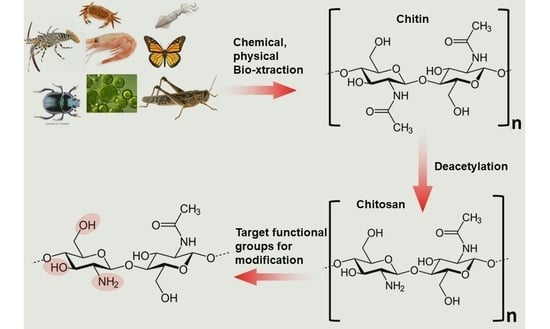
Chitosan: Sources, Processing and Modification Techniques
Abstract
:1. Introduction
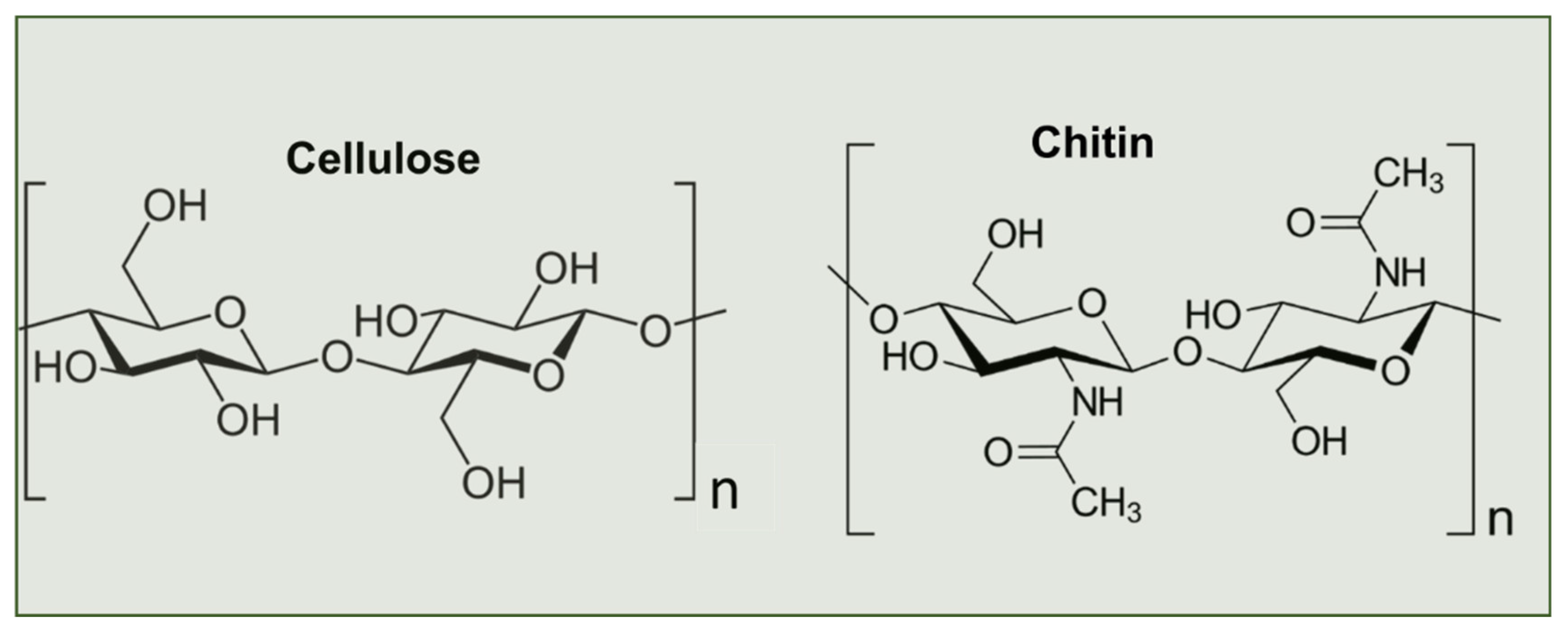
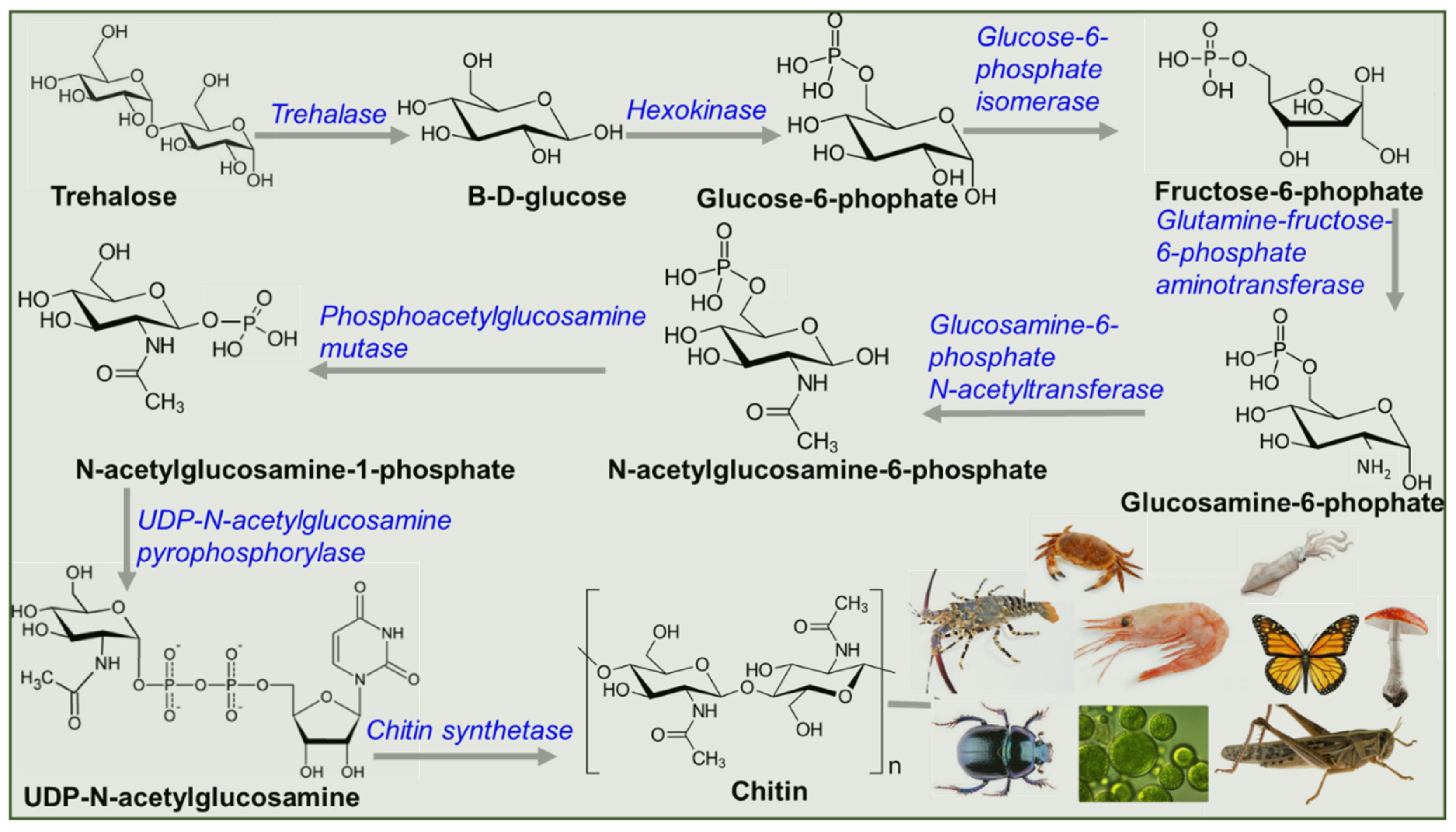
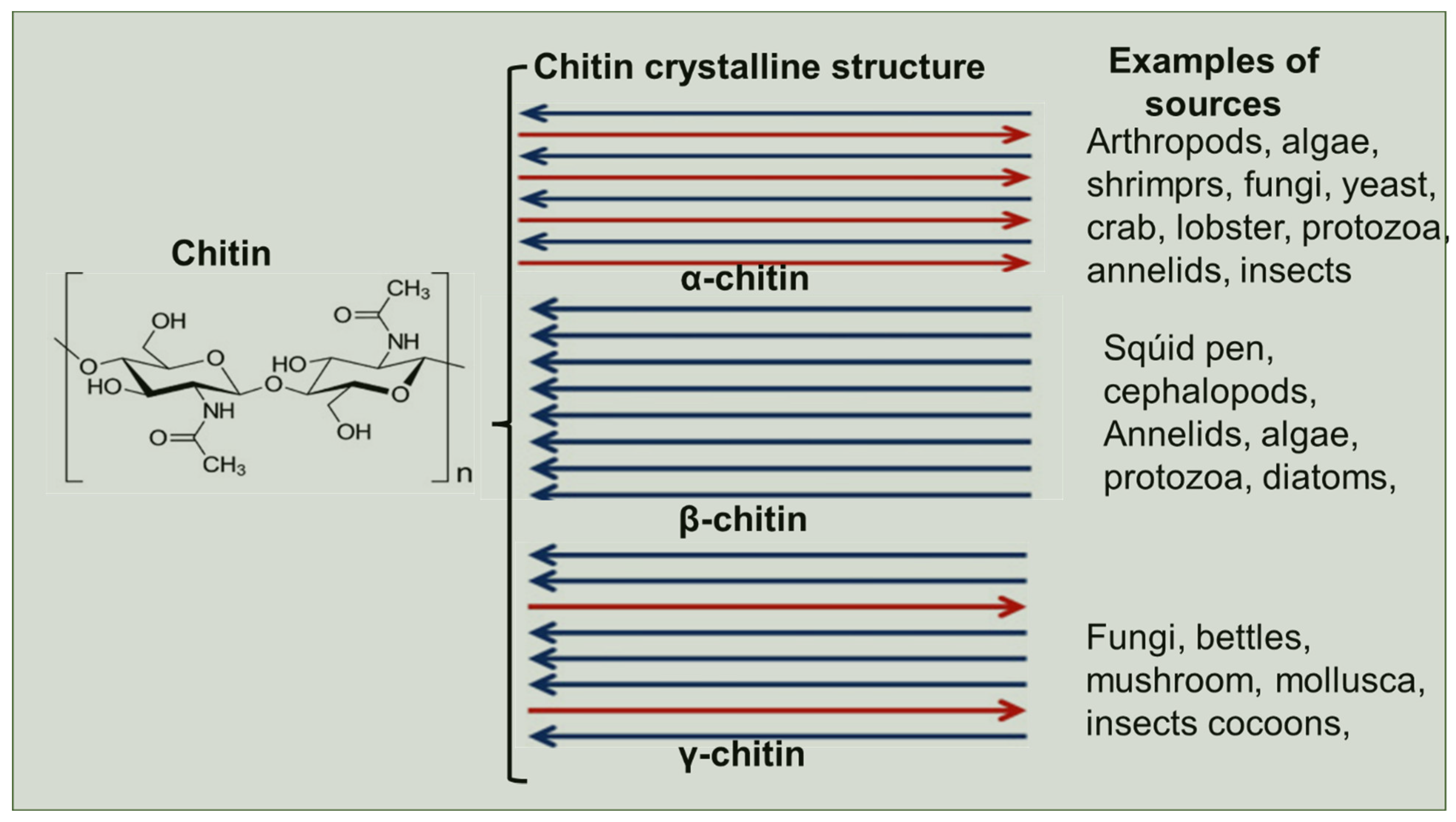
2. Biosynthesis of Chitin
3. Chitin Extraction Techniques
4. Chitin Deacetylation Techniques
5. Structure-Function Properties of Chitosan
5.1. Influence of DDA and Molecular Weight (Mw) on Chitosan Properties and Applications
5.2. Influence of Origin of Chitosan
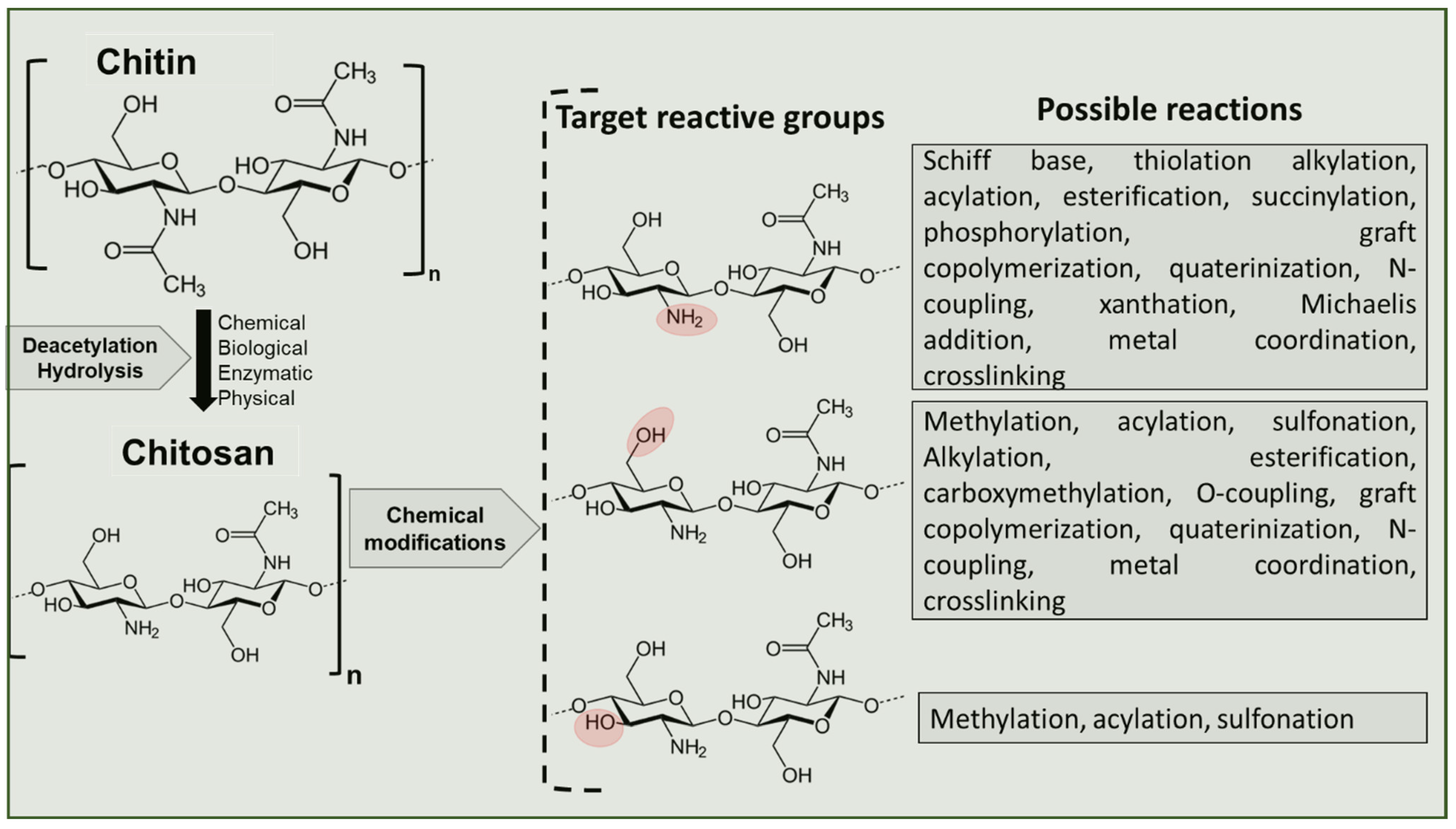
6. Tailoring Chitosan for Specific Applications
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kaya, M.; Lelešius, E.; Nagrockaitė, R.; Sargin, I.; Arslan, G.; Mol, A.; Baran, T.; Can, E.; Bitim, B. Differentiations of Chitin Content and Surface Morphologies of Chitins Extracted from Male and Female Grasshopper Species. PLoS ONE 2015, 10, e0115531. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.M.; Krishnamoorthy, S.; Paranthaman, R.; Moses, J.; Anandharamakrishnan, C. A review on source-specific chemistry, functionality, and applications of chitin and chitosan. Carbohydr. Polym. Technol. Appl. 2021, 2, 100036. [Google Scholar] [CrossRef]
- Crognale, S.; Russo, C.; Petruccioli, M.; D’Annibale, A. Chitosan Production by Fungi: Current State of Knowledge, Future Opportunities and Constraints. Fermentation 2022, 8, 76. [Google Scholar] [CrossRef]
- Yeul, V.S.; Rayalu, S.S. Unprecedented Chitin and Chitosan: A Chemical Overview. J. Polym. Environ. 2012, 21, 606–614. [Google Scholar] [CrossRef]
- Abidin, N.Z.; Kormin, F.; Abidin, N.Z.; Anuar, N.M.; Abu Bakar, M. The Potential of Insects as Alternative Sources of Chitin: An Overview on the Chemical Method of Extraction from Various Sources. Int. J. Mol. Sci. 2020, 21, 4978. [Google Scholar] [CrossRef]
- Elsoud, M.M.A.; El Kady, E.M. Current trends in fungal biosynthesis of chitin and chitosan. Bull. Natl. Res. Cent. 2019, 43, 59. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, S.I.; Ahmad, R.; Khan, M.S.; Kant, R.; Shahid, S.; Gautam, L.; Hasan, G.M.; Hassan, I. Chitin and its derivatives: Structural properties and biomedical applications. Int. J. Biol. Macromol. 2020, 164, 526–539. [Google Scholar] [CrossRef]
- Dutta, P.K.; Dutta, J.; Tripathi, V.S. Chitin and chitosan: Chemistry, properties and applications. J. Sci. Ind. Res. 2004, 63, 20–31. [Google Scholar]
- Dhillon, G.S.; Kaur, S.; Brar, S.K.; Verma, M. Green synthesis approach: Extraction of chitosan from fungus mycelia. Crit. Rev. Biotechnol. 2012, 33, 379–403. [Google Scholar] [CrossRef]
- Philibert, T.; Lee, B.H.; Fabien, N. Current Status and New Perspectives on Chitin and Chitosan as Functional Biopolymers. Appl. Biochem. Biotechnol. 2017, 181, 1314–1337. [Google Scholar] [CrossRef]
- Guebitz, G.M.; Pellis, A.; Nyanhongo, G.S. Delivery of Biomolecules Using Chitosan Wound Dressings. In Chitosan for Biomaterials IV: Biomedical Applications; Jayakumar, R., Prabaharan, M., Eds.; Springer: Cham, Switzerland, 2021; pp. 447–467. [Google Scholar] [CrossRef]
- Yang, E.; Hou, W.; Liu, K.; Yang, H.; Wei, W.; Kang, H.; Dai, H. A multifunctional chitosan hydrogel dressing for liver hemostasis and infected wound healing. Carbohydr. Polym. 2022, 291, 119631. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, Y.; Jiang, Z.; Hu, H.; Wang, S.; Chi, J.; Qiao, J.; Zhang, W.; Wang, Z.; Liu, W.; et al. Multifunctional effects of wound dressing based on chitosan-coordinated argentum with resistant bacterial penetration. Carbohydr. Polym. 2022, 288, 119329. [Google Scholar] [CrossRef]
- Hasibuan, P.A.Z.; Yuandani; Tanjung, M.; Gea, S.; Pasaribu, K.M.; Harahap, M.; Perangin-Angin, Y.A.; Prayoga, A.; Ginting, J.G. Antimicrobial and antihemolytic properties of a CNF/AgNP-chitosan film: A potential wound dressing material. Heliyon 2021, 7, e08197. [Google Scholar] [CrossRef]
- Chowdhury, F.; Ahmed, S.; Rahman, M.; Ahmed, A.; Hossain, D.; Reza, H.M.; Park, S.Y.; Sharker, S.M. Chronic Wound-dressing Chitosan-Polyphenolic Patch for pH Responsive Local Antibacterial Activity. Mater. Today Commun. 2022, 31, 103310. [Google Scholar] [CrossRef]
- Tamer, T.; Kenawy, E.; Agwa, M.; Sabra, S.; El-Meligy, M.; Mohy-Eldin, M. Wound dressing membranes based on immobilized Anisaldehyde onto (chitosan-GA-gelatin) copolymer: In-vitro and in-vivo evaluations. Int. J. Biol. Macromol. 2022, 211, 94–106. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, K.; Peng, X.; Zhang, L. Chitosan-based drug delivery systems: Current strategic design and potential application in human hard tissue repair. Eur. Polym. J. 2022, 166, 110979. [Google Scholar] [CrossRef]
- Kulkarni, N.; Jain, P.; Shindikar, A.; Suryawanshi, P.; Thorat, N. Advances in the colon-targeted chitosan based multiunit drug delivery systems for the treatment of inflammatory bowel disease. Carbohydr. Polym. 2022, 288, 119351. [Google Scholar] [CrossRef]
- Liu, L.; Gao, Q.; Lu, X.; Zhou, H. In situ forming hydrogels based on chitosan for drug delivery and tissue regeneration. Asian J. Pharm. Sci. 2016, 11, 673–683. [Google Scholar] [CrossRef] [Green Version]
- Singha, I.; Basu, A. Chitosan based injectable hydrogels for smart drug delivery applications. Sensors Int. 2022, 3, 100168. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, K.; Liang, J.; Jin, J.; Wang, X.; Yan, S. Chitosan-tripolyphosphate nanoparticles-mediated co-delivery of MTHFD1L shRNA and 5-aminolevulinic acid for combination photodynamic-gene therapy in oral cancer. Photodiagnosis Photodyn. Ther. 2021, 36, 102581. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Yang, X.-Q.; Gao, Z.; Li, Q.; Meng, T.-T.; Wu, J.-Y.; Yuan, H.; Hu, F.-Q. Redox-responsive chitosan oligosaccharide-SS-Octadecylamine polymeric carrier for efficient anti-Hepatitis B Virus gene therapy. Carbohydr. Polym. 2019, 212, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Kurakula, M.; Gorityala, S.; Moharir, K. Recent trends in design and evaluation of chitosan-based colon targeted drug delivery systems: Update 2020. J. Drug Deliv. Sci. Technol. 2021, 64, 102579. [Google Scholar] [CrossRef]
- Ahmed, S.; Ikram, S. Chitosan Based Scaffolds and Their Applications in Wound Healing. Achiev. Life Sci. 2016, 10, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Lodhi, G.; Kim, Y.-S.; Hwang, J.-W.; Kim, S.-K.; Jeon, Y.-J.; Je, J.-Y.; Ahn, C.-B.; Moon, S.-H.; Jeon, B.-T.; Park, P.-J. Chitooligosaccharide and Its Derivatives: Preparation and Biological Applications. BioMed Res. Int. 2014, 2014, 654913. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A Natural Biopolymer with a Wide and Varied Range of Applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef]
- Pita-López, M.L.; Fletes-Vargas, G.; Espinosa-Andrews, H.; Rodríguez-Rodríguez, R. Physically cross-linked chitosan-based hydrogels for tissue engineering applications: A state-of-the-art review. Eur. Polym. J. 2021, 145, 110176. [Google Scholar] [CrossRef]
- Kazemi-Aghdam, F.; Jahed, V.; Dehghan-Niri, M.; Ganji, F.; Vasheghani-Farahani, E. Injectable chitosan hydrogel embedding modified Halloysite nanotubes for bone tissue engineering. Carbohydr. Polym. 2021, 269, 118311. [Google Scholar] [CrossRef]
- Davachi, S.M.; Haramshahi, S.M.A.; Akhavirad, S.A.; Bahrami, N.; Hassanzadeh, S.; Ezzatpour, S.; Hassanzadeh, N.; Kebria, M.M.; Khanmohammadi, M.; Bagher, Z. Development of chitosan/hyaluronic acid hydrogel scaffolds via enzymatic reaction for cartilage tissue engineering. Mater. Today Commun. 2022, 30, 103230. [Google Scholar] [CrossRef]
- Valachová, K.; El Meligy, M.A.; Šoltés, L. Hyaluronic acid and chitosan-based electrospun wound dressings: Problems and solutions. Int. J. Biol. Macromol. 2022, 206, 74–91. [Google Scholar] [CrossRef]
- Zeng, X.; Chen, B.; Wang, L.; Sun, Y.; Jin, Z.; Liu, X.; Ouyang, L.; Liao, Y. Chitosan@Puerarin hydrogel for accelerated wound healing in diabetic subjects by miR-29ab1 mediated inflammatory axis suppression. Bioact. Mater. 2022, 19, 653–665. [Google Scholar] [CrossRef]
