Aerogel Assembled by Two Types of Carbon Nanoparticles for Efficient Removal of Heavy Metal Ions
Abstract
:1. Introduction
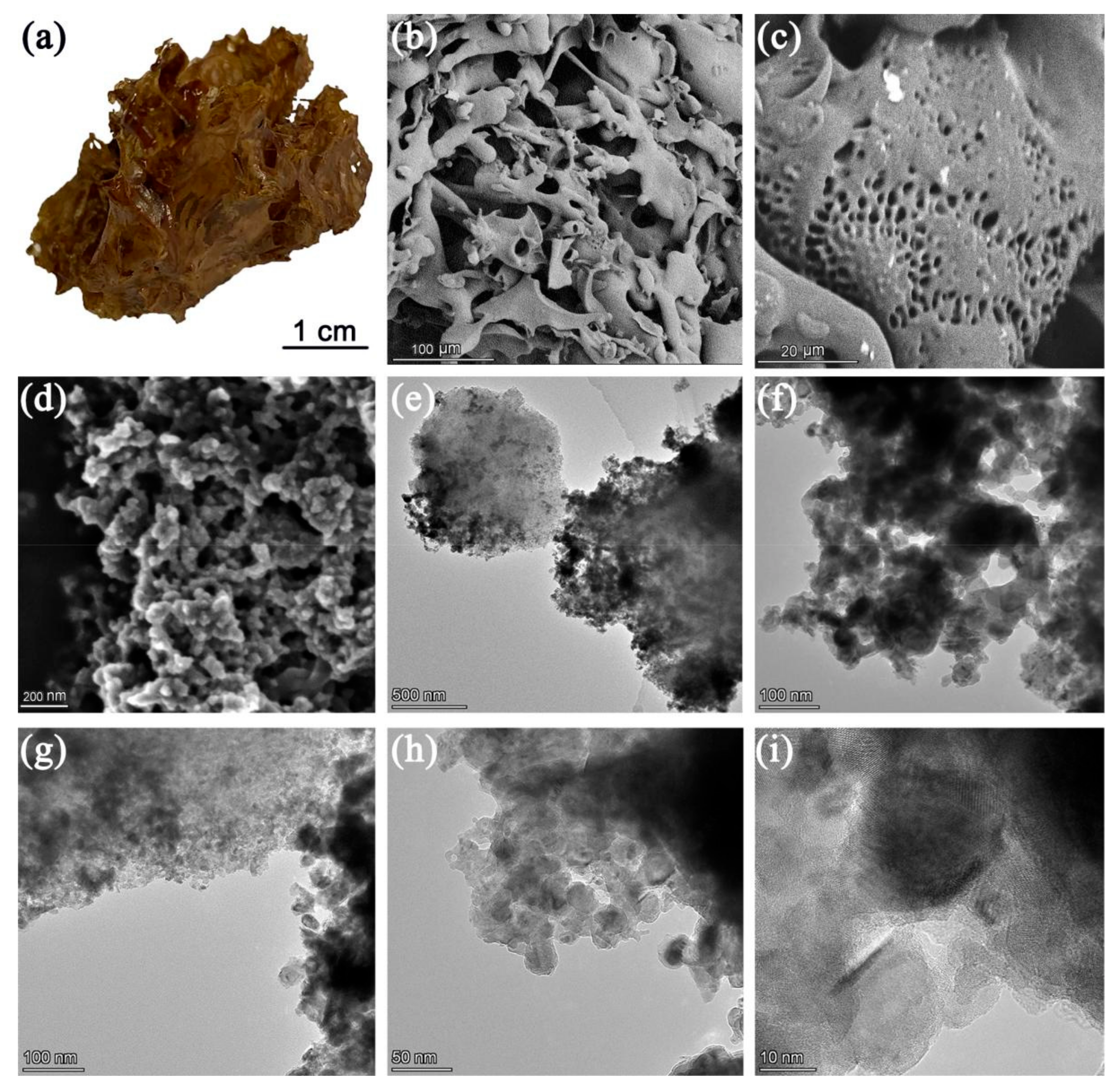
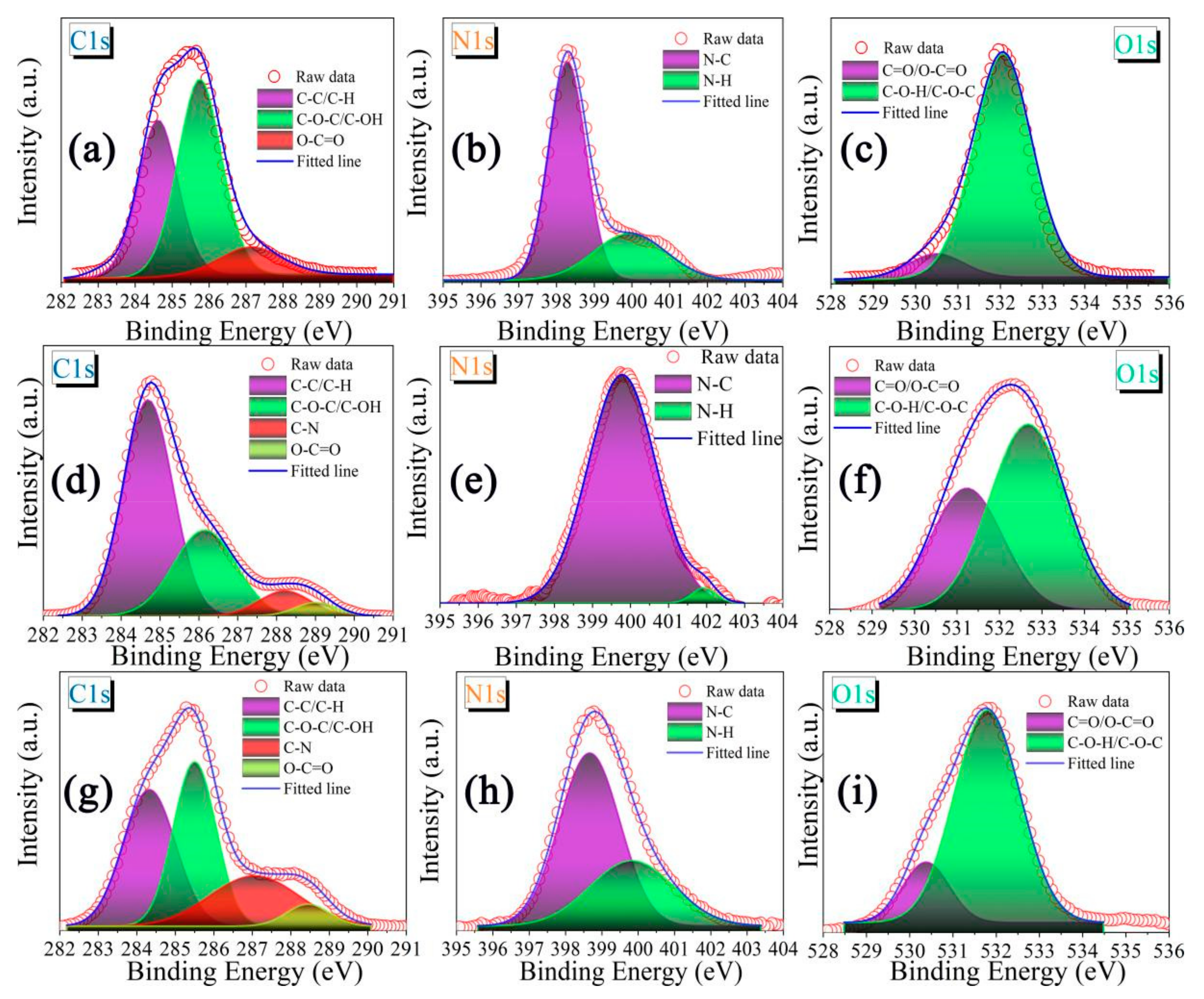
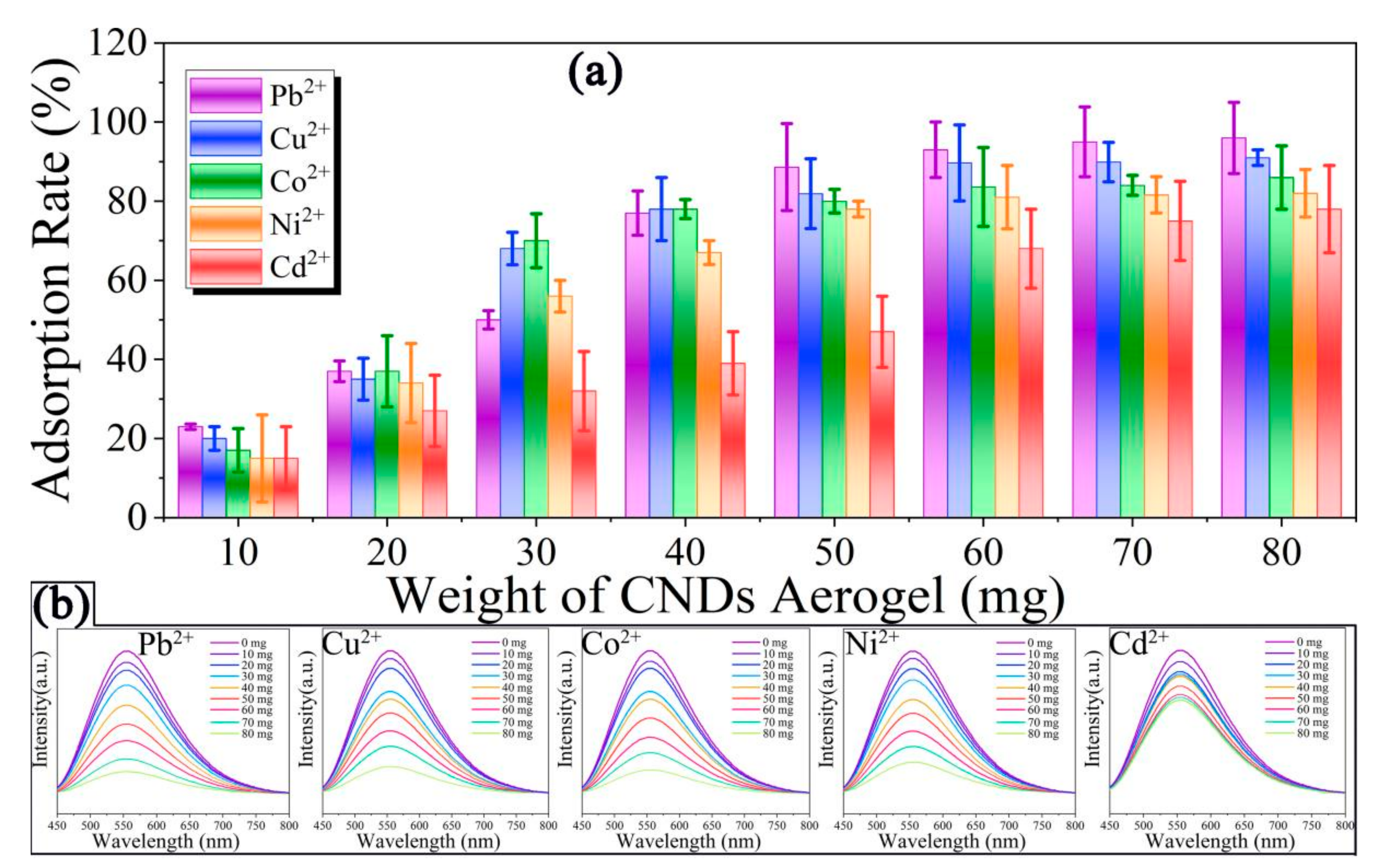
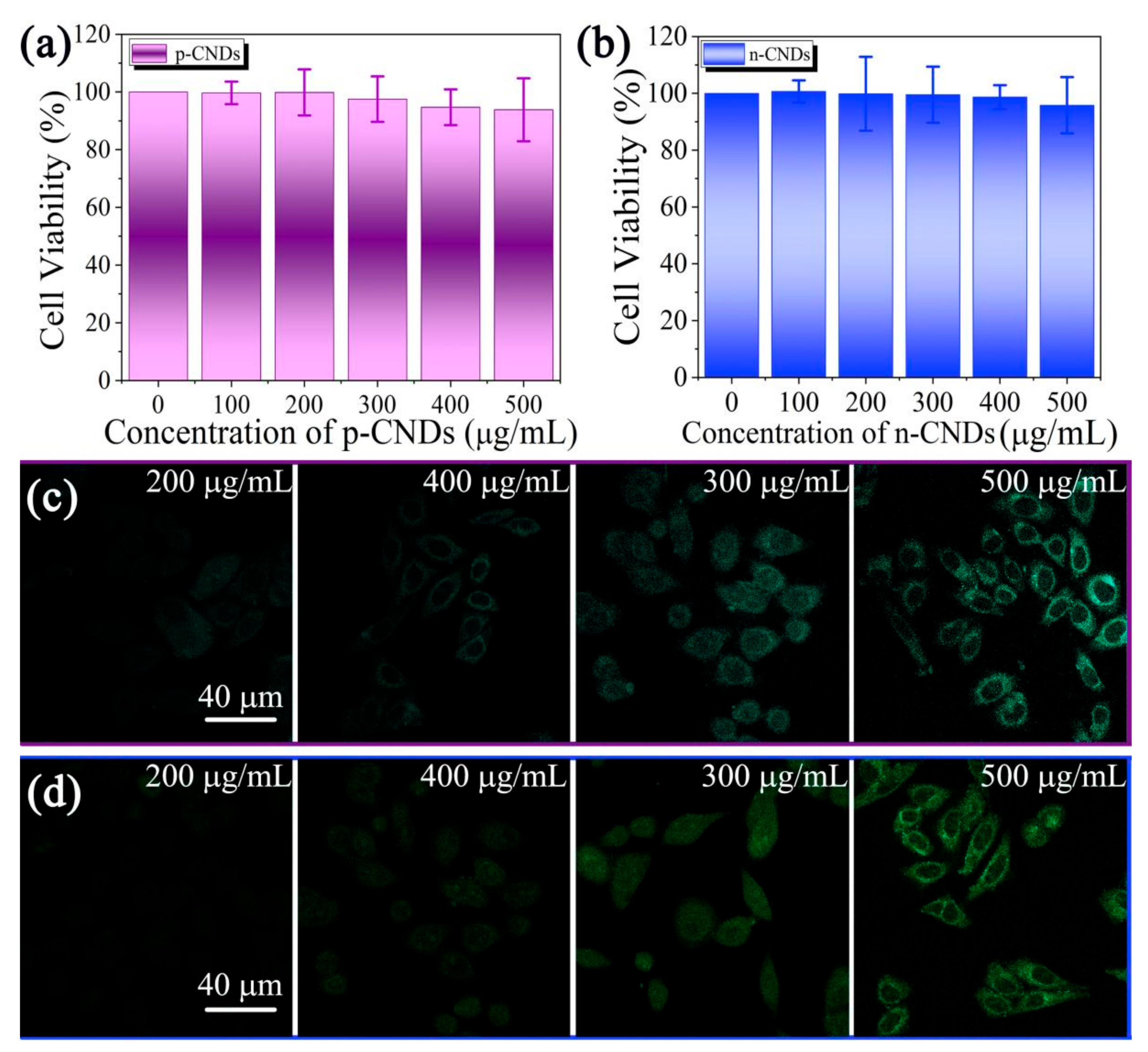
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Synthesis of p-CNDs and n-CNDs
4.2.2. Synthesis of CNDs Aerogel
4.2.3. Cytotoxicity Test
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.; Peng, D.; Liang, R.P.; Qiu, J.D. Graphene-based optical nanosensors for detection of heavy metal ions. Trend. Anal. Chem. 2018, 102, 280–289. [Google Scholar] [CrossRef]
- Zhang, J.; Han, J.; Wang, M.; Guo, R. Fe3O4/PANI/MnO2 core-shell hybrids as advanced adsorbents for heavy metal ions. J. Mater. Chem. A 2017, 5, 4058–4066. [Google Scholar] [CrossRef]
- Peng, Y.; Huang, H.; Zhang, Y.; Kang, C.; Chen, S.; Song, L.; Liu, D.; Zhong, C. A versatile MOF-based trap for heavy metal ion capture and dispersion. Nat. Commun. 2018, 1, 9. [Google Scholar] [CrossRef] [PubMed]
- Naina, A.; Tseng, Y.T.; Lin, Y.S.; Wei, S.C.; Mandal, R.P.; Unnikrishnan, B.; Huang, C.C.; Tseng, F.G.; Chang, H.T. Tuning the photoluminescence of metal nanoclusters for selective detection of multiple heavy metal ions. Sens. Actuators B-Chem. 2020, 321, 128539. [Google Scholar] [CrossRef]
