Glaucoma Treatment and Hydrogel: Current Insights and State of the Art
Abstract
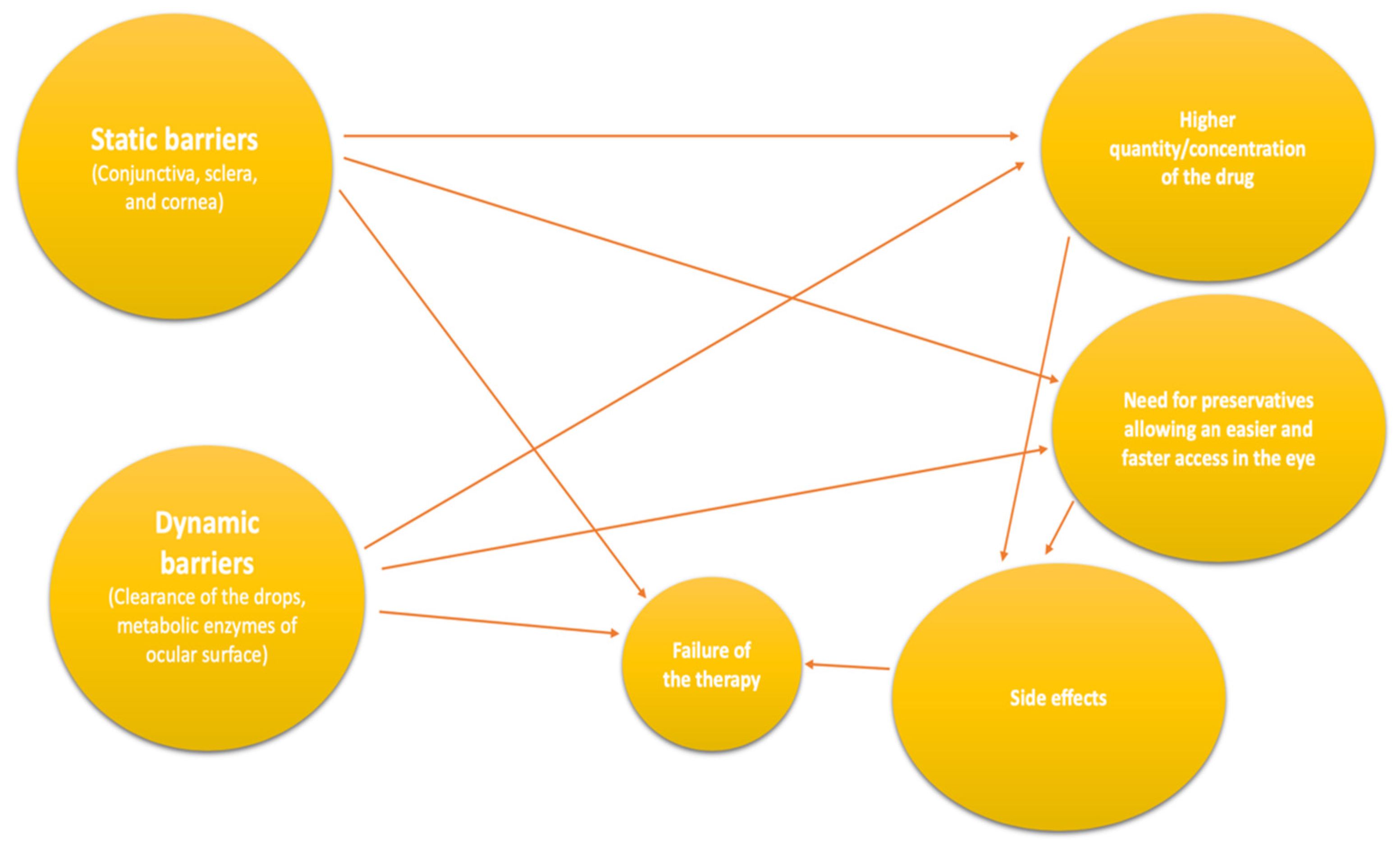
:1. Introduction
Polymers and Hydrogel in Ocular Drugs Delivery
2. Drug Delivery
2.1. Contact Lens
2.2. Hydrogel as an Ocular Drug Delivery System for Glaucoma Treatment
2.2.1. Pilocarpine
2.2.2. Timolol Maleate
2.2.3. Brimonidine Tartrate
2.2.4. Latanoprost
2.2.5. Other Drugs
| Author | Year | Drug | Group | Polymer Name | IOP Decrease (Mean Value) | Duration | Administration |
|---|---|---|---|---|---|---|---|
| Bellotti et al. [131] | 2019 | Brimonidine tartrate | α2 agonist | pNIPAAm hydrogels (PEG) | / | Eye drop | |
| Fedorchak et al. [128] | 2017 | Brimonidine tartrate | α2 agonist | Poly(lactic-co-glycolic) acid microspheres microspheres incorporated into the pNIPAAM gel | / | 28 days | Eye drop |
| Wang et al. [129] | 2017 | Brimonidine tartrate | α2 agonist | Linked dendrimer hydrogel via addition of polyamidoamine (PAMAM) dendrimer G5 and polyethylene glycol diacrylate (PEG) | / | 48 h | AC filling |
| Wang et al. [130] | 2018 | Brimonidine tartrate | α2 agonist | (a-CD/4-PEG hydrogels) hydrogel made of 4-arm polyethylene glycol (4-PEG) and a-cyclodextrin (a-CD) | / | 24 h | / |
| Dubey et al. [122] | 2014 | Timolol maleate- brimonidine tartrate | β-blockers- α2 agonist | Stimuli-sensitive hydrogel with Carbopol (poly(acrylic acid) | 14 mmHg (mean IOP after treatment) | 8 h | Eye drop |
| Holden et al. [119] | 2012 | Brimonidine-timolol maleate | α2 agonist- β-blockers | Polyamidoamine dendrimer hydrogel linked with polyethylene glycol (PEG)-acrylate chains | / | 6–72 h | / |
| Taka et al. [105] | 2020 | Timolol maleate-brimonidine tartrate | β-blockers- α2 agonist | Self-assembling peptide ac-(RADA)4-CONH2 | / | 8 h | / |
| Wang et al. [125] | 2021 | Brimonidine tartrate-and timolol maleate | α2 agonist β-blockers | Nano-in-nano dendrimer hydrogel particles −200 nm (nDHP) | 18.68 ± 1.35 mmHg (mean IOP after treatment) | / | Eye suspension |
| Yang et al. [99] | 2013 | Brimonidine-timolol maleate | α2 agonist- β-blockers | Hybrid dendrimer hydrogel/poly(lactic-co-glycolic acid) nanoparticle platform | 29.5% | 4 days | / |
| Cheng et al. [133] | 2016 | Latanoprost | Prostaglandin | Thermosensitive chitosan/gelatin | / | 7 days | Eye drop |
| Cheng et al. [97] | 2019 | Latanoprost | Prostaglandin | Thermosensitive hydrogel containing latanoprost and curcumin-loaded nanoparticles | / | 7 days | Eye drop |
| Cheng et al. [98] | 2014 | Latanoprost | Prostaglandin | Thermosensitive chitosan/gelatin/glycerol phosphate (C/G/GP) hydrogel | 2.4 mmHg (9.2%) | 31 days | Subconjunctival injection |
| Hsiao et al. [106] | 2014 | Latanoprost | Prostaglandin | Amphiphilic chitosan-based thermogelling | 10 mmHg | 39 days | Subconjunctival injection |
| Abu Hashim et al. [137] | 2014 | 0.5% atenolol | β1 adrenoceptor blocker | Niosomal Hydrogel containing atenolol | / | 8 h | Eye drop |
| Hsiue et al. [135] | 2002 | epinephrine | Catecholamine | Thermosensitive poly-N-isopropylacrylamide (PNIPAAm) | 8.9 mmHg (maximum) | 24 h | Eye drop |
| Prasannan et al. [136] | 2014 | epinephrine | Catecholamine | PAAc-g-PNIPAAm (PNIPAAm) | / | / | Eye drop |
| Chou et al. [107] | 2017 | pilocarpine | Cholinergic | Pilocarpine-loaded gallic acid (GA)-grafted gelatin-g-poly(N-isopropylacrylamide) (GN) | 5 mm Hg | 28 days | / |
| Lai et al. [111] | 2013 | pilocarpine | Cholinergic | Carboxyl- terminated PNIPAAm | / | 12 h | / |
| Luo et al. [113] | 2020 | pilocarpine and RGFP966 | Cholinergic | 4-hydroxy-3,5-dimethoxybenzoic acid (p-DMB)-modified chitosan-g-poly(N-isopropylacrylamide) | / | 70 days | / |
| Natu et al. [110] | 2007 | Pilocarpine hydrochloride | Cholinergic | Linking reaction of gelatin in N,N-(3dimethylaminopropyl)-N′-ethyl carbodiimide and N-hydroxy succinimide | / | 8 h | / |
| Nguyen et al. [112] | 2019 | Pilocarpine and ascorbic acid | Cholinergic | PAMAM dendrimers bearing amine surface groups (-NH2) linked with gelatin hydrogel and poly(N-isopropyl acrylamide) | / | 84 days | / |
| El-Feky et al. [108] | 2018 | Timolol Maleate | β-blockers | Chitosan-gelatin hydrogel linked with oxidized sucrose | / | 8 h | Eye drop |
| Esteban-Pérez et al. [117] | 2020 | Timolol maleate | β-blockers | Gelatin nanoparticles in a hydroxypropyl methylcellulose viscous solution | 4.33 ± 0.30 | 8 h | Eye drop |
| Fernandez-Colino et al. [114] | 2017 | Timolol maleate | β-blockers | Self-assembling elastin-like (EL) and silk-elastin-like hydrogels | / | 8 h | Eye drop |
| Karavasili et al. [121] | 2017 | Timolol maleate | β-blockers | Self-assembling peptides Ac-(RADA)4-CONH2 and Ac-(IEIK)3I-CONH2 | / | 24 h | Eye drop |
| Kulkarni et al. [120] | 2016 | Timolol maleate | β-blockers | Natural hydrogel from Tamarindus indica | / | 24 h | Eye drop |
| Pakzad et al. [124] | 2020 | Timolol maleate | β-blockers | N-(2-hydroxy-3-trimethylammonium) propyl chitosan chloride glycerophosphate (HTCC/GP) | / | 1 week | / |
| Wang et al. [126] | 2021 | Timolol maleate (TM) and levofloxacin | β-blockers | Multilayered sodium alginate-chitosan (SA-CS) hydrogel ball (HB) decorated by zinc oxide-modified biochar (ZnO-BC) (‘lollipop inspired’) | / | 2 weeks | / |
| Zhang et al. [118] | 2011 | Timolol maleate | β-blockers | Liposomal-hydrogel | / | 6 h | Eye drop |
3. Hydrogel Formulation after Glaucoma Surgery
3.1. Anti-Scarring Hydrogel
3.2. Management of Others Post or Intraoperative Complications
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Biggerstaff, K.S.; Lin, A. Glaucoma and Quality of Life. Int. Ophthalmol. Clin. 2018, 58, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A.; Broman, A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Tham, Y.-C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.-Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Alnwisi, S.; Ke, M. The impact of mild, moderate, and severe visual field loss in glaucoma on patients’ quality of life measured via the Glaucoma Quality of Life-15 Questionnaire: A meta-analysis. Medicine 2017, 96, e8019. [Google Scholar] [CrossRef]