- Mondéjar-López, M.; López-Jimenez, A.J.; Martínez, J.C.G.; Ahrazem, O.; Gómez-Gómez, L.; Niza, E. Comparative evaluation of carvacrol and eugenol chitosan nanoparticles as eco-friendly preservative agents in cosmetics. Int. J. Biol. Macromol. 2022, 206, 288–297. [Google Scholar] [CrossRef]
- Ta, Q.; Ting, J.; Harwood, S.; Browning, N.; Simm, A.; Ross, K.; Olier, I.; Al-Kassas, R. Chitosan nanoparticles for enhancing drugs and cosmetic components penetration through the skin. Eur. J. Pharm. Sci. 2021, 160, 105765. [Google Scholar] [CrossRef]
- Morsy, R.; Ali, S.S.; El-Shetehy, M. Development of hydroxyapatite-chitosan gel sunscreen combating clinical multidrug-resistant bacteria. J. Mol. Struct. 2017, 1143, 251–258. [Google Scholar] [CrossRef]
- Resende, A.H.M.; Farias, J.M.; Silva, D.D.; Rufino, R.D.; Luna, J.M.; Stamford, T.C.M.; Sarubbo, L.A. Application of biosurfactants and chitosan in toothpaste formulation. Colloids Surf. B Biointerfaces 2019, 181, 77–84. [Google Scholar] [CrossRef]
- Tabatabaei, M.; Rajaei, A.; Hosseini, E.; Aghbashlo, M.; Gupta, V.K.; Lam, S.S. Effect of type of fatty acid attached to chitosan on walnut oil-in-water Pickering emulsion properties. Carbohydr. Polym. 2022, 291, 119566. [Google Scholar] [CrossRef]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Rajaei, A.; Hadian, M.; Mohsenifar, A.; Rahmani-Cherati, T.; Tabatabaei, M. A coating based on clove essential oils encapsulated by chitosan-myristic acid nanogel efficiently enhanced the shelf-life of beef cutlets. Food Packag. Shelf Life 2017, 14, 137–145. [Google Scholar] [CrossRef]
- Chen, S.; Han, Y.; Jian, L.; Liao, W.; Zhang, Y.; Gao, Y. Fabrication, characterization, physicochemical stability of zein-chitosan nanocomplex for co-encapsulating curcumin and resveratrol. Carbohydr. Polym. 2020, 236, 116090. [Google Scholar] [CrossRef]
- Tanpichai, S.; Srimarut, Y.; Woraprayote, W.; Malila, Y. Chitosan coating for the preparation of multilayer coated paper for food-contact packaging: Wettability, mechanical properties, and overall migration. Int. J. Biol. Macromol. 2022, 213, 534–545. [Google Scholar] [CrossRef]
- Zhao, W.; Liang, X.; Wang, X.; Wang, S.; Wang, L.; Jiang, Y. Chitosan based film reinforced with EGCG loaded melanin-like nanocomposite (EGCG@MNPs) for active food packaging. Carbohydr. Polym. 2022, 290, 119471. [Google Scholar] [CrossRef]
- Maleki, G.; Woltering, E.J.; Mozafari, M. Applications of chitosan-based carrier as an encapsulating agent in food industry. Trends Food Sci. Technol. 2022, 120, 88–99. [Google Scholar] [CrossRef]
- Flórez, M.; Guerra-Rodríguez, E.; Cazón, P.; Vázquez, M. Chitosan for food packaging: Recent advances in active and intelligent films. Food Hydrocoll. 2021, 124, 107328. [Google Scholar] [CrossRef]
- Qu, B.; Luo, Y. Chitosan-based hydrogel beads: Preparations, modifications and applications in food and agriculture sectors—A review. Int. J. Biol. Macromol. 2020, 152, 437–448. [Google Scholar] [CrossRef]
- Yuan, X.; Zheng, J.; Jiao, S.; Cheng, G.; Feng, C.; Du, Y.; Liu, H. A review on the preparation of chitosan oligosaccharides and application to human health, animal husbandry and agricultural production. Carbohydr. Polym. 2019, 220, 60–70. [Google Scholar] [CrossRef]
- Paulraj, M.G.; Ignacimuthu, S.; Gandhi, M.R.; Shajahan, A.; Ganesan, P.; Packiam, S.M.; Al-Dhabi, N.A. Comparative studies of tripolyphosphate and glutaraldehyde cross-linked chitosan-botanical pesticide nanoparticles and their agricultural applications. Int. J. Biol. Macromol. 2017, 104, 1813–1819. [Google Scholar] [CrossRef]
- Masteri-Farahani, M.; Shahsavarifar, S. Chemical functionalization of chitosan biopolymer and chitosan-magnetite nanocomposite with sulfonic acid for acid-catalyzed reactions. Chin. J. Chem. Eng. 2021, 39, 154–161. [Google Scholar] [CrossRef]
- Kumari, S.; Tiyyagura, H.R.; Pottathara, Y.B.; Sadasivuni, K.K.; Ponnamma, D.; Douglas, T.E.; Skirtach, A.G.; Mohan, M. Surface functionalization of chitosan as a coating material for orthopaedic applications: A comprehensive review. Carbohydr. Polym. 2020, 255, 117487. [Google Scholar] [CrossRef]
- Verma, M.L.; Dhanya, B.; Sukriti; Rani, V.; Thakur, M.; Jeslin, J.; Kushwaha, R. Carbohydrate and protein based biopolymeric nanoparticles: Current status and biotechnological applications. Int. J. Biol. Macromol. 2020, 154, 390–412. [Google Scholar] [CrossRef]
- Yamazaki, S.; Takegawa, A.; Kaneko, Y.; Kadokawa, J.-I.; Yamagata, M.; Ishikawa, M. An acidic cellulose–chitin hybrid gel as novel electrolyte for an electric double layer capacitor. Electrochem. Commun. 2009, 11, 68–70. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, X.; Xie, Y.; Shen, Z.; Ling, Y.; Xue, X.; Tong, Y.; Wang, J.; Zhang, W.; Zhao, J. A gel polymer electrolyte film based on chitosan derivative and ionic liquid for the LiFePO4 cathode solid Li metal battery. Mater. Today Commun. 2022, 31, 103597. [Google Scholar] [CrossRef]
- Hamza, M.F.; Mira, H.; Wei, Y.; Aboelenin, S.M.; Guibal, E.; Salem, W.M. Sulfonation of chitosan for enhanced sorption of Li(I) from acidic solutions—Application to metal recovery from waste Li-ion mobile battery. Chem. Eng. J. 2022, 441, 135941. [Google Scholar] [CrossRef]
- Feng, J.; Yi, H.; Lei, Z.; Wang, J.; Zeng, H.; Deng, Y.; Wang, C. A three-dimensional crosslinked chitosan sulfate network binder for high-performance Li–S batteries. J. Energy Chem. 2020, 56, 171–178. [Google Scholar] [CrossRef]
- Chai, L.; Qu, Q.; Zhang, L.; Shen, M.; Zhang, L.; Zheng, H. Chitosan, a new and environmental benign electrode binder for use with graphite anode in lithium-ion batteries. Electrochimica Acta 2013, 105, 378–383. [Google Scholar] [CrossRef]
- Reshad, R.A.I.; Alam Jishan, T.; Chowdhury, N.N. Chitosan and its Broad Applications: A Brief Review. J. Clin. Exp. Investig. 2021, 12, em00779. [Google Scholar] [CrossRef]
- Shang, J.; Shao, Z.; Chen, X. Chitosan-based electroactive hydrogel. Polymer 2008, 49, 5520–5525. [Google Scholar] [CrossRef]
- Mourya, V.; Inamdar, N.N. Chitosan-modifications and applications: Opportunities galore. React. Funct. Polym. 2008, 68, 1013–1051. [Google Scholar] [CrossRef]
- Chungsiriporn, J.; Khunthongkaew, P.; Wongnoipla, Y.; Sopajarn, A.; Karrila, S.; Iewkittayakorn, J. Fibrous packaging paper made of oil palm fiber with beeswax-chitosan solution to improve water resistance. Ind. Crop. Prod. 2022, 177, 114541. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Bhardwaj, N.K.; Negi, Y.S. Surface coating of chitosan of different degree of acetylation on non surface sized writing and printing grade paper. Carbohydr. Polym. 2021, 269, 117674. [Google Scholar] [CrossRef]
- Parween, S.; Bhatnagar, I.; Bhosale, S.; Paradkar, S.; Michael, I.J.; Rao, C.M.; Asthana, A. Cross-linked chitosan biofunctionalized paper-based microfluidic device towards long term stabilization of blood typing antibodies. Int. J. Biol. Macromol. 2020, 163, 1233–1239. [Google Scholar] [CrossRef]
- Mohan V, L.; Shiva Nagendraa, S.M.; Maiyab, M.P. Photocatalytic degradation of gaseous toluene using self-assembled air filter based on chitosan/activated carbon/TiO2. J. Environ. Chem. Eng. 2019, 7, 103455. [Google Scholar] [CrossRef]
- Ribeiro, E.S.; de Farias, B.S.; Junior, T.R.S.C.; Pinto, L.A.D.A.; Diaz, P.S. Chitosan–based nanofibers for enzyme immobilization. Int. J. Biol. Macromol. 2021, 183, 1959–1970. [Google Scholar] [CrossRef]
- Ji, S.; Liu, W.; Su, S.; Gan, C.; Jia, C. Chitosan derivative functionalized carbon nanotubes as carriers for enzyme immobilization to improve synthetic efficiency of ethyl caproate. LWT 2021, 149, 111897. [Google Scholar] [CrossRef]
- Nouri, M.; Khodaiyan, F. Green synthesis of chitosan magnetic nanoparticles and their application with poly-aldehyde kefiran cross-linker to immobilize pectinase enzyme. Biocatal. Agric. Biotechnol. 2020, 29, 101681. [Google Scholar] [CrossRef]
- Ji, X.; Guo, M. Preparation and properties of a chitosan-lignin wood adhesive. Int. J. Adhes. Adhes. 2018, 82, 8–13. [Google Scholar] [CrossRef]
- Xi, X.; Pizzi, A.; Lei, H.; Zhang, B.; Chen, X.; Du, G. Environmentally friendly chitosan adhesives for plywood bonding. Int. J. Adhes. Adhes. 2021, 112, 103027. [Google Scholar] [CrossRef]
