Dextrin-Based Nanohydrogels for Rokitamycin Prolonged Topical Delivery
Abstract
:1. Introduction
2. Results and Discussion
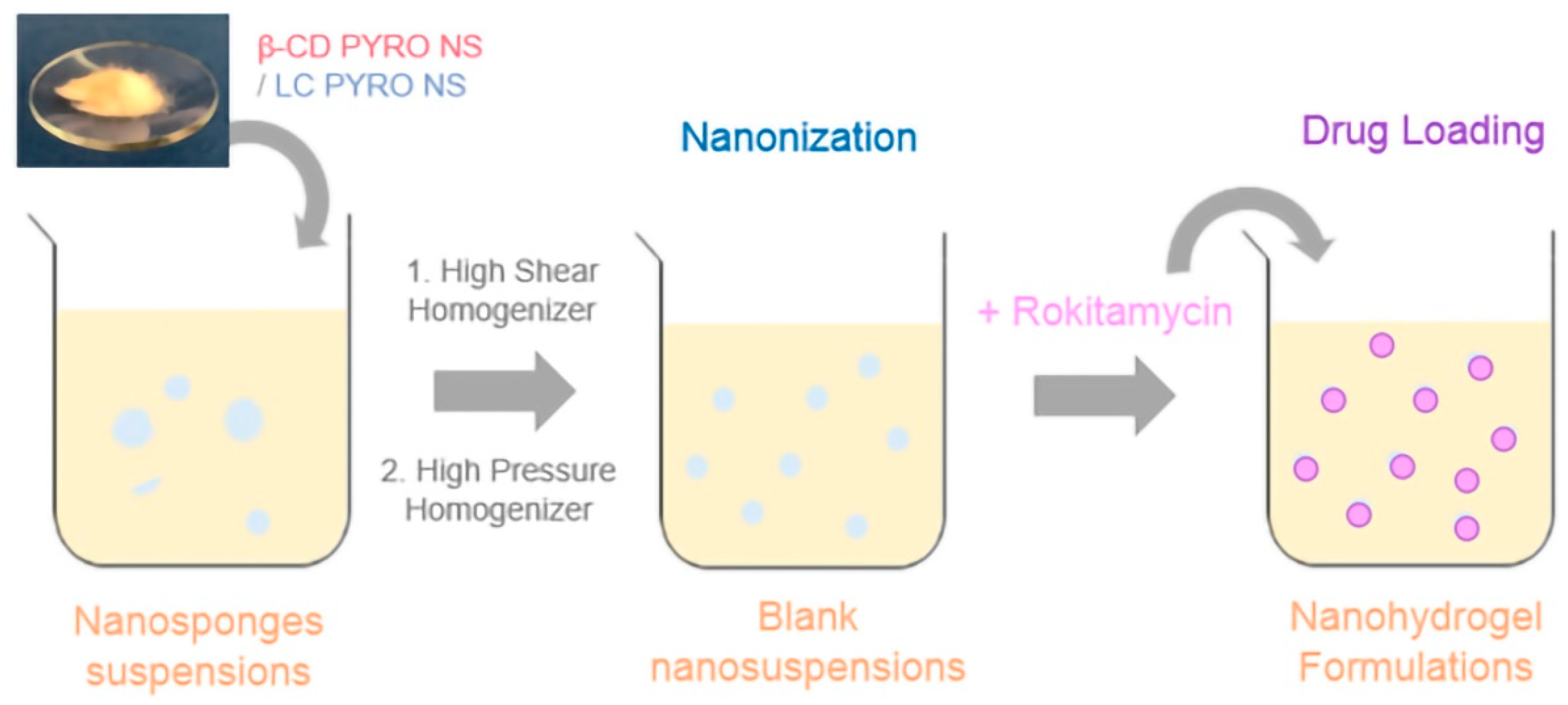
2.1. Physicochemical Characterization and Loading of Nanohydrogels
2.2. Swelling Capacity
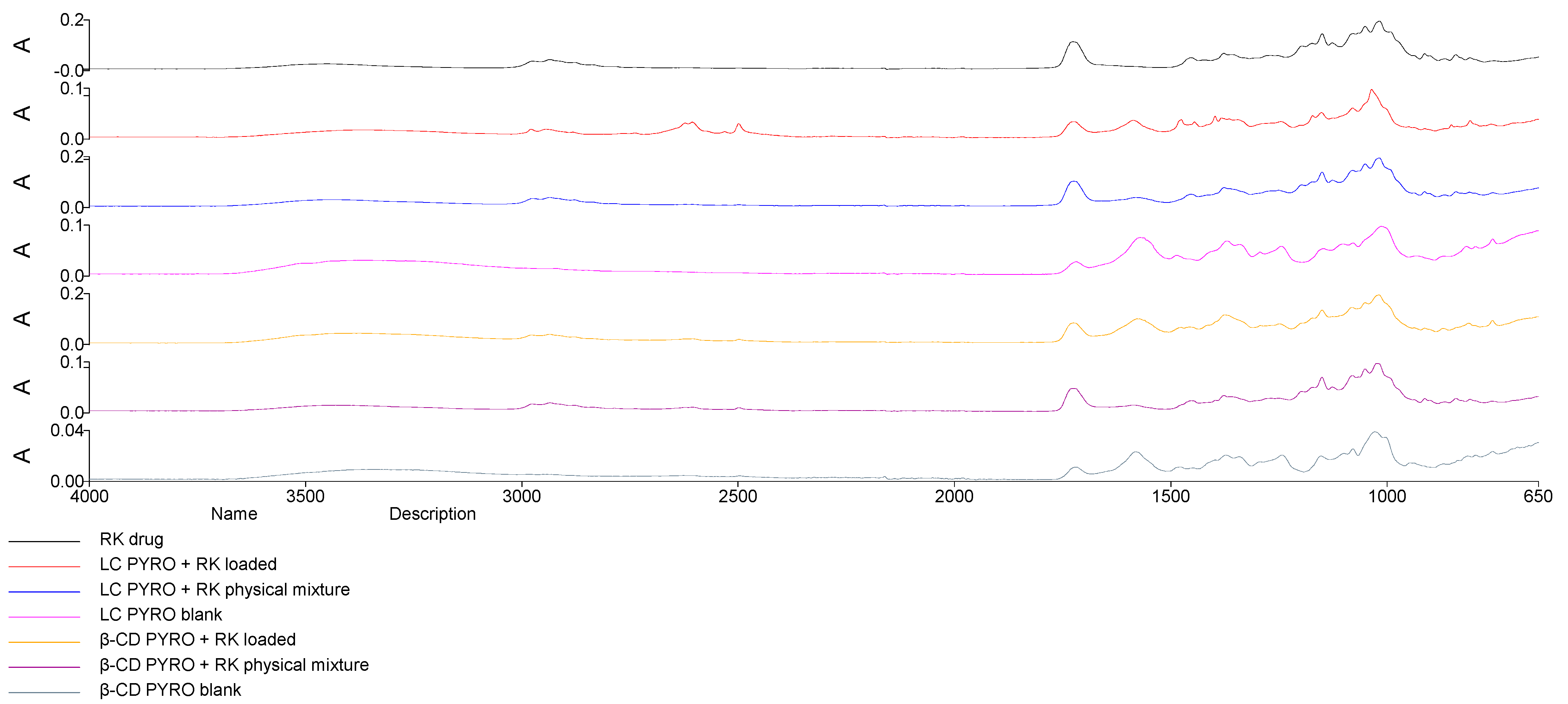
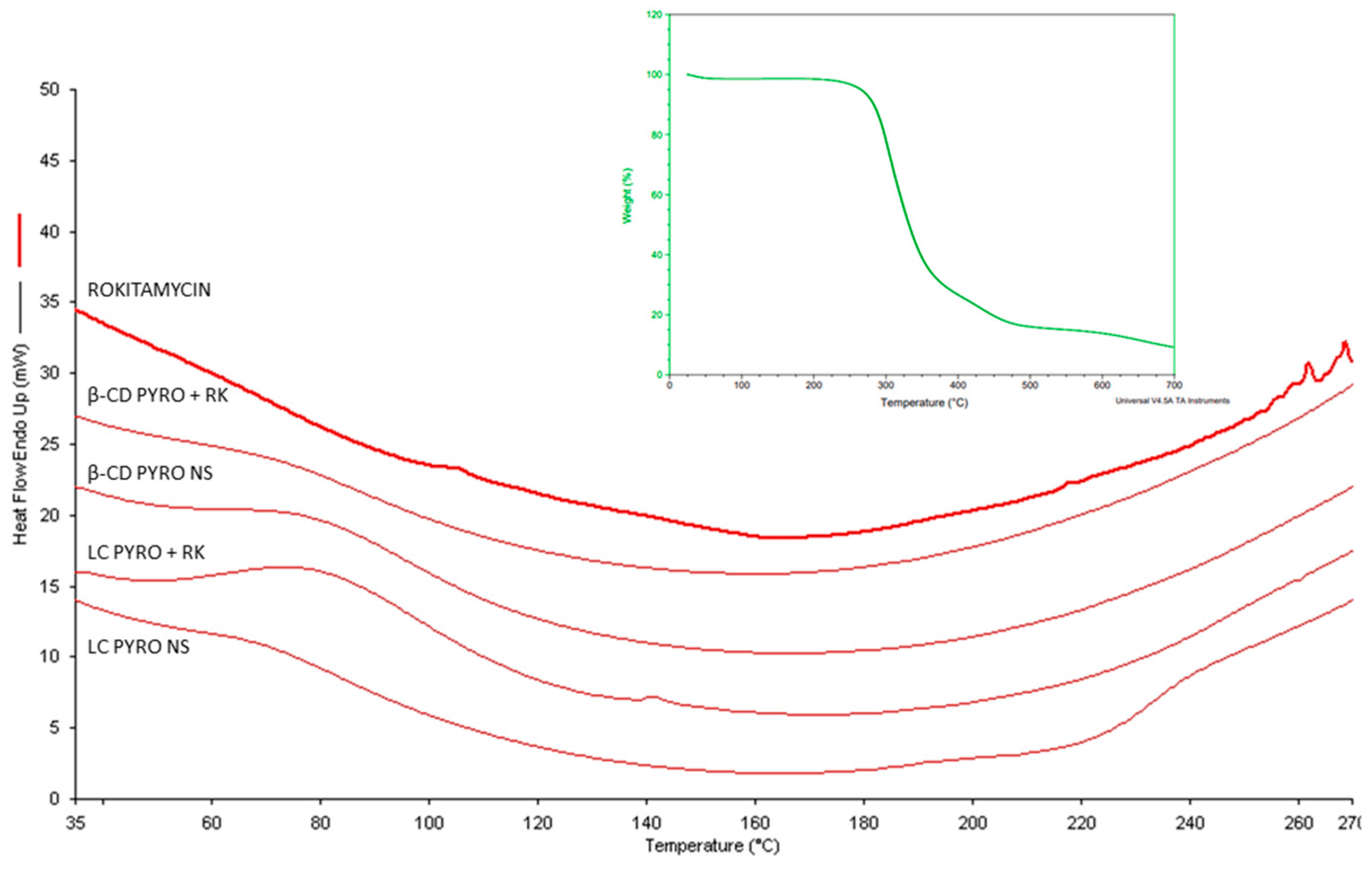
2.3. Structural Analysis: Fourier Transformed Infrared and Differential Scanning Calorimetry
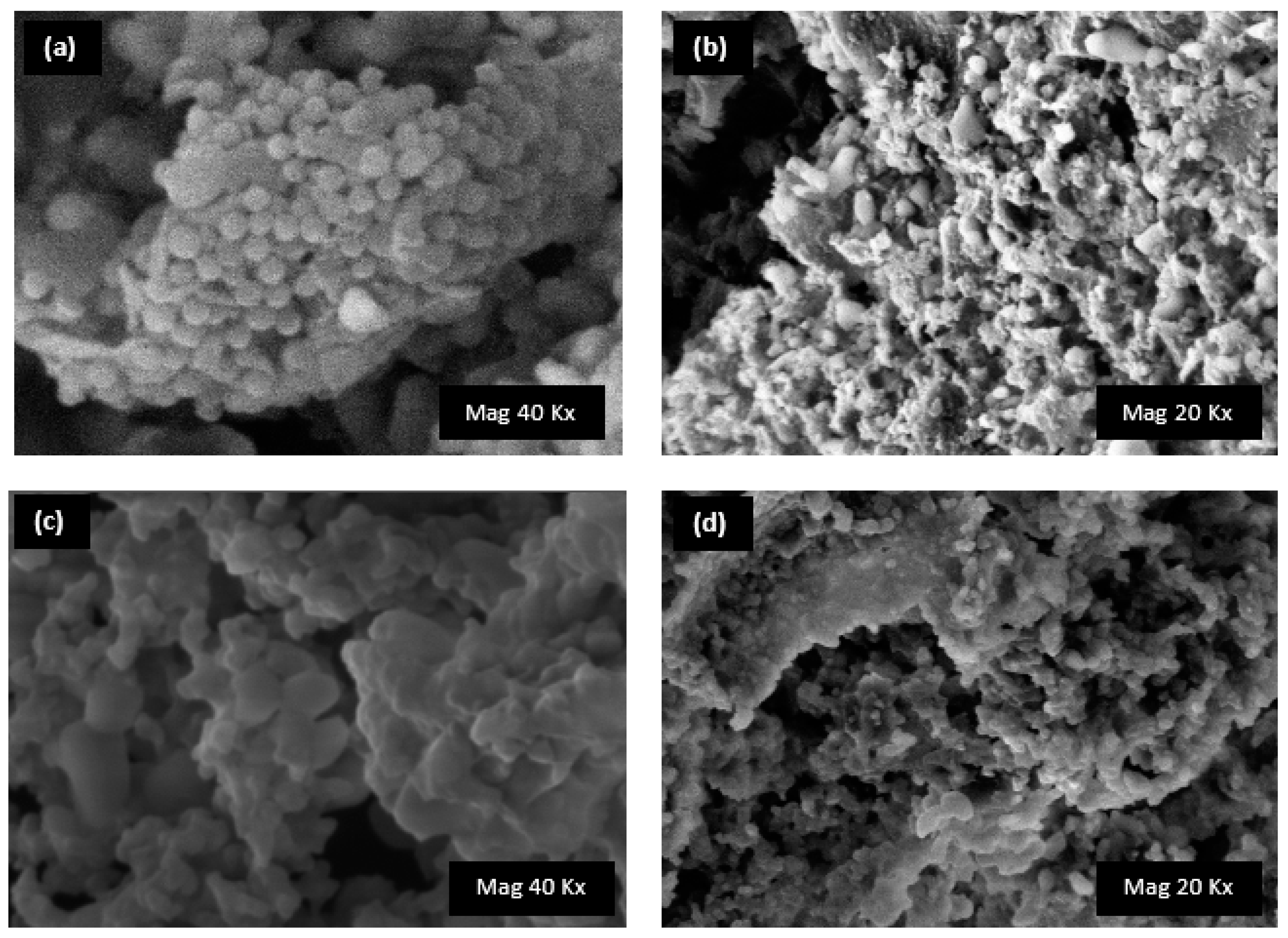
2.4. Morphology Analysis: Scanning Electron Microscopy
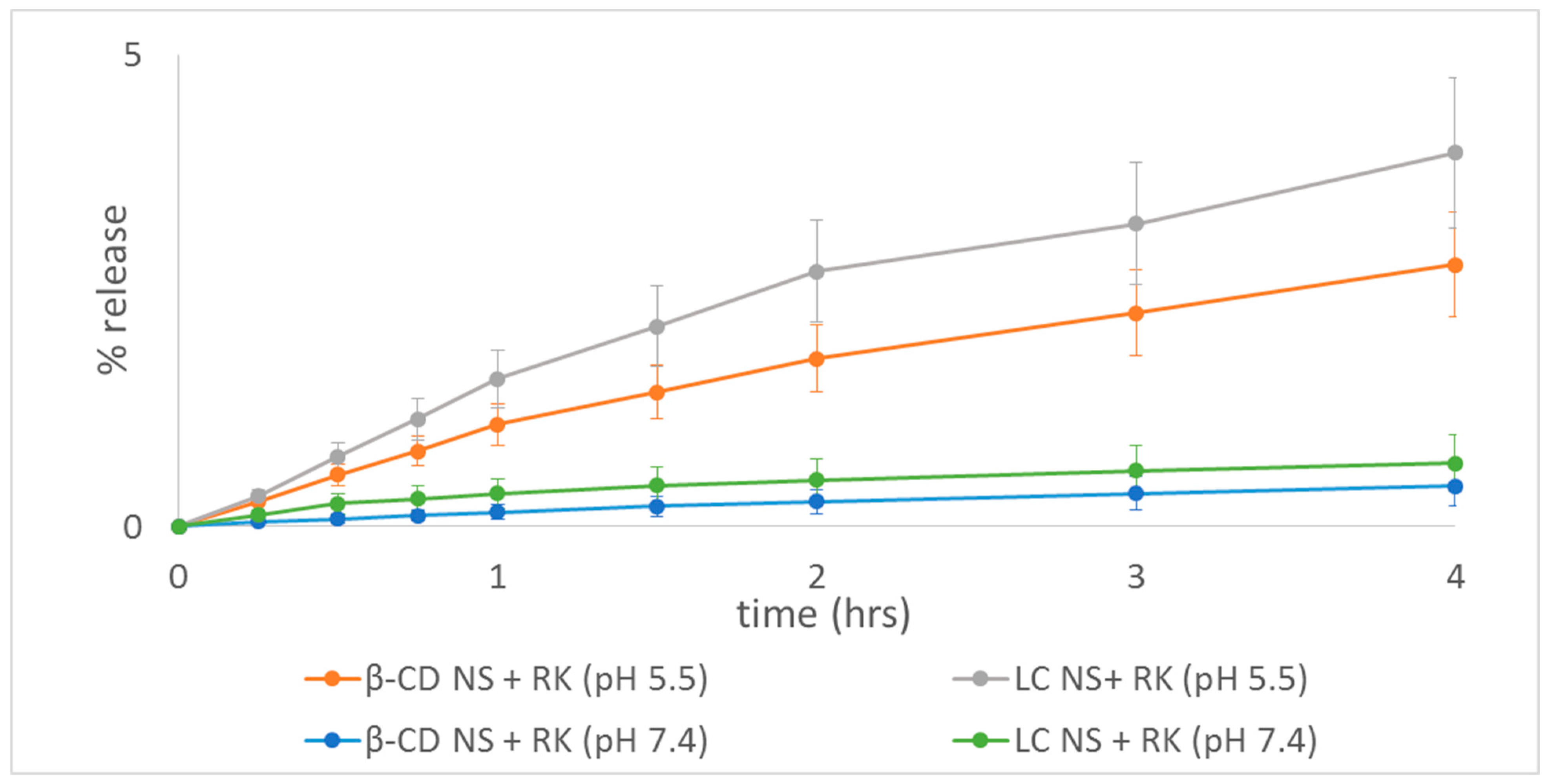
2.5. Rokitamycin Release Studies
2.6. Mathmatical Model Fitting
2.7. Evaluation of Biocompatibility
3. Conclusions
4. Materials and Methods
4.1. Chemicals and Reagents
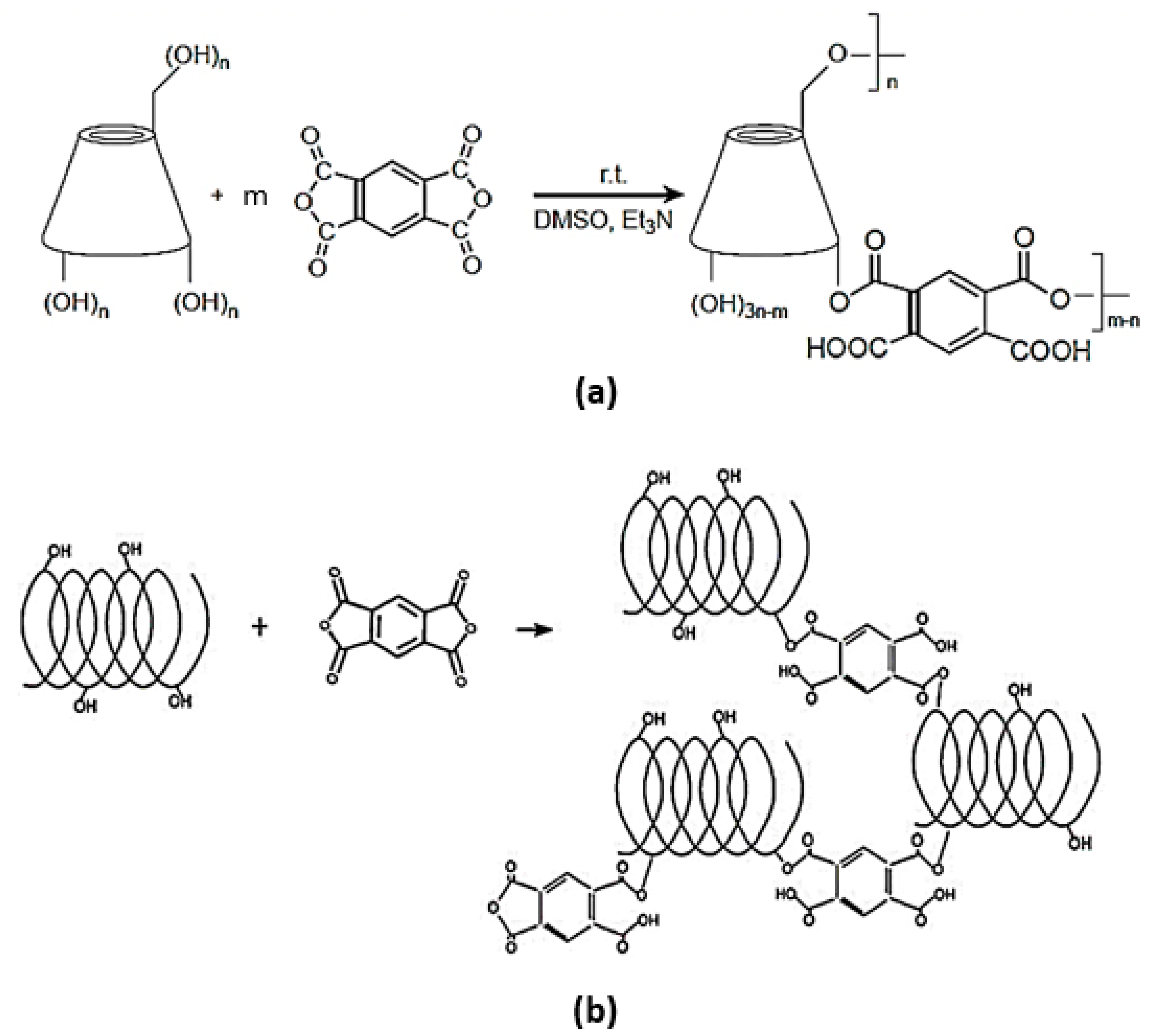
4.2. Synthesis of Dextrin-Based Nanosponges
- β-CD PYRO(1:4) NSs was prepared as follows: 14.10 g of dehydrated β-CD was added to 16.6 mL of DMSO inside a round bottom flask and left stirring until a colorless homogeneous solution is obtained. TEA (4.17 mL) was then pipetted into the flask followed by addition of 3.13 g of PYRO. At the end of the process a dark amorphous solid-like gel was formed and left overnight to solidify. The resulting nanosponge was removed and ground in a mortar and pestle and then washed in a Buchner with 500 mL of distilled water, and 500 mL of acetone. Once again it was left to dry and then extracted with acetone in a Soxhlet at 60–70 °C for 48 h. Finally, the coarse powder was packed and stored in a dry medium.
