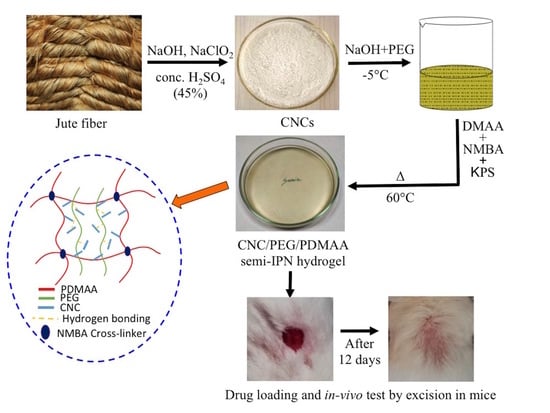
Advanced CNC/PEG/PDMAA Semi-IPN Hydrogel for Drug Delivery Management in Wound Healing
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Cellulose Nanocrystal (CNC) from Jute Fiber
2.3. Dissolution of CNC in PEG/NaOH Solvent System
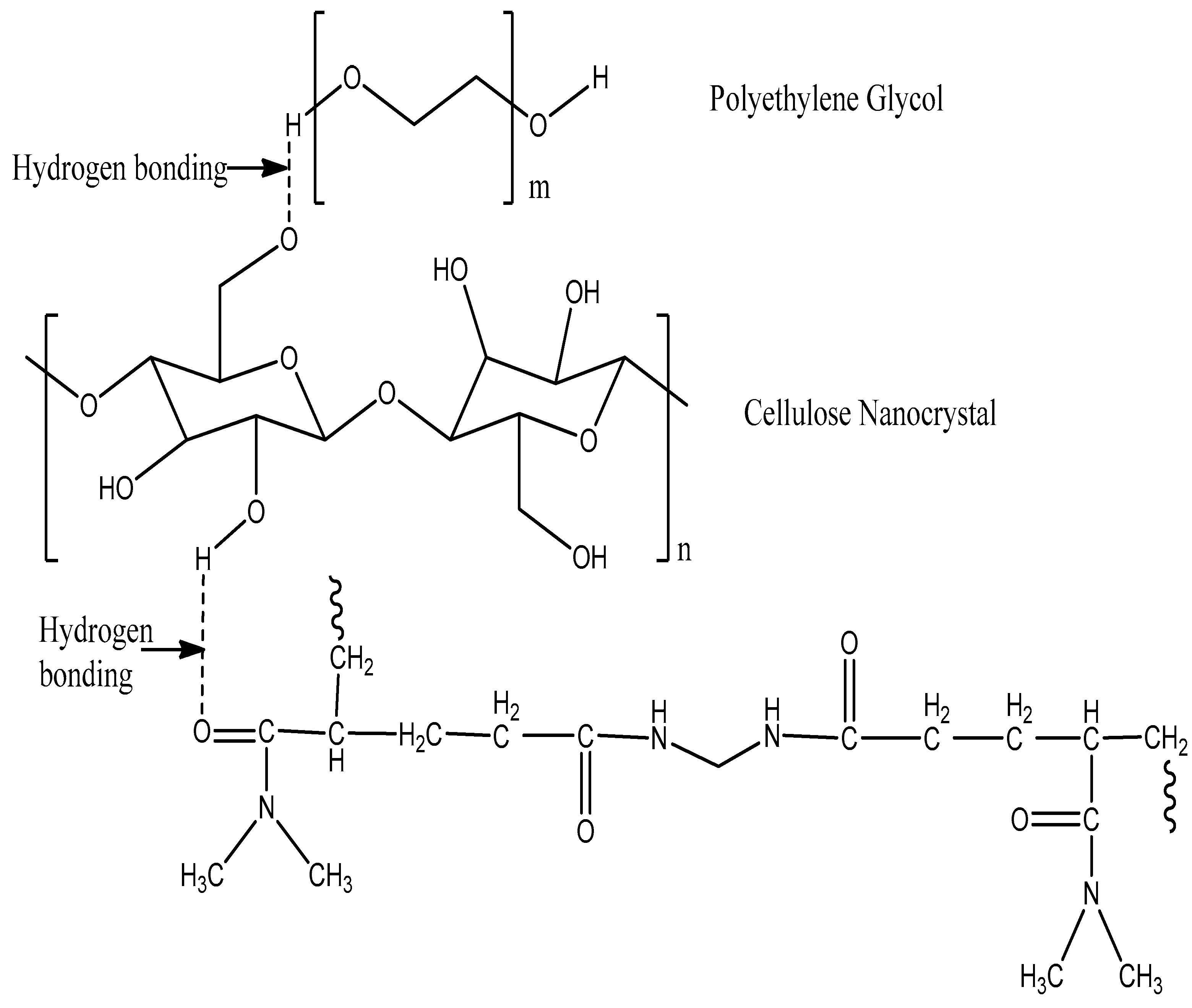
2.4. Synthesis of CNC/PEG/PDMAA Semi-IPN Hydrogel
2.5. Characterization Techniques
2.6. Swelling Test of CNC/PEG/PDMAA Semi-IPN Hydrogel
2.7. Gentamicin–Ninhydrin Assay
2.8. Loading of Gentamicin Sulphate on CNC/PEG/PDMAA Semi-IPN Hydrogel Film
2.9. In Vitro Cytotoxicity and Biocompatibility Study by Cell Culture
2.10. Antimicrobial Activity and In Vitro Release Profile
2.11. In Vivo Wound Healing Evaluation
3. Results and Discussion
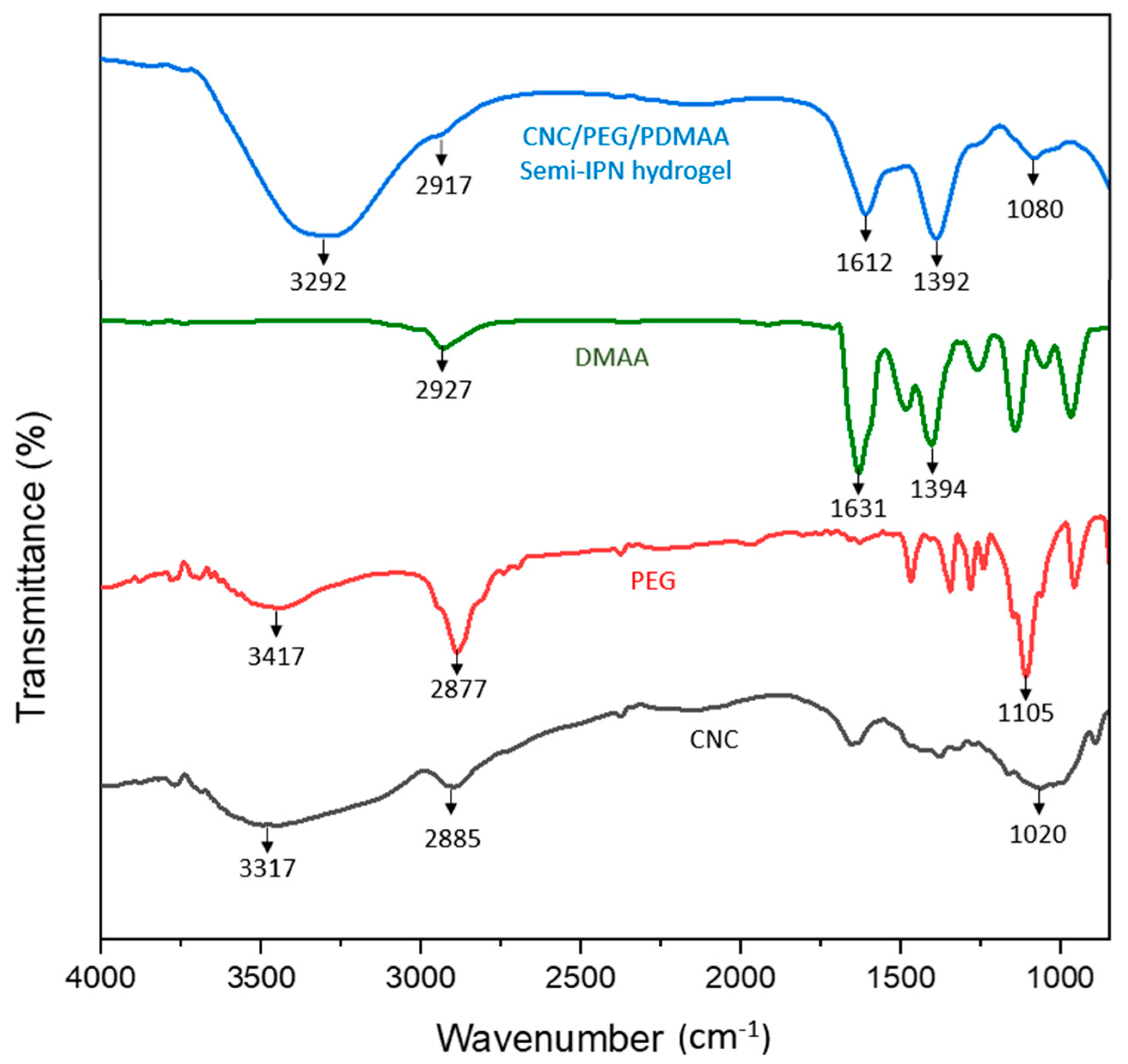
3.1. ATR Spectra Analysis
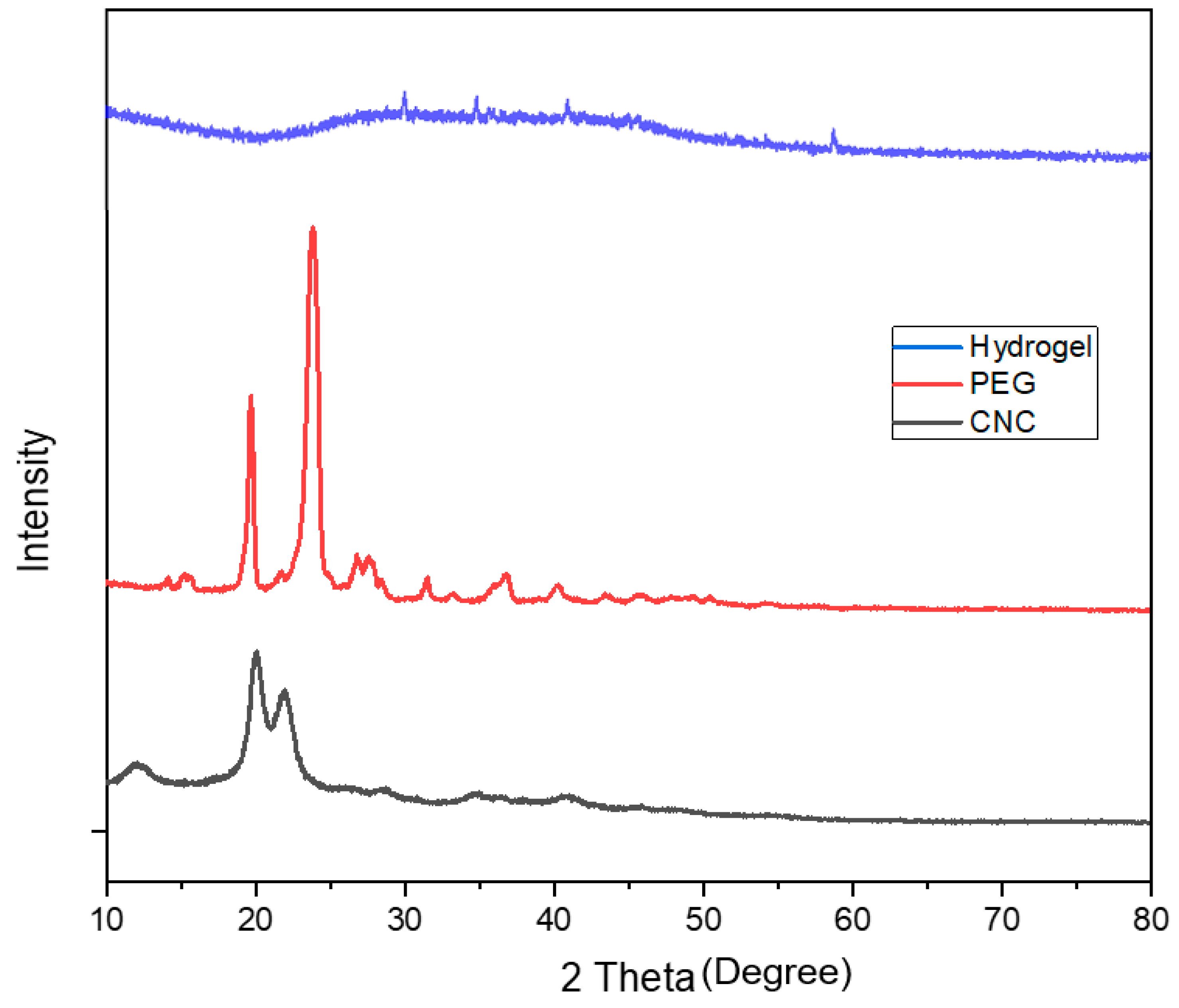
3.2. X-ray Diffraction Pattern Analysis
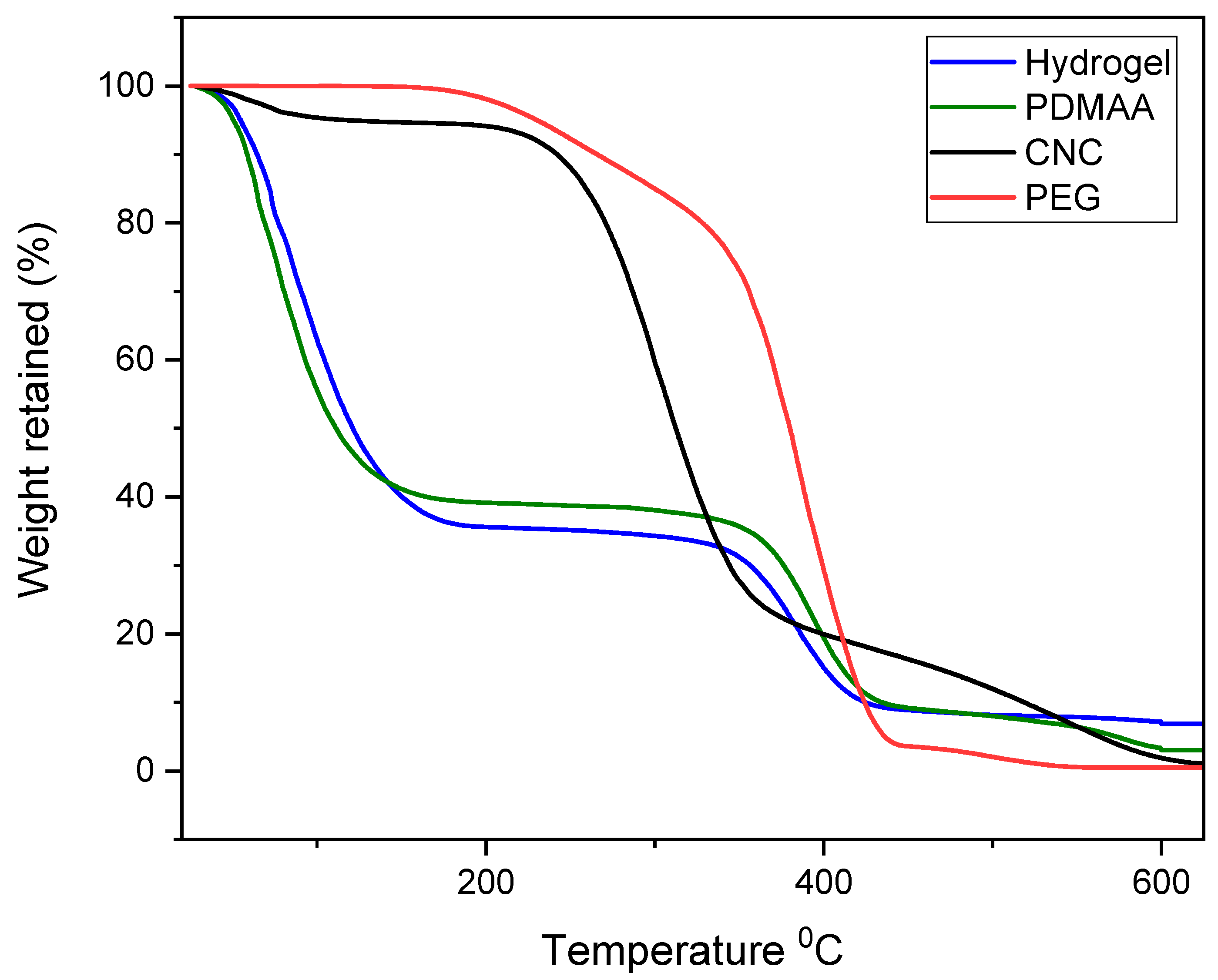
3.3. Thermogravimetric Analysis
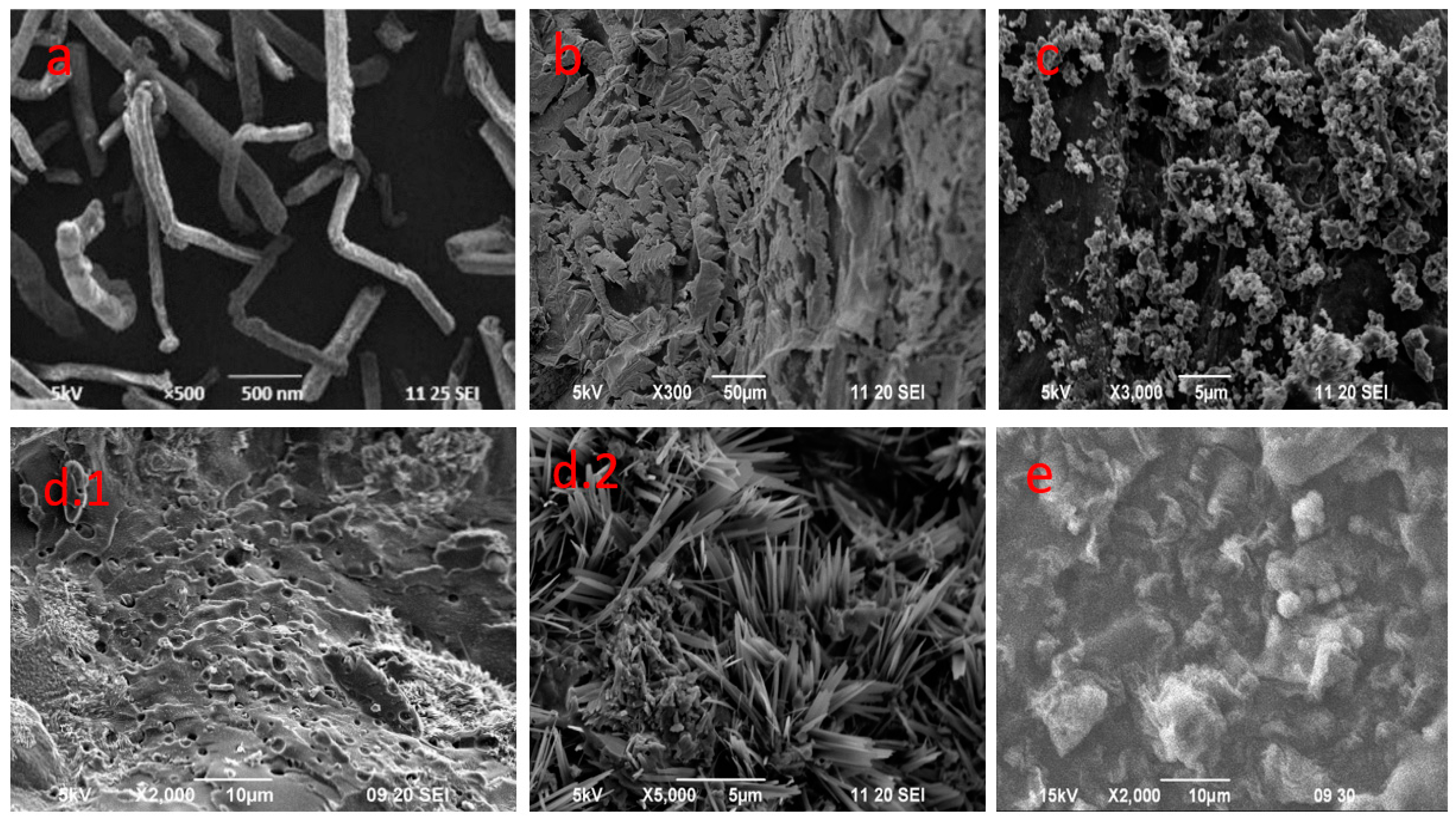
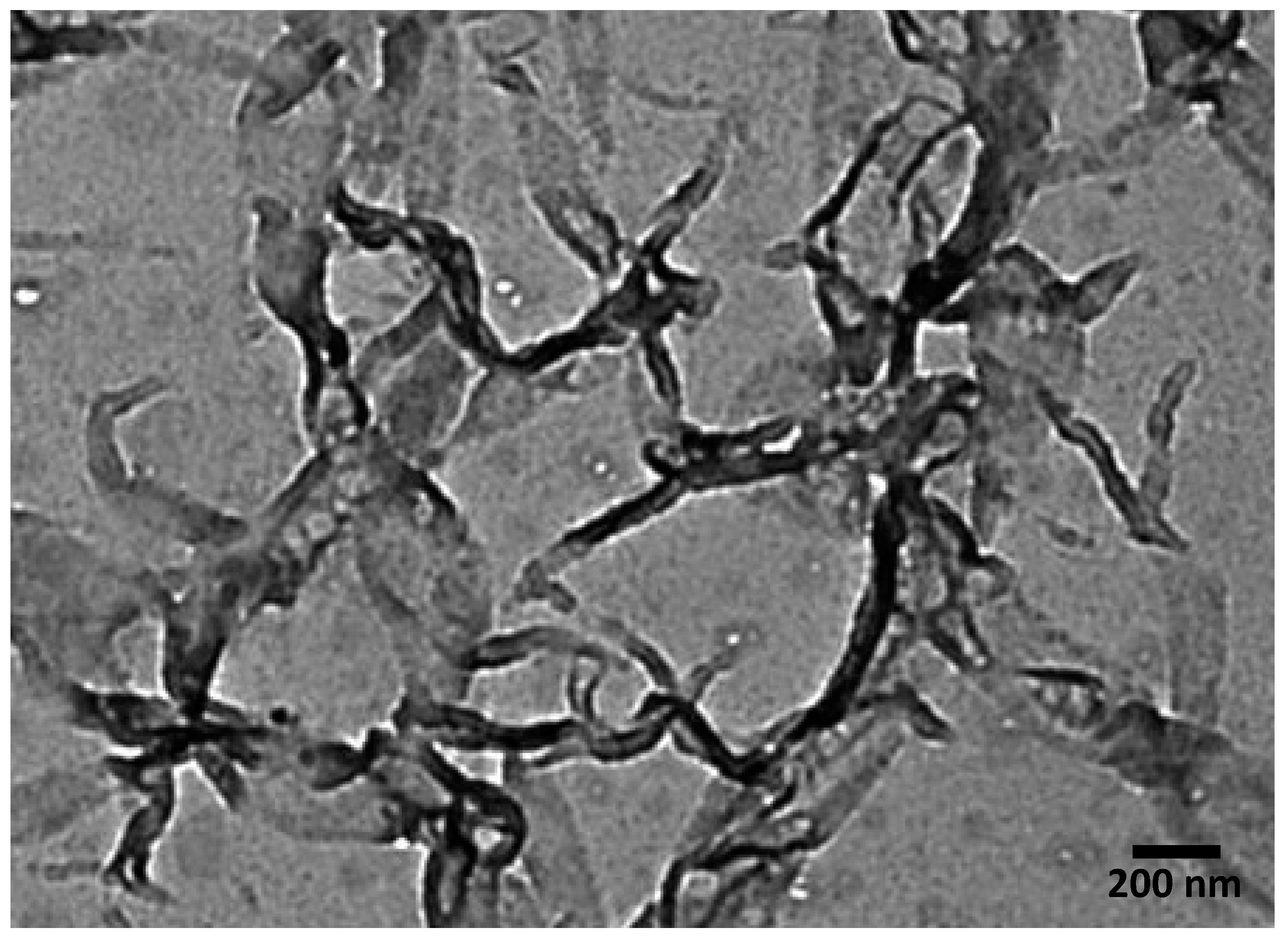
3.4. Morphology and Particle Size Analysis
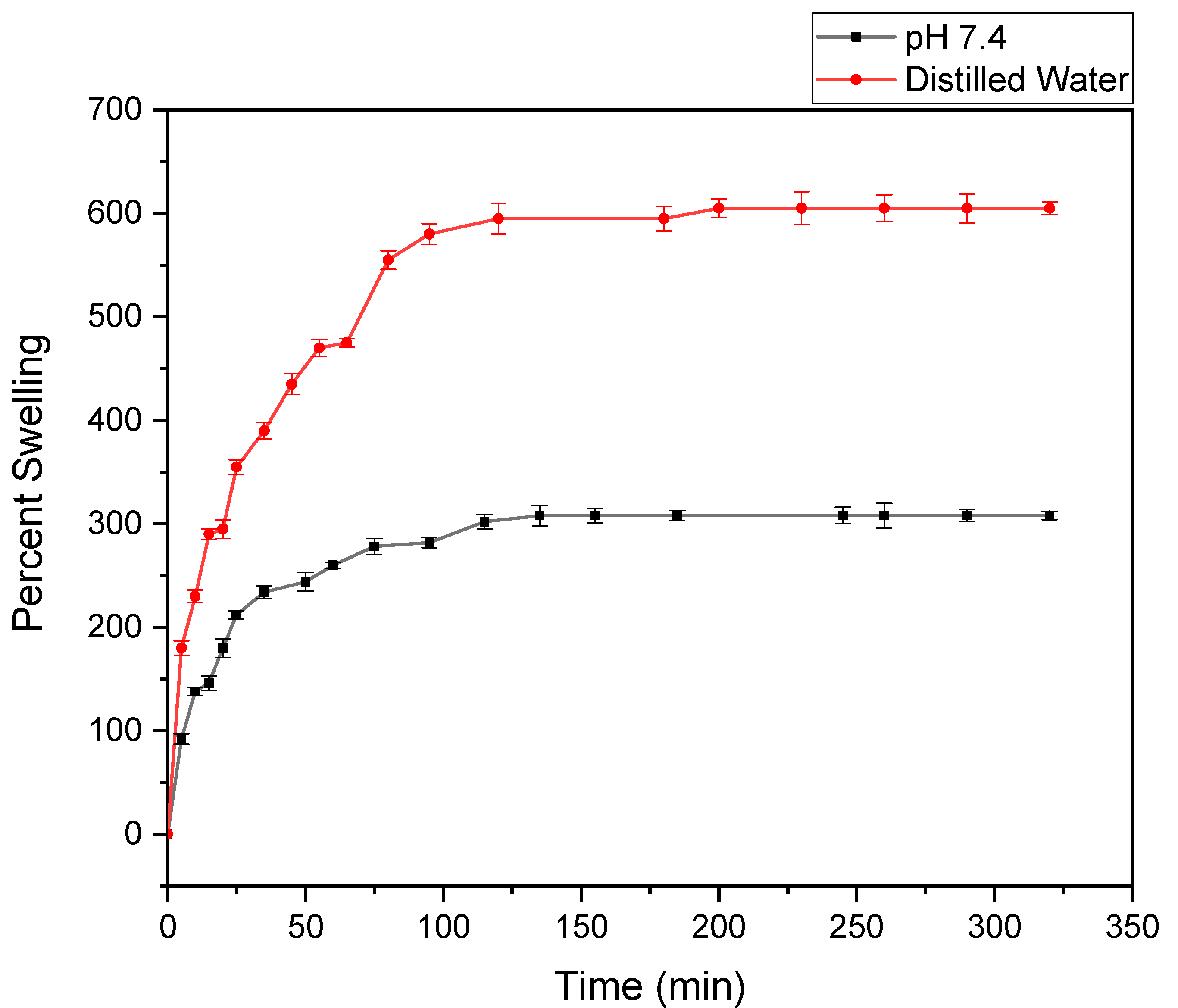
3.5. Swelling Study
3.6. Cytotoxic Effect Analysis
3.7. Gentamicin Drug Loading
3.7.1. Standard Curve of Gentamicin Sulphate
3.7.2. Drug Loading Performance and Optimum Drug Loading Efficiency
