In Vitro and Biological Characterization of Dexamethasone Sodium Phosphate Laden pH-Sensitive and Mucoadhesive Hydroxy Propyl β-Cyclodextrin-g-poly(acrylic acid)/Gelatin Semi-Interpenetrating Networks
Abstract
:1. Introduction
2. Methodology
2.1. Chemicals
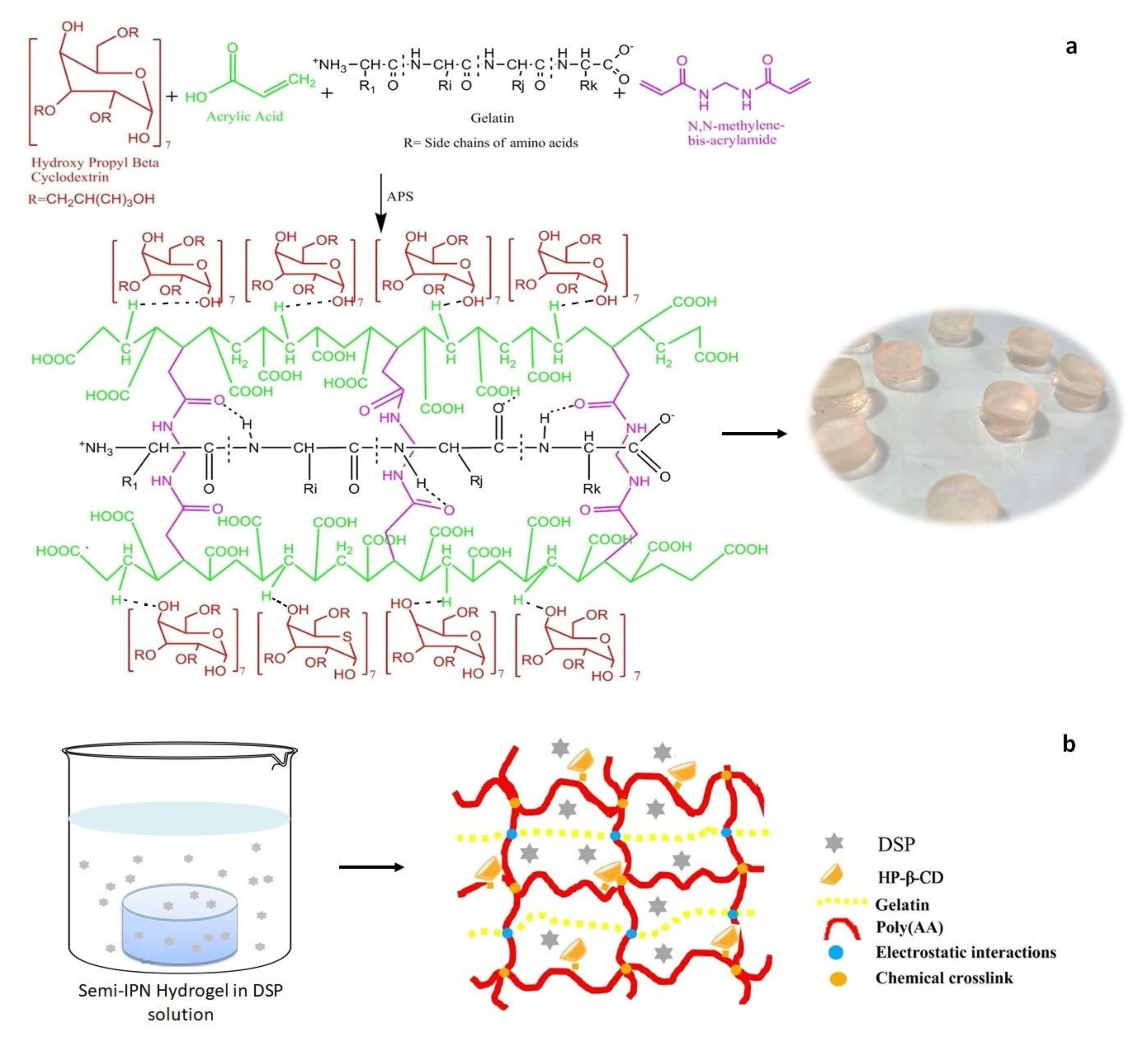
2.2. Synthesis of HP-β-CD-g-poly(AA)/Gelatin Semi-IPN Hydrogels
2.3. Swelling Study
2.4. Mucoadhesive Strength
2.5. Sol–Gel Analysis
2.6. Solid-State Characterization
2.7. Drug Loading
2.8. In Vitro Drug Release
2.9. Hemocompatibility Testing
2.10. Toxicity Testing
2.11. Statistical Analysis
3. Results and Discussion
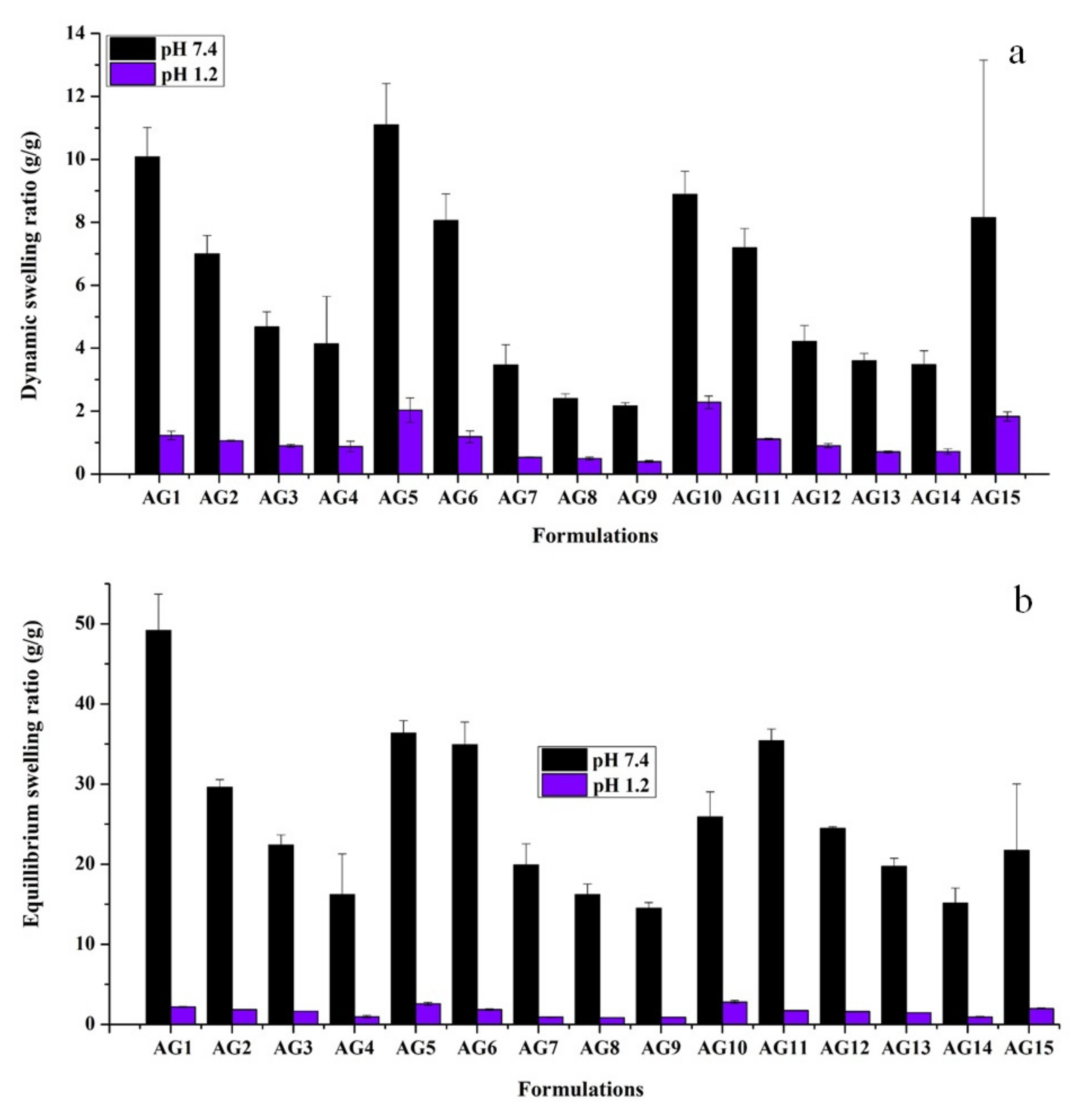
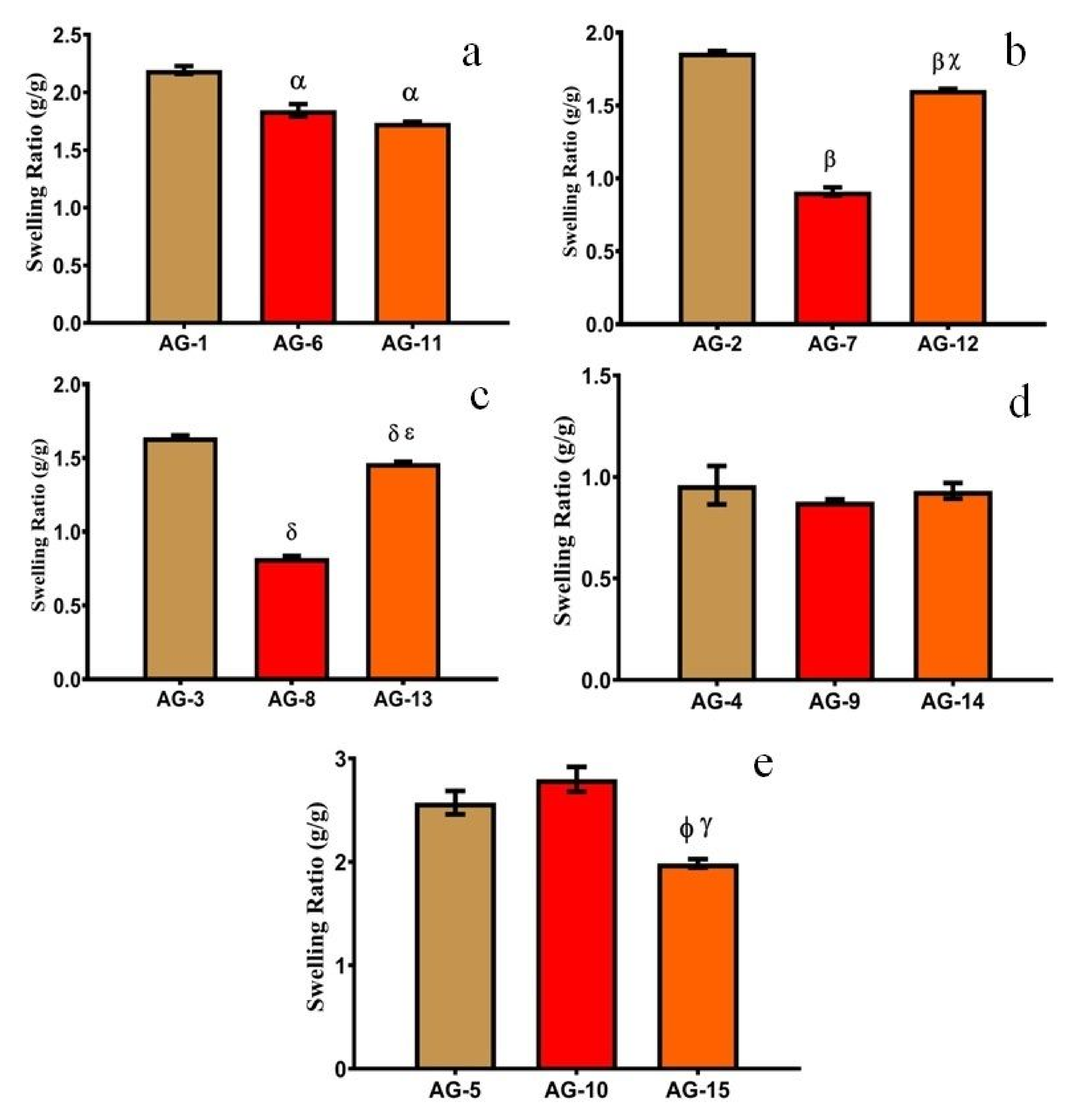
3.1. Swelling Studies
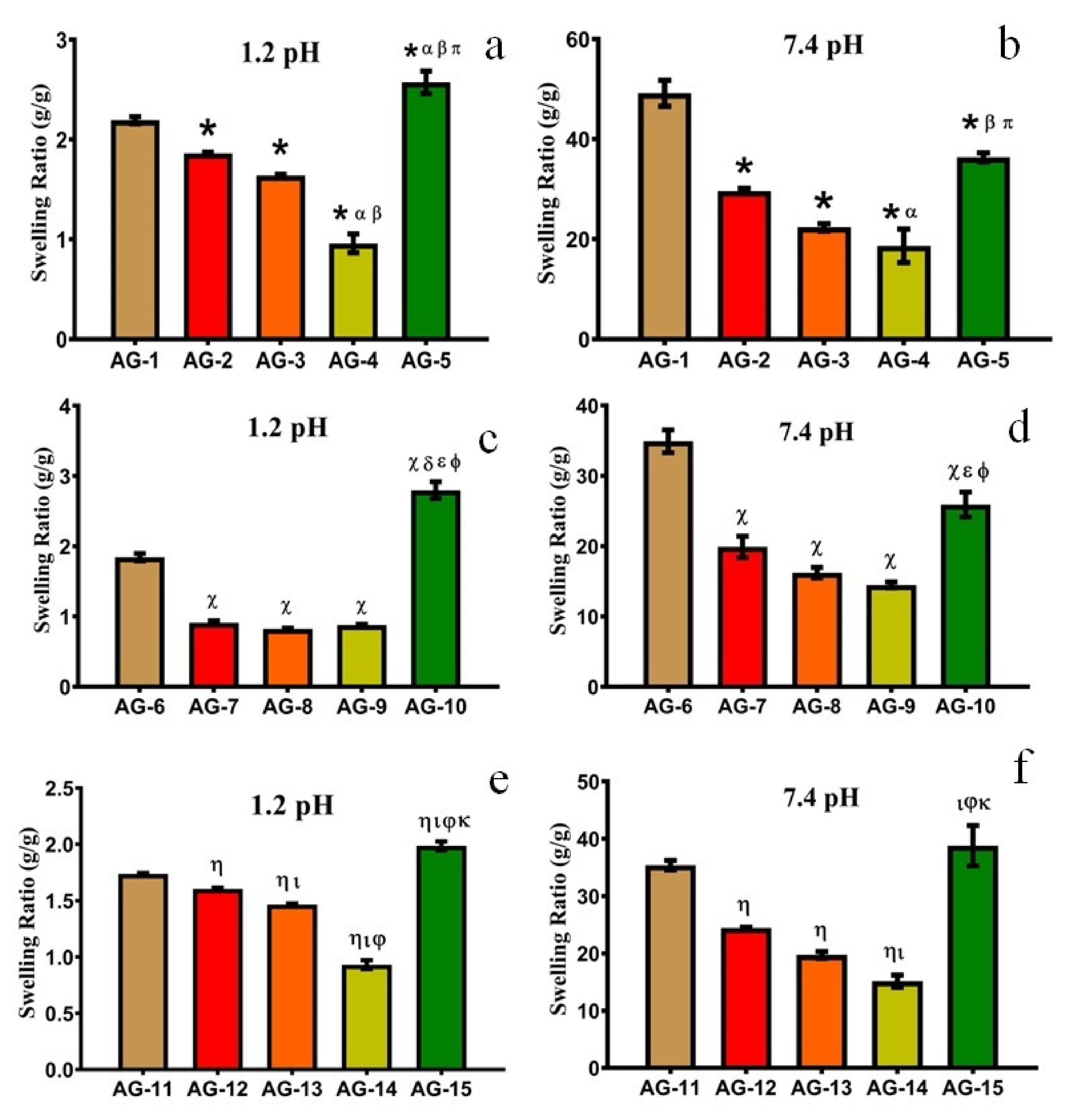
3.1.1. Effect of pH
3.1.2. Effect of AA
3.1.3. Effect of Gelatin
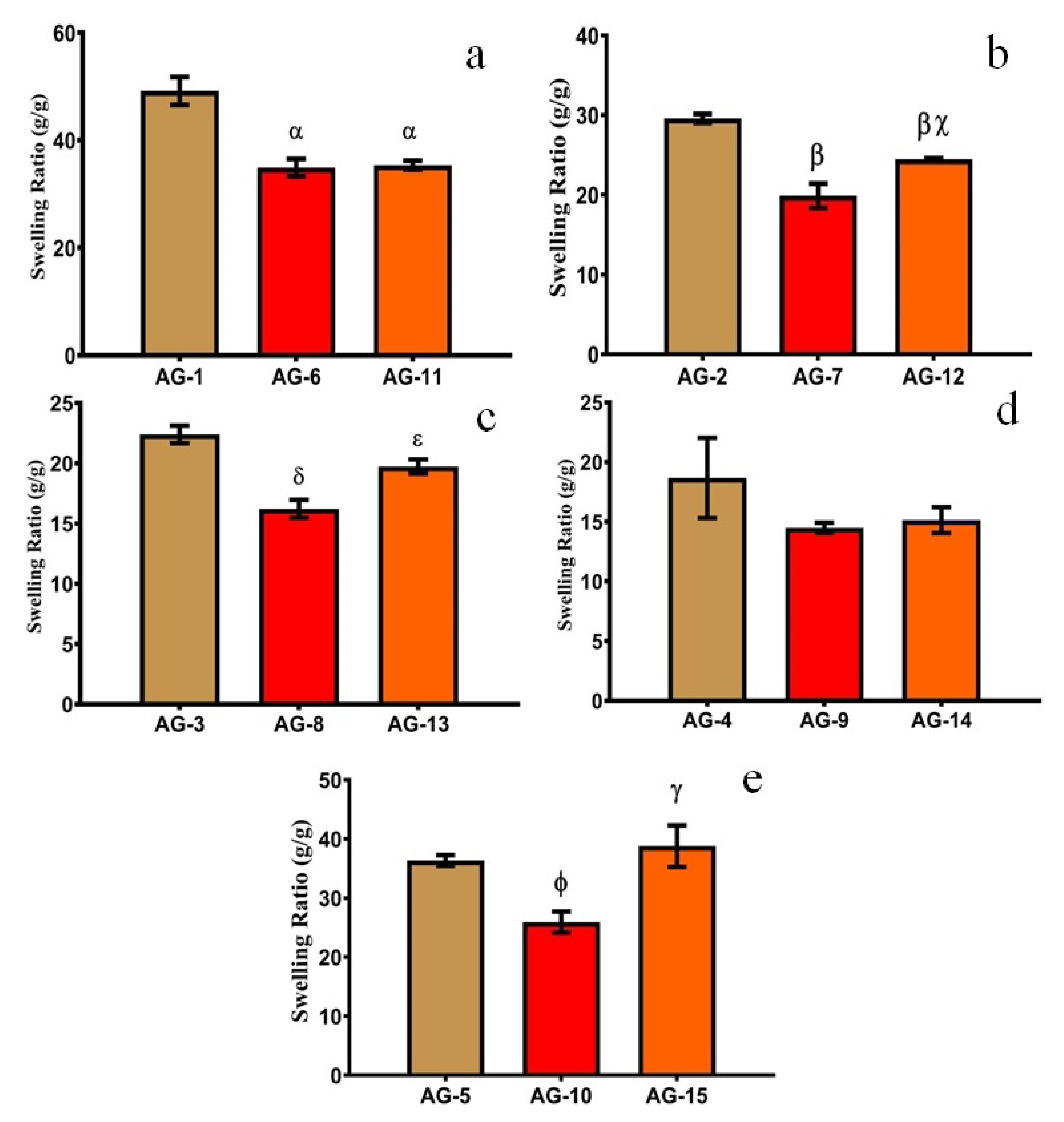
3.2. Mucoadhesive Strength
3.3. Sol–Gel Fraction
3.4. Solid-State Characterization
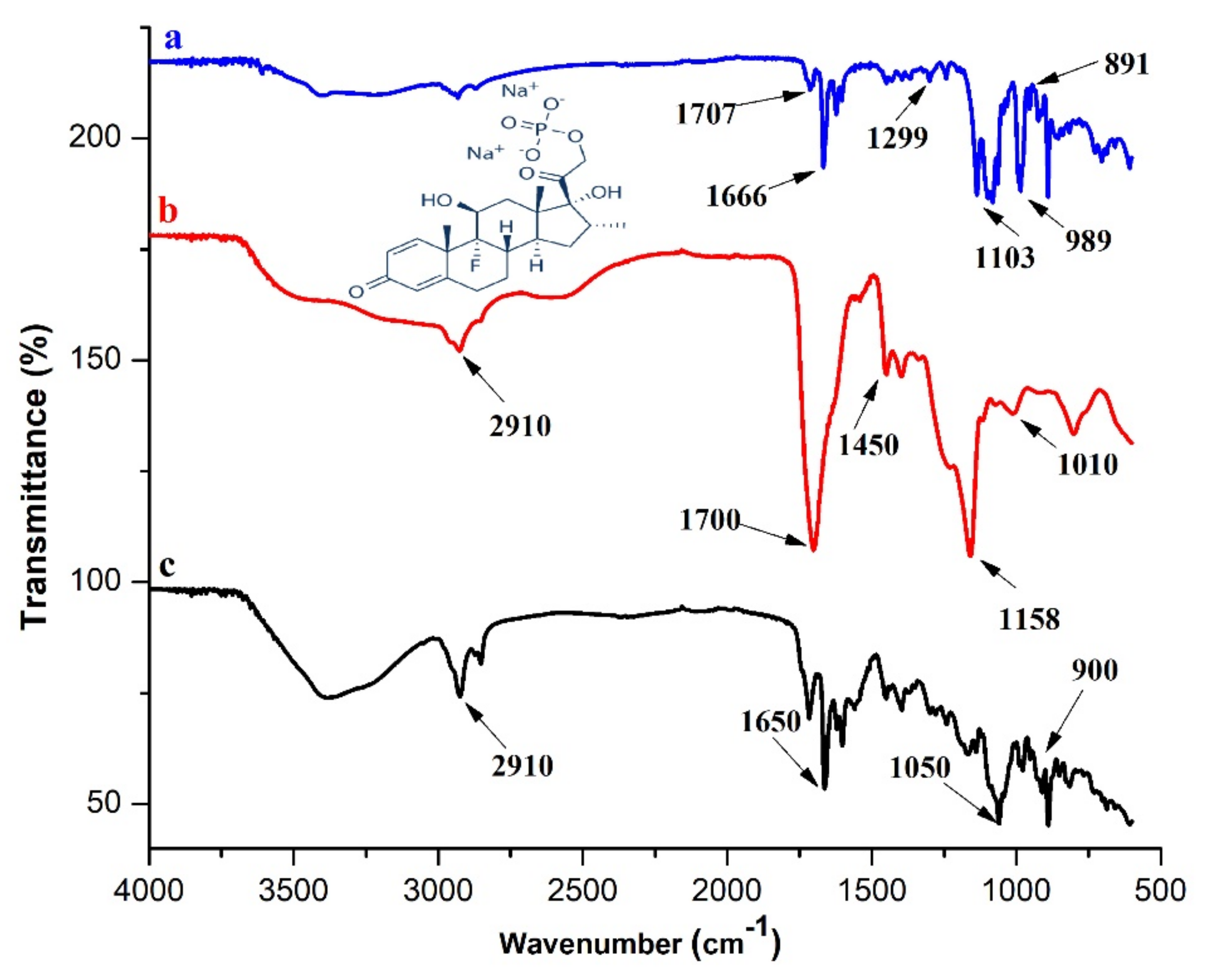
3.4.1. FTIR
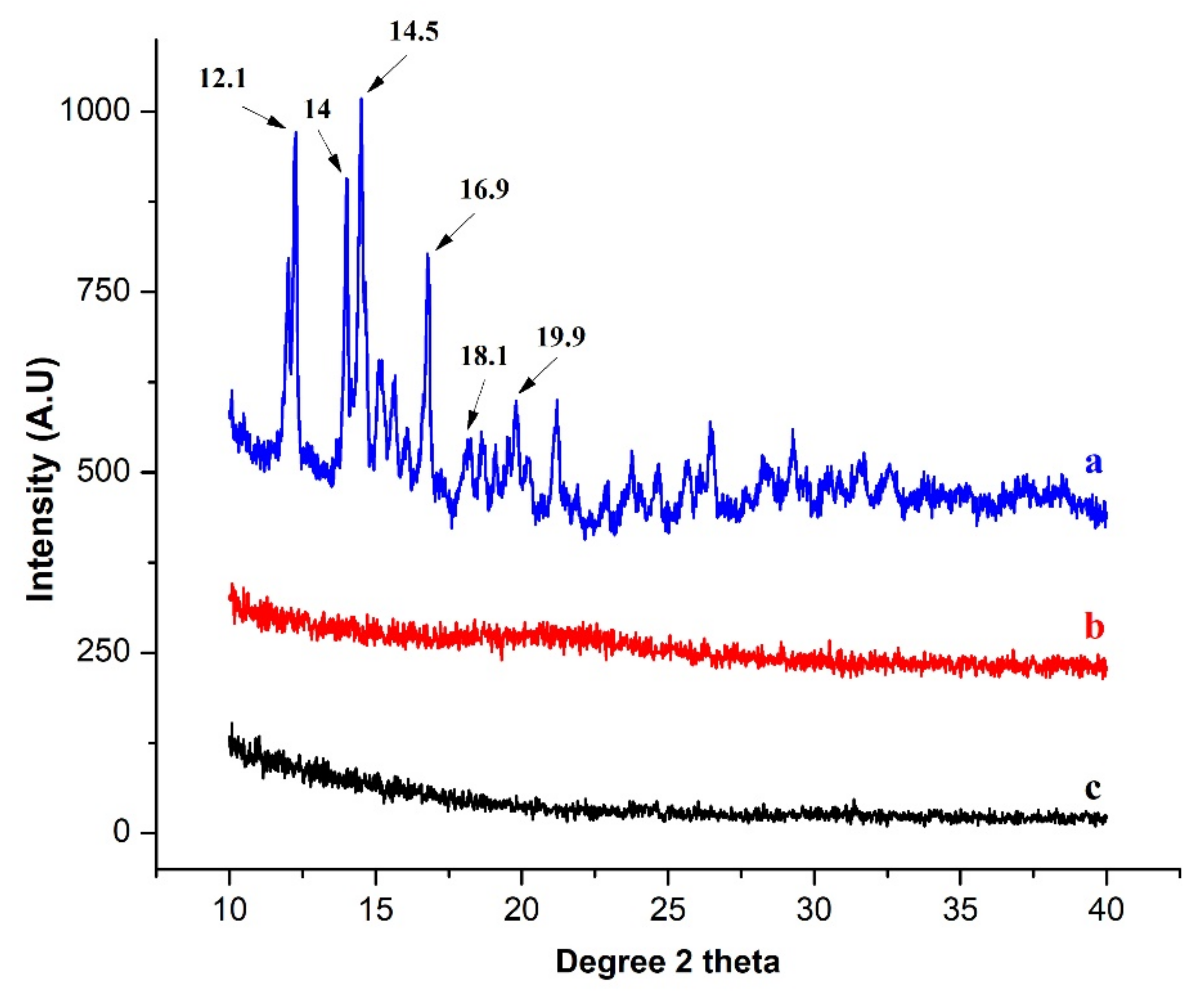
3.4.2. XRD
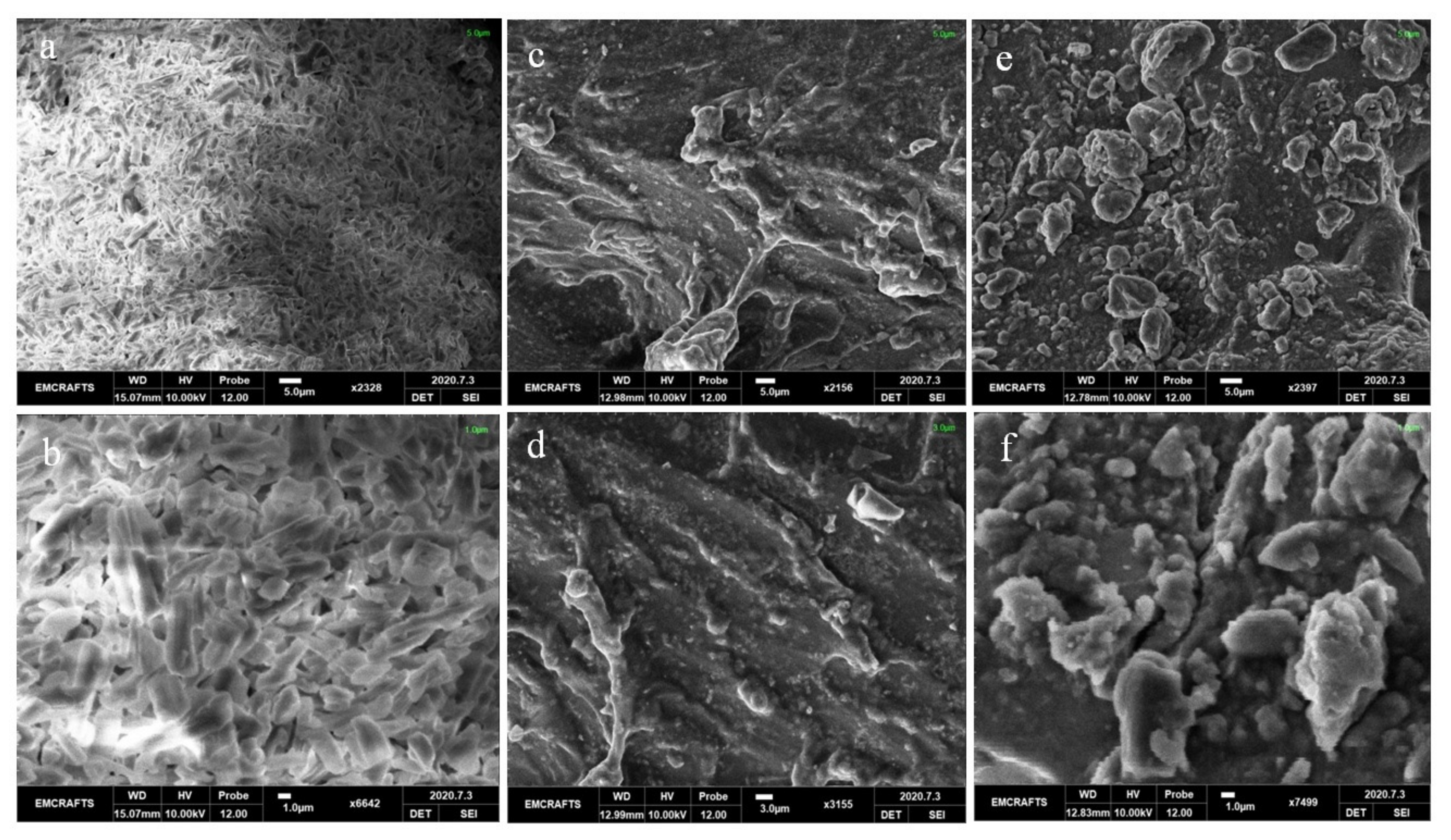
3.4.3. SEM
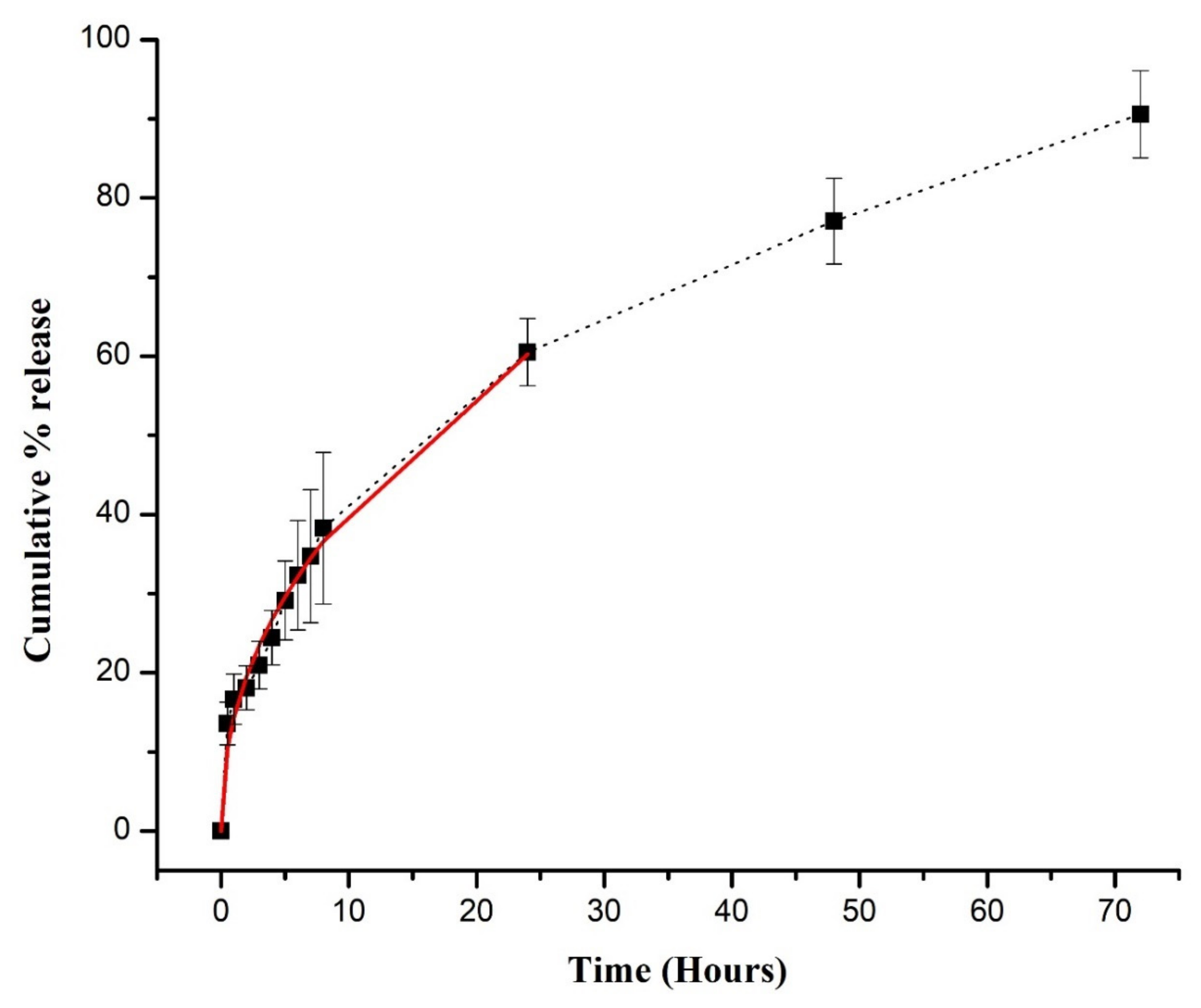
3.5. DSP Loading and In Vitro Release Study
3.6. Drug Release Modeling
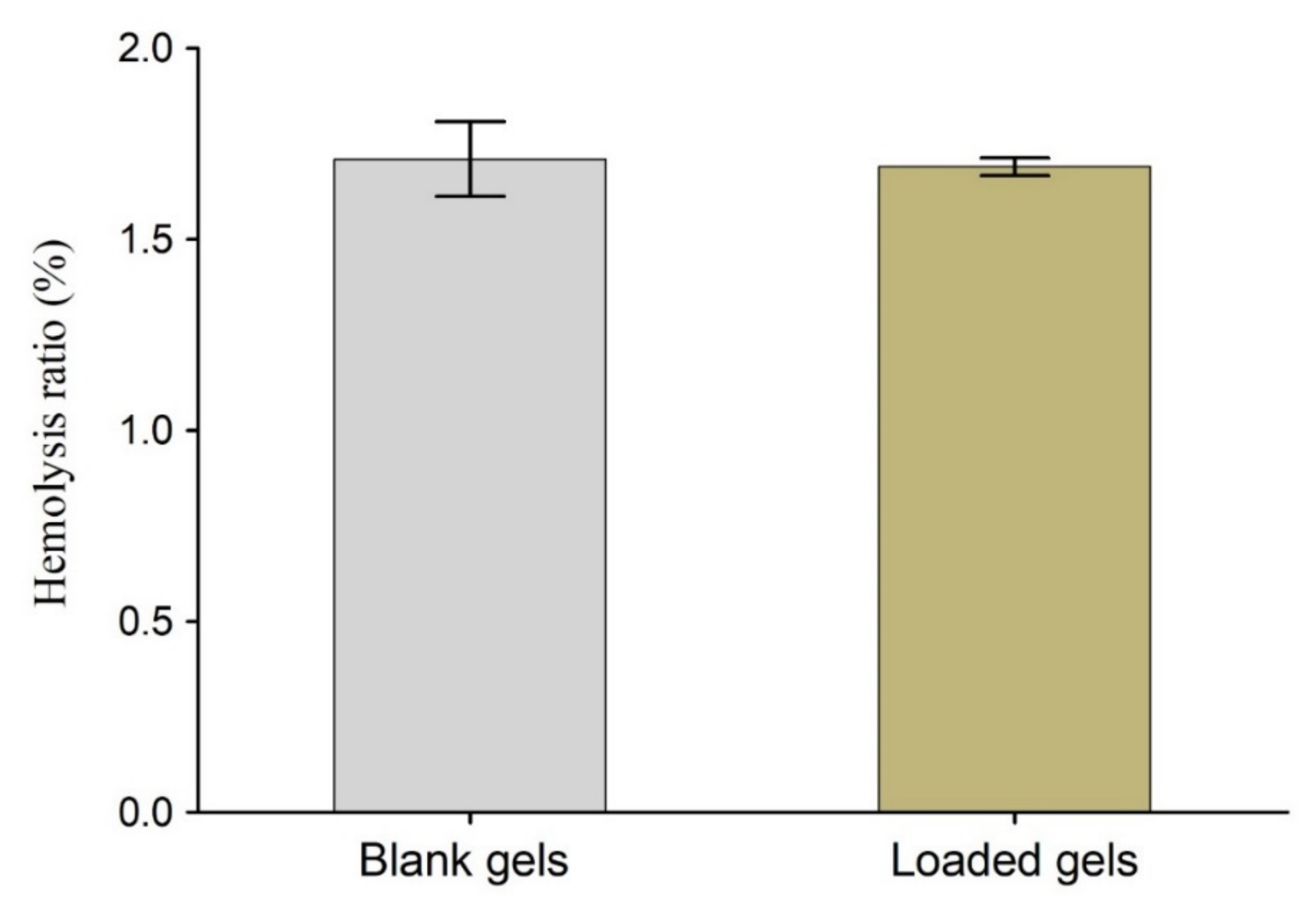
3.7. Hemocompatibility Test
3.8. Toxicity Testing
3.8.1. Monitoring the General Conditions of the Rabbits
3.8.2. Biochemical and Hematological Observation
3.8.3. Histopathological Examination
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ekici, S.; Saraydin, D. Interpenetrating polymeric network hydrogels for potential gastrointestinal drug release. Polym. Int. 2007, 56, 1371–1377. [Google Scholar] [CrossRef]
- Kulkarni, S.J. Interpenetrating Polymer Network-A Promising Method for Widening Applications of Polymers. Int. J. Res. Rev. 2020, 7, 74–79. [Google Scholar]
- Nosenko, T.; Sitnikova, V.; Uspenskaya, M. Effect of sodium hyaluronat on mechanical and sorption properties of IPN hydrogels based on acrylic acid and acrylamide. Mater. Today Proc. 2020, 30, 564–567. [Google Scholar] [CrossRef]
- Nosenko, T.N.; Sitnikova, V.E.; Uspenskaya, M.V. Sorption of human serum albumin on surface IPN acrylic hydrogels filled with sodium hyaluronate. Mater. Today Proc. 2020, 30, 596–598. [Google Scholar] [CrossRef]
- Chandel, A.K.S.; Kannan, D.; Nutan, B.; Singh, S.; Jewrajka, S.K. Dually crosslinked injectable hydrogels of poly(ethylene glycol) and poly[(2-dimethylamino)ethyl methacrylate]-b-poly(N-isopropyl acrylamide) as a wound healing promoter. J. Mater. Chem. B 2017, 5, 4955–4965. [Google Scholar] [CrossRef]
- Chandel, A.K.S.; Nutan, B.; Raval, I.H.; Jewrajka, S.K. Self-Assembly of Partially Alkylated Dextran-graft-poly[(2-dimethylamino)ethyl methacrylate] Copolymer Facilitating Hydrophobic/Hydrophilic Drug Delivery and Improving Conetwork Hydrogel Properties. Biomacromolecules 2018, 19, 1142–1153. [Google Scholar] [CrossRef]
- Chandel, A.K.S.; Bera, A.; Nutan, B.; Jewrajka, S.K. Reactive compatibilizer mediated precise synthesis and application of stimuli responsive polysaccharides-polycaprolactone amphiphilic co-network gels. Polymer 2016, 99, 470–479. [Google Scholar] [CrossRef]
- Ullah, K.; Sohail, M.; Mannan, A.; Rashid, H.; Shah, A.; Murtaza, G.; Khan, S.A. Facile Synthesis of Chitosan Based-(AMPS-co-AA) Semi-IPNs as a Potential Drug Carrier: Enzymatic Degradation, Cytotoxicity, and Preliminary Safety Evaluation. Curr. Drug Deliv. 2019, 16, 242–253. [Google Scholar] [CrossRef]
- Stella, V.J.; He, Q. Cyclodextrins. Toxicol. Pathol. 2008, 36, 30–42. [Google Scholar] [CrossRef]