- Ting, N.; Li, Y.J.; Ng, J. Different strategies and cost-effectiveness in the treatment of primary open angle glaucoma. Clin. Outcomes Res. 2014, 6, 523–530. [Google Scholar] [CrossRef]
- European Glaucoma Society. European Glaucoma Society Terminology and Guidelines for Glaucoma, 4th Edition—Chapter 3: Treatment principles and options Supported by the EGS Foundation: Part 1: Foreword; Introduction; Glossary; Chapter 3 Treatment principles and options. Br. J. Ophthalmol. 2017, 101, 130–195. [Google Scholar] [CrossRef]
- Prum, B.E.; Rosenberg, L.F.; Gedde, S.J.; Mansberger, S.L.; Stein, J.D.; Moroi, S.E.; Herndon, L.W.; Lim, M.C.; Williams, R.D. Primary Open-Angle Glaucoma Preferred Practice Pattern(®) Guidelines. Ophthalmology 2016, 123, P41–P111. [Google Scholar] [CrossRef]
- Kass, M.A.; Heuer, D.K.; Higginbotham, E.J.; Johnson, C.A.; Keltner, J.L.; Miller, J.P.; Parrish, R.K.; Wilson, M.R.; Gordon, M.O.; Ocular Hypertension Treatment Study Group. The Ocular Hypertension Treatment Study: A randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch. Ophthalmol. 2002, 120, 701–730. [Google Scholar] [CrossRef]
- The AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am. J. Ophthalmol. 2000, 130, 429–440. [Google Scholar] [CrossRef]
- Collaborative Normal-Tension Glaucoma Study Group. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am. J. Ophthalmol. 1998, 126, 498–505. [Google Scholar] [CrossRef]
- Heijl, A.; Leske, M.C.; Bengtsson, B.; Hyman, L.; Bengtsson, B.; Hussein, M. Reduction of intraocular pressure and glaucoma progression: Results from the Early Manifest Glaucoma Trial. Arch. Ophthalmol. 2002, 120, 1268–1279. [Google Scholar] [CrossRef] [PubMed]
- Nordstrom, B.L.; Friedman, D.S.; Mozaffari, E.; Quigley, H.A.; Walker, A.M. Persistence and adherence with topical glaucoma therapy. Am. J. Ophthalmol. 2005, 140, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Belhassen, M.; Laforest, L.; Licaj, I.; Van Ganse, É. Early adherence to antiglaucoma therapy: An observational study. Therapie 2016, 71, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Thrimawithana, T.R.; Young, S.; Bunt, C.R.; Green, C.; Alany, R.G. Drug delivery to the posterior segment of the eye. Drug Discov. Today 2011, 16, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.C. Ocular drug delivery conventional ocular formulations. Adv. Drug Deliv. Rev. 1995, 16, 39–43. [Google Scholar] [CrossRef]
- Bourlais, C.L.; Acar, L.; Zia, H.; Sado, P.A.; Needham, T.; Leverge, R. Ophthalmic drug delivery systems--recent advances. Prog. Retin. Eye Res. 1998, 17, 33–58. [Google Scholar] [CrossRef]
- Patel, A.; Cholkar, K.; Agrahari, V.; Mitra, A.K. Ocular drug delivery systems: An overview. World J. Pharm. 2013, 2, 47–64. [Google Scholar] [CrossRef]
- Elsaid, N.; Jackson, T.L.; Gunic, M.; Somavarapu, S. Positively charged amphiphilic chitosan derivative for the transscleral delivery of rapamycin. Investig. Ophthalmol. Vis. Sci. 2012, 53, 8105–8111. [Google Scholar] [CrossRef]
- Elsaid, N.; Somavarapu, S.; Jackson, T.L. Cholesterol-poly(ethylene) glycol nanocarriers for the transscleral delivery of sirolimus. Exp. Eye Res. 2014, 121, 121–129. [Google Scholar] [CrossRef]
- Wichterle, O.; Lím, D. Hydrophilic Gels for Biological Use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Allyn, M.M.; Luo, R.H.; Hellwarth, E.B.; Swindle-Reilly, K.E. Considerations for Polymers Used in Ocular Drug Delivery. Front. Med. 2022, 8, 787644. [Google Scholar] [CrossRef] [PubMed]
- Akulo, K.A.; Adali, T.; Moyo, M.T.G.; Bodamyali, T. Intravitreal Injectable Hydrogels for Sustained Drug Delivery in Glaucoma Treatment and Therapy. Polymers 2022, 14, 2359. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.R.; Kondiah, P.P.D.; Choonara, Y.E.; du Toit, L.C.; Ally, N.; Pillay, V. Hydrogel Biomaterials for Application in Ocular Drug Delivery. Front. Bioeng. Biotechnol. 2020, 8, 228. [Google Scholar] [CrossRef]
- Kumar, S.; Haglund, B.O.; Himmelstein, K.J. In situ-forming gels for ophthalmic drug delivery. J. Ocul. Pharm. 1994, 10, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Srivastava, A.; Galaev, I.Y.; Mattiasson, B. Smart polymers: Physical forms and bioengineering applications. Prog. Polym. Sci. 2007, 32, 1205–1237. [Google Scholar] [CrossRef]
- Ma, W.; Xu, H.; Nie, S.; Pan, W. Temperature-responsive, Pluronic-g-poly(acrylic acid) copolymers in situ gels for ophthalmic drug delivery: Rheology, in vitro drug release, and in vivo resident property. Drug Dev. Ind. Pharm. 2008, 34, 258–266. [Google Scholar] [CrossRef]
- Makwana, S.B.; Patel, V.A.; Parmar, S.J. Development and characterization of in-situ gel for ophthalmic formulation containing ciprofloxacin hydrochloride. Results Pharma Sci. 2016, 6, 1–6. [Google Scholar] [CrossRef]
- Gan, L.; Gan, Y.; Zhu, C.; Zhang, X.; Zhu, J. Novel microemulsion in situ electrolyte-triggered gelling system for ophthalmic delivery of lipophilic cyclosporine A: In vitro and in vivo results. Int. J. Pharm. 2009, 365, 143–149. [Google Scholar] [CrossRef]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2001, 53, 321–339. [Google Scholar] [CrossRef]
- Blanchard, J. Controlled Drug Delivery: Challenges and Strategies Edited by Kinam Park (Purdue University). American Chemical Society: Washington, DC. 1997. xvii + 629 pp. $145.95. ISBN 0-8412-3418-3. J. Am. Chem. Soc. 1998, 120, 4554–4555. [Google Scholar] [CrossRef]
- Schnyder, A.; Huwyler, J. Drug transport to brain with targeted liposomes. NeuroRx 2005, 2, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Winfield, A.J.; Jessiman, D.; Williams, A.; Esakowitz, L. A study of the causes of non-compliance by patients prescribed eyedrops. Br. J. Ophthalmol. 1990, 74, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Ghate, D.; Edelhauser, H.F. Barriers to glaucoma drug delivery. J. Glaucoma 2008, 17, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, A.L.; Katz, J.; Covert, D.; Kelly, C.A.; Suan, E.P.; Speicher, M.A.; Sund, N.J.; Robin, A.L. A video study of drop instillation in both glaucoma and retina patients with visual impairment. Am. J. Ophthalmol. 2011, 152, 982–988. [Google Scholar] [CrossRef]