- Shalbafan, A.; Hassannejad, H.; Rahmaninia, M. Formaldehyde adsorption capacity of chitosan derivatives as bio-adsorbents for wood-based panels. Int. J. Adhes. Adhes. 2020, 102, 102669. [Google Scholar] [CrossRef]
- Sarode, S.; Upadhyay, P.; Khosa, M.; Mak, T.; Shakir, A.; Song, S.; Ullah, A. Overview of wastewater treatment methods with special focus on biopolymer chitin-chitosan. Int. J. Biol. Macromol. 2018, 121, 1086–1100. [Google Scholar] [CrossRef]
- Sakib, M.N.; Mallik, A.K.; Rahman, M.M. Update on chitosan-based electrospun nanofibers for wastewater treatment: A review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100064. [Google Scholar] [CrossRef]
- Youcefi, F.; Ouahab, L.W.; Borsali, L.; Bengherbi, S.E.-I. Heavy metal removal efficiency and antibacterial activity of chitosan beads prepared from crustacean waste. Mater. Today Proc. 2022, 53, 265–268. [Google Scholar] [CrossRef]
- Ekka, B.; Mieriņa, I.; Juhna, T.; Kokina, K.; Turks, M. Synergistic effect of activated charcoal and chitosan on treatment of dairy wastewaters. Mater. Today Commun. 2022, 31, 103477. [Google Scholar] [CrossRef]
- Iber, B.T.; Okomoda, V.T.; Rozaimah, S.A.; Kasan, N.A. Eco-friendly approaches to aquaculture wastewater treatment: Assessment of natural coagulants vis-a-vis chitosan. Bioresour. Technol. Rep. 2021, 15, 100702. [Google Scholar] [CrossRef]
- Hou, J.; Aydemir, B.E.; Dumanli, A.G. Understanding the structural diversity of chitins as a versatile biomaterial. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 2021, 379, 20200331. [Google Scholar] [CrossRef]
- Santos, V.P.; Marques, N.S.S.; Maia, P.C.S.V.; De Lima, M.A.B.; de Oliveira Franco, L.; De Campos-Takaki, G.M. Seafood Waste as Attractive Source of Chitin and Chitosan Production and Their Applications. Int. J. Mol. Sci. 2020, 21, 4290. [Google Scholar] [CrossRef]
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials. Int. J. Adv. Res. 2016, 4, 411–427. [Google Scholar]
- Liu, X.; Cooper, A.M.; Zhang, J.; Zhu, K.Y. Biosynthesis, modifications and degradation of chitin in the formation and turnover of peritrophic matrix in insects. J. Insect Physiol. 2019, 114, 109–115. [Google Scholar] [CrossRef]
- Merzendorfer, H. The cellular basis of chitin synthesis in fungi and insects: Common principles and differences. Eur. J. Cell Biol. 2011, 90, 759–769. [Google Scholar] [CrossRef]
- Bastiaens, L.; Soetemans, L.; D’Hondt, E.; Elst, K. Sources of Chitin and Chitosan and their Isolation. In Chitin and Chitosan: Properties and Applications; Wiley: Hoboken, NJ, USA, 2019; pp. 1–34. [Google Scholar] [CrossRef]
- Kumirska, J.; Weinhold, M.X.; Thöming, J.; Stepnowski, P. Biomedical Activity of Chitin/Chitosan Based Materials—Influence of Physicochemical Properties Apart from Molecular Weight and Degree of N-Acetylation. Polymers 2011, 3, 1875–1901. [Google Scholar] [CrossRef]
- Zhu, K.Y.; Merzendorfer, H.; Zhang, W.; Zhang, J.; Muthukrishnan, S. Biosynthesis, Turnover, and Functions of Chitin in Insects. Annu. Rev. Entomol. 2016, 61, 177–196. [Google Scholar] [CrossRef]
- Ru, G.; Wu, S.; Yan, X.; Liu, B.; Gong, P.; Wang, L.; Feng, J. Inverse solubility of chitin/chitosan in aqueous alkali solvents at low temperature. Carbohydr. Polym. 2018, 206, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Hahn, T.; Tafi, E.; Paul, A.; Salvia, R.; Falabella, P.; Zibek, S. Current state of chitin purification and chitosan production from insects. J. Chem. Technol. Biotechnol. 2020, 95, 2775–2795. [Google Scholar] [CrossRef]
- Casadidio, C.; Peregrina, D.V.; Gigliobianco, M.R.; Deng, S.; Censi, R.; Di Martino, P. Chitin and Chitosans: Characteristics, Eco-Friendly Processes, and Applications in Cosmetic Science. Mar. Drugs 2019, 17, 369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gow, N.A.R.; Latge, J.-P.; Munro, C.A. The Fungal Cell Wall: Structure, Biosynthesis, and Function. Microbiol. Spectr. 2017, 5, 28513415. [Google Scholar] [CrossRef] [Green Version]
- Kjartansson, G.T.; Zivanovic, S.; Kristbergsson, A.K.; Weiss, J. Sonication-Assisted Extraction of Chitin from North Atlantic Shrimps (Pandalus borealis). J. Agric. Food Chem. 2006, 54, 5894–5902. [Google Scholar] [CrossRef]
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, chemical modification and characterization of chitin and chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. [Google Scholar] [CrossRef]
- Kurita, K. Chitin and Chitosan: Functional Biopolymers from Marine Crustaceans. Mar. Biotechnol. 2006, 8, 203–226. [Google Scholar] [CrossRef]
- Kaur, S.; Dhillon, G.S. Recent trends in biological extraction of chitin from marine shell wastes: A review. Crit. Rev. Biotechnol. 2015, 35, 44–61. [Google Scholar] [CrossRef]
- Bajaj, M.; Winter, J.; Gallert, C. Effect of deproteination and deacetylation conditions on viscosity of chitin and chitosan extracted from Crangon crangon shrimp waste. Biochem. Eng. J. 2011, 56, 51–62. [Google Scholar] [CrossRef]
- Spranghers, T.; Ottoboni, M.; Klootwijk, C.; Ovyn, A.; Deboosere, S.; De Meulenaer, B.; Michiels, J.; Eeckhout, M.; De Clercq, P.; De Smet, S. Nutritional composition of black soldier fly (Hermetia illucens) prepupae reared on different organic waste substrates. J. Sci. Food Agric. 2017, 97, 2594–2600. [Google Scholar] [CrossRef]
- Kaur, S.; Dhillon, G.S. The versatile biopolymer chitosan: Potential sources, evaluation of extraction methods and applications. Crit. Rev. Microbiol. 2013, 40, 155–175. [Google Scholar] [CrossRef]
- Srinivasan, H.; Kanayairam, V.; Ravichandran, R. Chitin and chitosan preparation from shrimp shells Penaeus monodon and its human ovarian cancer cell line, PA-1. Int. J. Biol. Macromol. 2018, 107, 662–667. [Google Scholar] [CrossRef]
- Mohan, K.; Ganesan, A.R.; Muralisankar, T.; Jayakumar, R.; Sathishkumar, P.; Uthayakumar, V.; Chandirasekar, R.; Revathi, N. Recent insights into the extraction, characterization, and bioactivities of chitin and chitosan from insects. Trends Food Sci. Technol. 2020, 105, 17–42. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [Green Version]
- Lopes, C.; Antelo, L.T.; Franco-Uría, A.; Alonso, A.; Perez-Martin, R. Chitin production from crustacean biomass: Sustainability assessment of chemical and enzymatic processes. J. Clean. Prod. 2018, 172, 4140–4151. [Google Scholar] [CrossRef] [Green Version]
- Tasar, O.C.; Erdal, S.; Taskin, M. Chitosan production by psychrotolerant Rhizopus oryzae in non-sterile open fermentation conditions. Int. J. Biol. Macromol. 2016, 89, 428–433. [Google Scholar] [CrossRef]
- Huq, T.; Khan, A.; Brown, D.; Dhayagude, N.; He, Z.; Ni, Y. Sources, production and commercial applications of fungal chitosan: A review. J. Bioresour. Bioprod. 2022, 7, 85–98. [Google Scholar] [CrossRef]
- Kasongo, K.J.; Tubadi, D.J.; Bampole, L.D.; Kaniki, T.A.; Kanda, N.J.M.; Lukumu, M.E. Extraction and characterization of chitin and chitosan from Termitomyces titanicus. SN Appl. Sci. 2020, 2, 406. [Google Scholar] [CrossRef] [Green Version]
- Namboodiri, M.M.T.; Pakshirajan, K. Chapter 10—Valorization of waste biomass for chitin and chitosan production. In Waste Biorefinery; Elsevier: Amsterdam, The Netherlands, 2020; pp. 241–266. [Google Scholar] [CrossRef]
- Li, B.; Zhang, J.; Dai, F.; Xia, W. Purification of chitosan by using sol–gel immobilized pepsin deproteinization. Carbohydr. Polym. 2011, 88, 206–212. [Google Scholar] [CrossRef]
- Kumari, S.; Rath, P.; Kumar, A.S.H.; Tiwari, T. Extraction and characterization of chitin and chitosan from fishery waste by chemical method. Environ. Technol. Innov. 2015, 3, 77–85. [Google Scholar] [CrossRef]
- Morgan, K.; Conway, C.; Faherty, S.; Quigley, C. A Comparative Analysis of Conventional and Deep Eutectic Solvent (DES)-Mediated Strategies for the Extraction of Chitin from Marine Crustacean Shells. Molecules 2021, 26, 7603. [Google Scholar] [CrossRef] [PubMed]