- He, Z.X.; Wang, Z.H.; Zhang, H.H.; Pan, X.; Su, W.R.; Liang, D.; Wu, C.B. Doxycycline and hydroxypropyl-β-cyclodextrin complex in poloxamer thermal sensitive hydrogel for ophthalmic delivery. Acta Pharm. Sin. B 2011, 1, 254–260. [Google Scholar] [CrossRef] [Green Version]
- Saghebasl, S.; Davaran, S.; Rahbarghazi, R.; Montaseri, A.; Salehi, R.; Ramazani, A. Synthesis and in vitro evaluation of thermosensitive hydrogel scaffolds based on (PNIPAAm-PCL-PEG-PCL-PNIPAAm)/Gelatin and (PCL-PEG-PCL)/Gelatin for use in cartilage tissue engineering. J. Biomater. Sci. Polym. Ed. 2018, 29, 1185–1206. [Google Scholar] [CrossRef] [PubMed]
- Ullah, K.; Khan, S.A.; Murtaza, G.; Sohail, M.; Azizullah; Manan, A.; Afzal, A. Gelatin-based hydrogels as potential biomaterials for colonic delivery of oxaliplatin. Int. J. Pharm. 2019, 556, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Afzal, S.; Khan, S.; Ranjha, N.M.; Jalil, A.; Riaz, A.; Haider, M.S.; Sarwar, S.; Saher, F.; Naeem, F. The Structural, Crystallinity and Thermal Properties of pH responsive Interpenetrating Gelatin/Sodium Alginate Based Polymeric Composites for the Controlled Delivery of Cetirizine HCl. Turk. J. Pharm. Sci. 2018, 15, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Kaith, B.S.; Kaur, M.; Sharma, N.; Khullar, S. A hydrogel based on dialdehyde carboxymethyl cellulose–gelatin and its utilization as a bio adsorbent. J. Chem. Sci. 2019, 132, 15. [Google Scholar] [CrossRef]
- Bakravi, A.; Ahamadian, Y.; Hashemi, H.; Namazi, H. Synthesis of gelatin-based biodegradable hydrogel nanocomposite and their application as drug delivery agent. Adv. Polym. Technol. 2018, 37, 2625–2635. [Google Scholar] [CrossRef]
- Mishra, R.K.; Majeed, A.B.A.; Banthia, A.K. Development and characterization of pectin/gelatin hydrogel membranes for wound dressing. Int. J. Plast. Technol. 2011, 15, 82–95. [Google Scholar] [CrossRef]
- Xing, Q.; Yates, K.F.; Vogt, C.; Qian, Z.; Frost, M.; Zhao, F. Increasing Mechanical Strength of Gelatin Hydrogels by Divalent Metal Ion Removal. Sci. Rep. 2015, 4, 4706. [Google Scholar] [CrossRef] [Green Version]
- Bonferoni, M.C.; Chetoni, P.; Giunchedi, P.; Rossi, S.; Ferrari, F.; Burgalassi, S.; Caramella, C. Carrageenan–gelatin mucoadhesive systems for ion-exchange based ophthalmic delivery: In vitro and preliminary in vivo studies. Eur. J. Pharm. Biopharm. 2004, 57, 465–472. [Google Scholar] [CrossRef]
- Khade, S.M.; Behera, B.; Sagiri, S.S.; Singh, V.K.; Thirugnanam, A.; Pal, K.; Ray, S.S.; Pradhan, D.K.; Bhattacharya, M.K. Gelatin–PEG based metronidazole-loaded vaginal delivery systems: Preparation, characterization and in vitro antimicrobial efficiency. Iran. Polym. J. 2014, 23, 171–184. [Google Scholar] [CrossRef]
- Singh, V.K.; Sagiri, S.S.; Khade, S.M.; Bhattacharya, M.K.; Pal, K. Development and characterization of gelatin-polysaccharide based phase-separated hydrogels for prevention of sexually transmitted diseases. J. Appl. Polym. Sci. 2014, 132, 41785. [Google Scholar] [CrossRef]
- Zheng, Y.; Liang, Y.; Zhang, D.; Sun, X.; Liang, L.; Li, J.; Liu, Y.-N. Gelatin-Based Hydrogels Blended with Gellan as an Injectable Wound Dressing. ACS Omega 2018, 3, 4766–4775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guizzardi, R.; Vaghi, L.; Marelli, M.; Natalello, A.; Andreosso, I.; Papagni, A.; Cipolla, L. Gelatin-Based Hydrogels through Homobifunctional Triazolinediones Targeting Tyrosine Residues. Molecules 2019, 24, 589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosseini, S.M.; Shahrousvand, M.; Shojaei, S.; Khonakdar, H.A.; Asefnejad, A.; Goodarzi, V. Preparation of superabsorbent eco-friendly semi-interpenetrating network based on cross-linked poly acrylic acid/xanthan gum/graphene oxide (PAA/XG/GO): Characterization and dye removal ability. Int. J. Biol. Macromol. 2020, 152, 884–893. [Google Scholar] [CrossRef] [PubMed]