- Stone, J.L.; Robin, A.L.; Novack, G.D.; Covert, D.W.; Cagle, G.D. An objective evaluation of eyedrop instillation in patients with glaucoma. Arch. Ophthalmol. 2009, 127, 732–736. [Google Scholar] [CrossRef]
- Sleath, B.; Blalock, S.; Covert, D.; Stone, J.L.; Skinner, A.C.; Muir, K.; Robin, A.L. The relationship between glaucoma medication adherence, eye drop technique, and visual field defect severity. Ophthalmology 2011, 118, 2398–2402. [Google Scholar] [CrossRef]
- Morse, A.R. Improving Medication Adherence to Reduce Vision Loss in Patients with Glaucoma: Low Hanging Fruit? Ophthalmology 2015, 122, 1280–1282. [Google Scholar] [CrossRef]
- Quigley, H.A. Glaucoma. Lancet 2011, 377, 1367–1377. [Google Scholar] [CrossRef]
- Schwartz, G.F.; Quigley, H.A. Adherence and persistence with glaucoma therapy. Surv. Ophthalmol. 2008, 53 (Suppl. S1), S57–S68. [Google Scholar] [CrossRef]
- Novack, G.D. Ophthalmic drug delivery: Development and regulatory considerations. Clin. Pharm. Ther. 2009, 85, 539–543. [Google Scholar] [CrossRef]
- Gupta, S.K.; Galpalli, N.D.; Agrawal, S.S.; Srivastava, S.; Saxena, R. Recent advances in pharmacotherapy of glaucoma. Indian J. Pharm. 2008, 40, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Abou-Jaoude, M.; Carbia, B.E.; Plummer, C.; Chauhan, A. Glaucoma therapy by extended release of timolol from nanoparticle loaded silicone-hydrogel contact lenses. J. Control. Release 2013, 165, 82–89. [Google Scholar] [CrossRef] [PubMed]
- McNamara, N.A.; Polse, K.A.; Brand, R.J.; Graham, A.D.; Chan, J.S.; McKenney, C.D. Tear mixing under a soft contact lens: Effects of lens diameter. Am. J. Ophthalmol. 1999, 127, 659–665. [Google Scholar] [CrossRef]
- Creech, J.L.; Chauhan, A.; Radke, C.J. Dispersive Mixing in the Posterior Tear Film Under a Soft Contact Lens. Ind. Eng. Chem. Res. 2001, 40, 3015–3026. [Google Scholar] [CrossRef]
- Zhu, H.; Chauhan, A. Effect of viscosity on tear drainage and ocular residence time. Optom. Vis. Sci. Off. Publ. Am. Acad. Optom. 2008, 85, 715–725. [Google Scholar] [CrossRef]
- Li, C.-C.; Chauhan, A. Modeling Ophthalmic Drug Delivery by Soaked Contact Lenses. Ind. Eng. Chem. Res. 2006, 45, 3718–3734. [Google Scholar] [CrossRef]
- Kwon, S.; Kim, S.H.; Khang, D.; Lee, J.Y. Potential Therapeutic Usage of Nanomedicine for Glaucoma Treatment. Int. J. Nanomed. 2020, 15, 5745–5765. [Google Scholar] [CrossRef]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Jin, R.; Dijkstra, P.J. Hydrogels for Tissue Engineering Applications. In Biomedical Applications of Hydrogels Handbook; Ottenbrite, R.M., Okano, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]
- Efron, N.; Brennan, N.A.; Chalmers, R.L.; Jones, L.; Lau, C.; Morgan, P.B.; Nichols, J.J.; Szczotka-Flynn, L.B.; Willcox, M.D. Thirty years of ‘quiet eye’ with etafilcon A contact lenses. Contact Lens Anterior Eye 2020, 43, 285–297. [Google Scholar] [CrossRef]
- Peng, H.T.; Shek, P.N. Novel wound sealants: Biomaterials and applications. Expert Rev. Med. Devices 2010, 7, 639–659. [Google Scholar] [CrossRef]
- Taniguchi, E.V.; Kalout, P.; Pasquale, L.R.; Kohane, D.S.; Ciolino, J.B. Clinicians’ perspectives on the use of drug-eluting contact lenses for the treatment of glaucoma. Ther. Deliv. 2014, 5, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Xinming, L.; Yingde, C.; Lloyd, A.W.; Mikhalovsky, S.V.; Sandeman, S.R.; Howel, C.A.; Liewen, L. Polymeric hydrogels for novel contact lens-based ophthalmic drug delivery systems: A review. Contact Lens Anterior Eye 2008, 31, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, X.; Sun, F. In vitro and in vivo evaluation of ketotifen fumarate-loaded silicone hydrogel contact lenses for ocular drug delivery. Drug Deliv. 2011, 18, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Ciolino, J.B.; Ross, A.E.; Tulsan, R.; Watts, A.C.; Wang, R.F.; Zurakowski, D.; Serle, J.B.; Kohane, D.S. Latanoprost-Eluting Contact Lenses in Glaucomatous Monkeys. Ophthalmology 2016, 123, 2085–2092. [Google Scholar] [CrossRef]
- Guzman-Aranguez, A.; Colligris, B.; Pintor, J. Contact lenses: Promising devices for ocular drug delivery. J. Ocul. Pharmacol. Ther. 2013, 29, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.-H.; Gause, S.; Chauhan, A. Review of ophthalmic drug delivery by contact lenses. J. Drug Deliv. Sci. Technol. 2014, 24, 123–135. [Google Scholar] [CrossRef]
- White, C.J.; Byrne, M.E. Molecularly imprinted therapeutic contact lenses. Expert Opin. Drug Deliv. 2010, 7, 765–780. [Google Scholar] [CrossRef]
- Yan, F.; Liu, Y.; Han, S.; Zhao, Q.; Liu, N. Bimatoprost Imprinted Silicone Contact Lens to Treat Glaucoma. AAPS PharmSciTech 2020, 21, 63. [Google Scholar] [CrossRef]
- Cegielska, O.; Sajkiewicz, P. Targeted Drug Delivery Systems for the Treatment of Glaucoma: Most Advanced Systems Review. Polymers 2019, 11, 1742. [Google Scholar] [CrossRef]
- Peng, C.-C.; Burke, M.T.; Carbia, B.E.; Plummer, C.; Chauhan, A. Extended drug delivery by contact lenses for glaucoma therapy. J. Control. Release 2012, 162, 152–158. [Google Scholar] [CrossRef]
- Al-Kinani, A.A.; Zidan, G.; Elsaid, N.; Seyfoddin, A.; Alani, A.W.G.; Alany, R.G. Ophthalmic gels: Past, present and future. Adv. Drug Deliv. Rev. 2018, 126, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.K.; Dilnawaz, F.; Krishnakumar, S. Nanotechnology in ocular drug delivery. Drug Discov. Today 2008, 13, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, X.; Sun, F. Preparation and evaluation of a contact lens vehicle for puerarin delivery. J. Biomater. Sci. Polym. Ed. 2010, 21, 271–288. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; Kim, J. Therapeutic contact lenses with polymeric vehicles for ocular drug delivery: A review. Materials 2018, 11, 1125. [Google Scholar] [CrossRef]
- Gulsen, D.; Chauhan, A. Ophthalmic drug delivery through contact lenses. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2342–2347. [Google Scholar] [CrossRef]
- Gulsen, D.; Li, C.-C.; Chauhan, A. Dispersion of DMPC liposomes in contact lenses for ophthalmic drug delivery. Curr. Eye Res. 2005, 30, 1071–1080. [Google Scholar] [CrossRef]
- Gulsen, D.; Chauhan, A. Dispersion of microemulsion drops in HEMA hydrogel: A potential ophthalmic drug delivery vehicle. Int. J. Pharm. 2005, 292, 95–117. [Google Scholar] [CrossRef]