- Kou, S.; Peters, L.M.; Mucalo, M.R. Chitosan: A review of sources and preparation methods. Int. J. Biol. Macromol. 2020, 169, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Mohan, K.; Ganesan, A.R.; Ezhilarasi, P.; Kondamareddy, K.K.; Rajan, D.K.; Sathishkumar, P.; Rajarajeswaran, J.; Conterno, L. Green and eco-friendly approaches for the extraction of chitin and chitosan: A review. Carbohydr. Polym. 2022, 287, 119349. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.C.; Zanette, C.; Grade, S.T.; Schaffer, J.V.; Alves, H.J.; Arantes, M.K. Optimization of lactic fermentation for extraction of chitin from freshwater shrimp waste. Acta Sci. Technol. 2017, 39, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Deng, J.-J.; Mao, H.; Fang, W.; Li, Z.-Q.; Shi, D.; Li, Z.-W.; Zhou, T.; Luo, X.-C. Enzymatic conversion and recovery of protein, chitin, and astaxanthin from shrimp shell waste. J. Clean. Prod. 2020, 271, 122655. [Google Scholar] [CrossRef]
- Chakravarty, J.; Yang, C.-L.; Palmer, J.; Brigham, C.J. Chitin Extraction from Lobster Shell Waste using Microbial Culture-based Methods. Appl. Food Biotechnol. 2018, 5, 141–154. [Google Scholar] [CrossRef]
- Rakshit, S.; Mondal, S.; Pal, K.; Jana, A.; Soren, J.P.; Barman, P.; Mondal, K.C.; Halder, S.K. Extraction of chitin from Litopenaeus vannamei shell and its subsequent characterization: An approach of waste valorization through microbial bioprocessing. Bioprocess Biosyst. Eng. 2021, 44, 1943–1956. [Google Scholar] [CrossRef]
- Castro, R.; Guerrero-Legarreta, I.; Bórquez, R. Chitin extraction from Allopetrolisthes punctatus crab using lactic fermentation. Biotechnol. Rep. 2018, 20, e00287. [Google Scholar] [CrossRef]
- Masselin, A.; Rousseau, A.; Pradeau, S.; Fort, L.; Gueret, R.; Buon, L.; Armand, S.; Cottaz, S.; Choisnard, L.; Fort, S. Optimizing Chitin Depolymerization by Lysozyme to Long-Chain Oligosaccharides. Mar. Drugs 2021, 19, 320. [Google Scholar] [CrossRef]
- Guan, F.; Han, Y.; Yan, K.; Zhang, Y.; Zhang, Z.; Wu, N.; Tian, J. Highly efficient production of chitooligosaccharides by enzymes mined directly from the marine metagenome. Carbohydr. Polym. 2020, 234, 115909. [Google Scholar] [CrossRef]
- Synowiecki, J.; Al-Khateeb, N.A.A.Q. The recovery of protein hydrolysate during enzymatic isolation of chitin from shrimp Crangon crangon processing discards. Food Chem. 2000, 68, 147–152. [Google Scholar] [CrossRef]
- Hongkulsup, C.; Khutoryanskiy, V.V.; Niranjan, K. Enzyme assisted extraction of chitin from shrimp shells (Litopenaeus vannamei). J. Chem. Technol. Biotechnol. 2015, 91, 1250–1256. [Google Scholar] [CrossRef]
- Xin, R.; Xie, W.; Xu, Z.; Che, H.; Zheng, Z.; Yang, X. Efficient extraction of chitin from shrimp waste by mutagenized strain fermentation using atmospheric and room-temperature plasma. Int. J. Biol. Macromol. 2019, 155, 1561–1568. [Google Scholar] [CrossRef]
- Taokaew, S.; Zhang, X.; Chuenkaek, T.; Kobayashi, T. Chitin from fermentative extraction of crab shells using okara as a nutrient source and comparative analysis of structural differences from chemically extracted chitin. Biochem. Eng. J. 2020, 159, 107588. [Google Scholar] [CrossRef]
- Liu, Y.; Xing, R.; Yang, H.; Liu, S.; Qin, Y.; Li, K.; Yu, H.; Li, P. Chitin extraction from shrimp (Litopenaeus vannamei) shells by successive two-step fermentation with Lactobacillus rhamnoides and Bacillus amyloliquefaciens. Int. J. Biol. Macromol. 2020, 148, 424–433. [Google Scholar] [CrossRef]
- Liu, P.; Liu, S.; Guo, N.; Mao, X.; Lin, H.; Xue, C.; Wei, D. Cofermentation of Bacillus licheniformis and Gluconobacter oxydans for chitin extraction from shrimp waste. Biochem. Eng. J. 2014, 91, 10–15. [Google Scholar] [CrossRef]
- Aranday-García, R.; Saimoto, H.; Shirai, K.; Ifuku, S. Chitin biological extraction from shrimp wastes and its fibrillation for elastic nanofiber sheets preparation. Carbohydr. Polym. 2019, 213, 112–120. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, A.; Li, Z.; Wang, J.; Zhang, S. Influence of anionic structure on the dissolution of chitosan in 1-butyl-3-methylimidazolium-based ionic liquids. Green Chem. 2011, 13, 3446–3452. [Google Scholar] [CrossRef]
- Morais, E.S.; Lopes, A.M.D.C.; Freire, M.G.; Freire, C.S.R.; Coutinho, J.A.P.; Silvestre, A.J.D. Use of Ionic Liquids and Deep Eutectic Solvents in Polysaccharides Dissolution and Extraction Processes towards Sustainable Biomass Valorization. Molecules 2020, 25, 3652. [Google Scholar] [CrossRef]
- Huang, W.-C.; Zhao, D.; Guo, N.; Xue, C.; Mao, X. Green and Facile Production of Chitin from Crustacean Shells Using a Natural Deep Eutectic Solvent. J. Agric. Food Chem. 2018, 66, 11897–11901. [Google Scholar] [CrossRef]
- Tolesa, L.D.; Gupta, B.S.; Lee, M.-J. Chitin and chitosan production from shrimp shells using ammonium-based ionic liquids. Int. J. Biol. Macromol. 2019, 130, 818–826. [Google Scholar] [CrossRef]
- Ma, Q.; Gao, X.; Bi, X.; Xia, M.; Han, Q.; Peng, M.; Tu, L.; Yang, Y.; Shen, Y.; Wang, M. Combination of steam explosion and ionic liquid pretreatments for efficient utilization of fungal chitin from citric acid fermentation residue. Biomass Bioenergy 2021, 145, 105967. [Google Scholar] [CrossRef]
- Kadokawa, J.-I. Dissolution, derivatization, and functionalization of chitin in ionic liquid. Int. J. Biol. Macromol. 2018, 123, 732–737. [Google Scholar] [CrossRef]
- Feng, M.; He, B.; Chen, X.; Xu, J.; Lu, X.; Jia, C.; Sun, J. Separation of chitin from shrimp shells enabled by transition metal salt aqueous solution and ionic liquid. Chin. J. Chem. Eng. 2022; In press. [Google Scholar] [CrossRef]
- Berton, P.; Shamshina, J.L.; Ostadjoo, S.; King, C.A.; Rogers, R.D. Enzymatic hydrolysis of ionic liquid-extracted chitin. Carbohydr. Polym. 2018, 199, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, C.; Hong, S.; Lian, H.; Mei, C.; Lee, J.; Wu, Q.; Hubbe, M.A.; Li, M.-C. Recent advances in extraction and processing of chitin using deep eutectic solvents. Chem. Eng. J. 2022, 446, 136953. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Wang, R.; Zhu, Y.; Yang, P.; Lin, Z.; Wang, Z.; Cong, W. Efficient extraction of chitin from crustacean waste via a novel ternary natural deep eutectic solvents. Carbohydr. Polym. 2022, 286, 119281. [Google Scholar] [CrossRef]
- Saravana, P.S.; Ho, T.C.; Chae, S.-J.; Cho, Y.-J.; Park, J.-S.; Lee, H.-J.; Chun, B.-S. Deep eutectic solvent-based extraction and fabrication of chitin films from crustacean waste. Carbohydr. Polym. 2018, 195, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Mukesh, C.; Mondal, D.; Prasad, K. Dissolution of α-chitin in deep eutectic solvents. RSC Adv. 2013, 3, 18149–18155. [Google Scholar] [CrossRef]
- Wang, J.; Teng, C.; Yan, L. Applications of deep eutectic solvents in the extraction, dissolution, and functional materials of chitin: Research progress and prospects. Green Chem. 2021, 24, 552–564. [Google Scholar] [CrossRef]
- Sun, X.; Wei, Q.; Yang, Y.; Xiao, Z.; Ren, X. In-depth study on the extraction and mechanism of high-purity chitin based on NADESs method. J. Environ. Chem. Eng. 2021, 10, 106859. [Google Scholar] [CrossRef]
- Suryawanshi, N.; Ayothiraman, S.; Eswari, J.S. Ultrasonication mode for the expedition of extraction process of chitin from the maritime shrimp shell waste. Indian J. Biochem. Biophys. 2020, 57, 431–438. [Google Scholar]
- Singh, A.; Benjakul, S.; Prodpran, T. Ultrasound-Assisted Extraction of Chitosan from Squid Pen: Molecular Characterization and Fat Binding Capacity. J. Food Sci. 2019, 84, 224–234. [Google Scholar] [CrossRef]
- Wardhono, E.Y.; Pinem, M.P.; Kustiningsih, I.; Effendy, M.; Clausse, D.; Saleh, K.; Guénin, E. Heterogeneous deacetylation reaction of chitin under low-frequency ultrasonic irradiation. Carbohydr. Polym. 2021, 267, 118180. [Google Scholar] [CrossRef]
- Sebastian, J.; Rouissi, T.; Brar, S.K.; Hegde, K.; Verma, M. Microwave-assisted extraction of chitosan from Rhizopus oryzae NRRL 1526 biomass. Carbohydr. Polym. 2019, 219, 431–440. [Google Scholar] [CrossRef]
- Prajapat, A.L.; Gogate, P.R. Depolymerization of guar gum solution using different approaches based on ultrasound and microwave irradiations. Chem. Eng. Process. Process Intensif. 2015, 88, 1–9. [Google Scholar] [CrossRef]
- El Knidri, H.; El Khalfaouy, R.; Laajeb, A.; Addaou, A.; Lahsini, A. Eco-friendly extraction and characterization of chitin and chitosan from the shrimp shell waste via microwave irradiation. Process Saf. Environ. Prot. 2016, 104, 395–405. [Google Scholar] [CrossRef]