3.8. Antimicrobial Activity Study
3.9. In Vitro Release Profile
3.10. In Vivo Wound Healing in Mice Model
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, J.; Han, C.R.; Duan, J.F.; Ma, M.G.; Zhang, X.M.; Xu, F.; Sun, R.C. Synthesis and characterization of mechanically flexible and tough cellulose nanocrystals–polyacrylamide nanocomposite hydrogels. Cellulose 2013, 20, 227–237. [Google Scholar] [CrossRef]
- Mardali, M.; Sarraf-Mamoory, R.; Sadeghi, B.; Safarbali, B. Acrylamide route for the co-synthesis of tungsten carbide–cobalt nanopowders with additives. Ceram. Int. 2016, 42, 9382–9386. [Google Scholar] [CrossRef]
- Saraydın, D.; Karadag, E.; Işıkver, Y.; Şahiner, N.; Güven, O. The influence of preparation methods on the swelling and network properties of acrylamide hydrogels with crosslinkers. J. Macromol. Sci. Part A 2004, 41, 419–431. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, S.S.; Lee, Y.M. Interpenetrating polymer network hydrogels based on poly (ethylene glycol) macromer and chitosan. Carbohydr. Polym. 2000, 41, 197–205. [Google Scholar] [CrossRef]
- Thakur, A.; Wanchoo, R.K.; Singh, P. Hydrogels of poly (acrylamide-co-acrylic acid): In-vitro study on release of gentamicin sulfate. Chem. Biochem. Eng. Q. 2011, 25, 471–482. [Google Scholar]