- Pourbashir, S.; Shahrousvand, M.; Ghaffari, M. Preparation and characterization of semi-IPNs of polycaprolactone/poly(acrylic acid)/cellulosic nanowhisker as artificial articular cartilage. Int. J. Biol. Macromol. 2020, 142, 298–310. [Google Scholar] [CrossRef]
- Boztepe, C.; Yüceer, M.; Künkül, A.; Şölener, M.; Kabasakal, O.S. Prediction of the deswelling behaviors of pH-and temperature-responsive poly (NIPAAm-co-AAc) IPN hydrogel by artificial intelligence techniques. Res. Chem. Intermed. 2020, 46, 409–428. [Google Scholar] [CrossRef]
- Chourasia, M.K.; Jain, S.K. Pharmaceutical approaches to colon targeted drug delivery systems. J. Pharm. Pharm. Sci. 2003, 6, 33–66. [Google Scholar]
- Patel, M.M. Cutting-edge technologies in colon-targeted drug delivery systems. Expert Opin. Drug Deliv. 2011, 8, 1247–1258. [Google Scholar] [CrossRef]
- Lee, S.H.; Bajracharya, R.; Min, J.Y.; Han, J.-W.; Park, B.J.; Han, H.-K. Strategic Approaches for Colon Targeted Drug Delivery: An Overview of Recent Advancements. Pharmaceutics 2020, 12, 68. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.; Kim, B. Mucoadhesive and pH-responsive behavior of gelatin containing hydrogels for protein drug delivery applications. Korea-Aust. Rheol. J. 2020, 32, 41–46. [Google Scholar] [CrossRef]
- Kesavan, K.; Kant, S.; Singh, P.N.; Pandit, J.K. Effect of hydroxypropyl-β-cyclodextrin on the ocular bioavailability of dexamethasone from a pH-induced mucoadhesive hydrogel. Curr. Eye Res. 2011, 36, 918–929. [Google Scholar] [CrossRef]
- Bukhari, S.M.H.; Khan, S.; Rehanullah, M.; Ranjha, N.M. Synthesis and Characterization of Chemically Cross-Linked Acrylic Acid/Gelatin Hydrogels: Effect of pH and Composition on Swelling and Drug Release. Int. J. Polym. Sci. 2015, 2015, 187961. [Google Scholar] [CrossRef]
- Hajikarimi, A.; Sadeghi, M. Free radical synthesis of cross-linking gelatin base poly NVP/acrylic acid hydrogel and nanoclay hydrogel as cephalexin drug deliver. J. Polym. Res. 2020, 27, 57. [Google Scholar] [CrossRef]
- Hago, E.-E.; Li, X. Interpenetrating Polymer Network Hydrogels Based on Gelatin and PVA by Biocompatible Approaches: Synthesis and Characterization. Adv. Mater. Sci. Eng. 2013, 2013, 328763. [Google Scholar] [CrossRef] [Green Version]
- Eid, M.; El-Arnaouty, M. Kinetic degradation and controlled drug delivery system studies for sensitive hydrogels prepared by gamma irradiation. J. Appl. Polym. Sci. 2009, 112, 1745–1754. [Google Scholar] [CrossRef]
- Shah, S.; Ranjha, N.M.; Javaid, Z. Development and evaluation of pH-dependent interpenetrating network of acrylic acid/polyvinyl alcohol. Iran. Polym. J. 2013, 22, 811–820. [Google Scholar] [CrossRef]
- Boztepe, C.; Künkül, A.; Yüceer, M. Application of artificial intelligence in modeling of the doxorubicin release behavior of pH and temperature responsive poly(NIPAAm-co-AAc)-PEG IPN hydrogel. J. Drug Deliv. Sci. Technol. 2020, 57, 101603. [Google Scholar] [CrossRef]
- Cheng, X.; Liu, J.; Wang, L.; Wang, R.; Liu, Z.; Zhuo, R. An enzyme-mediated in situ hydrogel based on polyaspartamide derivatives for localized drug delivery and 3D scaffolds. RSC Adv. 2016, 6, 101334–101346. [Google Scholar] [CrossRef]
- Ali, A.; Khalid, I.; Minhas, M.U.; Barkat, K.; Khan, I.U.; Syed, H.K.; Umar, A. Preparation and in vitro evaluation of Chondroitin sulfate and carbopol based mucoadhesive controlled release polymeric composites of Loxoprofen using factorial design. Eur. Polym. J. 2019, 121, 109312. [Google Scholar] [CrossRef]