- Kapoor, Y.; Chauhan, A. Ophthalmic delivery of Cyclosporine A from Brij-97 microemulsion and surfactant-laden p-HEMA hydrogels. Int. J. Pharm. 2008, 361, 222–229. [Google Scholar] [CrossRef]
- Kapoor, Y.; Chauhan, A. Drug and surfactant transport in Cyclosporine A and Brij 98 laden p-HEMA hydrogels. J. Colloid Interface Sci. 2008, 322, 624–633. [Google Scholar] [CrossRef]
- Goyal, G.; Garg, T.; Rath, G.; Goyal, A.K. Current nanotechnological strategies for treating glaucoma. Crit. Rev. Ther. Drug Carr. Syst. 2014, 31, 365–405. [Google Scholar] [CrossRef]
- Pita-Thomas, D.W.; Goldberg, J.L. Nanotechnology and glaucoma: Little particles for a big disease. Curr. Opin. Ophthalmol. 2013, 24, 130–135. [Google Scholar] [CrossRef]
- Lloyd, A.W.; Faragher, R.G.; Denyer, S.P. Ocular biomaterials and implants. Biomaterials 2001, 22, 769–785. [Google Scholar] [CrossRef]
- Hiratani, H.; Fujiwara, A.; Tamiya, Y.; Mizutani, Y.; Alvarez-Lorenzo, C. Ocular release of timolol from molecularly imprinted soft contact lenses. Biomaterials 2005, 26, 1293–1298. [Google Scholar] [CrossRef] [PubMed]
- Hiratani, H.; Mizutani, Y.; Alvarez-Lorenzo, C. Controlling drug release from imprinted hydrogels by modifying the characteristics of the imprinted cavities. Macromol. Biosci. 2005, 5, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Lorenzo, C.; Hiratani, H.; Gómez-Amoza, J.L.; Martínez-Pacheco, R.; Souto, C.; Concheiro, A. Soft contact lenses capable of sustained delivery of timolol. J. Pharm. Sci. 2002, 91, 2182–2192. [Google Scholar] [CrossRef] [PubMed]
- Hiratani, H.; Alvarez-Lorenzo, C. Timolol uptake and release by imprinted soft contact lenses made of N,N-diethylacrylamide and methacrylic acid. J. Control Release 2002, 83, 223–230. [Google Scholar] [CrossRef]
- Venkatesh, S.; Sizemore, S.P.; Byrne, M.E. Biomimetic hydrogels for enhanced loading and extended release of ocular therapeutics. Biomaterials 2007, 28, 717–724. [Google Scholar] [CrossRef]
- Hiratani, H.; Alvarez-Lorenzo, C. The nature of backbone monomers determines the performance of imprinted soft contact lenses as timolol drug delivery systems. Biomaterials 2004, 25, 1105–1113. [Google Scholar] [CrossRef]
- Jung, H.J.; Chauhan, A. Temperature sensitive contact lenses for triggered ophthalmic drug delivery. Biomaterials 2012, 33, 2289–2300. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Lakdawala, D.H.; Shaikh, A.A.; Desai, A.R.; Choksi, H.H.; Vaidya, R.J.; Ranch, K.M.; Koli, A.R.; Vyas, B.A.; Shah, D.O. In vitro and in vivo evaluation of novel implantation technology in hydrogel contact lenses for controlled drug delivery. J. Control. Release 2016, 226, 47–56. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Soni, P.D.; Patel, P.J.; Desai, A.R.; Desai, D.T.; Shukla, M.R.; Shah, S.A.; Shah, D.O.; Willcox, M.D. Controlled bimatoprost release from graphene oxide laden contact lenses: In vitro and in vivo studies. Colloids Surf. B Biointerfaces 2021, 208, 112096. [Google Scholar] [CrossRef] [PubMed]
- Ciolino, J.B.; Stefanescu, C.F.; Ross, A.E.; Salvador-Culla, B.; Cortez, P.; Ford, E.M.; Wymbs, K.A.; Sprague, S.L.; Mascoop, D.R.; Rudina, S.S.; et al. In vivo performance of a drug-eluting contact lens to treat glaucoma for a month. Biomaterials 2014, 35, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Sekar, P.; Chauhan, A. Effect of vitamin-E integration on delivery of prostaglandin analogs from therapeutic lenses. J. Colloid Interface Sci. 2019, 539, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.-C.; Kim, J.; Chauhan, A. Extended delivery of hydrophilic drugs from silicone-hydrogel contact lenses containing vitamin E diffusion barriers. Biomaterials 2010, 31, 4032–4047. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.-H.; Carbia, B.E.; Plummer, C.; Chauhan, A. Dual drug delivery from vitamin E loaded contact lenses for glaucoma therapy. Eur. J. Pharm. Biopharm. 2015, 94, 312–321. [Google Scholar] [CrossRef]
- Nicolson, P.C. Continuous Wear Contact Lens Surface Chemistry and Wearability. Eye Contact Lens 2003, 29, S30–S32. Available online: https://journals.lww.com/claojournal/Fulltext/2003/01001/Continuous_Wear_Contact_Lens_Surface_Chemistry_and.9.aspx (accessed on 1 April 2022). [CrossRef]
- Soluri, A.; Hui, A.; Jones, L. Delivery of ketotifen fumarate by commercial contact lens materials. Optom. Vis. Sci. 2012, 89, 1140–1149. [Google Scholar] [CrossRef]
- Tran, N.P.D.; Yang, M.C.; Tran-Nguyen, P.L. Evaluation of silicone hydrogel contact lenses based on poly(dimethylsiloxane) dialkanol and hydrophilic polymers. Colloids Surf. B Biointerfaces 2021, 206, 111957. [Google Scholar] [CrossRef]
- Chen, J.; Wang, D.; Wang, F.; Shi, S.; Chen, Y.; Yang, B.; Tang, Y.; Huang, C. Exendin-4 inhibits structural remodeling and improves Ca2+ homeostasis in rats with heart failure via the GLP-1 receptor through the eNOS/cGMP/PKG pathway. Peptides 2017, 90, 69–77. [Google Scholar] [CrossRef]
- Pitt, W.G.; Jack, D.R.; Zhao, Y.; Nelson, J.L.; Pruitt, J.D. Loading and release of a phospholipid from contact lenses. Optom. Vis. Sci. 2011, 88, 502–506. [Google Scholar] [CrossRef]
- Rykowska, I.; Nowak, I.; Nowak, R. Soft Contact Lenses as Drug Delivery Systems: A Review. Molecules 2021, 26, 5577. [Google Scholar] [CrossRef] [PubMed]
- García-Fernández, M.J.; Tabary, N.; Martel, B.; Cazaux, F.; Oliva, A.; Taboada, P.; Concheiro, A.; Alvarez-Lorenzo, C. Poly-(Cyclo)Dextrins as Ethoxzolamide Carriers in Ophthalmic Solutions and in Contact Lenses. Carbohydr Polym 2013, 98, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Jones, L.; Gorbet, M. Extended Latanoprost Release from Commercial Contact Lenses: In Vitro Studies Using Corneal Models. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.C.; Ben-Shlomo, A.; MacKay, E.O.; Plummer, C.E.; Chauhan, A. Drug Delivery by Contact Lens in Spontaneously Glaucomatous Dogs. Curr Eye Res 2012, 37, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Saha, D.; Majumdar, S.; Giri, L. Imaging Methods for the Assessment of a Complex Hydrogel as an Ocular Drug Delivery System for Glaucoma Treatment: Opportunities and Challenges in Preclinical Evaluation. Mol. Pharm. 2022, 19, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-H.; Ko, Y.-C.; Chang, Y.-F.; Huang, S.-H.; Liu, C.J.-L. Thermosensitive chitosan-gelatin-based hydrogel containing curcumin-loaded nanoparticles and latanoprost as a dual-drug delivery system for glaucoma treatment. Exp. Eye Res. 2019, 179, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-H.; Hung, K.H.; Tsai, T.H.; Lee, C.J.; Ku, R.Y.; Chiu, A.W.H.; Chiou, S.H.; Liu, C.J.L. Sustained delivery of latanoprost by thermosensitive chitosan-gelatin-based hydrogel for controlling ocular hypertension. Acta Biomater. 2014, 10, 4360–4366. [Google Scholar] [CrossRef]
