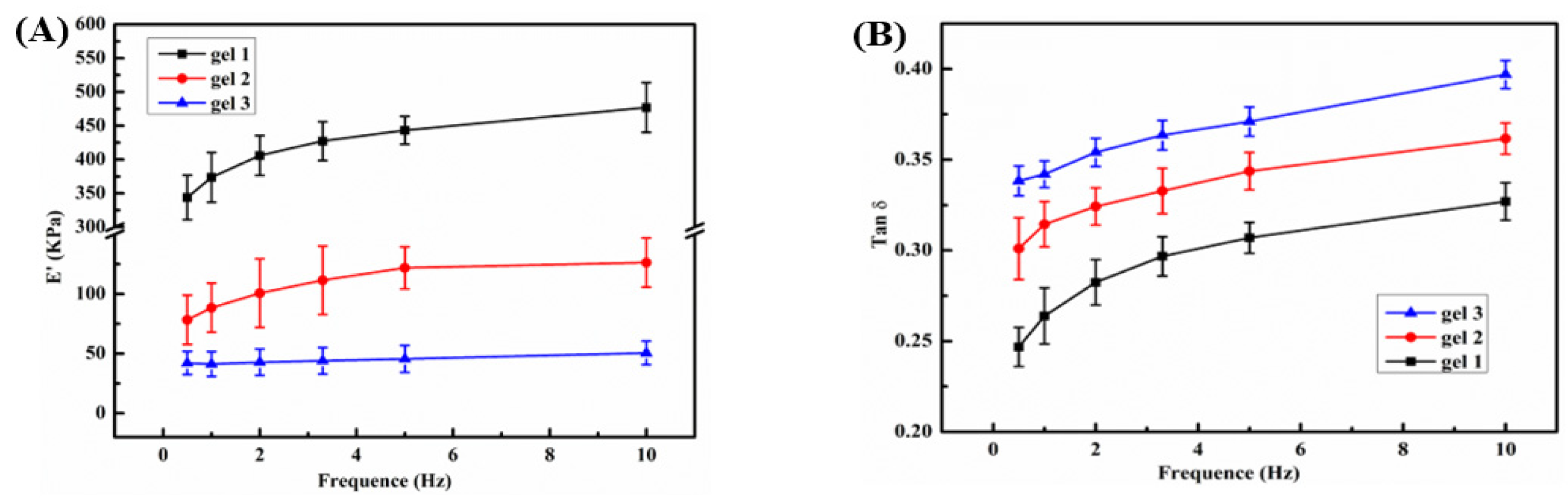
2.5. Mechanical Properties of the Hydrogel
2.6. Thermogravimetric Analysis (TGA) of Hydrogels
2.7. Environmental Scanning Electron Microscope (ESEM) Analysis of Hydrogels
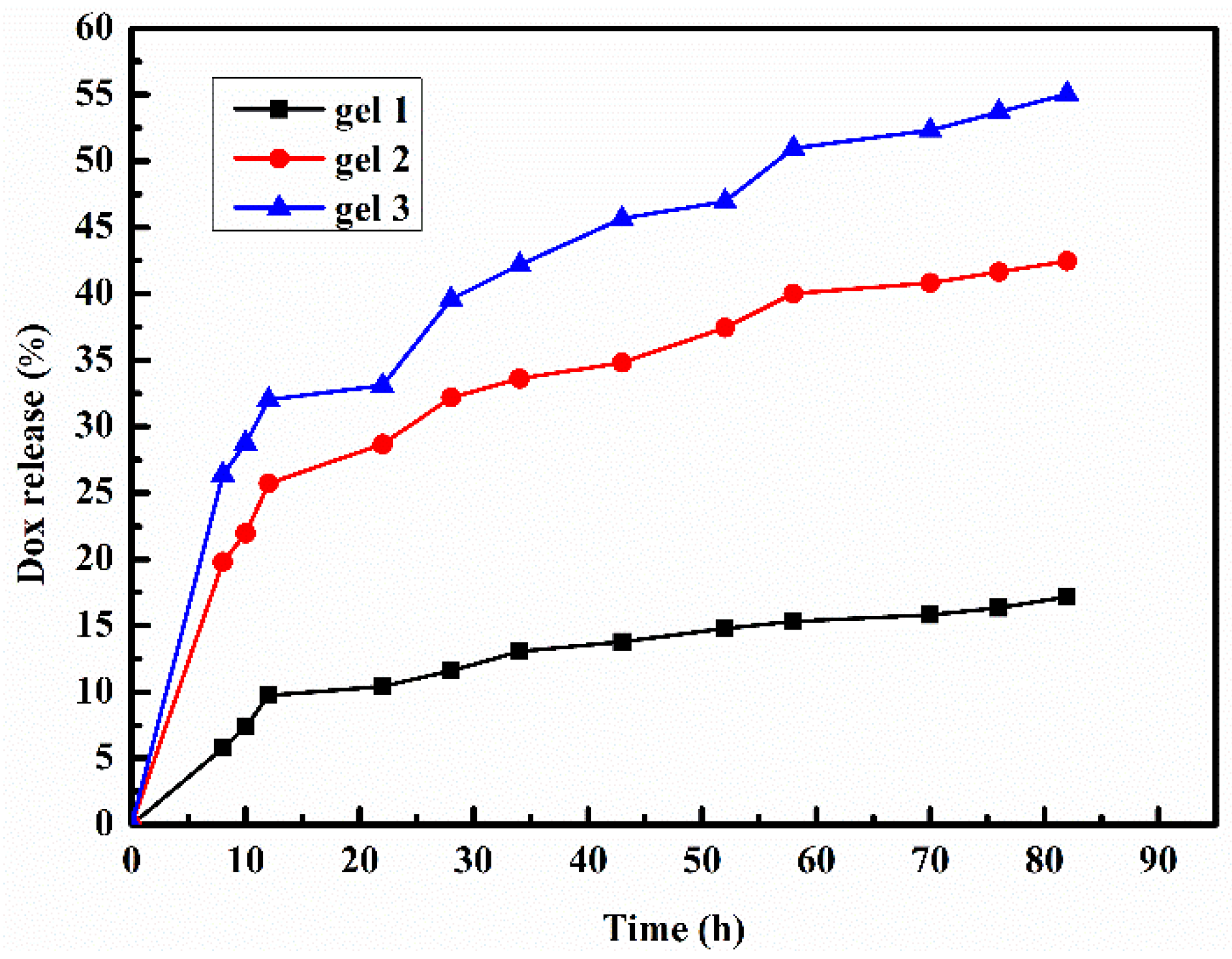
2.8. Drug Release of the Hydrogel
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis
4.2.1. Synthesis of HEMA-PLLA30 and HEMA-PDLA30 Macromonomers
4.2.2. Synthesis of Mist Monomer
4.2.3. Synthesis of Hydrogen-Bonded Hydrogel
4.2.4. Synthesis of a Coordination-Bond Hydrogel
4.2.5. Synthesis of Double-Crosslinked Hydrogel
4.3. Methods
4.3.1. Spectral Characterization
4.3.2. Thermogravimetric Analysis of the Hydrogels
4.3.3. ESEM Analysis of the Hydrogels
4.3.4. Mechanical Properties Analysis of the Hydrogels
4.3.5. Swelling−Deswelling Behavior of the Hydrogels
4.3.6. Drug Release of the Hydrogels
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, X.; Wei, C.; Wu, C.; Zhang, W. Customized Hydrogel for Sustained Release of Highly Water-Soluble Drugs. ACS Omega 2022, 7, 8493–8497. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, T.; Peng, L.; Sun, Q.; Wei, Y.; Han, B. Advancements in Hydrogel-Based Drug Sustained Release Systems for Bone Tissue Engineering. Front. Pharmacol. 2020, 11, 622. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Du, J.; Sun, J.; Guo, J.; Hu, X.; Wang, C. Transparent and UV Blocking Structural Colored Hydrogel for Contact Lenses. ACS Appl. Mater. Interfaces 2020, 12, 39639–39648. [Google Scholar] [CrossRef]
- Ma, S.; Hu, H.; Wu, J.; Li, X.; Ma, X.; Zhao, Z.; Liu, Z.; Wu, C.; Zhao, B.; Wang, Y.; et al. Functional Extracellular Matrix Hydrogel Modified with MSC- Derived Small Extracellular Vesicles for Chronic Wound Healing. Cell Prolif. 2022, 55, 13196. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Chang, S.S.; Lee, J.S. Anti-Aging Potential of Substance P-Based Hydrogel for Human Skin Longevity. Int. J. Mol. Sci. 2019, 20, 4453. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Yang, F.; Hu, L.; Wang, H.; Wang, Y.; Wang, Z.; Pan, S. Construction of Polysaccharide Based Physically Crosslinked Double-Network Antibacterial Hydrogel. Mater. Lett. 2022, 316, 132048. [Google Scholar] [CrossRef]