- Apriyanti, D.T.; Susanto, H.; Rokhati, N. Influence of Microwave Irradiation on Extraction of Chitosan from Shrimp Shell Waste. Reaktor 2018, 18, 45–50. [Google Scholar] [CrossRef]
- EL Knidri, H.; Dahmani, J.; Addaou, A.; Laajeb, A.; Lahsini, A. Rapid and efficient extraction of chitin and chitosan for scale-up production: Effect of process parameters on deacetylation degree and molecular weight. Int. J. Biol. Macromol. 2019, 139, 1092–1102. [Google Scholar] [CrossRef]
- Hajji, S.; Ghorbel-Bellaaj, O.; Younes, I.; Jellouli, K.; Nasri, M. Chitin extraction from crab shells by Bacillus bacteria. Biological activities of fermented crab supernatants. Int. J. Biol. Macromol. 2015, 79, 167–173. [Google Scholar] [CrossRef]
- Younes, I.; Hajji, S.; Frachet, V.; Rinaudo, M.; Jellouli, K.; Nasri, M. Chitin extraction from shrimp shell using enzymatic treatment. Antitumor, antioxidant and antimicrobial activities of chitosan. Int. J. Biol. Macromol. 2014, 69, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Hou, F.; Ma, X.; Fan, L.; Wang, D.; Ding, T.; Ye, X.; Liu, D. Enhancement of chitin suspension hydrolysis by a combination of ultrasound and chitinase. Carbohydr. Polym. 2019, 231, 115669. [Google Scholar] [CrossRef] [PubMed]
- Achinivu, E.C.; Shamshina, J.L.; Rogers, R.D. Chitin extracted from various biomass sources: It’s not the same. Fluid Phase Equilibria 2021, 552, 113286. [Google Scholar] [CrossRef]
- Qin, Y.; Lu, X.; Sun, N.; Rogers, R.D. Dissolution or extraction of crustacean shells using ionic liquids to obtain high molecular weight purified chitin and direct production of chitin films and fibers. Green Chem. 2010, 12, 968–971. [Google Scholar] [CrossRef]
- Hong, S.; Yuan, Y.; Yang, Q.; Zhu, P.; Lian, H. Versatile acid base sustainable solvent for fast extraction of various molecular weight chitin from lobster shell. Carbohydr. Polym. 2018, 201, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, J.C.; Ruiz, A.T.; Morales-Ramos, J.; Thomas, M.; Rojas, M.; Tomberlin, J. Insects as Sustainable Food Ingredients. Insects as Sustain Food Ingredients; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Battampara, P.; Sathish, T.N.; Reddy, R.; Guna, V.; Nagananda, G.; Reddy, N.; Ramesha, B.; Maharaddi, V.; Rao, A.P.; Ravikumar, H.; et al. Properties of chitin and chitosan extracted from silkworm pupae and egg shells. Int. J. Biol. Macromol. 2020, 161, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Sun, J.; Yu, L.; Zhang, C.; Bi, J.; Zhu, F.; Qu, M.; Jiang, C.; Yang, Q. Extraction and Characterization of Chitin from the Beetle Holotrichia parallela Motschulsky. Molecules 2012, 17, 4604–4611. [Google Scholar] [CrossRef]
- Kaya, M.; Erdogan, S.; Mol, A.; Baran, T. Comparison of chitin structures isolated from seven Orthoptera species. Int. J. Biol. Macromol. 2015, 72, 797–805. [Google Scholar] [CrossRef]
- De Queiroz Antonino, R.S.C.M.; Lia Fook, B.R.P.; De Oliveira Lima, V.A.; De Farias Rached, R.Í.; Lima, E.P.N.; Da Silva Lima, R.J.; Peniche Covas, C.A.; Lia Fook, M.V. Preparation and Characterization of Chitosan Obtained from Shells of Shrimp (Litopenaeus vannamei Boone). Mar. Drugs 2017, 15, 141. [Google Scholar] [CrossRef] [Green Version]
- Pakizeh, M.; Moradi, A.; Ghassemi, T. Chemical extraction and modification of chitin and chitosan from shrimp shells. Eur. Polym. J. 2021, 159, 110709. [Google Scholar] [CrossRef]
- Batista, I.; Roberts, G.A.F. A novel, facile technique for deacetylating chitin. Die Makromol. Chemie 1990, 191, 429–434. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.; Pan, W.; Wu, Q. Absorption behaviors and structure changes of chitin in alkali solution. Carbohydr. Polym. 2008, 72, 235–239. [Google Scholar] [CrossRef]
- Jung, J.; Zhao, Y. Alkali- or acid-induced changes in structure, moisture absorption ability and deacetylating reaction of β-chitin extracted from jumbo squid (Dosidicus gigas) pens. Food Chem. 2014, 152, 355–362. [Google Scholar] [CrossRef]
- Tan, T.S.; Chin, H.Y.; Tsai, M.-L.; Liu, C.-L. Structural alterations, pore generation, and deacetylation of α- and β-chitin submitted to steam explosion. Carbohydr. Polym. 2015, 122, 321–328. [Google Scholar] [CrossRef]
- Younes, I.; Ghorbel-Bellaaj, O.; Nasri, R.; Chaabouni, M.; Rinaudo, M.; Nasri, M. Chitin and chitosan preparation from shrimp shells using optimized enzymatic deproteinization. Process Biochem. 2012, 47, 2032–2039. [Google Scholar] [CrossRef]
- Suresh, P.V.; Sakhare, P.Z.; Sachindra, N.M.; Halami, P.M. Extracellular chitin deacetylase production in solid state fermentation by native soil isolates of Penicillium monoverticillium and Fusarium oxysporum. J. Food Sci. Technol. 2012, 51, 1594–1599. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Park, R.-D.; Muzzarelli, R. Chitin Deacetylases: Properties and Applications. Mar. Drugs 2010, 8, 24–46. [Google Scholar] [CrossRef]
- Schmitz, C.; Auza, L.G.; Koberidze, D.; Rasche, S.; Fischer, R.; Bortesi, L. Conversion of Chitin to Defined Chitosan Oligomers: Current Status and Future Prospects. Mar. Drugs 2019, 17, 452. [Google Scholar] [CrossRef] [Green Version]
- Hembach, L.; Cord-Landwehr, S.; Moerschbacher, B.M. Enzymatic production of all fourteen partially acetylated chitosan tetramers using different chitin deacetylases acting in forward or reverse mode. Sci. Rep. 2017, 7, 17692. [Google Scholar] [CrossRef] [Green Version]
- Hamer, S.N.; Cord-Landwehr, S.; Biarnés, X.; Planas, A.; Waegeman, H.; Moerschbacher, B.; Kolkenbrock, S. Enzymatic production of defined chitosan oligomers with a specific pattern of acetylation using a combination of chitin oligosaccharide deacetylases. Sci. Rep. 2015, 5, srep08716. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Lin, Q.L.; Chen, Z.X.; Wu, W.; Xiao, H.X. Preparation of chitosan oligomers COS and their effect on the retrogradation of intermediate amylose rice starch. J. Food Sci. Technol. 2011, 49, 695–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daraghmeh, N.; Chowdhry, B.Z.; Leharne, S.A.; Al Omari, M.M.H.; Badwan, A.A. Co-Processed Chitin-Mannitol as a New Excipient for Oro-Dispersible Tablets. Mar. Drugs 2015, 13, 1739–1764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Moran, H.B.T.; Turley, J.L.; Andersson, M.; Lavelle, E.C. Immunomodulatory properties of chitosan polymers. Biomaterials 2018, 184, 1–9. [Google Scholar] [CrossRef]
- Haske-Cornelius, O.; Bischof, S.; Beer, B.; Bartolome, M.J.; Olakanmi, E.O.; Mokoba, M.; Guebitz, G.; Nyanhongo, G. Enzymatic synthesis of highly flexible lignin cross-linked succinyl-chitosan hydrogels reinforced with reed cellulose fibres. Eur. Polym. J. 2019, 120, 109201. [Google Scholar] [CrossRef]
- Dongre, R.S. Introductory Chapter: Multitask Portfolio of Chitin/Chitosan: Biomatrix to Quantum Dot. In Chitin-Chitosan—Myriad Functionalities in Science and Technology; InTechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Chang, T.; Dong, S.; Zhang, D.; Ma, C.; Chen, S.; Li, H. Biopolymer films based on chitosan/potato protein/linseed oil/ZnO NPs to maintain the storage quality of raw meat. Food Chem. 2020, 332, 127375. [Google Scholar] [CrossRef]
- Grobler, S.; Perchyonok, V. Cytotoxicity of low, medium and high molecular weight chitosan’s on balb/c 3t3 mouse fibroblast cells at a 75–85% de-acetylation degree. Mater. Sci. Eng. Adv. Res. 2018, 2, 27–30. [Google Scholar] [CrossRef]
- Tan, G.; Kaya, M.; Tevlek, A.; Sargin, I.; Baran, T. Antitumor activity of chitosan from mayfly with comparison to commercially available low, medium and high molecular weight chitosans. In Vitro Cell. Dev. Biol. Anim. 2018, 54, 366–374. [Google Scholar] [CrossRef]
- Sivashankari, P.; Prabaharan, M. Deacetylation modification techniques of chitin and chitosan. In Chitosan Based Biomaterials Volume 1; Elsevier: Amsterdam, The Netherlands, 2017; pp. 117–133. [Google Scholar] [CrossRef]
- Ahmadi, F.; Oveisi, Z.; Samani, S.M.; Amoozgar, Z. Chitosan based hydrogels: Characteristics and pharmaceutical applications. Res. Pharm. Sci. 2015, 10, 1–16. [Google Scholar]