- Yang, H.; Leffler, C.T. Hybrid dendrimer hydrogel/poly(lactic-co-glycolic acid) nanoparticle platform: An advanced vehicle for topical delivery of antiglaucoma drugs and a likely solution to improving compliance and adherence in glaucoma management. J. Ocul. Pharmacol. Ther. 2013, 29, 166–172. [Google Scholar] [CrossRef]
- Yadav, K.S.; Rajpurohit, R.; Sharma, S. Glaucoma: Current treatment and impact of advanced drug delivery systems. Life Sci. 2019, 221, 362–376. [Google Scholar] [CrossRef]
- Kabiri, M.; Kamal, S.H.; Pawar, S.V.; Roy, P.R.; Derakhshandeh, M.; Kumar, U.; Hatzikiriakos, S.G.; Hossain, S.; Yadav, V.G. A stimulus-responsive, in situ-forming, nanoparticle-laden hydrogel for ocular drug delivery. Drug Deliv. Transl. Res. 2018, 8, 484–495. [Google Scholar] [CrossRef]
- Gupta, S.; Samanta, M.K.; Raichur, A.M. Dual-drug delivery system based on in situ gel-forming nanosuspension of forskolin to enhance antiglaucoma efficacy. AAPS PharmSciTech 2010, 11, 322–335. [Google Scholar] [CrossRef]
- Almeida, J.F.; Fonseca, A.; Baptista, C.M.S.G.; Leite, E.; Gil, M.H. Immobilization of drugs for glaucoma treatment. J. Mater. Sci. Mater. Med. 2007, 18, 2309–2317. [Google Scholar] [CrossRef]
- Patel, P.; Patel, G. Formulation, ex-vivo and preclinical in-vivo studies of combined ph and ion-sensitive ocular sustained in situ hydrogel of timolol maleate for the treatment of glaucoma. Biointerface Res. Appl. Chem. 2021, 11, 8242–8265. [Google Scholar] [CrossRef]
- Taka, E.; Karavasili, C.; Bouropoulos, N.; Moschakis, T.; Andreadis, D.D.; Zacharis, C.K.; Fatouros, D.G. Ocular co-Delivery of Timolol and Brimonidine from a Self-Assembling Peptide Hydrogel for the Treatment of Glaucoma: In Vitro and Ex Vivo Evaluation. Pharmaceuticals 2020, 13, 126. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, M.-H.; Chiou, S.H.; Larsson, M.; Hung, K.H.; Wang, Y.L.; Liu, C.J.L.; Liu, D.M. A temperature-induced and shear-reversible assembly of latanoprost-loaded amphiphilic chitosan colloids: Characterization and in vivo glaucoma treatment. Acta Biomater. 2014, 10, 3188–3196. [Google Scholar] [CrossRef]
- Chou, S.-F.; Luo, L.-J.; Lai, J.-Y. In vivo Pharmacological Evaluations of Pilocarpine-Loaded Antioxidant-Functionalized Biodegradable Thermogels in Glaucomatous Rabbits. Sci. Rep. 2017, 7, 42344. [Google Scholar] [CrossRef] [PubMed]
- El-Feky, G.S.; Zayed, G.M.; Elshaier, Y.A.M.M.; Alsharif, F.M. Chitosan-Gelatin Hydrogel Crosslinked With Oxidized Sucrose for the Ocular Delivery of Timolol Maleate. J. Pharm. Sci. 2018, 107, 3098–3104. [Google Scholar] [CrossRef]
- Review, C. Glaucoma and its treatment. Hosp. Pharm. 1979, 14, 90–101. [Google Scholar]
- Natu, M.V.; Sardinha, J.P.; Correia, I.J.; Gil, M.H. Controlled release gelatin hydrogels and lyophilisates with potential application as ocular inserts. Biomed. Mater. 2007, 2, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.-Y. Biodegradable in situ gelling delivery systems containing pilocarpine as new antiglaucoma formulations: Effect of a mercaptoacetic acid/N-isopropylacrylamide molar ratio. Drug Des. Dev. Ther. 2013, 7, 1273–1285. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Luo, L.-J.; Lai, J.-Y. Dendritic Effects of Injectable Biodegradable Thermogels on Pharmacotherapy of Inflammatory Glaucoma-Associated Degradation of Extracellular Matrix. Adv. Healthc. Mater. 2019, 8, e1900702. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.-J.; Nguyen, D.D.; Lai, J.-Y. Benzoic acid derivative-modified chitosan-g-poly(N-isopropylacrylamide): Methoxylation effects and pharmacological treatments of Glaucoma-related neurodegeneration. J. Control. Release 2020, 317, 246–258. [Google Scholar] [CrossRef]
- Fernández-Colino, A.; Quinteros, D.A.; Allemandi, D.A.; Girotti, A.; Palma, S.D.; Arias, F.J. Self-Assembling Elastin-Like Hydrogels for Timolol Delivery: Development of an Ophthalmic Formulation Against Glaucoma. Mol. Pharm. 2017, 14, 4498–4508. [Google Scholar] [CrossRef]
- Marquis, R.E.; Whitson, J.T. Management of glaucoma: Focus on pharmacological therapy. Drugs Aging 2005, 22, 1–21. [Google Scholar] [CrossRef]
- Schmidl, D.; Schmetterer, L.; Garhöfer, G.; Popa-Cherecheanu, A. Pharmacotherapy of glaucoma. J. Ocul. Pharmacol. Ther. 2015, 31, 63–77. [Google Scholar] [CrossRef]
- Esteban-Pérez, S.; Andrés-Guerrero, V.; López-Cano, J.J.; Molina-Martínez, I.; Herrero-Vanrell, R.; Bravo-Osuna, I. Gelatin Nanoparticles-HPMC Hybrid System for Effective Ocular Topical Administration of Antihypertensive Agents. Pharmaceutics 2020, 12, 306. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, Q.; Yang, Z.; Pan, W.; Nie, S. Novel ophthalmic timolol meleate liposomal-hydrogel and its improved local glaucomatous therapeutic effect in vivo. Drug Deliv. 2011, 18, 502–510. [Google Scholar] [CrossRef]
- Holden, C.A.; Tyagi, P.; Thakur, A.; Kadam, R.; Jadhav, G.; Kompella, U.B.; Yang, H. Polyamidoamine dendrimer hydrogel for enhanced delivery of antiglaucoma drugs. Nanomedicine 2012, 8, 776–783. [Google Scholar] [CrossRef]
- Kulkarni, G.T.; Sethi, N.; Awasthi, R.; Pawar, V.K.; Pahuja, V. Development of Ocular Delivery System for Glaucoma Therapy Using Natural Hydrogel as Film Forming Agent and Release Modifier. Polim. Med. 2016, 46, 25–33. [Google Scholar] [CrossRef]
- Karavasili, C.; Komnenou, A.; Katsamenis, O.L.; Charalampidou, G.; Kofidou, E.; Andreadis, D.; Koutsopoulos, S.; Fatouros, D.G. Self-Assembling Peptide Nanofiber Hydrogels for Controlled Ocular Delivery of Timolol Maleate. ACS Biomater. Sci. Eng. 2017, 3, 3386–3394. [Google Scholar] [CrossRef]
