Synthesis and Properties of Hydrogels on Medical Titanium Alloy Surface by Modified Dopamine Adhesion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of TC4-OH
2.2.2. Preparation of TC4-PDA and TC4-Initiator
2.2.3. Preparation of Hydrogels on TC4
2.3. Structure Characterization
2.4. Swelling and Deswelling Properties of the Hydrogel
2.5. The Scanning Electron Microscope (SEM) Analysis of the Hydrogel
2.6. Thermal Analysis of the Hydrogel
2.7. Mechanical Properties Analysis of the Hydrogel
3. Results and Discussion
3.1. Synthesis
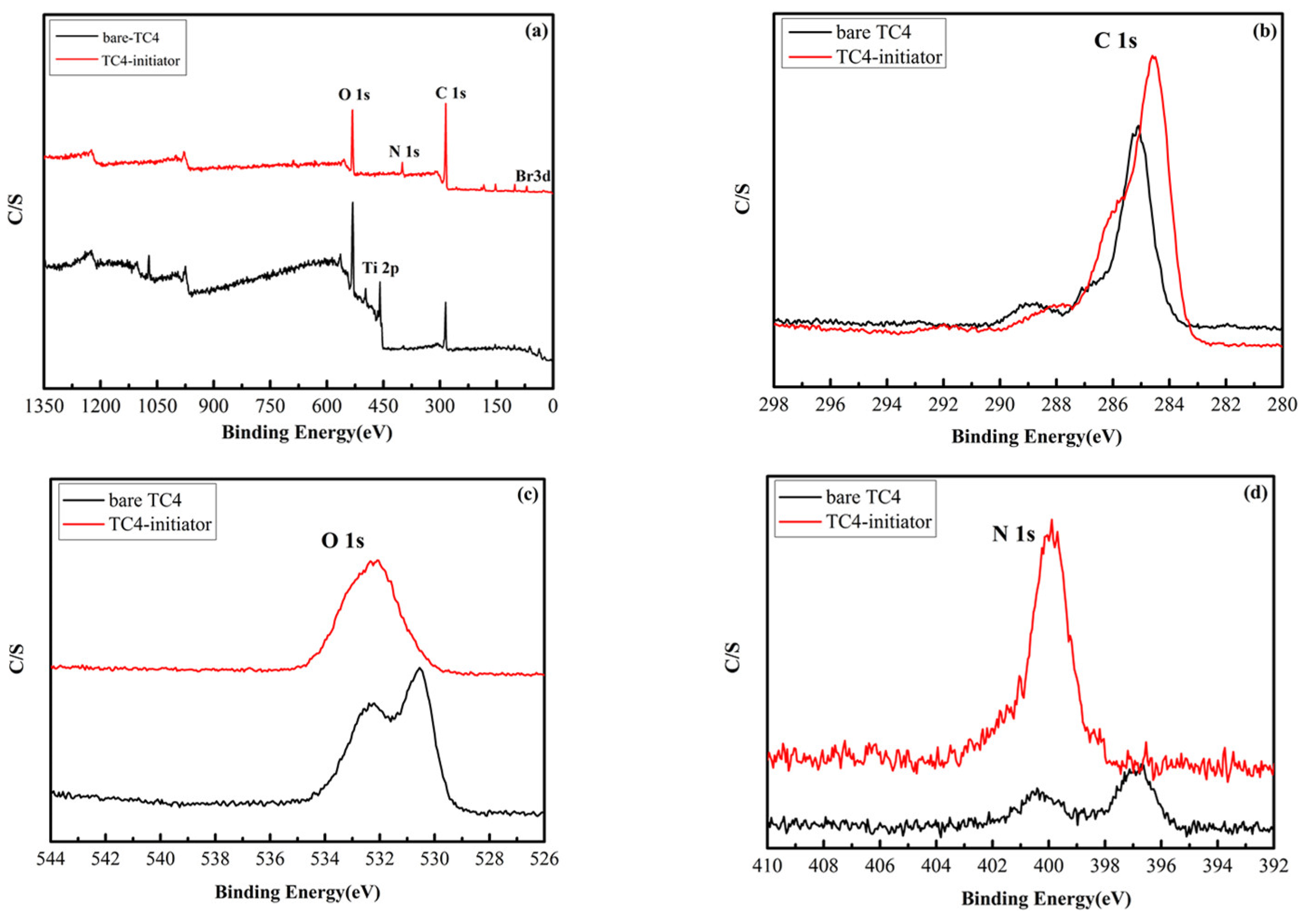
3.2. Structure Characterization
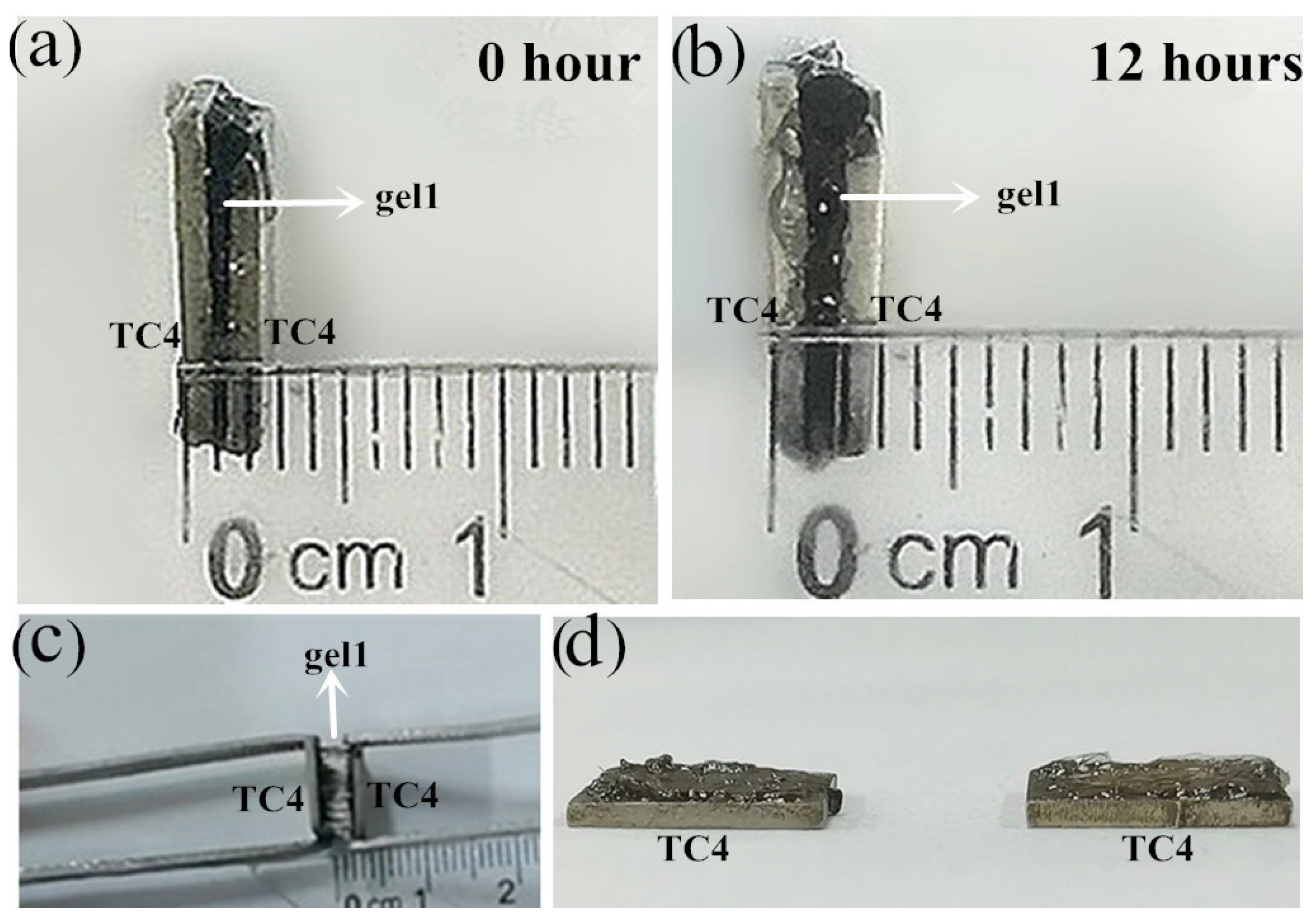
3.3. Hydrogel Bonding
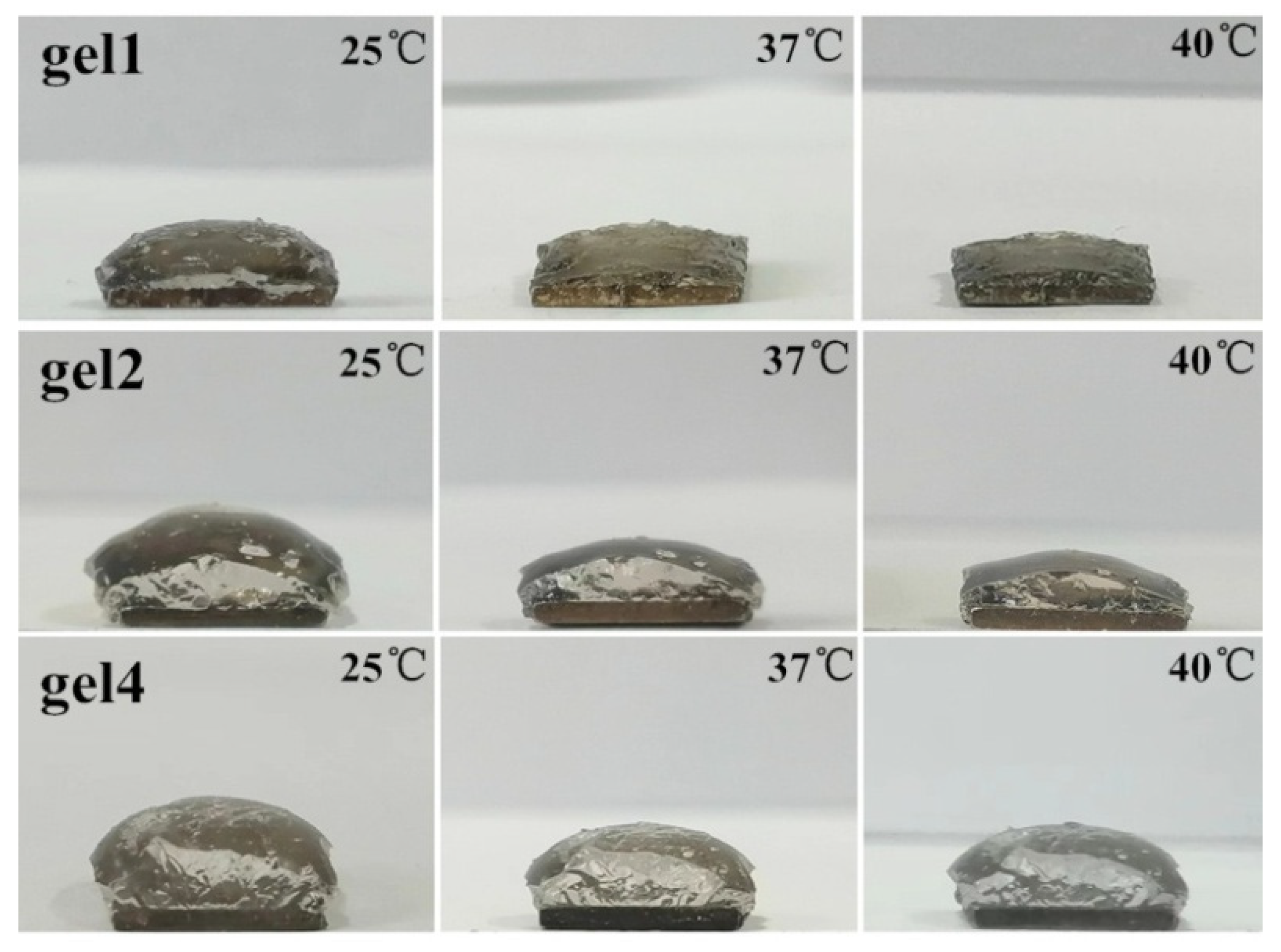
3.4. Thermosensitivity and Reversibility of Hydrogels
3.5. Scanning Electron Microscope
3.6. Thermogravimetric Analysis
3.7. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Manivasagam, V.K.; Popat, K.C. In Vitro Investigation of Hemocompatibility of Hydrothermally Treated Titanium and Titanium Alloy Surfaces. ACS Omega 2020, 5, 8108–8120. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Lee, T.; Kwon, I.K.; Kim, H.S.; Hahn, S.K.; Lee, C.S. Surface Modification of Multipass Caliber-Rolled Ti Alloy with Dexamethasone-Loaded Graphene for Dental Applications. ACS Appl. Mater. Interfaces 2015, 7, 9598–9607. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhong, X.B.; Fu, X.M. Enhanced Bone Remodeling Effects of Low-Modulus Ti−5Zr−3Sn−5Mo−25Nb Alloy Implanted in the Mandible of Beagle Dogs under Delayed Loading. ACS Omega 2019, 4, 18653–18662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wisdom, E.C.; Zhou, Y.; Chen, C.; Tamerler, C.; Snead, M.L. Mitigation of Peri-implantitis by Rational Design of Bifunctional Peptides with Antimicrobial Properties. ACS Biomater. Sci. Eng. 2020, 6, 2682–2695. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.H.; Han, Q.; Wang, J.C.; Li, D.D.; Song, Z.M.; Yu, J.H. Promotion of Osseointegration between Implant and Bone Interface by Titanium Alloy Porous Scaffolds Prepared by 3D Printing. ACS Biomater. Sci. Eng. 2020, 6, 5181–5190. [Google Scholar] [CrossRef]
- Wang, C.C.; Hu, H.X.; Li, Z.P.; Shen, Y.F.; Xu, Y.; Zhang, G.Q.; Zeng, X.Q.; Deng, J.; Zhao, S.C.; Ren, T.H.; et al. Enhanced Osseointegration of Titanium Alloy Implants with Laser Microgrooved Surfaces and Graphene Oxide Coating. ACS Appl. Mater. Interfaces 2019, 11, 39470–39483. [Google Scholar] [CrossRef]
- Zhao, Y.; Wong, S.M.; Wong, H.M.; Wu, S.; Hu, T.; Yeung, K.W.K.; Chu. P.K. Effects of Carbon and Nitrogen Plasma Immersion Ion Implantationon In vitro and In vivo Biocompatibility of Titanium Alloy. ACS Appl. Mater. Interfaces 2013, 5, 1510–1516. [Google Scholar] [CrossRef]
- Wang, S.X.; Li, Q.R.; Wang, B.; Hou, Y.T.; Zhang, T. Recognition of Different Rough Surface Based Highly Sensitive Silver Nanowire-Graphene Flexible Hydrogel Skin. Ind. Eng. Chem. Res. 2019, 58, 21553–21561. [Google Scholar] [CrossRef]
- Wei, P.; Chen, T.; Chen, G.; Liu, H.; Mugaanire, I.T.; Hou, K.; Zhu, M. Conductive Self-Healing Nanocomposite Hydrogel Skin Sensors with Antifreezing and Thermoresponsive Properties. ACS Appl. Mater. Interfaces 2020, 12, 3068–3079. [Google Scholar] [CrossRef]
- Cui, X.; Murakami, T.; Tamura, Y.; Aoki, K.; Hoshino, Y.; Miura, Y. Bacterial Inhibition and Osteoblast Adhesion on Ti Alloy Surfaces Modified by Poly(PEGMA-r-Phosmer) Coating. ACS Appl. Mater. Interfaces 2018, 10, 23674–23681. [Google Scholar] [CrossRef]