- LC PYRO (1:4) NSs: Inside a dry clean round bottom flask, 9.78 g of LC was solubilized under continuous stirring in 40 mL of DMSO and then 10 mL of TEA and 7.55 g of PYRO were added. The molar ratio used between Linecaps and cross-linker was 1:0.57, expressed as molar ratio of one mole of condensed glucose of maltodextrin (molar mass of 162.15 g/mol) with respect to 0.57 moles of cross-linker. After 24 h, the reaction was complete and the nanosponges were ground and washed with deionized water in a Buchner funnel and then left for drying. The purification was carried out by means of Soxhlet extraction with acetone for 14 h.
4.3. Preparation of Blank Nanosuspensions
4.4. Rokitamycin Loading Procedure
4.5. Physicochemical Characterizations
4.5.1. Dynamic Light Scattering (DLS)
4.5.2. Fourier Transformed Infrared (FTIR)
4.5.3. Thermogravimetric Analysis (TGA)
4.5.4. Differential Scanning Calorimetry (DSC)
4.5.5. Scanning Electron Microscopy (SEM)
4.5.6. High Performance Liquid Chromatography (HPLC)
4.6. Encapsulation Efficiency and Loading Capacity
4.7. Mucoadhesion Capability Evaluation
4.8. Swelling Degree Evaluation
4.9. Viscosity Determination
4.10. Rokitmycin Release Studies
4.11. Mathmatical Models Fitting
4.12. Evaluation of Biocompatibility
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Braga, P.C.; Bovio, C.; Culici, M.; Dal Sasso, M. Flow cytometric assessment of susceptibilities of Streptococcus pyogenes to erythromycin and rokitamycin. Antimicrob. Agents Chemother. 2003, 47, 408–412. [Google Scholar] [CrossRef] [Green Version]
- Dinos, G.P. The macrolide antibiotic renaissance. Br. J. Pharmacol. 2017, 174, 2967–2983. [Google Scholar] [CrossRef]