- Dubey, A.; Prabhu, P. Formulation and evaluation of stimuli-sensitive hydrogels of timolol maleate and brimonidine tartrate for the treatment of glaucoma. Int. J. Pharm. Investig. 2014, 4, 112–118. [Google Scholar] [CrossRef]
- Singh, V.; Bushetti, S.S.; Appala, R.; Shareef, A.; Imam, S.S.; Singh, M. Original Article Stimuli-sensitive hydrogels: A novel ophthalmic drug delivery system. Indian J. Ophthalmol. 2010, 58, 477–481. [Google Scholar] [CrossRef]
- Pakzad, Y.; Fathi, M.; Omidi, Y.; Mozafari, M.; Zamanian, A. Synthesis and characterization of timolol maleate-loaded quaternized chitosan-based thermosensitive hydrogel: A transparent topical ocular delivery system for the treatment of glaucoma. Int. J. Biol. Macromol. 2020, 159, 117–128. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Huang, D.; Norat, P.; Grannonico, M.; Cooper, R.C.; Gui, Q.; Chow, W.N.; Liu, X.; Yang, H. Nano-in-Nano Dendrimer Gel Particles for Efficient Topical Delivery of Antiglaucoma Drugs into the Eye. Chem. Eng. J. 2021, 425, 130498. [Google Scholar] [CrossRef]
- Wang, F.; Song, Y.; Huang, J.; Wu, B.; Wang, Y.; Pang, Y.; Zhang, W.; Zhu, Z.; Ma, F.; Wang, X. Lollipop-Inspired Multilayered Drug Delivery Hydrogel for Dual Effective, Long-Term, and NIR-Defined Glaucoma Treatment. Macromol. Biosci. 2021, 21, e2100202. [Google Scholar] [CrossRef]
- Rahman, M.Q.; Ramaesh, K.; Montgomery, D.M. Brimonidine for glaucoma. Expert Opin. Drug Saf. 2010, 9, 483–491. [Google Scholar] [CrossRef]
- Fedorchak, M.V.; Conner, I.P.; Schuman, J.S.; Cugini, A.; Little, S.R. Long Term Glaucoma Drug Delivery Using a Topically Retained Gel/Microsphere Eye Drop. Sci. Rep. 2017, 7, 8639. [Google Scholar] [CrossRef]
- Wang, J.; Williamson, G.S.; Lancina Iii, M.G.; Yang, H. Mildly Cross-Linked Dendrimer Hydrogel Prepared via Aza-Michael Addition Reaction for Topical Brimonidine Delivery. J. Biomed. Nanotechnol. 2017, 13, 1089–1096. [Google Scholar] [CrossRef]
- Wang, J.; Williamson, G.S.; Yang, H. Branched polyrotaxane hydrogels consisting of alpha-cyclodextrin and low-molecular-weight four-arm polyethylene glycol and the utility of their thixotropic property for controlled drug release. Colloids Surf. B Biointerfaces 2018, 165, 144–149. [Google Scholar] [CrossRef]
- Bellotti, E.; Fedorchak, M.V.; Velankar, S.; Little, S.R. Tuning of thermoresponsive pNIPAAm hydrogels for the topical retention of controlled release ocular therapeutics. J. Mater. Chem. B 2019, 7, 1276–1283. [Google Scholar] [CrossRef]
- Digiuni, M.; Fogagnolo, P.; Rossetti, L. A review of the use of latanoprost for glaucoma since its launch. Expert Opin. Pharmacother. 2012, 13, 723–745. [Google Scholar] [CrossRef]
- Cheng, Y.-H.; Tsai, T.H.; Jhan, Y.Y.; Chiu, A.W.H.; Tsai, K.L.; Chien, C.S.; Chiou, S.H.; Liu, C.J.L. Thermosensitive chitosan-based hydrogel as a topical ocular drug delivery system of latanoprost for glaucoma treatment. Carbohydr. Polym. 2016, 144, 390–399. [Google Scholar] [CrossRef]
- Podos, S.M. 2. Epinephrine. Ophthalmology 1980, 87, 721–723. [Google Scholar] [CrossRef]
- Hsiue, G.-H.; Hsu, S.; Yang, C.-C.; Lee, S.-H.; Yang, I.-K. Preparation of controlled release ophthalmic drops, for glaucoma therapy using thermosensitive poly-N-isopropylacrylamide. Biomaterials 2002, 23, 457–462. [Google Scholar] [CrossRef]
- Prasannan, A.; Tsai, H.-C.; Chen, Y.-S.; Hsiue, G.-H. A thermally triggered in situ hydrogel from poly(acrylic acid-co-N-isopropylacrylamide) for controlled release of antiglaucoma drugs. J. Mater. Chem. B 2014, 2, 1988–1997. [Google Scholar] [CrossRef]
- Abu Hashim, I.I.; El-Dahan, M.S.; Yusif, R.M.; Abd-Elgawad, A.-E.H.; Arima, H. Potential use of niosomal hydrogel as an ocular delivery system for atenolol. Biol. Pharm. Bull. 2014, 37, 541–551. [Google Scholar] [CrossRef]
- Chang, M.R.; Cheng, Q.; Lee, D.A. Basic science and clinical aspects of wound healing in glaucoma filtering surgery. J. Ocul. Pharm. Ther. 1998, 14, 75–95. [Google Scholar] [CrossRef]
- Li, Z.; Van Bergen, T.; Van de Veire, S.; Van de Vel, I.; Moreau, H.; Dewerchin, M.; Maudgal, P.C.; Zeyen, T.; Spileers, W.; Moons, L.; et al. Inhibition of vascular endothelial growth factor reduces scar formation after glaucoma filtration surgery. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5217–5225. [Google Scholar] [CrossRef]
- Schlunck, G.; Meyer-ter-Vehn, T.; Klink, T.; Grehn, F. Conjunctival fibrosis following filtering glaucoma surgery. Exp. Eye Res. 2016, 142, 76–82. [Google Scholar] [CrossRef]
- Greenfidd, D.S.; Liebmann, J.M.; Jee, J.; Ritch, R. Late-onset bleb leaks after glaucoma filtering surgery. Arch. Ophthalmol. 1998, 116, 443–447. [Google Scholar] [CrossRef]
- Kitazawa, Y.; Taniguchi, T.; Nakano, Y.; Shirato, S.; Yamamoto, T. 5-Fluorouracil for trabeculectomy in glaucoma. Graefe’s Arch. Clin. Exp. Ophthalmol. 1987, 225, 403–405. [Google Scholar] [CrossRef]
- Mochizuki, K.; Jikihara, S.; Ando, Y.; Hori, N.; Yamamoto, T.; Kitazawa, Y. Incidence of delayed onset infection after trabeculectomy with adjunctive mitomycin C or 5-fluorouracil treatment. Br. J. Ophthalmol. 1997, 81, 877–883. [Google Scholar] [CrossRef]
- Yamamoto, T.; Sawada, A.; Mayama, C.; Araie, M.; Ohkubo, S.; Sugiyama, K.; Kuwayama, Y.; Treatment Study Group. The 5-year incidence of bleb-related infection and its risk factors after filtering surgeries with adjunctive mitomycin C: Collaborative bleb-related infection incidence and treatment study 2. Ophthalmology 2014, 121, 1001–1006. [Google Scholar] [CrossRef]
- Blake, D.A.; Sahiner, N.; John, V.T.; Clinton, A.D.; Galler, K.E.; Walsh, M.; Arosemena, A.; Johnson, P.Y.; Ayyala, R.S. Inhibition of cell proliferation by mitomycin C incorporated into P(HEMA) hydrogels. J. Glaucoma 2006, 15, 291–298. [Google Scholar] [CrossRef]
- Miyata, T.; Asami, N.; Uragani, T. A reversibly antigen-responsive hydrogel. Nature 1999, 399, 766–768. [Google Scholar] [CrossRef]