- Wang, S.S.; Song, J.X.; Li, Y.C.; Zhao, X.C.; Chen, L.; Li, G.; Wang, L.P.; Jia, Z.F.; Ge, X.C. Grafting antibacterial polymer brushes from titanium surface via polydopamine chemistry and activators regenerated by electron transfer ATRP. React. Funct. Polym. 2019, 140, 48–55. [Google Scholar] [CrossRef]
- Timimi, Z.J.; Tammemi, Z. Polymer Blends and Nanocomposite Materials Based on Polymethyl Methacrylate (PMMA) for Bone Regeneration and Repair: Characterization and Preparation. J. Sustain. Mater. Process. Manag. 2022, 2, 15–23. [Google Scholar]
- Fu, L.Y.; Omi, M.; Sun, M.K.; Cheng, B.; Mao, G.; Liu, T.; Mendonça, G.; Averick, S.E.; Mishina, Y.; Matyjaszewski, K. Covalent Attachment of P15 Peptide to Ti Alloy Surface Modified with Polymer to Enhance Osseointegration of Implants. ACS Appl. Mater. Interfaces 2019, 11, 38531–38536. [Google Scholar] [CrossRef] [PubMed]
- Minet, I.; Delhalle, J.; Hevesi, L.; Mekhalif, Z. Surface-initiated ATRP of PMMA, PS and diblock PS-b-PMMA copolymers from stainless steel modified by 11-(2-bromoisobutyrate)-undecyl-1-phosphonic acid. J. Colloid Interface Sci. 2009, 332, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Su, Q.; Ye, Q.; Zhou, F.; Zhang, Y. Polyelectrolyte brushes as efficient platform for synthesis of Cu and Pt bimetallic nanocrystals onto TiO2 nanowires. Surf. Interface Anal. 2017, 49, 904–909. [Google Scholar] [CrossRef]
- Yu, B.-Y.; Zheng, J.; Chang, Y.; Sin, M.-C.; Chang, C.-H.; Higuchi, A.; Sun, Y.-M. Surface Zwitterionization of Titanium for a General Bio-Inert Control of Plasma Proteins, Blood Cells, Tissue Cells, and Bacteria. Langmuir 2014, 30, 7502–7512. [Google Scholar] [CrossRef]