- Rassu, G.; Gavini, E.; Mattana, A.; Giunchedi, P. Improvement of Antiamoebic Activity of Rokitamycin Loaded in Chitosan Microspheres. Open Drug Deliv. J. 2008, 2, 38–43. [Google Scholar] [CrossRef] [Green Version]
- Vázquez-Laslop, N.; Mankin, A.S. How Macrolide Antibiotics Work. Trends Biochem. Sci. 2018, 43, 668–684. [Google Scholar] [CrossRef] [PubMed]
- Venditto, V.J.; Feola, D.J. Delivering macrolide antibiotics to heal a broken heart—And other inflammatory conditions. Adv. Drug Deliv. Rev. 2022, 184, 114252. [Google Scholar] [CrossRef]
- Scutera, S.; Argenziano, M.; Sparti, R.; Bessone, F.; Bianco, G.; Bastiancich, C.; Castagnoli, C.; Stella, M.; Musso, T.; Cavalli, R. Enhanced Antimicrobial and Antibiofilm Effect of New Colistin-Loaded Human Albumin Nanoparticles. Antibiotics 2021, 10, 57. [Google Scholar] [CrossRef]
- Braga, P.C. Rokitamycin: Bacterial Resistance to a 16-Membered Ring Macrolide Differs from that to 14- and 15-Membered Ring Macrolides. J. Chemother. 2013, 14, 115–131. [Google Scholar] [CrossRef]
- Gavini, E.; Rassu, G.; Ferraro, L.; Generosi, A.; Rau, J.V.; Brunetti, A.; Giunchedi, P.; Dalpiaz, A. Influence of Chitosan Glutamate on the in vivo Intranasal Absorption of Rokitamycin from Microspheres. J. Pharm. Sci. 2011, 100, 1488–1502. [Google Scholar] [CrossRef]
- Orlando, R.; Fragasso, A.; Benvenuti, C.; Okolicsanyi, L. Pharmacokinetics of Rokitamycin after Single and Repeated Dose Oral Administration to Patients with Liver Cirrhosis. Drug Investig. 1991, 33, 162–166. [Google Scholar] [CrossRef]