- Guo, T.C.; Qian, Z.Y.; Huang, M.J.; Kan, B.; Gu, Y.C.; Gong, C.Y.; Yang, J.L.; Wang, K.; Dai, M.; Li, X.Y.; et al. Synthesis, characterization, and hydrolytic degradation behavior of a novel biodegradable pH-sensitive hydrogel based on polycaprolactone, methacrylic acid, and poly(ethylene glycol). J. Biomed. Mater. Res. A 2008, 85, 36–46. [Google Scholar] [CrossRef]
- Branco, M.C.; Pochan, D.J.; Wagner, N.J.; Schneider, J.P. Macromolecular diffusion and release from self-assembled β-hairpin peptide hydrogels. Biomaterials 2009, 30, 1339–1347. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, W.L.; Wang, J.C.; Zhang, X.; Zhang, H.; Wang, X.Q.; Zhou, T.Y.; Zhang, Q. Controlled delivery of recombinant hirudin based on thermo-sensitive Pluronic® F127 hydrogel for subcutaneous administration: In vitro and in vivo characterization. J. Control. Release 2007, 117, 387–395. [Google Scholar] [CrossRef]
- Han, Q.; Wang, Y.; Li, X.; Peng, R.; Li, A.; Qian, Z.; Yu, L. Effects of bevacizumab loaded PEG-PCL-PEG hydrogel intracameral application on intraocular pressure after glaucoma filtration surgery. J. Mater. Sci. Mater. Med. 2015, 26, 225. [Google Scholar] [CrossRef]
- Liang, L.; Xu, X.-D.; Zhang, X.-Z.; Feng, M.; Peng, C.; Jiang, F.-G. Prevention of filtering surgery failure by subconjunctival injection of a novel peptide hydrogel into rabbit eyes. Biomed. Mater. 2010, 5, 45008. [Google Scholar] [CrossRef]
- Martin, G.; Lübke, J.; Schefold, S.; Jordan, J.F.; Schlunck, G.; Reinhard, T.; Kanokwijitsilp, T.; Prucker, O.; Rühe, J.; Anton, A. Prevention of Ocular Tenon Adhesion to Sclera by a PDMAA Polymer to Improve Results after Glaucoma Surgery. Macromol. Rapid Commun. 2020, 41, 1900352. [Google Scholar] [CrossRef]
- Frazier, K.; Williams, S.; Kothapalli, D.; Klapper, H.; Grotendorst, G.R. Stimulation of Fibroblast Cell Growth, Matrix Production, and Granulation Tissue Formation by Connective Tissue Growth Factor. J. Investig. Dermatol. 1996, 107, 404–411. [Google Scholar] [CrossRef]
- Chen, B.; Wu, P.; Liang, L.; Zhao, C.; Wang, Z.; He, L.; Zhang, R.; Xu, N. Inhibited effect of an RGD peptide hydrogel on the expression of β1-integrin, FAK, and Akt in Tenon’s capsule fibroblasts. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2021, 109, 1857–1865. [Google Scholar] [CrossRef]
- Nagata, T.; Harada, Y.; Arai, M.; Hirose, T.; Kondo, H. Polyethylene Glycol-Based Synthetic Hydrogel Sealant for Filtration Bleb Leaks: An In Vivo and Histologic Study. Transl. Vis. Sci. Technol. 2020, 9, 24. [Google Scholar] [CrossRef]
- Xi, L.; Wang, T.; Zhao, F.; Zheng, Q.; Li, X.; Luo, J.; Liu, J.; Quan, D.; Ge, J. Evaluation of an injectable thermosensitive hydrogel as drug delivery implant for ocular glaucoma surgery. PLoS ONE 2014, 9, e100632. [Google Scholar] [CrossRef] [PubMed]
- Kojima, S.; Sugiyama, T.; Takai, S.; Jin, D.; Ueki, M.; Oku, H.; Tabata, Y.; Ikeda, T. Effects of gelatin hydrogel loading mitomycin C on conjunctival scarring in a canine filtration surgery model. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2601–2605. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.Q.; Lan, Y.Q.; Guo, H.; Cheng, L.Z.; Fan, J.Z.; Cai, X.; Zhang, L.M.; Chen, R.F.; Zhou, H.S. Ophthalmic drug-loaded N,O-carboxymethyl chitosan hydrogels: Synthesis, in vitro and in vivo evaluation. Acta Pharmacol. Sin. 2010, 31, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.; Qin, G.; Li, X.; Lv, H.; Qian, Z.; Yu, L. The PEG-PCL-PEG hydrogel as an implanted ophthalmic delivery system after glaucoma filtration surgery; a pilot study. Med. Hypothesis Discov. Innov. Ophthalmol. 2014, 3, 3–8. [Google Scholar]
- Wilgus, T.A.; Ferreira, A.M.; Oberyszyn, T.M.; Bergdall, V.K.; DiPietro, L.A. Regulation of scar formation by vascular endothelial growth factor. Lab. Investig. 2008, 88, 579–590. [Google Scholar] [CrossRef]
- Kojima, S.; Sugiyama, T.; Takai, S.; Jin, D.; Shibata, M.; Oku, H.; Tabata, Y.; Ikeda, T. Effects of Gelatin Hydrogel Containing Chymase Inhibitor on Scarring in a Canine Filtration Surgery Model. Invest Ophthalmol Vis Sci 2011, 52, 7672–7680. [Google Scholar] [CrossRef]
- He, S.; Walls, A.F. Human mast cell chymase induces the accumulation of neutrophils, eosinophils and other in¯ammatory cells in vivo. Br. J. Pharm. 1998, 125, 1491–1500. [Google Scholar] [CrossRef]
- Maruichi, M.; Takai, S.; Sugiyama, T.; Ueki, M.; Oku, H.; Sakaguchi, M.; Okamoto, Y.; Muramatsu, M.; Ikeda, T.; Miyazaki, M. Role of chymase on growth of cultured canine Tenon’s capsule fibroblasts and scarring in a canine conjunctival flap model. Exp. Eye Res. 2004, 79, 111–118. [Google Scholar] [CrossRef]
- Maeda, M.; Kojima, S.; Sugiyama, T.; Jin, D.; Takai, S.; Oku, H.; Kohmoto, R.; Ueki, M.; Ikeda, T. Effects of gelatin hydrogel containing anti-transforming growth factor-β antibody in a canine filtration surgery model. Int. J. Mol. Sci. 2017, 18, 985. [Google Scholar] [CrossRef]
- Jampel, H.D.; Roche, N.; Stark, W.J.; Roberts, A.B. Transforming growth factor-β in human aqueous humor. Curr. Eye Res. 1990, 9, 963–969. [Google Scholar] [CrossRef]
- Sun, J.; Liu, X.; Lei, Y.; Tang, M.; Dai, Z.; Yang, X.; Yu, X.; Yu, L.; Sun, X.; Ding, J. Sustained subconjunctival delivery of cyclosporine A using thermogelling polymers for glaucoma filtration surgery. J. Mater. Chem. B 2017, 5, 6400–6411. [Google Scholar] [CrossRef]
- Qiao, X.; Peng, X.; Qiao, J.; Jiang, Z.; Han, B.; Yang, C.; Liu, W. Evaluation of a photocrosslinkable hydroxyethyl chitosan hydrogel as a potential drug release system for glaucoma surgery. J. Mater. Sci. Mater. Med. 2017, 28, 149. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.Q.; Qin, L.Y.; Cai, J.L.; Zhu, G.Y.; Bin, X.; Yan, H.S. Effect of heparin on production of basic fibroblast growth factor and transforming growth factor-beta1 by human normal skin and hyperplastic scar fibroblasts. J. Burn Care Res. 2007, 28, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.Y.; Yap, Z.L.; Seet, L.F.; Chan, H.H.; Toh, L.Z.; Chu, S.W.; Lee, Y.S.; Wong, T.T.; Tan, T.T. Positive-charge tuned gelatin hydrogel-siSPARC injectable for siRNA anti-scarring therapy in post glaucoma filtration surgery. Sci. Rep. 2021, 11, 1470. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Qin, G.; Yu, L. The triblock copolymers hydrogel through intracameral injection may be a new potential ophthalmic drug delivery with antiscaring drugs after glaucoma filtration surgery. Med. Hypotheses 2013, 80, 23–25. [Google Scholar] [CrossRef]