- Zarida, C.N.; Fauziah, O.; Arifah, A.K.; Nazri, M.Y.; Rusnah, M.; GK, M.A.K. In vitro elution and dissolution of tobramycin and gentamicin from calcium phosphate. Afr. J. Pharm. Pharmacol. 2011, 5, 2283–2291. [Google Scholar]
- Murray, P.R. Mandell, Douglas and Bennett’s Principles and Practice of Infectious Diseases. Clin. Microbiol. Rev. 2015, 1, 191–223. [Google Scholar]
- Sarwar, M.S.; Huang, Q.; Ghaffar, A.; Abid, M.A.; Zafar, M.S.; Khurshid, Z.; Latif, M. A smart drug delivery system based on biodegradable chitosan/poly (allylamine hydrochloride) blend films. Pharmaceutics 2020, 12, 131. [Google Scholar] [CrossRef] [Green Version]
- Balakrishnan, B.; Mohanty, M.; Umashankar, P.R.; Jayakrishnan, A. Evaluation of an in situ forming hydrogel wound dressing based on oxidized alginate and gelatin. Biomaterials 2005, 26, 6335–6342. [Google Scholar] [CrossRef]
- Hwang, M.R.; Kim, J.O.; Lee, J.H.; Kim, Y.I.; Kim, J.H.; Chang, S.W.; Jin, S.G.; Kim, J.; Lyoo, W.S.; Han, S.S.; et al. Gentamicin-loaded wound dressing with polyvinyl alcohol/dextran hydrogel: Gel characterization and in vivo healing evaluation. AAPS Pharm. Sci. Tech. 2010, 11, 1092–1103. [Google Scholar] [CrossRef] [Green Version]