- Cheng, H.; Yue, K.; Kazemzadeh-Narbat, M.; Liu, Y.; Khalilpour, A.; Li, B.; Zhang, Y.S.; Annabi, N.; Khademhosseini, A. Mussel-Inspired Multifunctional Hydrogel Coating for Prevention of Infections and Enhanced Osteogenesis. ACS Appl. Mater. Interfaces 2017, 9, 11428–11439. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Yuk, H.; Parada, G.A.; Wu, Y.; Liu, X.; Nabzdyk, C.S.; Youcef-Toumi, K.; Zang, J.; Zhao, X. Multifunctional “Hydrogel Skins” on Diverse Polymers with Arbitrary Shapes. Adv. Mater. 2019, 31, 1807101. [Google Scholar] [CrossRef]
- Kolewe, W.K.; Zhu, J.; Mako, N.R.; Nonnenmann, S.S.; Schiffman, J.D. Bacterial Adhesion Is Affected by the Thickness and Stiffness of Poly(ethylene glycol) Hydrogels. ACS Appl. Mater. Interfaces 2018, 10, 2275–2281. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Xu, T.; Chen, W.; Wang, R.; Xu, Z.; Ye, Z.; Chi, B. Improvement of toughness for the hyaluronic acid and adipic acid dihydrazide hydrogel by PEG. Fibers Polym. 2017, 18, 817–824. [Google Scholar] [CrossRef]
- Muir, B.V.O.; Myung, D.; Knoll, W.; Frank, C.W. Grafting of Cross-Linked Hydrogel Networks to Titanium Surfaces. ACS Appl. Mater. Interfaces 2014, 6, 958–966. [Google Scholar] [CrossRef]
- Bahrami, M.; Houérou, V.L.; Rühe, J. Lubrication of surfaces covered by surface-attached hydrogel layers. Tribol. Int. 2020, 149, 105637. [Google Scholar] [CrossRef]
- Ma, A.Y.; Jiang, C.J.; Li, M.N.; Cao, L.L.; Deng, Z.H.; Bai, L.J.; Wang, W.X.; Chen, H.; Yang, H.W.; Wei, D.L. Surface-initiated photoinduced electron transfer ATRP and mussel-inspired chemistry: Surface engineering of graphene oxide for self-healing hydrogels. React. Funct. Polym. 2020, 150, 104547. [Google Scholar] [CrossRef]