- Rassu, G.; Gavini, E.; Jonassen, H.; Zambito, Y.; Fogli, S.; Breschi, M.C.; Giunchedi, P. New chitosan derivatives for the preparation of rokitamycin loaded microspheres designed for ocular or nasal administration. J. Pharm. Sci. 2009, 98, 4852–4865. [Google Scholar] [CrossRef] [PubMed]
- Moya-Ortega, M.D.; Alvarez-Lorenzo, C.; Concheiro, A.; Loftsson, T. Cyclodextrin-based nanogels for pharmaceutical and biomedical applications. Int. J. Pharm. 2012, 428, 152–163. [Google Scholar] [CrossRef]
- Argenziano, M.; Dianzani, C.; Ferrara, B.; Swaminathan, S.; Manfredi, A.; Ranucci, E.; Cavalli, R.; Ferruti, P. Cyclodextrin-Based Nanohydrogels Containing Polyamidoamine Units: A New Dexamethasone Delivery System for Inflammatory Diseases. Gels 2017, 3, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shende, P.; Kulkarni, Y.A.; Gaud, R.S.; Deshmukh, K.; Cavalli, R.; Trotta, F.; Caldera, F. Acute and Repeated Dose Toxicity Studies of Different β-Cyclodextrin-Based Nanosponge Formulations. J. Pharm. Sci. 2015, 104, 1856–1863. [Google Scholar] [CrossRef]
- Tannous, M.; Trotta, F.; Cavalli, R. Nanosponges for combination drug therapy: State-of-the-art and future directions. Nanomedicine 2020, 15, 643–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caldera, F.; Tannous, M.; Cavalli, R.; Zanetti, M.; Trotta, F. Evolution of Cyclodextrin Nanosponges. Int. J. Pharm. 2017, 531, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, R.; Trotta, F.; Tumiatti, W. Cyclodextrin-based Nanosponges for Drug Delivery. J. Incl. Phenom. Macrocycl. Chem. 2006, 56, 209–213. [Google Scholar] [CrossRef]