- Niu, Y.; Hu, W.; Guo, M.; Wang, Y.; Jia, J.; Hu, Z. Preparation of cotton-based fibrous adsorbents for the removal of heavy metal ions. Carbohyd. Polym. 2019, 225, 115218. [Google Scholar] [CrossRef]
- Li, F.; Wang, X.; Yuan, T.; Sun, R. A lignosulfonate-modified graphene hydrogel with ultrahigh adsorption capacity for Pb (II) removal. J. Mater. Chem. A 2016, 4, 11888–19630. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, H.; Zhang, H.; Ma, L.; Wang, Z. Polyacrylamide-phytic acid-polydopamine conducting porous hydrogel for rapid detection and removal of copper (II) ions. Biosens. Bioelectron. 2017, 91, 306–312. [Google Scholar] [CrossRef]
- Liu, L.; Luo, X.B.; Ding, L.; Luo, S.L. Application of nanotechnology in the removal of heavy metal from water. In Nanomaterials for the Removal of Pollutants and Resource Reutilization; Elsevier: Amsterdam, The Netherlands, 2019; pp. 83–147. [Google Scholar]
- Wang, R.; Liang, R.; Dai, T.; Chen, J.; Shuai, X.; Liu, C. Pectin-based adsorbents for heavy metal ions: A review. Trends Food Sci. Tech. 2019, 91, 319–329. [Google Scholar] [CrossRef]
- Wu, Y.; Pang, H.; Liu, Y.; Wang, X.; Yu, S.; Fu, D.; Chen, J.; Wang, X. Environmental remediation of heavy metal ions by novel-nanomaterials: A review. Environ. Pollut. 2019, 246, 608–620. [Google Scholar] [CrossRef]