- Asha, A.B.; Chen, Y.J.; Zhang, H.X.; Ghaemi, S.; Ishihara, K.; Liu, Y.; Narain, R. Rapid Mussel-Inspired Surface Zwitteration for Enhanced Antifouling and Antibacterial Properties. Langmuir 2019, 35, 1621–1630. [Google Scholar] [CrossRef]
- Zhang, B.; Yan, Q.; Yuan, S.J.; Zhuang, X.D.; Zhang, F. Enhanced Antifouling and Anticorrosion Properties of Stainless Steel by Biomimetic Anchoring PEGDMA-Cross-Linking Polycationic Brushes. Ind. Eng. Chem. Res. 2019, 58, 7107–7119. [Google Scholar] [CrossRef]
- Li, N.; Li, T.; Qiao, X.-Y.; Li, R.; Yao, Y.; Gong, Y.-K. Universal Strategy for Efficient Fabrication of Blood Compatible Surfaces via Polydopamine-Assisted Surface-Initiated Activators Regenerated by Electron Transfer Atom-Transfer Radical Polymerization of Zwitterions. ACS Appl. Mater. Interfaces 2020, 12, 12337–12344. [Google Scholar] [CrossRef]
- Zhang, C.; Li, H.-N.; Du, Y.; Ma, M.-Q.; Xu, Z.-K. CuSO4/H2O2-Triggered Polydopamine/Poly(sulfobetainemethacrylate) Coatings for Antifouling Membrane Surfaces. Langmuir 2017, 33, 1210–1216. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Edmondson, S. Polydopamine-melanin initiators for Surface-initiated ATRP. Polymer 2011, 52, 2141–2149. [Google Scholar] [CrossRef]
- Dong, L.; Liu, X.D.; Xiong, Z.G.; Sheng, D.K.; Lin, C.H.; Zhou, Y.; Yang, Y. Preparation of UV-Blocking Poly(vinylidene fluoride) Films through SI-AGET ATRP Using a Colorless Polydopamine Initiator Layer. Ind. Eng. Chem. Res. 2018, 57, 12662–12669. [Google Scholar] [CrossRef]
- Hsueh, N.; Chai, C.L.L. Evaluation of 2-Bromoisobutyryl Catechol Derivatives for Atom Transfer Radical Polymerization-Functionalized Polydopamine Coatings. Langmuir 2021, 37, 8811–8820. [Google Scholar] [CrossRef]