- Swaminathan, S.; Cavalli, R.; Trotta, F. Cyclodextrin-based nanosponges: A versatile platform for cancer nanotherapeutics development. Wiley Interdiscip. Rev. Nanomed. Nanobiotech. 2016, 8, 579–601. [Google Scholar] [CrossRef] [PubMed]
- Appleton, S.L.; Tannous, M.; Argenziano, M.; Muntoni, E.; Rosa, A.C.; Rossi, D.; Caldera, F.; Scomparin, A.; Trotta, F.; Cavalli, R. Nanosponges as protein delivery systems: Insulin, a case study. Int. J. Pharm. 2020, 590, 119888. [Google Scholar] [CrossRef]
- Yi, P.; Wang, Y.; He, P.; Zhan, Y.; Sun, Z.; Li, Y.; Zhang, Y. Study on β-cyclodextrin-complexed nanogels with improved thermal response for anticancer drug delivery. Mater. Sci. Eng. C 2017, 78, 773–779. [Google Scholar] [CrossRef]
- Trotta, F.; Dianzani, C.; Caldera, F.; Mognetti, B.; Cavalli, R. The application of nanosponges to cancer drug delivery. Expert Opin. Drug Deliv. 2014, 11, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Ciesielska, A.; Ciesielski, W.; Girek, B.; Girek, T.; Koziel, K.; Kulawik, D.; Lagiewka, J. Biomedical Application of Cyclodextrin Polymers Cross-Linked via Dianhydrides of Carboxylic Acids. Appl. Sci. 2020, 10, 8463. [Google Scholar] [CrossRef]
- Nabawy, A.; Makabenta, J.M.; Li, C.H.; Park, J.; Chattopadhyay, A.N.; Schmidt-Malan, S.; Gupta, A.; Patel, R.; Rotello, V.M. Activity of biodegradable polymeric nanosponges against dual-species bacterial biofilms. ACS Biomater. Sci. Eng. 2021, 7, 1780–1786. [Google Scholar] [CrossRef]