- Hasanpour, M.; Hatami, M. Application of three dimensional porous aerogels as adsorbent for removal of heavy metal ions from water/wastewater: A review study. Adv. Colloid Inter. Sci. 2020, 284, 102247. [Google Scholar] [CrossRef]
- Liu, C.; Lei, X.; Wang, L.; Jia, J.; Liang, X.; Zhao, X.; Zhu, H. Investigation on the removal performances of heavy metal ions with the layer-by-layer assembled forward osmosis membranes. Chem. Eng. J. 2017, 327, 60–70. [Google Scholar] [CrossRef]
- Mukherjee, R.; Bhunia, P.; De, S. Impact of graphene oxide on removal of heavy metals using mixed matrix membrane. Chem. Eng. J. 2016, 292, 284–297. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, G.; Ye, X.; Wang, G.; Zhang, H.; Zhou, H.; Zhang, Y.; Zhao, H. 3D graphene/δ-MnO2 aerogels for highly efficient and reversible removal of heavy metal ions. J. Mater. Chem. A 2016, 4, 1970–1979. [Google Scholar] [CrossRef]
- Du, B.; Chai, L.; Li, W.; Wang, X.; Chen, X.; Zhou, J.; Sun, R. Preparation of functionalized magnetic graphene oxide/lignin composite nanoparticles for adsorption of heavy metal ions and reuse as electromagnetic wave absorbers. Sep. Purif. Technol. 2022, 297, 121509. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, H.; Li, J.; Yang, K.; Zhang, Z.; Chen, F.; Wang, B. High-throughput metal trap: Sulfhydryl-functionalized wood membrane stacks for rapid and highly efficient heavy metal ion removal. ACS Appl. Mater. Inter. 2020, 12, 15002–15011. [Google Scholar] [CrossRef]
- Yang, X.C.; Li, Q.; Tang, M.; Yang, Y.L.; Yang, W.; Hu, J.F.; Pu, X.L.; Liu, J.; Zhao, J.T.; Zhang, Z.J. One stone, two birds: pH- and temperature-sensitive nitrogendoped carbon dots for multiple anticounterfeiting and multiple cell imaging. ACS Appl. Mater. Inter. 2020, 12, 20849–20858. [Google Scholar] [CrossRef]
- Li, H.; Kang, Z.; Liu, Y.; Lee, S.-T. Carbon nanodots: Synthesis, properties and applications. J. Mater. Chem. 2012, 22, 24230–24253. [Google Scholar] [CrossRef]
- Mou, Z.; Wang, B.; Lu, H.; Dai, S.; Huang, Z. Synthesis of poly(ionic liquid)s brush-grafted carbon dots for high-performance lubricant additives of polyethylene glycol. Carbon 2019, 154, 301–312. [Google Scholar] [CrossRef]
- Yue, J.; Li, L.; Cao, L.; Zan, M.; Yang, D.; Wang, Z.; Chang, Z.; Mei, Q.; Miao, P.; Dong, W.-F. Two-step hydrothermal preparation of carbon dots for calcium ion detection. ACS Appl. Mater. Inter. 2019, 11, 44566–44572. [Google Scholar] [CrossRef]
- Jiang, L.; Ding, H.; Lu, S.; Geng, T.; Xiao, G.; Zou, B.; Bi, H. Photoactivated fluorescence enhancement in F,N-doped carbon dots with piezochromic behavior. Angew. Chem. Int. Ed. 2020, 59, 9986–9991. [Google Scholar] [CrossRef]