- Ajaz, N.; Khan, I.U.; Asghar, S.; Khalid, S.H.; Irfan, M.; Asif, M.; Chatha, S.A.S. Assessing the pH responsive and mucoadhesive behavior of dexamethasone sodium phosphate loaded itaconic acid-grafted-poly(acrylamide)/carbopol semi-interpenetrating networks. J. Polym. Res. 2021, 28, 278. [Google Scholar] [CrossRef]
- Bashir, S.; Teo, Y.Y.; Naeem, S.; Ramesh, S.; Ramesh, K. pH responsive N-succinyl chitosan/Poly(acrylamide-co-acrylic acid) hydrogels and in vitro release of 5-fluorouracil. PLoS ONE 2017, 12, e0179250. [Google Scholar]
- Flores-Arriaga, J.C.; de Jesús Pozos-Guillén, A.; Escobar-García, D.M.; Grandfils, C.; Cerda-Cristerna, B.I. Cell viability and hemocompatibility evaluation of a starch-based hydrogel loaded with hydroxyapatite or calcium carbonate for maxillofacial bone regeneration. Odontology 2017, 105, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.N.; Varaprasad, K.; Ravindra, S.; Reddy, G.S.; Reddy, K.; Raju, K.M. Evaluation of blood compatibility and drug release studies of gelatin based magnetic hydrogel nanocomposites. Colloids Surf. A Physicochem. Eng. Asp. 2011, 385, 20–27. [Google Scholar] [CrossRef]
- Rizwan, M.; Yahya, R.; Hassan, A.; Yar, M.; Azzahari, A.D.; Selvanathan, V.; Sonsudin, F.; Abouloula, C.N. pH Sensitive Hydrogels in Drug Delivery: Brief History, Properties, Swelling, and Release Mechanism, Material Selection and Applications. Polymers 2017, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Malik, N.S.; Ahmad, M.; Minhas, M.U. Cross-linked β-cyclodextrin and carboxymethyl cellulose hydrogels for controlled drug delivery of acyclovir. PLoS ONE 2017, 12, e0172727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jabbari, E.; Nozari, S. Swelling behavior of acrylic acid hydrogels prepared by γ-radiation crosslinking of polyacrylic acid in aqueous solution. Eur. Polym. J. 2000, 36, 2685–2692. [Google Scholar] [CrossRef]
- Huang, X.; Xu, S.; Zhong, M.; Wang, J.; Feng, S.; Shi, R. Modification of Na-bentonite by polycations for fabrication of amphoteric semi-IPN nanocomposite hydrogels. Appl. Clay Sci. 2009, 42, 455–459. [Google Scholar] [CrossRef]
- Kowalski, G.; Kijowska, K.; Witczak, M.; Kuterasiński, Ł.; Łukasiewicz, M. Synthesis and Effect of Structure on Swelling Properties of Hydrogels Based on High Methylated Pectin and Acrylic Polymers. Polymers 2019, 11, 114. [Google Scholar] [CrossRef] [Green Version]
- Gupta, B.; Tummalapalli, M.; Deopura, B.; Alam, M. Preparation and characterization of in-situ crosslinked pectin–gelatin hydrogels. Carbohydr. Polym. 2014, 106, 312–318. [Google Scholar] [CrossRef]
- Alsarra, I.A.; Hamed, A.Y.; Mahrous, G.M.; El Maghraby, G.M.; Al-Robayan, A.A.; Alanazi, F.K. Mucoadhesive polymeric hydrogels for nasal delivery of acyclovir. Drug Dev. Ind. Pharm. 2009, 35, 352–362. [Google Scholar] [CrossRef]
- Ahmad, N.; Amin, M.C.I.M.; Mahali, S.M.; Ismail, I.; Chuang, V.T.G. Biocompatible and Mucoadhesive Bacterial Cellulose-g-Poly(acrylic acid) Hydrogels for Oral Protein Delivery. Mol. Pharm. 2014, 11, 4130–4142. [Google Scholar] [CrossRef]
- Ramadan, A.A.; Elbakry, A.M.; Esmaeil, A.H.; Khaleel, S.A. Pharmaceutical and pharmacokinetic evaluation of novel rectal mucoadhesive hydrogels containing tolmetin sodium. J. Pharm. Investig. 2017, 48, 673–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jovanović, M.; Tomić, N.; Cvijić, S.; Stojanović, D.; Ibrić, S.; Uskoković, P. Mucoadhesive Gelatin Buccal Films with Propranolol Hydrochloride: Evaluation of Mechanical, Mucoadhesive, and Biopharmaceutical Properties. Pharmaceutics 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Minhas, M.U.; Ahmad, M.; Khan, K.U.; Sohail, M.; Khalid, I. Functionalized pectin hydrogels by cross-linking with monomer: Synthesis, characterization, drug release and pectinase degradation studies. Polym. Bull. 2019, 77, 339–356. [Google Scholar] [CrossRef]