- Shah, R.A.; Runge, T.; Ostertag, T.W.; Tang, S.; Dziubla, T.D.; Hilt, J.Z. Development of Temperature-Responsive Polymeric Gels with Physical Crosslinking Due to Tntermolecular π–π Interactions. Polym. Int. 2022, 71, 292–300. [Google Scholar] [CrossRef]
- Tong, J.; Zhou, H.; Zhou, J.; Chen, Y.; Shi, J.; Zhang, J.; Liang, X.; Du, T. Design and Evaluation of Chitosan-Amino Acid Thermosensitive Hydrogel. Mar. Life Sci. Technol. 2022, 4, 74–87. [Google Scholar] [CrossRef]
- Che, Y.; Li, D.; Liu, Y.; Ma, Q.; Tan, Y.; Yue, Q.; Meng, F. Physically Cross-Linked pH-Responsive Chitosan-Based Hydrogels with Enhanced Mechanical Performance for Controlled Drug Delivery. RSC Adv. 2016, 6, 106035–106045. [Google Scholar] [CrossRef]
- Lee, J.H.; Tachibana, T.; Yamana, K.; Kawasaki, R.; Yabuki, A. Simple Formation of Cancer Drug-Containing Self-Assembled Hydrogels with Temperature and pH-Responsive Release. Langmuir 2021, 37, 11269–11275. [Google Scholar] [CrossRef]
- Salawi, A.; Khan, A.; Zaman, A.; Riaz, T.; Ihsan, H.; Butt, M.H.; Aman, W.; Khan, R.; Majeed, I.; Almoshari, Y.; et al. Development of Statistically Optimized Chemically Cross-Linked Hydrogel for the Sustained-Release Delivery of Favipiravir. Polymers 2022, 14, 2369. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Kwon, M.; Choi, H.E.; Hahn, S.W.; Kim, K.S. Non-Invasive Topical Drug-Delivery System Using Hyaluronate Nanogels Crosslinked via Click Chemistry. Materials 2021, 14, 1504. [Google Scholar] [CrossRef] [PubMed]
- Hoang, H.T.; Vu, T.T.; Karthika, V.; Jo, S.H.; Jo, Y.J.; Seo, J.W.; Oh, C.W.; Park, S.H.; Lim, K.T. Dual Cross-Linked Chitosan/Alginate Hydrogels Prepared by Nb-Tz ‘Click’ Reaction for pH Responsive Drug Delivery. Carbohydr. Polym. 2022, 288, 119389. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Hu, S.; Zhang, S.; Dong, S.; Hu, J.; Kang, L.; Yang, X. A Double-Layer Hydrogel Based on Alginate-Carboxymethyl Cellulose and Synthetic Polymer as Sustained Drug Delivery System. Sci. Rep. 2021, 11, 9142. [Google Scholar] [CrossRef]
- Xian, S.; Webber, M.J. Temperature-Responsive Supramolecular Hydrogels. J. Mater. Chem. B 2020, 8, 9197–9211. [Google Scholar] [CrossRef]
- Hollingshead, S.; Liu, J.C. pH-Sensitive Mechanical Properties of Elastin-Based Hydrogels. Macromol. Biosci. 2020, 20, 1900369. [Google Scholar] [CrossRef]