- Verma, A.; Arshad, F.; Ahmad, K.; Goswami, U.; Samanta, S.K.; Sahoo, A.K.; Sk, M.P. Role of surface charge in enhancing antibacterial activity of fluorescent carbon dots. Nanotechnology 2019, 31, 095101. [Google Scholar] [CrossRef] [PubMed]
- Pudza, M.Y.; Abidin, Z.Z.; Rashid, S.A.; Yasin, F.M.; Noor, A.; Issa, M.A. Eco-friendly sustainable fluorescent carbon dots for the adsorption of heavy metal ions in aqueous environment. Nanomaterials 2020, 10, 315. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Chen, X.; Gong, X.; Gao, X.; Dai, Q.; Nguyen, T.T.; Guo, M. Luminescent carbon quantum dots/nanofibrillated cellulose composite aerogel for monitoring adsorption of heavy metal ions in water. Opt. Mater. 2020, 100, 109642. [Google Scholar] [CrossRef]
- Wang, L.; Chen, C.; Tapas, S.; Lei, J.; Matsuoka, M.; Zhang, J.; Zhang, J. Carbon dots modified mesoporous organosilica as an adsorbent for the removal of 2,4-dichlorophenol and heavy metal ions. J. Mater. Chem. A 2015, 3, 13357–13364. [Google Scholar] [CrossRef]
- Yang, X.C.; Yang, Y.L.; Xu, M.M.; Liang, S.S.; Pu, X.L.; Hu, J.F.; Li, Q.L.; Zhao, J.T.; Zhang, Z.J. Metal-ion-cross-linked nitrogen-doped carbon dot hydrogels for dual-spectral detection and extractable removal of divalent heavy metal ions. ACS Appl. Nano Mater. 2021, 4, 13986–13994. [Google Scholar] [CrossRef]