- Hamdi, M.; Nasri, R.; Ben Amor, I.; Li, S.; Gargouri, J.; Nasri, M. Structural features, anti-coagulant and anti-adhesive potentials of blue crab (Portunus segnis) chitosan derivatives: Study of the effects of acetylation degree and molecular weight. Int. J. Biol. Macromol. 2020, 160, 593–601. [Google Scholar] [CrossRef]
- Sukul, M.; Sahariah, P.; Lauzon, H.L.; Borges, J.; Másson, M.; Mano, J.F.; Haugen, H.J.; Reseland, J.E. In vitro biological response of human osteoblasts in 3D chitosan sponges with controlled degree of deacetylation and molecular weight. Carbohydr. Polym. 2020, 254, 117434. [Google Scholar] [CrossRef]
- Ivanova, D.G.; Yaneva, Z.L. Antioxidant Properties and Redox-Modulating Activity of Chitosan and Its Derivatives: Biomaterials with Application in Cancer Therapy. Biores. Open Access 2020, 9, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Hosseinnejad, M.; Jafari, S.M. Evaluation of different factors affecting antimicrobial properties of chitosan. Int. J. Biol. Macromol. 2016, 85, 467–475. [Google Scholar] [CrossRef]
- Meng, X.; Yang, L.; Kennedy, J.F.; Tian, S. Effects of chitosan and oligochitosan on growth of two fungal pathogens and physiological properties in pear fruit. Carbohydr. Polym. 2010, 81, 70–75. [Google Scholar] [CrossRef]
- Wu, S. Preparation of water soluble chitosan by hydrolysis with commercial α-amylase containing chitosanase activity. Food Chem. 2011, 128, 769–772. [Google Scholar] [CrossRef]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef] [Green Version]
- Bumgardner, J.; Murali, V.; Su, H.; Jenkins, O.; Velasquez-Pulgarin, D.; Jennings, J.; Sivashanmugam, A.; Jayakumar, R. Characterization of chitosan matters. In Chitosan Based Biomaterials Volume 1; Elsevier: Amsterdam, The Netherlands, 2017; pp. 81–114. [Google Scholar] [CrossRef]
- Kara, A.; Stevens, R. Characterisation of biscuit fired bone china body microstructure. Part I: XRD and SEM of crystalline phases. J. Eur. Ceram. Soc. 2002, 22, 731–736. [Google Scholar] [CrossRef]
- Pavinatto, A.; Pavinatto, F.J.; Delezuk, J.A.D.M.; Nobre, T.M.; Souza, A.L.; Campana-Filho, S.P.; Oliveira, O.N. Low molecular-weight chitosans are stronger biomembrane model perturbants. Colloids Surf. B Biointerfaces 2013, 104, 48–53. [Google Scholar] [CrossRef]
- Liang, T.-W.; Huang, C.-T.; Dzung, N.A.; Wang, S.-L. Squid Pen Chitin Chitooligomers as Food Colorants Absorbers. Mar. Drugs 2015, 13, 681–696. [Google Scholar] [CrossRef] [Green Version]
- Vázquez, J.A.; Rodríguez-Amado, I.; Montemayor, M.I.; Fraguas, J.; González, M.D.P.; Murado, M.A. Chondroitin Sulfate, Hyaluronic Acid and Chitin/Chitosan Production Using Marine Waste Sources: Characteristics, Applications and Eco-Friendly Processes: A Review. Mar. Drugs 2013, 11, 747–774. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, A.I.; Coutinho, A.J.; Costa Lima, S.A.; Reis, S. Marine Polysaccharides in Pharmaceutical Applications: Fucoidan and Chitosan as Key Players in the Drug Delivery Match Field. Mar. Drugs 2019, 17, 654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.; Bi, S.; Pang, J.; Sun, M.; Feng, C.; Chen, X. Preparation and characterization of chitosan from crab shell (Portunus trituberculatus) by NaOH/urea solution freeze-thaw pretreatment procedure. Int. J. Biol. Macromol. 2019, 147, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Jiang, F.; Xu, Q.; Chen, D.; Yu, B.; Huang, Z.; Mao, X.; Yu, J.; He, J. New insights into the role of chitosan oligosaccharide in enhancing growth performance, antioxidant capacity, immunity and intestinal development of weaned pigs. RSC Adv. 2017, 7, 9669–9679. [Google Scholar] [CrossRef] [Green Version]
- Ngo, D.-H.; Kim, S.-K. Chapter Two–Antioxidant Effects of Chitin, Chitosan, and Their Derivatives. Adv. Food Nutr. Res. 2014, 73, 15–31. [Google Scholar]
- Yen, M.-T.; Yang, J.-H.; Mau, J.-L. Antioxidant properties of chitosan from crab shells. Carbohydr. Polym. 2008, 74, 840–844. [Google Scholar] [CrossRef]
- Varun, T.K.; Senani, S.; Jayapal, N.; Chikkerur, J.; Roy, S.; Tekulapally, V.B.; Gautam, M.; Kumar, N. Extraction of chitosan and its oligomers from shrimp shell waste, their characterization and antimicrobial effect. Veter. World 2017, 10, 170–175. [Google Scholar] [CrossRef] [Green Version]
- Song, C.; Yu, H.; Zhang, M.; Yang, Y.; Zhang, G. Physicochemical properties and antioxidant activity of chitosan from the blowfly Chrysomya megacephala larvae. Int. J. Biol. Macromol. 2013, 60, 347–354. [Google Scholar] [CrossRef]
- Shin, C.-S.; Kim, D.-Y.; Shin, W.-S. Characterization of chitosan extracted from Mealworm Beetle (Tenebrio molitor, Zophobas morio) and Rhinoceros Beetle (Allomyrina dichotoma) and their antibacterial activities. Int. J. Biol. Macromol. 2018, 125, 72–77. [Google Scholar] [CrossRef]
- Kaya, M.; Baran, T.; Erdoğan, S.; Menteş, A.; Özüsağlam, M.A.; Çakmak, Y.S. Physicochemical comparison of chitin and chitosan obtained from larvae and adult Colorado potato beetle (Leptinotarsa decemlineata). Mater. Sci. Eng. C 2014, 45, 72–81. [Google Scholar] [CrossRef]
- Ibitoye, B.E.; Lokman, I.H.; Hezmee, M.N.M.; Goh, Y.M.; Zuki, A.B.Z.; Jimoh, A.A. Extraction and physicochemical characterization of chitin and chitosan isolated from house cricket. Biomed. Mater. 2017, 13, 025009. [Google Scholar] [CrossRef] [Green Version]
- Karimi, K.; Zamani, A. Mucor indicus: Biology and industrial application perspectives: A review. Biotechnol. Adv. 2013, 31, 466–481. [Google Scholar] [CrossRef]
- Ghormade, V.; Pathan, E.K.; Deshpande, M.V. Can fungi compete with marine sources for chitosan production? Int. J. Biol. Macromol. 2017, 104, 1415–1421. [Google Scholar] [CrossRef]
- Watts, P.; Smith, A.; Hinchcliffe, M. ChiSys® as a Chitosan-Based Delivery Platform for Nasal Vaccination. In Mucosal Delivery of Biopharmaceuticals; Springer: Boston, MA, USA, 2014; pp. 499–516. [Google Scholar] [CrossRef]
- Ghidelli, C.; Pérez-Gago, M.B. Recent advances in modified atmosphere packaging and edible coatings to maintain quality of fresh-cut fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2017, 58, 662–679. [Google Scholar] [CrossRef]
- Varelas, V.; Tataridis, P.; Liouni, M.; Nerantzis, E.T. Valorization of Winery Spent Yeast Waste Biomass as a New Source for the Production of β-Glucan. Waste Biomass Valorization 2016, 7, 807–817. [Google Scholar] [CrossRef]
- Satari, B.; Karimi, K.; Taherzadeh, M.J.; Zamani, A. Co-Production of Fungal Biomass Derived Constituents and Ethanol from Citrus Wastes Free Sugars without Auxiliary Nutrients in Airlift Bioreactor. Int. J. Mol. Sci. 2016, 17, 302. [Google Scholar] [CrossRef] [Green Version]
- Brasselet, C.; Pierre, G.; Dubessay, P.; Dols-Lafargue, M.; Coulon, J.; Maupeu, J.; Vallet-Courbin, A.; de Baynast, H.; Doco, T.; Michaud, P.; et al. Modification of Chitosan for the Generation of Functional Derivatives. Appl. Sci. 2019, 9, 1321. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.A.; Halfar, J. First evidence of chitin in calcified coralline algae: New insights into the calcification process of Clathromorphum compactum. Sci. Rep. 2014, 4, 6162. [Google Scholar] [CrossRef]
- Saito, T.; Kuramae, R.; Wohlert, J.; Berglund, L.A.; Isogai, A. An Ultrastrong Nanofibrillar Biomaterial: The Strength of Single Cellulose Nanofibrils Revealed via Sonication-Induced Fragmentation. Biomacromolecules 2012, 14, 248–253. [Google Scholar] [CrossRef]
- Alves, N.; Mano, J. Chitosan derivatives obtained by chemical modifications for biomedical and environmental applications. Int. J. Biol. Macromol. 2008, 43, 401–414. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhuang, S. Chitosan-based materials: Preparation, modification and application. J. Clean. Prod. 2022, 355, 131825. [Google Scholar] [CrossRef]
- Nunes, Y.L.; de Menezes, F.L.; de Sousa, I.G.; Cavalcante, A.L.G.; Cavalcante, F.T.T.; Moreira, K.d.S.; de Oliveira, A.L.B.; Mota, G.F.; Souza, J.E.D.S.; Falcão, I.R.D.A.; et al. Chemical and physical Chitosan modification for designing enzymatic industrial biocatalysts: How to choose the best strategy? Int. J. Biol. Macromol. 2021, 181, 1124–1170. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Feng, C.; Chen, H.; Gao, Y. Chemical Modification of Chitosan for Developing Cancer Nanotheranostics. Biomacromolecules 2022, 23, 2197–2218. [Google Scholar] [CrossRef]