- Ranjha, N.M.; Madni, A.; Bakar, A.A.; Talib, N.; Ahmad, S.; Ahmad, H. Preparation and characterization of isosorbide mononitrate hydrogels obtained by free-radical polymerization for site-specific delivery. Trop. J. Pharm. Res. 2014, 13, 1979–1985. [Google Scholar] [CrossRef] [Green Version]
- Sohail, K.; Khan, I.U.; Shahzad, Y.; Hussain, T.; Ranjha, N.M. pH-sensitive polyvinylpyrrolidone-acrylic acid hydrogels: Impact of material parameters on swelling and drug release. Braz. J. Pharm. Sci. 2014, 50, 173–184. [Google Scholar] [CrossRef]
- Jin, S.; Liu, M.; Zhang, F.; Chen, S.; Niu, A. Synthesis and characterization of pH-sensitivity semi-IPN hydrogel based on hydrogen bond between poly (N-vinylpyrrolidone) and poly (acrylic acid). Polymer 2006, 47, 1526–1532. [Google Scholar] [CrossRef]
- Larrañaga, A.; Petisco, S.; Villanueva, R.; Iturri, J.J.; Moya, S.; Meaurio, E.; Sarasua, J.-R. Physicochemical properties of plasma polymerized acrylic acid, ε-caprolactone and lactic acid films. In Proceedings of the European Technical Conference-Society of Plastic Engineers, Boston, MA, USA, 1–5 May 2011. [Google Scholar]
- Umemura, J.; Hayashi, S. Infrared spectra and molecular configurations of liquid and crystalline acrylic acids. Bull. Inst. Chem. Res. Kyoto Univ. 1975, 52, 585–595. [Google Scholar]
- Buhus, G.; Peptu, C.; Popa, M.; Desbrieres, J. Controlled release of water soluble antibiotics by carboxymethylcellulose-and gelatin-based hydrogels crosslinked with epichlorohydrin. Cell. Chem. Technol. 2009, 43, 141–151. [Google Scholar]
- Chang, Y.; Xiao, L.; Tang, Q. Preparation and characterization of a novel thermosensitive hydrogel based on chitosan and gelatin blends. J. Appl. Polym. Sci. 2009, 113, 400–407. [Google Scholar] [CrossRef]
- Nawaz, S.; Khan, S.; Farooq, U.; Haider, M.S.; Ranjha, N.M.; Rasul, A.; Nawaz, A.; Arshad, N.; Hameed, R. Biocompatible hydrogels for the controlled delivery of anti-hypertensive agent: Development, characterization and in vitro evaluation. Des. Monomers Polym. 2018, 21, 18–32. [Google Scholar] [CrossRef] [Green Version]
- Ullah, A. Development and Evaluation of Self-Assembled Stabilized Supramolecular Hydrogels of Polyoxometalates; COMSATS Institute of Information Technology Islamabad: Islamabad, Pakistan, 2017. [Google Scholar]
- Su, J.; Chen, J.; Li, L.; Li, B.; Shi, L.; Zhang, H.; Ding, X. Preparation of Natural Borneol/2-Hydroxypropyl-β-cyclodextrin Inclusion Complex and Its Effect on the Absorption of Tetramethylpyrazine Phosphate in Mouse. Chem. Pharm. Bull. 2012, 60, 736–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soleimani, F.; Sadeghi, M.; Shahsavari, H. Graft copolymerization of gelatin-g-poly (acrylic acid-co-acrylamide) and calculation of grafting parameters. Indian J. Sci. Technol. 2012, 5, 2041–2046. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, J.; Zhou, Y.; Zhang, R.; Song, Q.; Lei, L.; Li, X. Supramolecular nanofibers of dexamethasone derivatives to form hydrogel for topical ocular drug delivery. Colloids Surf. B Biointerfaces 2018, 164, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-R.; Li, A.; Mei, W.; Zhu, R.-R.; Li, K.; Sun, X.-Y.; Qian, Y.-C.; Wang, S.-L. Dexamethasone sodium phosphate intercalated layered double hydroxides and their therapeutic efficacy in a murine asthma model. RSC Adv. 2015, 5, 23826–23834. [Google Scholar] [CrossRef]