- Baek, S.; Kim, D.; Jeon, S.L.; Seo, J. Preparation and characterization of pH-responsive poly (N, N-dimethyl acrylamide-co-methacryloyl sulfadimethoxine) hydrogels for application as food freshness indicators. React. Funct. Polym. 2017, 120, 57–65. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Ahmad, I.; Abdullah, I.; Dufresne, A.; Zainudin, S.Y.; Sheltami, R.M. Effects of hydrolysis conditions on the morphology, crystallinity, and thermal stability of cellulose nanocrystals extracted from kenaf bast fibers. Cellulose 2012, 19, 855–866. [Google Scholar] [CrossRef]
- Pourshahrestani, S.; Zeimaran, E.; Kadri, N.A.; Mutlu, N.; Boccaccini, A.R. Polymeric hydrogel systems as emerging biomaterial platforms to enable hemostasis and wound healing. Adv. Healthc. Mater. 2020, 9, 2000905. [Google Scholar] [CrossRef] [PubMed]
- Sahiner, M.; Sagbas, S.; Bitlisli, B.O. p (AAm/TA)-based IPN hydrogel films with antimicrobial and antioxidant properties for biomedical applications. J. Appl. Polym. Sci. 2015, 132, 41876. [Google Scholar] [CrossRef]
- Cidreira, A.C.M.; de Castro, K.C.; Hatami, T.; Linan, L.Z.; Mei, L.H.I. Cellulose nanocrystals-based materials as hemostatic agents for wound dressings: A review. Biomed. Microdevices 2021, 23, 1–23. [Google Scholar] [CrossRef]
- Hossain, S.; Shahruzzaman, M.; Kabir, S.F.; Rahman, M.S.; Sultana, S.; Mallik, A.K.; Haque, P.; Takafuji, M.; Rahman, M.M. Jute cellulose nanocrystal/poly (N, N-dimethylacrylamide-co-3-methacryloxypropyltrimethoxysilane) hybrid hydrogels for removing methylene blue dye from aqueous solution. J. Sci. Adv. Mater. Devices 2021, 6, 254–263. [Google Scholar] [CrossRef]