- Madera-Santana, T.J.; Herrera-Méndez, C.H.; Rodríguez-Núñez, J.R. An overview of the chemical modifications of chitosan and their advantages. Green Mater. 2018, 6, 131–142. [Google Scholar] [CrossRef] [Green Version]
- Illy, N.; Benyahya, S.; Durand, N.; Auvergne, R.; Caillol, S.; David, G.; Boutevin, B. The influence of formulation and processing parameters on the thermal properties of a chitosan-epoxy prepolymer system. Polym. Int. 2013, 63, 420–426. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Li, Q.; Shen, Y.; Ge, Z.; Zhang, W.; Chen, S. Enhanced water-solubility, antibacterial activity and biocompatibility upon introducing sulfobetaine and quaternary ammonium to chitosan. Carbohydr. Polym. 2016, 143, 246–253. [Google Scholar] [CrossRef]
- Pardeshi, C.V.; Belgamwar, V.S. Controlled synthesis of N,N,N-trimethyl chitosan for modulated bioadhesion and nasal membrane permeability. Int. J. Biol. Macromol. 2016, 82, 933–944. [Google Scholar] [CrossRef]
- Singh, G.; Nayal, A.; Malhotra, S.; Koul, V. Dual functionalized chitosan based composite hydrogel for haemostatic efficacy and adhesive property. Carbohydr. Polym. 2020, 247, 116757. [Google Scholar] [CrossRef]
- Sahariah, P.; Másson, M. Efficient synthesis of chitosan derivatives as clickable tools. Eur. Polym. J. 2022, 166, 111039. [Google Scholar] [CrossRef]
- Chen, C.; Tao, S.; Qiu, X.; Ren, X.; Hu, S. Long-alkane-chain modified N-phthaloyl chitosan membranes with controlled permeability. Carbohydr. Polym. 2013, 91, 269–276. [Google Scholar] [CrossRef]
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Muthumeenal, A.; Neelakandan, S.; Kanagaraj, P.; Nagendran, A. Synthesis and properties of novel proton exchange membranes based on sulfonated polyethersulfone and N-phthaloyl chitosan blends for DMFC applications. Renew. Energy 2016, 86, 922–929. [Google Scholar] [CrossRef]
- Alkabli, J. Progress in preparation of thiolated, crosslinked, and imino-chitosan derivatives targeting specific applications. Eur. Polym. J. 2022, 165, 110998. [Google Scholar] [CrossRef]
- Seedevi, P.; Moovendhan, M.; Vairamani, S.; Shanmugam, A. Evaluation of antioxidant activities and chemical analysis of sulfated chitosan from Sepia prashadi. Int. J. Biol. Macromol. 2017, 99, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Negm, N.A.; Hefni, H.; Abd-Elaal, A.A.; Badr, E.A.; Kana, M.T.A. Advancement on modification of chitosan biopolymer and its potential applications. Int. J. Biol. Macromol. 2020, 152, 681–702. [Google Scholar] [CrossRef] [PubMed]
- Bahramzadeh, E.; Yilmaz, E.; Adali, T. Chitosan-graft-poly(N-hydroxy ethyl acrylamide) copolymers: Synthesis, characterization and preliminary blood compatibility in vitro. Int. J. Biol. Macromol. 2018, 123, 1257–1266. [Google Scholar] [CrossRef]
- Dena-Aguilar, J.; Jaureguirincon, J.; Bonilla-Petriciolet, A.; Romero, J. Synthesis and characterization of aminated copolymers of polyacrylonitrile-graft-chitosan and their application for the removal of heavy metals from aqueous solution. J. Chil. Chem. Soc. 2015, 60, 2876–2880. [Google Scholar] [CrossRef]
- Wang, J.-P.; Chen, Y.-Z.; Wang, Y.; Yuan, S.-J.; Sheng, G.-P.; Yu, H.-Q. A novel efficient cationic flocculant prepared through grafting two monomers onto chitosan induced by Gamma radiation. RSC Adv. 2011, 2, 494–500. [Google Scholar] [CrossRef]
- Hassan, M.M. Enhanced antimicrobial activity and reduced water absorption of chitosan films graft copolymerized with poly(acryloyloxy)ethyltrimethylammonium chloride. Int. J. Biol. Macromol. 2018, 118, 1685–1695. [Google Scholar] [CrossRef]
- Khairkar, S.R.; Raut, A.R. Synthesis of chitosan-graft-polyaniline-based composites. Am. J. Mater. Sci. Eng. 2014, 2, 62–67. [Google Scholar] [CrossRef] [Green Version]
- Beer, B.; Bartolome, M.J.; Berndorfer, L.; Bochmann, G.; Guebitz, G.M.; Nyanhongo, G.S. Controlled enzymatic hydrolysis and synthesis of lignin cross-linked chitosan functional hydrogels. Int. J. Biol. Macromol. 2020, 161, 1440–1446. [Google Scholar] [CrossRef]
- Liu, Q.; Ji, N.; Xiong, L.; Sun, Q. Rapid gelling, self-healing, and fluorescence-responsive chitosan hydrogels formed by dynamic covalent crosslinking. Carbohydr. Polym. 2020, 246, 116586. [Google Scholar] [CrossRef]
- Huber, D.; Tegl, G.; Baumann, M.; Sommer, E.; Gorji, E.G.; Borth, N.; Schleining, G.; Nyanhongo, G.S.; Guebitz, G.M. Chitosan hydrogel formation using laccase activated phenolics as cross-linkers. Carbohydr. Polym. 2017, 157, 814–822. [Google Scholar] [CrossRef]
- Zhuang, S.; Yin, Y.; Wang, J. Removal of cobalt ions from aqueous solution using chitosan grafted with maleic acid by gamma radiation. Nucl. Eng. Technol. 2018, 50, 211–215. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, P.; Pandey, J. Binary grafted chitosan film: Synthesis, characterization, antibacterial activity and prospects for food packaging. Int. J. Biol. Macromol. 2018, 115, 341–348. [Google Scholar] [CrossRef]
- da Silva, S.B.; Krolicka, M.; van den Broek, L.A.M.; Frissen, A.E.; Boeriu, C.G. Water-soluble chitosan derivatives and pH-responsive hydrogels by selective C-6 oxidation mediated by TEMPO-laccase redox system. Carbohydr. Polym. 2018, 186, 299–309. [Google Scholar] [CrossRef]
- Huber, D.; Ortner, A.; Daxbacher, A.; Nyanhongo, G.S.; Bauer, W.; Guebitz, G.M. Influence of Oxygen and Mediators on Laccase-Catalyzed Polymerization of Lignosulfonate. ACS Sustain. Chem. Eng. 2016, 4, 5303–5310. [Google Scholar] [CrossRef]
- Kaneko, Y.; Matsuda, S.-I.; Kadokawa, J.-I. Chemoenzymatic Syntheses of Amylose-Grafted Chitin and Chitosan. Biomacromolecules 2007, 8, 3959–3964. [Google Scholar] [CrossRef]
| Field of Application | Applications | References |
|---|---|---|
| Biomedical and Pharmaceutical applications | Antioxidant: free radical scavenger/quencher Antimicrobial agent: positively charged chitosan-NH2 groups interact with negatively charged microbial cell membrane creating pores Drug delivery: mucoadhesive properties increase drug permeation of intestinal, nasal, and buccal epithelial cells, Gene therapy: Delivering various genes and siRNA Chitosan based drugs. For example, lowering effect of cholesterol for obesity treatment Regenerative technology/tissue engineering: bone, neural, cornea, cardiac and skin regenerative technology. Provides a three-dimensional tissue growth matrix, activate macrophage activity and stimulate cell proliferation Wound management: homeostatic agent, participate in repair, replacement, activation of humor immunity, complement system, and CD4+ cells, enhances granulation as well as the organization of the repaired tissues. It slowly degrades into N-acetyl-β-d-glucosamine that stimulates fibroblast proliferation, regular collagen deposition in addition to stimulating hyaluronic acid synthesis at the wound site. | [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33] |
| Health care products | Cosmetics formulations: Antimicrobial, antifungal, UV absorbing abilities exploited in various cosmetics formulations including in shampoos, rinses, colorants, hair lotions, spray, toothpaste formulations and tonics. Sunscreens, moisturizer foundation, eyeshadow, lipstick, cleansing materials, and bath agent, toothpaste, mouthwashes, and chewing gum as a dental filler. | [34,35,36,37] |
| Food Industry | Packaging, edible coatings, body filling, emulsifying agent, natural flavor extender, texture controlling, thickening and stabilizing agent, food preservation (antimicrobial agent), antioxidant agent. Flocculation/Clarification and deacilification of fruits and beverages | [38,39,40,41,42,43,44,45] |
| Agriculture | Antimicrobial activities against various plant pathogens. Fruit preservative. controlled delivery of fertilizers, pesticides, and insecticides. Increase in the auxin concentration and urea release in the soil, germination capacity, root length and activity, and seedling height | [46,47,48] |
| Industrial application | Functional materials: Graphitic carbon nanocapsules/composites, tungsten carbide chitin whiskers, etc. are used in the production of micro-electrochemical systems and 3D networks | [49,50,51] |