- Georgieva, D.; Kostova, B.; Ivanova, S.; Rachev, D.; Tzankova, V.; Kondeva-Burdina, M.; Christova, D. pH-Sensitive Cationic Copolymers of Different Macromolecular Architecture as Potential Dexamethasone Sodium Phosphate Delivery Systems. J. Pharm. Sci. 2014, 103, 2406–2413. [Google Scholar] [CrossRef]
- Dong, K.; Dong, Y.; You, C.; Xu, W.; Huang, X.; Yan, Y.; Zhang, L.; Wang, K.; Xing, J. Assessment of the drug loading, in vitro and in vivo release behavior of novel pH-sensitive hydrogel. Drug Deliv. 2016, 23, 174–184. [Google Scholar]
- Qiu, N.; Cheng, X.; Wang, G.; Wang, W.; Wen, J.; Zhang, Y.; Song, H.; Ma, L.; Wei, Y.; Peng, A.; et al. Inclusion complex of barbigerone with hydroxypropyl-β-cyclodextrin: Preparation and in vitro evaluation. Carbohydr. Polym. 2014, 101, 623–630. [Google Scholar] [CrossRef]
- Radev, L.; Fernandes, M.H.V.; Salvado, I.M.; Kovacheva, D. Organic/Inorganic bioactive materials Part III: In vitro bioactivity of gelatin/silicocarnotite hybrids. Open Chem. 2009, 7, 721–730. [Google Scholar] [CrossRef]
- Pal, K.; Banthia, A.K.; Majumdar, D.K. Preparation and characterization of polyvinyl alcohol-gelatin hydrogel membranes for biomedical applications. AAPS PharmSciTech 2007, 8, E142–E146. [Google Scholar] [CrossRef]
- Das, S.; Subuddhi, U. Controlled delivery of dexamethasone to the intestine from poly(vinyl alcohol)–poly(acrylic acid) microspheres containing drug-cyclodextrin complexes: Influence of method of preparation of inclusion complex. RSC Adv. 2014, 4, 24222–24231. [Google Scholar] [CrossRef]
- Ajaz, N.; Khan, I.U.; Khalid, I.; Khan, R.U.; Khan, H.A.; Asghar, S.; Khalid, S.H.; Shahzad, Y.; Yousaf, A.M.; Hussain, T.; et al. In vitro and toxicological assessment of dexamethasone sodium phosphate loaded pH sensitive Pectin-g-poly(AA)/PVP semi interpenetrating network. Mater. Today Commun. 2020, 25, 101325. [Google Scholar] [CrossRef]
- Shivakumar, H.; Gupta, N.; Satish, C. Preparation and characterization of gelatin-poly(methacrylic acid) interpenetrating polymeric network hydrogels as a ph-sensitive delivery system for glipizide. Indian J. Pharm. Sci. 2007, 69, 64–68. [Google Scholar] [CrossRef] [Green Version]
- Wu, I.Y.; Bala, S.; Škalko-Basnet, N.; di Cagno, M.P. Interpreting non-linear drug diffusion data: Utilizing Korsmeyer-Peppas model to study drug release from liposomes. Eur. J. Pharm. Sci. 2019, 138, 105026. [Google Scholar] [CrossRef] [PubMed]
- Picone, P.; Sabatino, M.A.; Ajovalasit, A.; Giacomazza, D.; Dispenza, C.; di Carlo, M. Biocompatibility, hemocompatibility and antimicrobial properties of xyloglucan-based hydrogel film for wound healing application. Int. J. Biol. Macromol. 2019, 121, 784–795. [Google Scholar] [CrossRef]
- Obiweluozor, F.O.; Maharjan, B.; Emechebe, A.G.; Park, C.H.; Kim, C.S. Mussel-inspired elastic interpenetrated network hydrogel as an alternative for anti-thrombotic stent coating membrane. Chem. Eng. J. 2018, 347, 932–943. [Google Scholar] [CrossRef]
- Alexandre, N.; Ribeiro, J.; Gärtner, A.; Pereira, T.; Amorim, I.; Fragoso, J.; Lopes, A.; Fernandes, J.; Costa, E.; Santos-Silva, A. Biocompatibility and hemocompatibility of polyvinyl alcohol hydrogel used for vascular grafting—In vitro and in vivo studies. J. Biomed. Mater. Res. Part A 2014, 102, 4262–4275. [Google Scholar]
- Mirzakhanian, Z.; Faghihi, K.; Barati, A.; Momeni, H.R. Synthesis and characterization of fast-swelling porous superabsorbent hydrogel based on starch as a hemostatic agent. J. Biomater. Sci. Polym. Ed. 2015, 26, 1439–1451. [Google Scholar] [CrossRef]