- Zou, J.; Bao, D.; Ma, R.; Zhu, Z.; Chen, X.; Zhu, J.; Fan, X.; Zhang, K.; Zheng, H.; Li, F.; et al. Green and sustainable self-assembly nanocomposite from gentamicin sulfate/lignosulfonate with efficient antibacterial and wound-healing activity. ACS Sustain. Chem. Eng. 2020, 8, 4931–4940. [Google Scholar] [CrossRef]
- Alemdar, A.; Sain, M. Isolation and characterization of nanofibers from agricultural residues–Wheat straw and soy hulls. Bioresour. Technol. 2008, 99, 1664–1671. [Google Scholar] [CrossRef]
- Rosli, N.A.; Ahmad, I.; Abdullah, I. Isolation and characterization of cellulose nanocrystals from Agave angustifolia fibre. BioResources 2013, 8, 1893–1908. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, R.; Ray, S.K. Removal of congo red and methyl violet from water using nano clay filled composite hydrogels of poly acrylic acid and polyethylene glycol. Chem. Eng. J. 2015, 260, 269–283. [Google Scholar] [CrossRef]
- Liu, M.; Wang, L.; Su, H.; Cao, H.; Tan, T. pH-sensitive IPN hydrogel based on poly (aspartic acid) and poly (vinyl alcohol) for controlled release. Polym. Bull. 2013, 70, 2815–2827. [Google Scholar] [CrossRef]