- Wang, F.; Gao, W.; Thamphiwatana, S.; Luk, B.T.; Angsantikul, P.; Zhang, Q.; Hu, C.M.J.; Fang, R.H.; Copp, J.A. Hydrogel Retaining Toxin-Absorbing Nanosponges for Local Treatment of Methicillin-Resistant Staphylococcus aureus Infection. Adv. Mater. 2015, 27, 3437–3443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khazaei Monfared, Y.; Mahmoudian, M.; Hoti, G.; Caldera, F.; López Nicolás, J.M.; Zakeri-Milani, P.; Matencio, A.; Trotta, F. Cyclodextrin-Based Nanosponges as Perse Antimicrobial Agents Increase the Activity of Natural Antimicrobial Peptide Nisin. Pharmaceutics 2022, 14, 685. [Google Scholar] [CrossRef] [PubMed]
- Matencio, A.; Hoti, J.; Monfared, Y.K.; Rezayat, A.; Pedrazzo, A.R.; Caldera, F.; Trotta, F. Cyclodextrin Monomers and Polymers for Drug Activity Enhancement. Polymers 2021, 13, 1684. [Google Scholar] [CrossRef]
- Mendes, C.; Meirelles, G.C.; Barp, C.G.; Assreuy, J.; Silva, M.A.S.; Ponchel, G. Cyclodextrin based nanosponge of norfloxacin: Intestinal permeation enhancement and improved antibacterial activity. Carbohydr. Polym. 2018, 195, 586–592. [Google Scholar] [CrossRef]
- Deshmukh, K.; Tanwar, Y.S.; Sharma, S.; Shende, P.; Cavalli, R. Functionalized nanosponges for controlled antibacterial and antihypocalcemic actions. Biomed. Pharmacother. 2016, 84, 485–494. [Google Scholar] [CrossRef]
- Evaluation of the Taste Masking Performance of New Maltodextrin KLEPTOSE® LINECAPS-Scientific Poster|Roquette. Available online: https://www.roquette.com/media-center/resources/pharma-poster-evaluation-of-the-taste-masking-performance-kleptose-linecaps (accessed on 27 June 2022).