- Anwar, M.; Pervaiz, F.; Shoukat, H.; Noreen, S.; Shabbir, K.; Majeed, A.; Ijaz, S. Formulation and evaluation of interpenetrating network of xanthan gum and polyvinylpyrrolidone as a hydrophilic matrix for controlled drug delivery system. Polym. Bull. 2020, 78, 59–80. [Google Scholar] [CrossRef]
- Khan, S.A.; Azam, W.; Ashames, A.; Fahelelbom, K.M.; Ullah, K.; Mannan, A.; Murtaza, G. β-Cyclodextrin-based (IA-co-AMPS) Semi-IPNs as smart biomaterials for oral delivery of hydrophilic drugs: Synthesis, characterization, in-Vitro and in-Vivo evaluation. J. Drug Deliv. Sci. Technol. 2020, 60, 101970. [Google Scholar] [CrossRef]
- Tan, L.; Xu, X.; Song, J.; Luo, F.; Qian, Z. Synthesis, characterization, and acute oral toxicity evaluation of pH-sensitive hydrogel based on MPEG, poly (ε-caprolactone), and itaconic acid. BioMed Res. Int. 2013, 2013, 239838. [Google Scholar] [CrossRef] [Green Version]




| Code | AA g/100 g | Gelatin g/100 g | Code | AA g/100 g | Gelatin g/100 g | Code | AA g/100 g | Gelatin g/100 g |
|---|---|---|---|---|---|---|---|---|
| AG-1 | 16.66 | 0.33 | AG-6 | 16.66 | 1.33 | AG-11 | 16.66 | 2.66 |
| AG-2 | 33.33 | AG-7 | 33.33 | AG-12 | 33.33 | |||
| AG-3 | 50 | AG-8 | 50 | AG-13 | 50 | |||
| AG-4 | 66.66 | AG-9 | 66.66 | AG-14 | 66.66 | |||
| AG-5 | 83.33 | AG-10 | 83.33 | AG-15 | 83.33 |
| Code | Gel (%) | Sol (%) | Code | Gel (%) | Sol (%) | Code | Gel (%) | Sol (%) |
|---|---|---|---|---|---|---|---|---|
| AG-1 | 90 | 10 | AG-6 | 89.47 | 10.52 | AG-11 | 87.09 | 12.90 |
| AG-2 | 91.42 | 8.57 | AG-7 | 90.62 | 9.37 | AG-12 | 89.09 | 10.90 |
| AG-3 | 92.42 | 7.57 | AG-8 | 91.78 | 8.21 | AG-13 | 90.58 | 9.41 |
| AG-4 | 94.28 | 5.71 | AG-9 | 92.78 | 7.21 | AG-14 | 90.62 | 9.37 |
| AG-5 | 95.68 | 4.31 | AG-10 | 93.05 | 6.94 | AG-15 | 91.26 | 8.73 |
| Model | Parameter | Value |
|---|---|---|
| Korsmeyer–Peppas | Correlation coefficient (R2) | 0.9810 |
| Diffusion exponent (n) | 0.453 | |
| Release rate constant (K) | 14.270 |
| Observations | Control Blood | Blood after Incubation | Blood with Blank Gels | Blood with Loaded Gels |
|---|---|---|---|---|
| Hemoglobin (10–15 g/dL) | 13.63 ± 0.05 | 13.66 ± 0.11 | 13.13 ± 0.25 | 13.2 ± 0.1 |
| P.V.C Hematocrit (%) | 39.66 ± 1.52 | 37.36 ± 0.85 | 37.63 ± 0.58 | 38 ± 1 |
| Total red blood cells (4.2–5.9 × 1012 L−1) | 7.68 ± 0.38 | 7.53 ± 0.31 | 7.3 ± 0.07 | 7.43 ± 0.15 |
| Mean corpuscular volume (80–96 fL) | 53.2 ± 0.85 | 53.1 ± 0.60 | 53.33 ± 1.52 | 53 ± 1 |
| Mean corpuscular hemoglobin (MCHb) (27–32 pg) | 21.03 ± 2.98 | 19.4 ± 0.55 | 18.1 ± 1.15 | 17.13 ± 1.02 |
| MCHb Concentration (g/dL) | 36.76 ± 1.34 | 39.96 ± 0.70 | 37.6 ± 0.4 | 35.1 ± 2.85 |
| Total white blood cells /cumm | 8800 ± 100 | 8866 ± 152 | 8800 ± 264 | 9100 ± 200 |
| Neutrophils (%) | 46 ± 1 | 48 ± 1 | 43.66 ± 2.08 | 44 ± 2.64 |
| Lymphocytes (%) | 38.66 ± 1.52 | 35.66 ± 1.52 | 39.33 ± 2.08 | 41 ± 2.64 |
| Monocytes (%) | 12.66 ± 1.52 | 8 ± 1 | 10.33 ± 2.08 | 12.33 ± 1.52 |
| Eosinophils (%) | 6 ± 1 | 6.33 ± 1.52 | 6.66 ± 0.57 | 5.33 ± 1.52 |
| Platelets count/cumm | 248,666 ± 5131 | 308,000 ± 7000 | 317,666 ± 20,404 | 315,666 ± 2516 |
| Observations | Control (Group I) | Treated (Group II) |
|---|---|---|
| Mortality rate | Zero | Zero |
| Ocular toxicity (Lacrimation, redness of conjunctiva) | No toxicity | No toxicity |
| Dermal toxicity (Erythema, swelling, wound formation) | No toxicity | No toxicity |
| Illness signs (Loss of activity) | No sign | No sign |
| Weight of Rabbits (kg) | ||
| Pretreatment | 1.66 ± 0.25 | 1.73 ± 0.25 |
| First Day | 1.5 ± 0.17 | 1.6 ± 0.20 |
| Seventh Day | 1.36 ± 0.11 | 1.43 ± 0.24 |