- Sarkar, K.; Devi, P.; Lata, A.; Ghosh, R.; Kumar, P. Engineering carbon quantum dots for enhancing the broadband photoresponse in a silicon process-line compatible photodetector. J. Mater. Chem. C 2019, 7, 13182–13191. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Peng, Z.; Liu, Z.; Chen, Z.; Shang, L.; Lu, S.; Zhang, T. Self-crosslinking carbon dots loaded ruthenium dots as an efficient and super-stable hydrogen production electrocatalyst at all pH values. Nano Energy 2019, 65, 104023. [Google Scholar] [CrossRef]
- Malina, T.; Poláková, K.; Skopalík, J.; Milotová, V.; Holá, K.; Havrdová, M.; Tománková, K.B.; Čmiel, V.; Šefc, L.; Zbořil, R. Carbon dots for in vivo fluorescence imaging of adipose tissuederived mesenchymal stromal cells. Carbon 2019, 152, 434–443. [Google Scholar] [CrossRef]
- Cao, L.; Wang, X.; Meziani, M.J.; Lu, F.; Wang, H.; Luo, P.G.; Lin, Y.; Harruff, B.A.; Veca, L.M.; Murray, D.; et al. Carbon dots for multiphoton bioimaging. J. Am. Chem. Soc. 2007, 129, 11318–11319. [Google Scholar] [CrossRef]
- Zheng, X.T.; Ananthanarayanan, A.; Luo, K.Q.; Chen, P. Glowing graphene quantum dots and carbon dots: Properties, syntheses, and biological applications. Small 2015, 11, 1620–1636. [Google Scholar] [CrossRef]