- Girek, T.; Koziel, K.; Girek, B.; Ciesielski, W. CD Oxyanions as a Tool for Synthesis of Highly Anionic Cyclodextrin Polymers. Polymers 2020, 12, 2845. [Google Scholar] [CrossRef]
- Shah, P.; Goodyear, B.; Dholaria, N.; Puri, V.; Michniak-Kohn, B. Nanostructured Non-Ionic Surfactant Carrier-Based Gel for Topical Delivery of Desoximetasone. Int. J. Mol. Sci. 2021, 22, 1535. [Google Scholar] [CrossRef]
- Momekova, D.; Danov, Y.; Momekov, G.; Ivanov, E.; Petrov, P. Polysaccharide Cryogels Containing β-Cyclodextrin for the Delivery of Cannabidiol. Pharmaceutics 2021, 13, 1774. [Google Scholar] [CrossRef]
- He, Y.; Zeng, S.; Abd El-Aty, A.M.; Hacımüftüoğlu, A.; Kalekristos Yohannes, W.; Khan, M.; She, Y. Development of Water-Compatible Molecularly Imprinted Polymers Based on Functionalized β-Cyclodextrin for Controlled Release of Atropine. Polymers 2020, 12, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castiglione, F.; Crupi, V.; Majolino, D.; Mele, A.; Rossi, B.; Trotta, F.; Venuti, V. Effect of cross-linking properties on the vibrational dynamics of cyclodextrins-based polymers: An experimental-numerical study. J. Phys. Chem. B 2012, 116, 7952–7958. [Google Scholar] [CrossRef]
- Hoti, G.; Caldera, F.; Cecone, C.; Rubin Pedrazzo, A.; Anceschi, A.; Appleton, S.L.; Khazaei Monfared, Y.; Trotta, F. Effect of the Cross-Linking Density on the Swelling and Rheological Behavior of Ester-Bridged β-Cyclodextrin Nanosponges. Materials 2021, 14, 478. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, S.; Cavalli, R.; Trotta, F.; Ferruti, P.; Ranucci, E.; Gerges, I.; Manfredi, A.; Marinotto, D.; Vavia, P.R. In vitro release modulation and conformational stabilization of a model protein using swellable polyamidoamine nanosponges of β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2010, 68, 183–191. [Google Scholar] [CrossRef]
- Tannous, M.; Caldera, F.; Hoti, G.; Dianzani, U.; Cavalli, R.; Trotta, F. Drug-Encapsulated Cyclodextrin Nanosponges; Springer: Berlin/Heidelberg, Germany, 2021; Volume 2207. [Google Scholar]
- Swaminathan, S.; Vavia, P.R.; Trotta, F.; Torne, S. Formulation of betacyclodextrin based nanosponges of itraconazole. J. Incl. Phenom. Macrocycl. Chem. 2007, 57, 89–94. [Google Scholar] [CrossRef]
- Dhakar, N.K.; Matencio, A.; Caldera, F.; Argenziano, M.; Cavalli, R.; Dianzani, C.; Zanetti, M.; López-Nicolás, J.M.; Trotta, F. Comparative Evaluation of Solubility, Cytotoxicity and Photostability Studies of Resveratrol and Oxyresveratrol Loaded Nanosponges. Pharmaceutics 2019, 11, 545. [Google Scholar] [CrossRef] [Green Version]
- Rizzi, V.; Gubitosa, J.; Signorile, R.; Fini, P.; Cecone, C.; Matencio, A.; Trotta, F.; Cosma, P. Cyclodextrin nanosponges as adsorbent material to remove hazardous pollutants from water: The case of ciprofloxacin. Chem. Eng. J. 2021, 411, 128514. [Google Scholar] [CrossRef]
- Khazaei Monfared, Y.; Mahmoudian, M.; Cecone, C.; Caldera, F.; Zakeri-Milani, P.; Matencio, A.; Trotta, F. Stabilization and Anticancer Enhancing Activity of the Peptide Nisin by Cyclodextrin-Based Nanosponges against Colon and Breast Cancer Cells. Polymers 2022, 14, 594. [Google Scholar] [CrossRef]
- Tod, M.; Biarez, O.; Nicolas, P.; Petitjean, O. Sensitive determination of josamycin and rokitamycin in plasma by high-performance liquid chromatography with fluorescence detection. J. Chromatogr. B Biomed. Sci. Appl. 1992, 575, 171–176. [Google Scholar] [CrossRef]