- Souza, A.D.; Marshall, L.R.; Yoon, J.; Kulesha, A.; Edirisinghe, D.I.U.; Chandrasekaran, S.; Rathee, P.; Prabhakar, R.; Makhlynets, O.V. Peptide Hydrogel with Self-Healing and Redox-Responsive Properties. Nano Converg. 2022, 9, 18. [Google Scholar]
- Wang, J.; Zhang, X.; Zhang, S.; Kang, J.; Guo, Z.; Feng, B.; Zhao, H.; Luo, Z.; Yu, J.; Song, W.; et al. Semi-Convertible Hydrogel Enabled Photoresponsive Lubrication. Matter 2021, 4, 675–687. [Google Scholar] [CrossRef]
- Xing, J.; Yang, B.; Dang, W.; Li, J.; Bai, B. Preparation of Photo/Electro-Sensitive Hydrogel and Its Adsorption/Desorption Behavior to Acid Fuchsine. Water Air Soil Pollut. 2020, 231, 231. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, M.; Li, H. A Transient Simulation to Predict the Kinetic Behavior of Magnetic-Sensitive Hydrogel Responsive to Magnetic Stimulus. Int. J. Mech. Sci. 2020, 182, 105765. [Google Scholar] [CrossRef]
- Yun, C.; Hwang, S.; Kwak, J. A Wet-Chemistry-Based Hydrogel Sensing Platform for 2D Imaging of Pressure, Chemicals and Temperature. Nanoscale 2018, 10, 13581–13588. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Ren, J.; Zhao, C.; Kong, W.; Dai, Q.; Chen, Q.; Liu, C.; Sun, R. Xylan-Based Temperature/pH Sensitive Hydrogels for Drug Controlled Release. Carbohydr. Polym. 2016, 151, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Liu, K.; Qian, X. A Facile Preparation of pH-Temperature Dual Stimuli-Responsive Supramolecular Hydrogel and Its Controllable Drug Release. J. Appl. Polym. Sci. 2016, 133, 43279. [Google Scholar] [CrossRef]
- Ning, W.; Shang, P.; Wu, J.; Shi, X.; Liu, S. Novel Amphiphilic, Biodegradable, Biocompatible, Thermo-Responsive ABA Triblock Copolymers Based on PCL and PEG Analogues via a Combination of ROP and RAFT: Synthesis, Characterization, and Sustained Drug Release from Self-Assembled Micelles. Polymers 2018, 10, 214. [Google Scholar] [CrossRef]
- Wang, D.; Xia, Y.; Zhang, D.; Sun, X.; Chen, X.; Oliver, S.; Shi, S.; Lei, L. Hydrogen-Bonding Reinforced Injectable Hydrogels: Application as a Thermo-Triggered Drug Controlled-Release System. ACS Appl. Energy Mater. 2020, 2, 1587–1596. [Google Scholar] [CrossRef]
- Wang, F.; Huang, K.; Xu, Z.; Shi, F.; Chen, C. Self-Healable Nanocellulose Composite Hydrogels Combining Multiple Dynamic Bonds for Drug Delivery. Int. J. Biol. Macromol. 2022, 203, 143–152. [Google Scholar] [CrossRef]