- Vales, T.P.; Jee, J.P.; Lee, W.Y.; Min, I.; Cho, S.; Kim, H.J. Protein Adsorption and Bacterial Adhesion Resistance of Cross-linked Copolymer Hydrogels Based on Poly(2-methacryloyloxyethyl phosphorylcholine) and Poly(2-hydroxyethyl methacrylate). Bull. Korean Chem. Soc. 2020, 41, 406–412. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, W.; Zhang, J.; Peng, X.; Li, G.; Zhang, L.-M.; Yang, L. Thermosensitive Hydrogels Based on Methylcellulose Derivatives for Prevention of Postoperative Adhesion. Cellulose 2020, 27, 1555–1571. [Google Scholar] [CrossRef]
- Goda, T.; Matsuno, R.; Konno, T.; Takai, M.; Ishihara, K. Protein Adsorption Resistance and Oxygen Permeability of Chemically Crosslinked Phospholipid Polymer Hydrogel for Ophthalmologic Biomaterials. J. Biomed. Mater. Res. 2009, 89B, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Macková, H.; Plichta, Z.; Hlídková, H.; Sedláček, O.; Konefal, R.; Sadakbayeva, Z.; Dušková-Smrčková, M.; Horák, D.; Kubinová, Š. Reductively Degradable Poly(2-hydroxyethyl methacrylate) Hydrogels with Oriented Porosity for Tissue Engineering. ACS Appl. Mater. Interfaces 2017, 9, 10544–10553. [Google Scholar] [CrossRef]
- Park, C.; Heo, J.; Lee, J.; Kim, T.; Kim, S.Y. Well-Defined Dual Light- and Thermo-Responsive Rod-Coil Block Copolymers Containing an Azobenzene, MEO2MA and OEGMA. Polymers 2020, 12, 284. [Google Scholar] [CrossRef] [Green Version]
- Hu, N.; Chen, C.; Tan, J.; Wang, W.J.; Wang, C.; Fan, H.Q.; Wang, J.P.; Müller-Buschbaum, P.; Zhong, Q. Enhanced Adsorption of Methylene Blue Triggered by the Phase Transition of Thermoresponsive Polymers in Hybrid Interpenetrating Polymer Network Hydrogels. ACS Appl. Polym. Mater. 2020, 2, 3674–3684. [Google Scholar] [CrossRef]