- Luo, P.G.; Sahu, S.; Yang, S.-T.; Sonkar, S.K.; Wang, J.; Wang, H.; LeCroy, G.E.; Cao, L.; Sun, Y.-P. Carbon “quantum” dots for optical bioimaging. J. Mater. Chem. B 2013, 1, 2116–2127. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.-C.; Gao, S.; Fu, S.-Q.; Yao, X.; Jiao, Z.; Zhao, J.-T.; Zhang, Z.-J.; Cheng, L.-L. Aerogel Assembled by Two Types of Carbon Nanoparticles for Efficient Removal of Heavy Metal Ions. Gels 2022, 8, 459. https://doi.org/10.3390/gels8080459
Yang X-C, Gao S, Fu S-Q, Yao X, Jiao Z, Zhao J-T, Zhang Z-J, Cheng L-L. Aerogel Assembled by Two Types of Carbon Nanoparticles for Efficient Removal of Heavy Metal Ions. Gels. 2022; 8(8):459. https://doi.org/10.3390/gels8080459
Chicago/Turabian StyleYang, Xue-Chun, Song Gao, Sha-Qi Fu, Xuan Yao, Zheng Jiao, Jing-Tai Zhao, Zhi-Jun Zhang, and Ling-Li Cheng. 2022. "Aerogel Assembled by Two Types of Carbon Nanoparticles for Efficient Removal of Heavy Metal Ions" Gels 8, no. 8: 459. https://doi.org/10.3390/gels8080459
APA StyleYang, X.-C., Gao, S., Fu, S.-Q., Yao, X., Jiao, Z., Zhao, J.-T., Zhang, Z.-J., & Cheng, L.-L. (2022). Aerogel Assembled by Two Types of Carbon Nanoparticles for Efficient Removal of Heavy Metal Ions. Gels, 8(8), 459. https://doi.org/10.3390/gels8080459