- Tang, Q.; Zhao, D.; Yang, H.; Wang, L.; Zhang, X. A pH-Responsive Self-Healing Hydrogel Based on Multivalent Coordination of Ni2+ with Polyhistidine-Terminated PEG and IDA-Modified Oligochitosan. J. Mater. Chem. B 2019, 7, 30–42. [Google Scholar] [CrossRef]
- Praveena, N.M.; Virat, G.; Krishnan, V.G.; Gowd, E.B. Stereocomplex Formation and Hierarchical Structural Changes During Heating of Supramolecular Gels Obtained by Polylactide Racemic Blends. Polymer 2022, 241, 124530. [Google Scholar] [CrossRef]
- Mao, H.; Wang, C.; Chang, X.; Cao, H.; Shan, G.; Bao, Y.; Pan, P. Poly(Lactic Acid)/Poly(Ethylene Glycol) Stereocomplexed Physical Hydrogels Showing Thermally-Induced Gel-Sol-Gel Multiple Phase Transitions. Mater. Chem. Front. 2018, 2, 313–322. [Google Scholar] [CrossRef]
- Sharma, B.; Singh, A.; Sarma, T.K.; Sardana, N.; Pal, A. Chirality Control of Multi-Stimuli Responsive and Self-Healing Supramolecular Metallo-Hydrogels. New J. Chem. 2018, 42, 6427–6432. [Google Scholar] [CrossRef]
- He, S.; Me, L.; Wu, C.; Tao, M.; Zhai, Z.; Xu, K.; Zhong, W. In SituhyHrogelation of Bicalutamide-Peptide Conjugates at Prostate Tissue for Smart Drug Release Based on pH and Enzymatic Activity. Nanoscale 2019, 11, 5030–5037. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Tseng, S.J.; Kempson, I.M.; Peng, S.; Wu, W.; Liu, J. Controlled Delivery of Recombinant Adeno-Associated Virus Serotype 2 Using pH-Sensitive Poly (Ethylene Glycol)-Poly-L-Histidine Hydrogels. Biomaterials 2012, 33, 9239–9245. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Takashima, Y.; Hashidzume, A.; Yamaguchi, H.; Harada, A. Reversible self-assembly of gels through metal-ligand interactions. Sci. Rep. 2013, 3, 1243. [Google Scholar] [CrossRef]
- Park, C.; Heo, J.; Lee, J.; Kim, T.; Kim, S.Y. Well-Defined Dual Light- and Thermo-Responsive Rod-Coil Block Copolymers Containing an Azobenzene, MEO2MA and OEGMA. Polymers 2020, 12, 284. [Google Scholar] [CrossRef]
- Li, X.; Xuan, Y.; Li, Q. Self-Adaptive Chip Cooling with Template-Fabricated Nanocomposite P (MEO2MA-co-OEGMA) Hydrogel. Int. J. Heat Mass Transf. 2021, 166, 120790. [Google Scholar] [CrossRef]
- Fan, X.; Wang, M.; Yuan, D.; He, C. Amphiphilic Conetworks and Gels Physically Cross-Linked via Stereocomplexation of Polylactide. Langmuir 2013, 29, 14307–14313. [Google Scholar] [CrossRef]