- Lin, A.P.; Chung, J.E.; Zhang, K.S.; Chang, M.M.; Orengo-Nania, S.; Gross, R.L.; Chang, P.T. Outcomes of surgical bleb revision for late-onset bleb leaks after trabeculectomy. J. Glaucoma 2013, 22, 21–25. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Quigley, H.A.; Jampel, H.D.; Friedman, D.S.; Ahmad, S.I.; Congdon, N.G.; McKinnon, S. Outcomes of surgical bleb revision for complications of trabeculectomy. Ophthalmology 2009, 116, 1713–1718. [Google Scholar] [CrossRef]
- Song, A.; Scott, I.U.; Flynn, H.W.J.; Budenz, D.L. Delayed-onset bleb-associated endophthalmitis: Clinical features and visual acuity outcomes. Ophthalmology 2002, 109, 985–991. [Google Scholar] [CrossRef]
- Georgoulas, S.; Dahlmann-Noor, A.; Brocchini, S.; Khaw, P.T. Modulation of wound healing during and after glaucoma surgery. Prog. Brain Res. 2008, 173, 237–254. [Google Scholar] [CrossRef]
- Tannenbaum, D.P.; Hoffman, D.; Greaney, M.J.; Caprioli, J. Outcomes of bleb excision and conjunctival advancement for leaking or hypotonous eyes after glaucoma filtering surgery. Br. J. Ophthalmol. 2004, 88, 99–103. [Google Scholar] [CrossRef]
- Calladine, D.; Ratnarajan, G.; McAllister, J. New polyethylene glycol ‘hydrogel bandage’ technique to cover the conjunctival-limbal wound in fornix-based trabeculectomy surgery. Clin. Exp. Ophthalmol. 2011, 39, 921–923. [Google Scholar] [CrossRef]
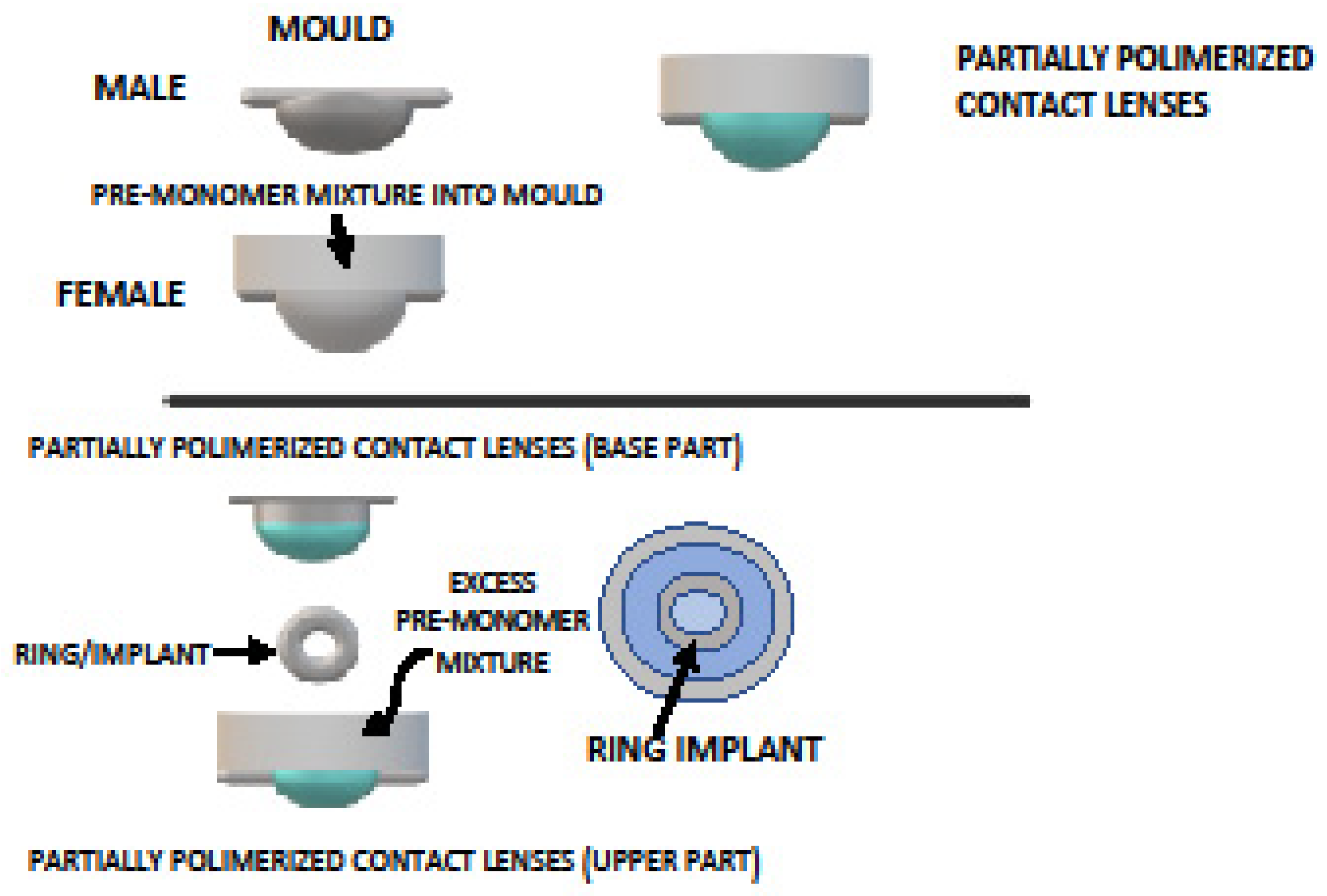
| Author | Year | Drug | Group | Polymer Name | Manufacturing | IOP Decrease | Duration |
|---|---|---|---|---|---|---|---|
| Maulvi F.A. [82] | 2021 | Bimatoprost | Prostaglandin | Graphene oxide | Bimatoprost before polymerization | / | |
| Garcia Fernandez M.J. [93] | 2013 | Ethoxzolamide | CAI | Poly-HEMA and poly-HEMA-co-APMA | Dry disk immersed into | / | 10 days |
| Jung H. J. [80] | 2012 | Timolol | B-blockers | Propoxylated glyceryl triacylate | Adding particles to the polymerization mixture | / | <=5 days |
| Maulvi F.A. [81] | 2016 | Timolol | B-blockers | Ethyl cellulose nanoparticle-laden ring | TM loaded ring in hydrogel contact lens | Decrease by 6.3 ± 1.92 mmHg after three hours | 8 days (in vivo) |
| Mohammadi S. [94] | 2014 | Latanoprost | Prostaglandin | Balaficon A/Senofilcon A | Incubation in drug solution | >24 h | |
| Peng C. C. [95] | 2012 | Timolol | B-blockers | NIGHT&DAY silicone hydrogel contact lenses With/without vit. E | Soaked | Decreased by 5 | / |
| Ciolino J.B. [55] | 2016 | Latanoprost | Prostaglandin | Methafilicon+ methacrylic acid (Hydrogel) | Photopolymerization | Low dose: decreased > 6. High dose decreased> 10 | / |
| Sekar P. [84] | 2019 | Bimatoprost and Latanoprost | Prostaglandin | Vit E added to ACUVUE OASIS and ACUVUE TRUE EYE | Soaked | / | >10 days |
| Yan F. [59] | 2020 | Bimatoprost | Prostaglandin | HEMA (hydroxyl ethylmethacrylate) | Imprinting vs. soaked | / | Imprinted 36–60 h |
| Xu J. [64] | 2010 | Puerarin | Chinese medicine ability to block b-receptors | pHEMA-NVP-MA | Soaked | / | 350 min |
| Author | In vitro/Vivo | Hydrogel | Function | Drug Delivered | Activation Mode | Administration Site |
|---|---|---|---|---|---|---|
| Blake et al. J Glaucoma, 2006 [145] | in vitro | P(HEMA) | Drug delivery system | Mitomycin C | / | / |
| Liang et al. Biomed Mater., 2010 [151] | in vivo | Peptide hydrogel with RGD sequence | Keeping tissues apart, inflammatory inhibition | / | / | Filtering bleb |
| Yang et al. Acta Pharmacol Sin., 2010 [158] | in vitro and vivo | CMCS | Drug delivery system | 5-fluorouracil, bevacizumab | / | Filtering bleb |
| Kojima et al. Invest Ophthalmol Vis Sci., 2011 [161] | in vivo | Gelatin-hydrogel | Drug delivery system | Chymase inhibitor | / | Filtering bleb |
| Xi et al. PLoS One., 2014 [156] | in vitro and vivo | PTMC15-F127-PTMC15 | Drug delivery system | Mitomycin C | Body temperature | Filtering bleb |
| Peng et al. Med Hypothesis Discov Innov Ophthalmol., 2014 [159] | in vivo | PECE | Drug delivery system | Bevacizumab | Body temperature | Anterior chamber |
| Han et al. J Mater Sci Mater Med., 2015 [150] | in vitro and vivo | PECE | Drug delivery system | Bevacizumab | Body temperature | Anterior chamber |
| Kojima et al. Invest Ophthalmol Vis Sci., 2015 [157] | in vivo | Gelatin-hydrogel | Drug delivery system | Mitomycin C | / | Filtering bleb |
| Sun et al. J Mater Chem B., 2017 [166] | in vitro and vivo | PLGA-PEG-PLGA | Drug delivery system | Cyclosporine A | Body temperature | Filtering bleb |
| Qiao et al. J Mater Sci Mater Med., 2017 [167] | in vivo | HECTS | Drug delivery system | Heparin | UV irradiation | Under scleral flap |
| Maeda et al. Int J Mol Sci., 2017 [164] | in vivo | Gelatin-hydrogel | Drug delivery system | TGF-β antibody | / | Filtering bleb |
| Martin et al. Macromol Rapid Commun., 2020 [152] | in vitro | DMAA + AOAQ | Fibroblast cells repellent | / | UV irradiation | / |
| Chen et al. J Biomed Mater Res B Appl Biomater., 2021 [154] | in vitro | Peptide hydrogel with RGD sequence | Peptide competition on protein binding site | / | / | / |
| Chun et al. Sci Rep., 2021 [169] | in vitro and vivo | Gelatin-tyramine | Drug delivery system | siRNA | Charge tunability | Filtering bleb |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fea, A.M.; Novarese, C.; Caselgrandi, P.; Boscia, G. Glaucoma Treatment and Hydrogel: Current Insights and State of the Art. Gels 2022, 8, 510. https://doi.org/10.3390/gels8080510
Fea AM, Novarese C, Caselgrandi P, Boscia G. Glaucoma Treatment and Hydrogel: Current Insights and State of the Art. Gels. 2022; 8(8):510. https://doi.org/10.3390/gels8080510
Chicago/Turabian StyleFea, Antonio Maria, Cristina Novarese, Paolo Caselgrandi, and Giacomo Boscia. 2022. "Glaucoma Treatment and Hydrogel: Current Insights and State of the Art" Gels 8, no. 8: 510. https://doi.org/10.3390/gels8080510
APA StyleFea, A. M., Novarese, C., Caselgrandi, P., & Boscia, G. (2022). Glaucoma Treatment and Hydrogel: Current Insights and State of the Art. Gels, 8(8), 510. https://doi.org/10.3390/gels8080510