- Zabaniotou, A.; Ioannidou, O.; Antonakou, E.; Lappas, A. Experimental study of pyrolysis for potential energy, hydrogen and carbon material production from lignocellulosic biomass. Int. J. Hydrog. Energy 2008, 33, 2433–2444. [Google Scholar] [CrossRef]
- Ali, A.E.H.; Shawky, H.A.; Abd El Rehim, H.A.; Hegazy, E.A. Synthesis and characterization of PVP/AAc copolymer hydrogel and its applications in the removal of heavy metals from aqueous solution. Eur. Polym. J. 2003, 39, 2337–2344. [Google Scholar]
- Hiremath, J.N.; Vishalakshi, B. Effect of Crosslinking on swelling behaviour of IPN hydrogels of Guar Gum & Polyacrylamide. Der. Pharma. Chem. 2012, 4, 946–955. [Google Scholar]
- Ibrahim, A.G.; Hai, F.A.; Wahab, H.A.; Mahmoud, H. Synthesis, characterization, swelling studies and dye removal of chemically crosslinked acrylic acid/acrylamide/N, N-dimethyl acrylamide hydrogels. Am. J. Appl. Chem. 2016, 4, 221–234. [Google Scholar]
- Hasan, M.M.; Khan, M.N.; Haque, P.; Rahman, M.M. Novel alginate-di-aldehyde cross-linked gelatin/nano-hydroxyapatite bioscaffolds for soft tissue regeneration. Int. J. Biol. Macromol. 2018, 117, 1110–1117. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Haque, P.; Rashid, T.U.; Khan, M.N.; Mallik, A.K.; Khan, M.N.I.; Khan, M.; Rahman, M.M. Core–shell drug carrier from folate conjugated chitosan obtained from prawn shell for targeted doxorubicin delivery. J. Mater. Sci. Mater. Med. 2017, 28, 1–10. [Google Scholar] [CrossRef]