- Shi, X.; Wu, J.; Wang, Z.; Song, F.; Liu, S. Synthesis and properties of a temperature-sensitive hydrogel based on physical crosslinking via stereocomplexation of PLLA-PDLA. RSC Adv. 2020, 10, 19759–19769. [Google Scholar] [CrossRef]
- Ishii, T.; Wada, A.; Tsuzuki, S.; Casolaro, M.; Ito, Y. Copolymers Including L-Histidine and Hydrophobic Moiety for Preparation of Nonbiofouling Surface. Biomacromolecules 2007, 8, 3340–3344. [Google Scholar] [CrossRef]
- Yi, X.; He, J.; Wang, X.; Zhang, Y.; Tan, G.; Zhou, Z.; Chen, J.; Chen, D.; Wang, R.; Tian, W.; et al. Tunable Mechanical, Antibacterial, and Cytocompatible Hydrogels Based on a Functionalized Dual Network of Metal Coordination Bonds and Covalent Crosslinking. ACS Appl. Mater. Interfaces 2018, 10, 6190–6198. [Google Scholar] [CrossRef]
- Shao, J.; Sun, J.; Bian, X.; Cui, Y.; Li, G.; Chen, X. Investigation of Poly (Lactide) Stereocomplexes: 3-Armed Poly(L-lactide) Blended with Linear and 3-Armed Enantiomers. J. Phys. Chem. B 2012, 116, 9983–9991. [Google Scholar] [CrossRef]
- Okamoto, Y. Hydrolyses of α-Amino Acid P-Nitro-Phenyl Esters by Poly(N-Methacryloyl-L-Histidine). J. Chem. Soc. Jpn. 1978, 6, 870–873. [Google Scholar]




| Sample | (HEMA-PLLA30):(HEMA-PDLA30) n:n | MEO2MA/OEGMA n:n | Mist (mmol) | cZn(Ⅱ) (mol/L) |
|---|---|---|---|---|
| gel 1 | 1:1 | 90:10 | 0 | 0 |
| gel 2 | 1:1 | 90:10 | 1.79 | 0.1 |
| gel 3 | 0:0 | 90:10 | 1.79 | 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Q.; Fu, Y.; Yang, W.; Liu, S. A Temperature/pH Double-Responsive and Physical Double-Crosslinked Hydrogel Based on PLA and Histidine. Gels 2022, 8, 570. https://doi.org/10.3390/gels8090570
Wu Q, Fu Y, Yang W, Liu S. A Temperature/pH Double-Responsive and Physical Double-Crosslinked Hydrogel Based on PLA and Histidine. Gels. 2022; 8(9):570. https://doi.org/10.3390/gels8090570
Chicago/Turabian StyleWu, Qingrong, Yu Fu, Wanying Yang, and Shouxin Liu. 2022. "A Temperature/pH Double-Responsive and Physical Double-Crosslinked Hydrogel Based on PLA and Histidine" Gels 8, no. 9: 570. https://doi.org/10.3390/gels8090570
APA StyleWu, Q., Fu, Y., Yang, W., & Liu, S. (2022). A Temperature/pH Double-Responsive and Physical Double-Crosslinked Hydrogel Based on PLA and Histidine. Gels, 8(9), 570. https://doi.org/10.3390/gels8090570