| Electrolyte: Sulfuric acid and chitosan combination has the ability to discharge high voltage Chitosan provides ionic conductivity and can be used in the production of solid-state batteries Photography: fixing agent for color prints | [52,53,54,55,56,57,58,59] | |
| Paper manufacture: Production of filter papers, water-resistant papers, biodegrading packages, water-resistant papers | [59,60,61,62,63] | |
| Enzyme carrier: immobilizing enzymes on solid materials | [64,65,66] | |
| Construction industry | wood adhesive, fungicide, wood quality enhancer, and preservative | [67,68,69] |
| Waste treatment | Flocculating, and negative charge (chelating agent), for dye, heavy metal ions removal and decontamination. Used for various processing plants such as whey, dairy, poultry, and seafood processing plants | [70,71,72,73,74,75] |
| Extraction Techniques | Process Conditions | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Chemical methods | Deproteinization conditions: NaOH, KOH, Na2SO3, Na2CO3 Temp: 25–100 °C, 30 min–72 h Demineralization: HCL, HNO3, CH3COOH, HCOOH Temp: 25–100 °C, 30 min–48 h Decolorization: organic solvents such as acetone, ethyl alcohol, diethyl ether Bleaching: KMnO4, NaCIO/H2O2; Temp: 20–60 °C, 25 min–12 h Recovery: precipitation with 5–10%NaOH Deacetylation: NaOH/KOH 30–50% w/v, Temp: 80–150 °C, Time 1–8 h | Short processing time Produces chitin with high DA% Accompanied by deacetylation Process used at industrial scale | Multistep process Deacetylation unavoidable Environmentally unfriendly generate large quantities of waste that cannot be used as human and animal nutrients. Calcium carbonate lost to waste stream | [88,104,105] |
| Biological and enzyme based methods | Demineralization: fermentation using lactic acid producing bacteria or lactic acid Deproteinization using enzymes (cellulases, pectinases, chitinases, lipases, papain, hemicellulases, pepsin and lysozyme produces chitooligosaccharides, lysozyme Protease deproteinization and demineralization: in (10% HCl solution at 20 °C for 30 min) at 55 °C and pH of 8.5 Combined deproteinization and demineralization: microorganisms producing proteases or proteases Protease demineralization at 25 °C for 20 min in the presence of lactic acid ratio of 1:1.1 w/w and acetic acid ratio of 1:1.2 w/w) Deproteinized with chitinase at 45 °C and a pH of 6.0 with shaking at 150 rpm Alcalase, esperase and neutrase in deproteinization, followed by deacetylation by alkaline treatment, reached the highest degrees of deacetylation with 61.0–63.7% NaOH for 14.9–16.4 h Combination of species, including Serratia marcescens and L. plantarum, increased deproteinization and demineralization activity Decoloration: acetone or organic solvent, Deacetylation: chitin deacetylase producing by bacteria Lactic acid ratio of 1:1.1 w/w and shells: acetic acid ratio of 1:1.2 w/w) had a maximum demineralization | High quality of final product Sustainable process Environmentally safe; specific, fast in action, reduces the use of energy, chemicals and/or water compared to conventional processes Regular deacetylation and MW | Long processing time (days) Process still under development enzymatic method had a higher degree of acetylation (19.4%) and viscosity than that prepared by chemical method (17.2%). | [106,107,108,109,110,111,112,113,114,115,116,117,118,119,120] |
| Ionic liquids | Complete dissolution followed by the selective precipitation of chitin. Treatment with [C2C1im] [CH3COO] [121]. causes swelling swell Ionic liquids 1-ethyl-3-methylimidazolium acetate [C2mim] [OAc], 1-butyl-3-methylimidazolium chloride [C4mim]Cl, [C2mim]Cl, [C2mim] [OAc], and 1-allyl-3-methylimidazolium acetate [Amim] [OAc], are effective against chitin from shrimp shells, crab shell waste, and squid pens. Combination of steam explosion and ionic liquid pretreatments for efficient utilization of fungal chitin | Scaling-up the process were successful leading to the establishment of a company 525 Solutions at industrial scale [122]. Dissolution and coagulation of the polymer combined with enzymatic hydrolysis, reduces its crystallinity, making the polymer more accessible to the enzyme | Harsh totally dissolves chitin Toxicity and nonbiodegradability DESs are the ability to perform a three-step process in single step, including demineralization, deproteinization and chitin dissolution | [121,122,123,124,125,126,127,128] |
| Deep eutectic solvents | Demineralization, deproteinization and chitin dissolution perform a three-step process in single step Mixture of hydrogen bond acceptor (HBA) and a hydrogen bond donor (HBD), choline chloride (ChCl) is commonly used as an HBA, while HBDs include lactic acid, malonic acid, and citric acid 150 °C Incubating different ratio mixtures of DESs (ChCl/citric acid, ChCl/L-lactic acid, and ChCl/malic acid) with chitin sources at temperatures between 50–150 °C for 2–6 h DES plus Microwave: DES ratios of 1:5, 1:10, and 1:20. Next, the mixture was heated under 700 W microwave irradiation (Haier MZC-2070M1) for different durations of time (1, 3, 7, and 9 min) Demineralization was carried out by the malic acids. When choline chloride–malic acid was applied to the shrimp shells, minerals, which are mostly in the form of crystalline CaCO3, were removed by the malic acid, leaving the proteins and chitin. The spacing between the chitin–protein fibers was filled with proteins and minerals; thus, the removal of minerals resulted in a weakening of the linkages within the inner structural organization of the shrimp shells. Since the minerals are removed by the malic acids, in order to conduct demineralization, one component of the DESs used in the chitin extraction should be an acid. | Single step for simultaneous removal of protein and minerals Demineralization, deproteinization and chitin dissolution perform a three-step process in single step Low melting temperature, non-flammability, highly chemical and thermal stability and superior biodegradability. No deacetylation Solvent recycling possible | High solvent viscosity causes difficulty at large scale DESs are a new class of ionic liquid analogues derived from inexpensive commercially available raw materials with a melting point lower than that of each individual component. DESs are biodegradable, cheap and easy to produce | [129,130,131,132,133,134] |
| Ultrasound extraction | Ultrasound’s cavitation effect solubilizes protein associated with chitin, dissociates covalent bonds in polymer chains and disperses aggregates Uses high-intensity Ultrasound signals at 750 W power and 20 kHz ± 50 Hz operating frequency to enhance the efficiency of extraction of chitin, | Reduces the extraction time and avoids the requirement of high temperatures. | [135,136,137] | |
| Microwave-assisted extraction | Microwave heating involves two main mechanisms: (i) dipolar polarization and (ii) ionic conduction Increasing the microwave irradiation to 130 watts of power for 15 min resulted in high deproteinization (11.46%) and a low ash content (5.4%) at 700 °C for 2 h using 50% of NaOH solution in a power range of 500–650 W resulted in a low DDA, and the deacetylation reaction was more than 80% completed after 10 min. MAE allowed the production of chitosan with medium and high MW (300–360 kDa). | Fast deacetylation of chitosan in 24 min, compared to conventional heating method that requires 6–7 h Upscaling possibility | [138,139,140,141,142] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellis, A.; Guebitz, G.M.; Nyanhongo, G.S. Chitosan: Sources, Processing and Modification Techniques. Gels 2022, 8, 393. https://doi.org/10.3390/gels8070393
Pellis A, Guebitz GM, Nyanhongo GS. Chitosan: Sources, Processing and Modification Techniques. Gels. 2022; 8(7):393. https://doi.org/10.3390/gels8070393
Chicago/Turabian StylePellis, Alessandro, Georg M. Guebitz, and Gibson Stephen Nyanhongo. 2022. "Chitosan: Sources, Processing and Modification Techniques" Gels 8, no. 7: 393. https://doi.org/10.3390/gels8070393
APA StylePellis, A., Guebitz, G. M., & Nyanhongo, G. S. (2022). Chitosan: Sources, Processing and Modification Techniques. Gels, 8(7), 393. https://doi.org/10.3390/gels8070393