- Schneider, L.A.; Korber, A.; Grabbe, S.; Dissemond, J. Influence of pH on wound-healing: A new perspective for wound-therapy? Arch. Dermatol. 2007, 298, 413–420. [Google Scholar] [CrossRef]
- Dwivedi, C.; Pandey, H.; Pandey, A.C.; Patil, S.; Ramteke, P.W.; Laux, P.; Luch, A.; Singh, A.V. In vivo biocompatibility of electrospun biodegradable dual carrier (antibiotic+ growth factor) in a mouse model—Implications for rapid wound healing. Pharmaceutics 2019, 11, 180. [Google Scholar] [CrossRef] [Green Version]


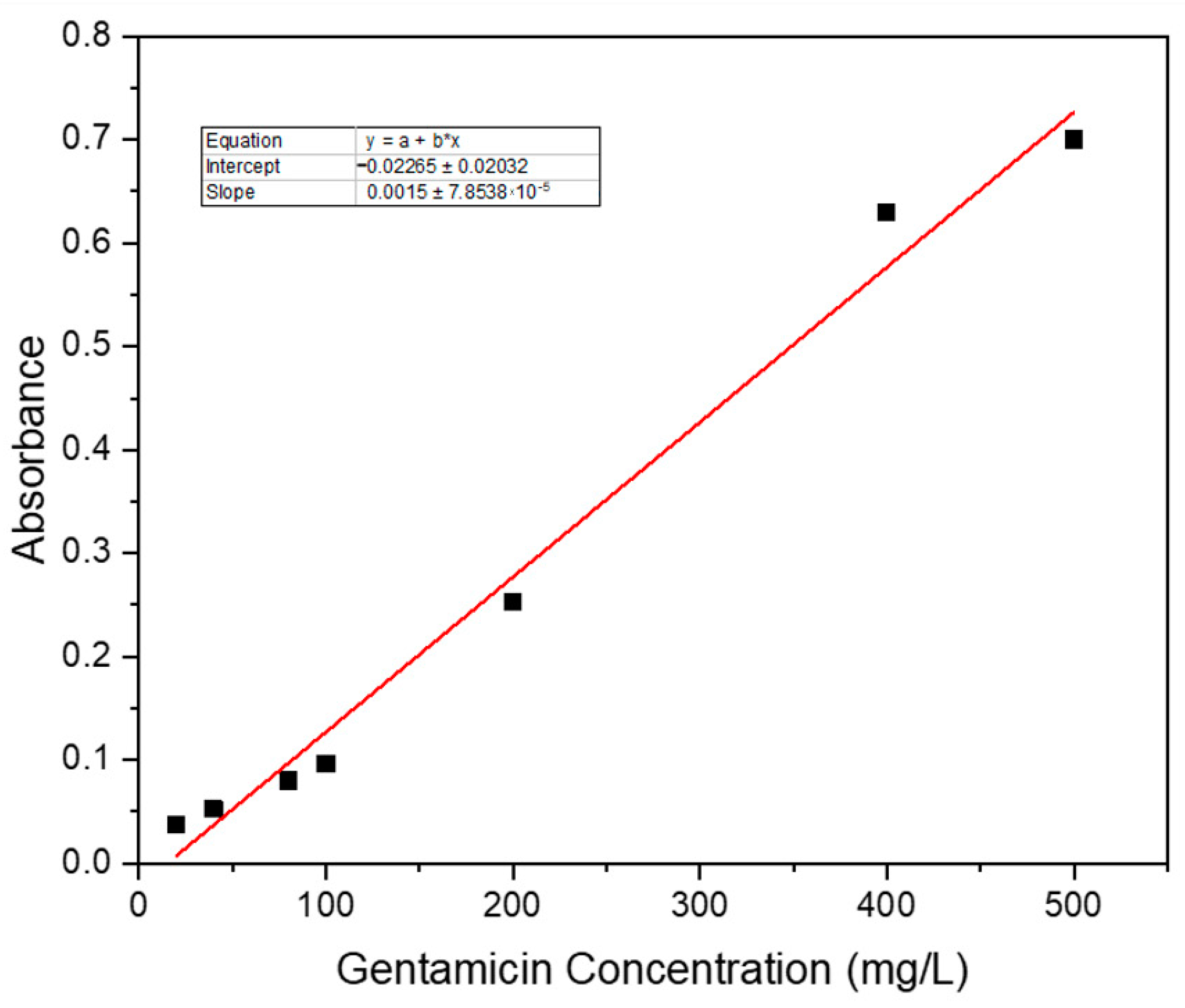
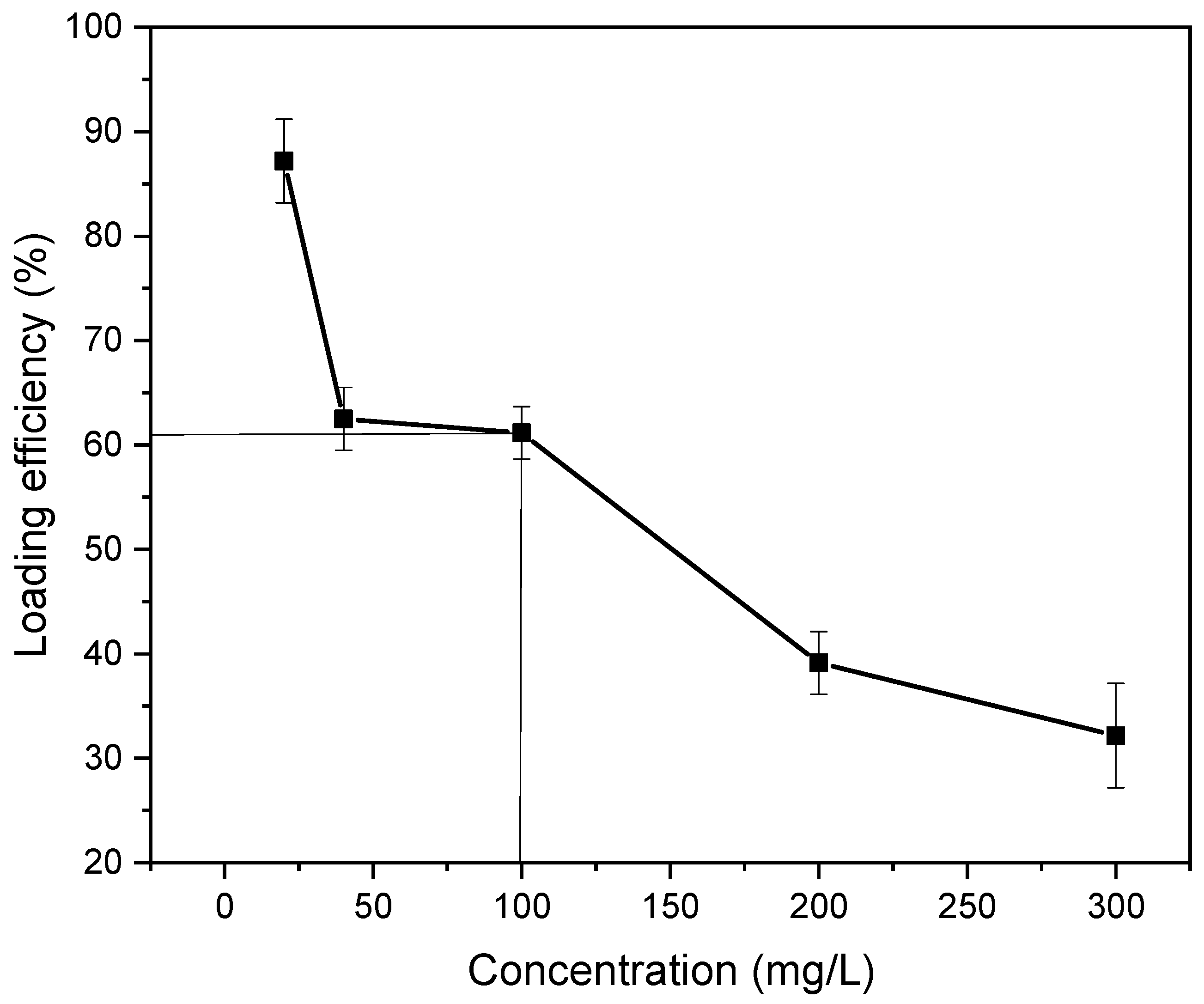


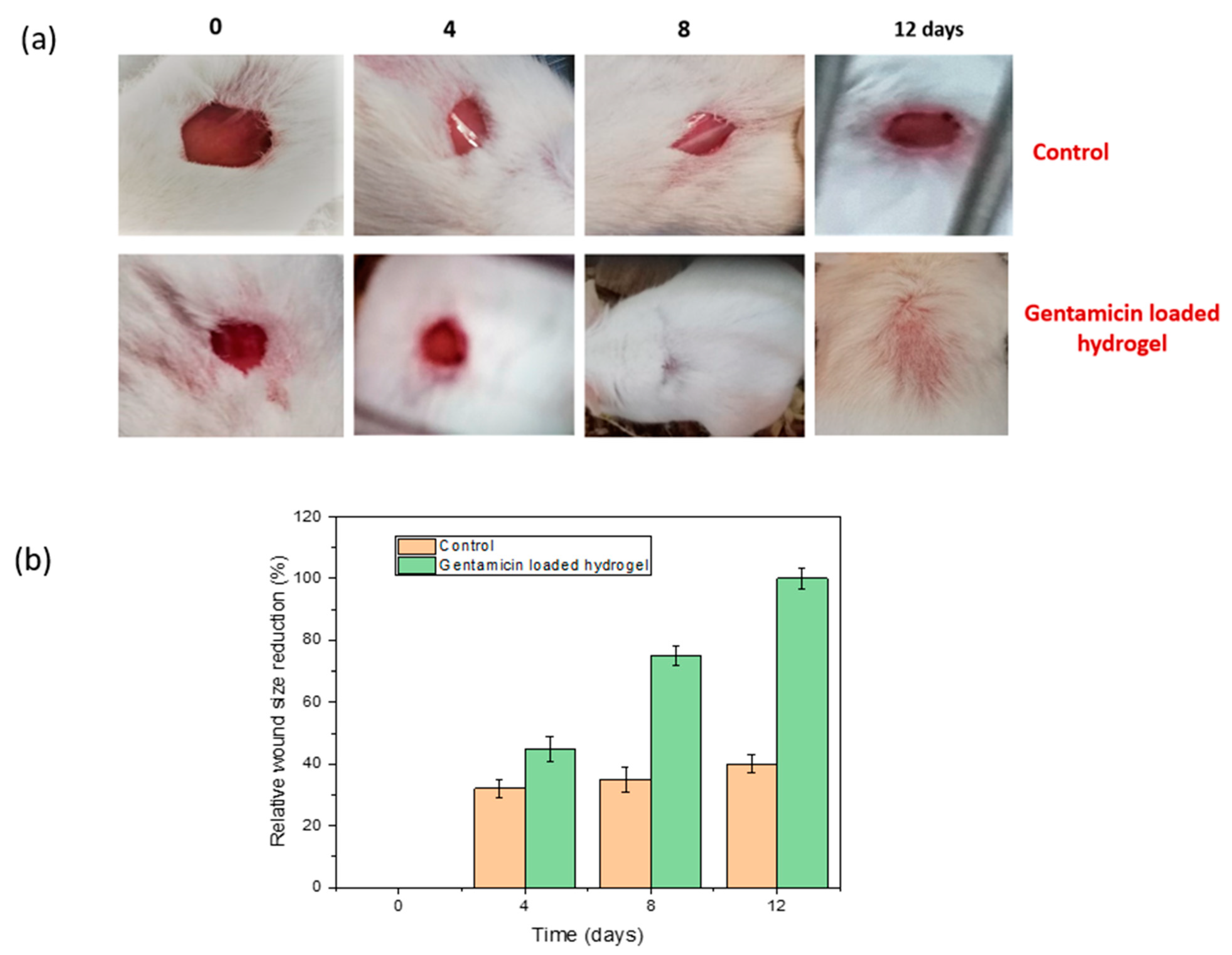
| Sample ID | Survival of BHK-21 Cell | Survival of Vero Cell | Remarks |
|---|---|---|---|
| Control (−) | 100% | 100% | No cytotoxicity |
| Control (+) | >95% | >95% | No cytotoxicity |
| Hydrogel sample | >95% | >95% | No cytotoxicity |
| Gentamicin Sulphate Concentration (mg/L) | Loaded Amount of Gentamicin Sulphate in 24 h (mg) | Loading Efficiency (%) | Standard Deviation(± %) |
|---|---|---|---|
| 20 | 17.43 | 87.17 | 3.26 |
| 40 | 25 | 62.5 | 2.44 |
| 100 | 61.16 | 61.16 | 2.04 |
| 200 | 80.3 | 40.15 | 2.67 |
| 300 | 96.57 | 32.19 | 4.08 |