- Ferjaoui, Z.; Dine, E.J.A.; Kulmukhamedova, A.; Bezdetnaya, L.; Chang, C.S.; Schneider, R.; Mutelet, F.; Mertz, D.; Bégin-Colin, S.; Quilès, F.; et al. Doxorubicin-Loaded Thermoresponsive Superparamagnetic Nanocarriers for Controlled Drug Delivery and Magnetic Hyperthermia Applications. ACS Appl. Mater. Interfaces 2019, 11, 30610–30620. [Google Scholar] [CrossRef]
- Yoon, J.A.; Kowalewski, T.; Matyjaszewski, K. Comparison of Thermoresponsive Deswelling Kinetics of Poly(oligo(ethylene oxide)methacrylate)-Based Thermoresponsive Hydrogels Prepared by “Graft-from” ATRP. Macromolecules 2011, 44, 2261–2268. [Google Scholar] [CrossRef]









| Sample | MPC (%) | n(MPC) (mmol) | n(MEO2MA) (mmol) | n(OEGMA) (mmol) | MBA (%) |
|---|---|---|---|---|---|
| gel1 | 0 | 0 | 2.358 | 0.125 | 1.5 |
| gel2 | 5 | 0.124 | 2.244 | 0.118 | 1.5 |
| gel3 | 10 | 0.248 | 2.125 | 0.111 | 1.5 |
| gel4 | 15 | 0.372 | 2.005 | 0.105 | 1.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Wu, Q.; Yang, W.; Liu, S. Synthesis and Properties of Hydrogels on Medical Titanium Alloy Surface by Modified Dopamine Adhesion. Gels 2022, 8, 458. https://doi.org/10.3390/gels8080458
Fu Y, Wu Q, Yang W, Liu S. Synthesis and Properties of Hydrogels on Medical Titanium Alloy Surface by Modified Dopamine Adhesion. Gels. 2022; 8(8):458. https://doi.org/10.3390/gels8080458
Chicago/Turabian StyleFu, Yu, Qingrong Wu, Wanying Yang, and Shouxin Liu. 2022. "Synthesis and Properties of Hydrogels on Medical Titanium Alloy Surface by Modified Dopamine Adhesion" Gels 8, no. 8: 458. https://doi.org/10.3390/gels8080458
APA StyleFu, Y., Wu, Q., Yang, W., & Liu, S. (2022). Synthesis and Properties of Hydrogels on Medical Titanium Alloy Surface by Modified Dopamine Adhesion. Gels, 8(8), 458. https://doi.org/10.3390/gels8080458