- Argenziano, M.; Haimhoffer, A.; Bastiancich, C.; Jicsinszky, L.; Caldera, F.; Trotta, F.; Scutera, S.; Alotto, D.; Fumagalli, M.; Musso, T.; et al. In vitro enhanced skin permeation and retention of imiquimod loaded in β-cyclodextrin nanosponge hydrogel. Pharmaceutics 2019, 11, 138. [Google Scholar] [CrossRef] [Green Version]
- Bastiancich, C.; Scutera, S.; Alotto, D.; Cambieri, I.; Fumagalli, M.; Casarin, S.; Rossi, S.; Trotta, F.; Stella, M.; Cavalli, R.; et al. Cyclodextrin-Based Nanosponges as a Nanotechnology Strategy for Imiquimod Delivery in Pathological Scarring Prevention and Treatment. J. Nanopharm. Drug Deliv. 2015, 2, 311–324. [Google Scholar] [CrossRef]
- Torne, S.J.; Ansari, K.A.; Vavia, P.R.; Trotta, F.; Cavalli, R. Enhanced oral paclitaxel bioavailability after administration of paclitaxel-loaded nanosponges. Drug Deliv. 2010, 17, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Bruschi, M.L. 5-Mathematical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Elsevier: Amsterdam, The Netherlands, 2015; pp. 63–86. [Google Scholar] [CrossRef]


| Sample | Blank β-CD PYRO | β-CD PYRO + RK | Blank LC PYRO | LC PYRO + RK |
|---|---|---|---|---|
| Average Diameter ± SD (nm) | 215.6 ± 2.54 | 256.3 ± 13.8 | 213.5 ± 2.43 | 250.7 ± 11.0 |
| PDI ± SD | 0.021 ± 0.015 | 0.023 ± 0.013 | 0.048 ± 0.031 | 0.050 ± 0.010 |
| Z-Potential ± SD (mV) | −28.45 ± 0.71 | −26.89 ± 0.34 | −27.15 ± 0.22 | −25.73 ± 0.33 |
| Mucoadhesion (%) | 85.72 | 76.93 | 84.59 | 72.39 |
| Sample | Blank β-CD PYRO | β-CD PYRO + RK | Blank LC PYRO | LC PYRO + RK |
|---|---|---|---|---|
| Average Diameter ± SD (nm) | 223.6 ± 92.0 | 267.3 ± 14.5 | 223.5 ± 2.43 | 244.7 ± 25.2 |
| PDI ± SD | 0.024 ± 0.013 | 0.024 ± 0.013 | 0.028 ± 0.013 | 0.022 ± 0.015 |
| Z-Potential ± SD (mV) | −28.38 ± 2.22 | −27.86 ± 0.24 | −26.15 ± 0.24 | −27.44 ± 0.21 |
| Sample | RK | β-CD PYRO + RK | LC PYRO + RK | RK | β-CD PYRO + RK | LC PYRO + RK |
|---|---|---|---|---|---|---|
| pH | 5.5 | 5.5 | 5.5 | 7.4 | 7.4 | 7.4 |
| Solubility (μg/mL) | 470.85 | 571.19 | 1455.13 | 450.44 | 525.21 | 1130.21 |
| Enhancement Factor | - | 1.21 | 3.09 | - | 1.17 | 2.51 |
| Sample | β-CD PYRO NS | LC PYRO NS | β-CD PYRO NS | LC PYRO NS |
|---|---|---|---|---|
| pH | 5.5 | 5.5 | 7.4 | 7.4 |
| Swelling Degree | 684.90 ± 10.62 | 892.45 ± 7.96 | 1182.69 ± 11.67 | 1105.76 ± 10.94 |
| Sample | β-CD PYRO + RK (pH 5.5) | β-CD PYRO + RK (pH 7.4) | LC PYRO + RK (pH 5.5) | LC PYRO + RK (pH 7.4) |
|---|---|---|---|---|
| Higuchi Model (r2) | 0.9957 | 0.9942 | 0.9951 | 0.9895 |
| Korsmeyer–Peppas Model (r2) | 0.9988 | 0.9781 | 0.9940 | 0.9756 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tannous, M.; Lucia Appleton, S.; Hoti, G.; Caldera, F.; Argenziano, M.; Monfared, Y.K.; Matencio, A.; Trotta, F.; Cavalli, R. Dextrin-Based Nanohydrogels for Rokitamycin Prolonged Topical Delivery. Gels 2022, 8, 490. https://doi.org/10.3390/gels8080490
Tannous M, Lucia Appleton S, Hoti G, Caldera F, Argenziano M, Monfared YK, Matencio A, Trotta F, Cavalli R. Dextrin-Based Nanohydrogels for Rokitamycin Prolonged Topical Delivery. Gels. 2022; 8(8):490. https://doi.org/10.3390/gels8080490
Chicago/Turabian StyleTannous, Maria, Silvia Lucia Appleton, Gjylije Hoti, Fabrizio Caldera, Monica Argenziano, Yousef Khazaei Monfared, Adrián Matencio, Francesco Trotta, and Roberta Cavalli. 2022. "Dextrin-Based Nanohydrogels for Rokitamycin Prolonged Topical Delivery" Gels 8, no. 8: 490. https://doi.org/10.3390/gels8080490