| Sample | Bacteria Specific Diameter (mm) of Inhibition Zone | |
|---|---|---|
| Staphylococcus aureus | Escherichia coli | |
| Drug loaded hydrogel film | 25 | 23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afrin, S.; Shahruzzaman, M.; Haque, P.; Islam, M.S.; Hossain, S.; Rashid, T.U.; Ahmed, T.; Takafuji, M.; Rahman, M.M. Advanced CNC/PEG/PDMAA Semi-IPN Hydrogel for Drug Delivery Management in Wound Healing. Gels 2022, 8, 340. https://doi.org/10.3390/gels8060340
Afrin S, Shahruzzaman M, Haque P, Islam MS, Hossain S, Rashid TU, Ahmed T, Takafuji M, Rahman MM. Advanced CNC/PEG/PDMAA Semi-IPN Hydrogel for Drug Delivery Management in Wound Healing. Gels. 2022; 8(6):340. https://doi.org/10.3390/gels8060340
Chicago/Turabian StyleAfrin, Samia, Md. Shahruzzaman, Papia Haque, Md. Sazedul Islam, Shafiul Hossain, Taslim Ur Rashid, Tanvir Ahmed, Makoto Takafuji, and Mohammed Mizanur Rahman. 2022. "Advanced CNC/PEG/PDMAA Semi-IPN Hydrogel for Drug Delivery Management in Wound Healing" Gels 8, no. 6: 340. https://doi.org/10.3390/gels8060340
APA StyleAfrin, S., Shahruzzaman, M., Haque, P., Islam, M. S., Hossain, S., Rashid, T. U., Ahmed, T., Takafuji, M., & Rahman, M. M. (2022). Advanced CNC/PEG/PDMAA Semi-IPN Hydrogel for Drug Delivery Management in Wound Healing. Gels, 8(6), 340. https://doi.org/10.3390/gels8060340