| Fourteen Day | 1.46 ± 0.15 | 1.58 ± 0.10 |
| Water Intake (mL) | ||
| Pretreatment | 184 ± 4.72 | 192 ± 4.93 |
| First Day | 182 ± 5.50 | 186 ± 3.21 |
| Seventh Day | 187 ± 4.93 | 189 ± 3.05 |
| Fourteen Day | 188 ± 1.52 | 190 ± 5.68 |
| Food Intake (g) | ||
| Pretreatment | 68.33 ± 3.21 | 60.33 ± 3.51 |
| First Day | 69.33 ± 0.57 | 60 ± 3.00 |
| Seventh Day | 68.33 ± 2.51 | 65.33 ± 3.05 |
| Fourteen Day | 68.66 ± 1.52 | 65.66 ± 4.16 |
| Hematology | Control (Group I) | Treated (Group II) |
|---|---|---|
| Hemoglobin (10–15 g/dL) | 13.7 ± 1.21 | 13.06 ± 0.80 |
| TLC (4.5–11 × 109 L−1) | 4.3 ± 0.81 | 5 ± 1 |
| Red Blood Cells (4.2–5.9 × 1012 L−1) | 5.49 ± 0.62 | 5.65 ± 0.35 |
| Platelets (150–400 × 109 L−1) | 166 ± 11.15 | 186 ± 38.21 |
| Monocytes (2–8%) | 5.33 ± 1.52 | 4.33 ± 2.30 |
| Neutrophils (40–60%) | 55 ± 4.35 | 41 ± 17.52 |
| Lymphocytes (20–40%) | 75.33 ± 4.61 | 76.66 ± 4.16 |
| Eosinophils (1–4%) | 4 ± 1 | 3.33 ± 1.52 |
| Mean Corpuscular Volume (80–96 fL) | 65.43 ± 3.98 | 70.2 ± 7.70 |
| MCHb (27–32 pg) | 25.16 ± 4.18 | 25.6 ± 2.95 |
| MCHb Concentration (32–36%) | 33.33 ± 2.89 | 35.46 ± 5.62 |
| Biochemical Analysis | Control (Group I) | Treated (Group II) |
|---|---|---|
| Lipid Profile | ||
| Cholesterol (10–80 mg/dL) | 63.93 ± 3.45 | 65.33 ± 7.23 |
| Triglycerides (46–68 mg/dL) | 54.28 ± 1.82 | 58.33 ± 8.02 |
| Renal Profile | ||
| Creatinine (0.2–0.9 mg/dL) | 0.83 ± 0.20 | 0.7 ± 0.1 |
| Urea (10–50 mg/dL) | 30 ± 10 | 24.66 ± 13.61 |
| Uric acid (3.4–7.1 mg/dL) | 5.13 ± 1.09 | 5.56 ± 0.51 |
| Liver Profile | ||
| Alanine aminotransferase (ALT) (17–77 IU/L) | 34.66 ± 5.03 | 32 ± 11.13 |
| Aspartate aminotransferase (AST) (54–298 IU/L) | 25.33 ± 11.71 | 21 ± 10.41 |
| Group | Stomach (g) | Kidney (g) | Heart (g) | Liver (g) | Lung (g) |
|---|---|---|---|---|---|
| Control | 12.33 ± 1.85 | 10.69 ± 1.16 | 4.08 ± 0.62 | 34.09 ± 3.57 | 16.31 ± 3.05 |
| Treated | 14.66 ± 3.51 | 8.34 ± 1.47 | 3.46 ± 0.47 | 44.15 ± 7.93 | 13.33 ± 4.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ajaz, N.; Khan, I.U.; Irfan, M.; Khalid, S.H.; Asghar, S.; Mehmood, Y.; Asif, M.; Usra; Hussain, G.; Shahzad, Y.; et al. In Vitro and Biological Characterization of Dexamethasone Sodium Phosphate Laden pH-Sensitive and Mucoadhesive Hydroxy Propyl β-Cyclodextrin-g-poly(acrylic acid)/Gelatin Semi-Interpenetrating Networks. Gels 2022, 8, 290. https://doi.org/10.3390/gels8050290
Ajaz N, Khan IU, Irfan M, Khalid SH, Asghar S, Mehmood Y, Asif M, Usra, Hussain G, Shahzad Y, et al. In Vitro and Biological Characterization of Dexamethasone Sodium Phosphate Laden pH-Sensitive and Mucoadhesive Hydroxy Propyl β-Cyclodextrin-g-poly(acrylic acid)/Gelatin Semi-Interpenetrating Networks. Gels. 2022; 8(5):290. https://doi.org/10.3390/gels8050290
Chicago/Turabian StyleAjaz, Nyla, Ikram Ullah Khan, Muhammad Irfan, Syed Haroon Khalid, Sajid Asghar, Yasir Mehmood, Muhammad Asif, Usra, Ghulam Hussain, Yasser Shahzad, and et al. 2022. "In Vitro and Biological Characterization of Dexamethasone Sodium Phosphate Laden pH-Sensitive and Mucoadhesive Hydroxy Propyl β-Cyclodextrin-g-poly(acrylic acid)/Gelatin Semi-Interpenetrating Networks" Gels 8, no. 5: 290. https://doi.org/10.3390/gels8050290
APA StyleAjaz, N., Khan, I. U., Irfan, M., Khalid, S. H., Asghar, S., Mehmood, Y., Asif, M., Usra, Hussain, G., Shahzad, Y., Shah, S. U., & Munir, M. U. (2022). In Vitro and Biological Characterization of Dexamethasone Sodium Phosphate Laden pH-Sensitive and Mucoadhesive Hydroxy Propyl β-Cyclodextrin-g-poly(acrylic acid)/Gelatin Semi-Interpenetrating Networks. Gels, 8(5), 290. https://doi.org/10.3390/gels8050290