Self-Assembly of Alkylamido Isophthalic Acids toward the Design of a Supergelator: Phase-Selective Gelation and Dye Adsorption
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. Gelation Behavior
2.3. Hansen Solubility Parameters
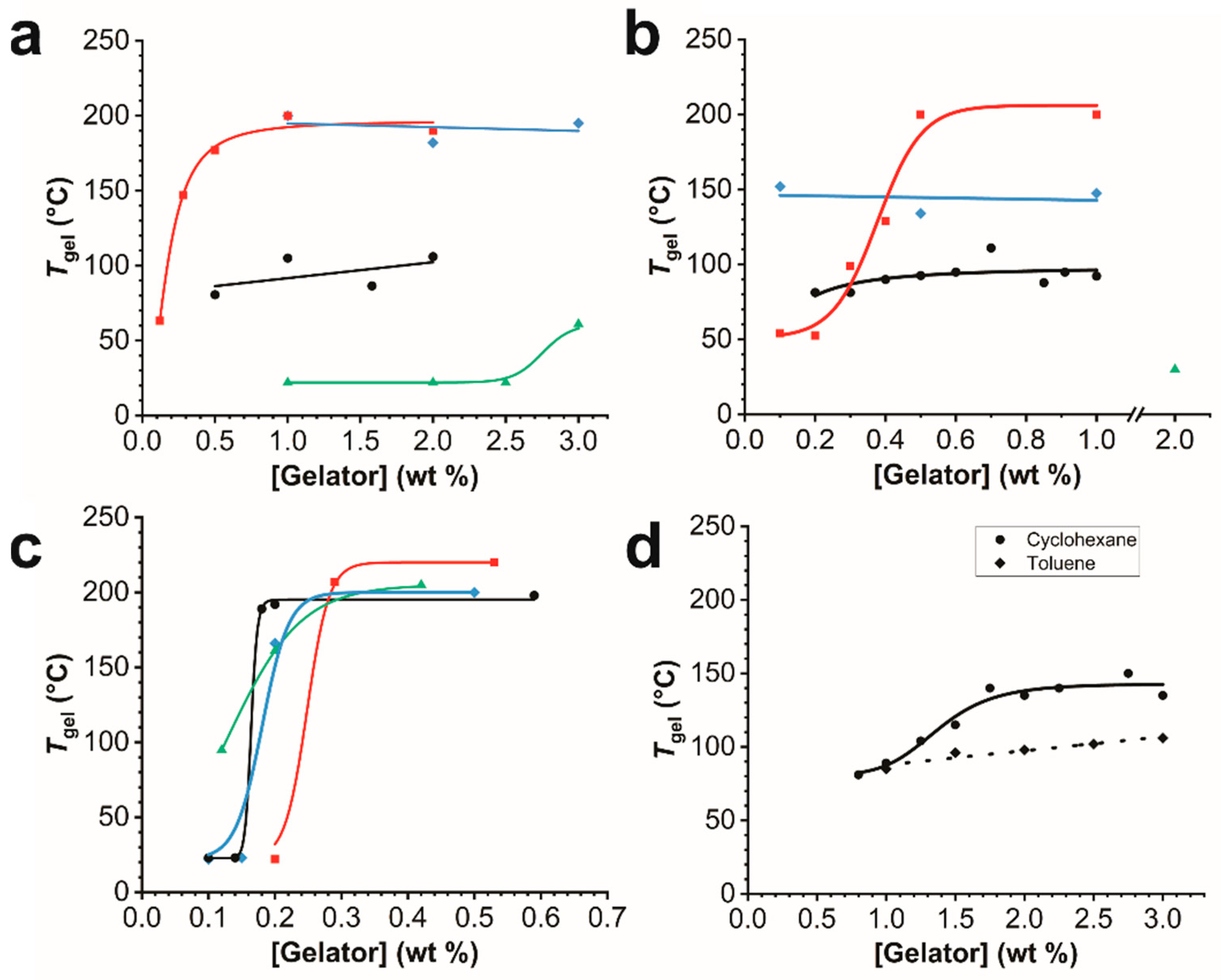
2.4. Thermal Stability
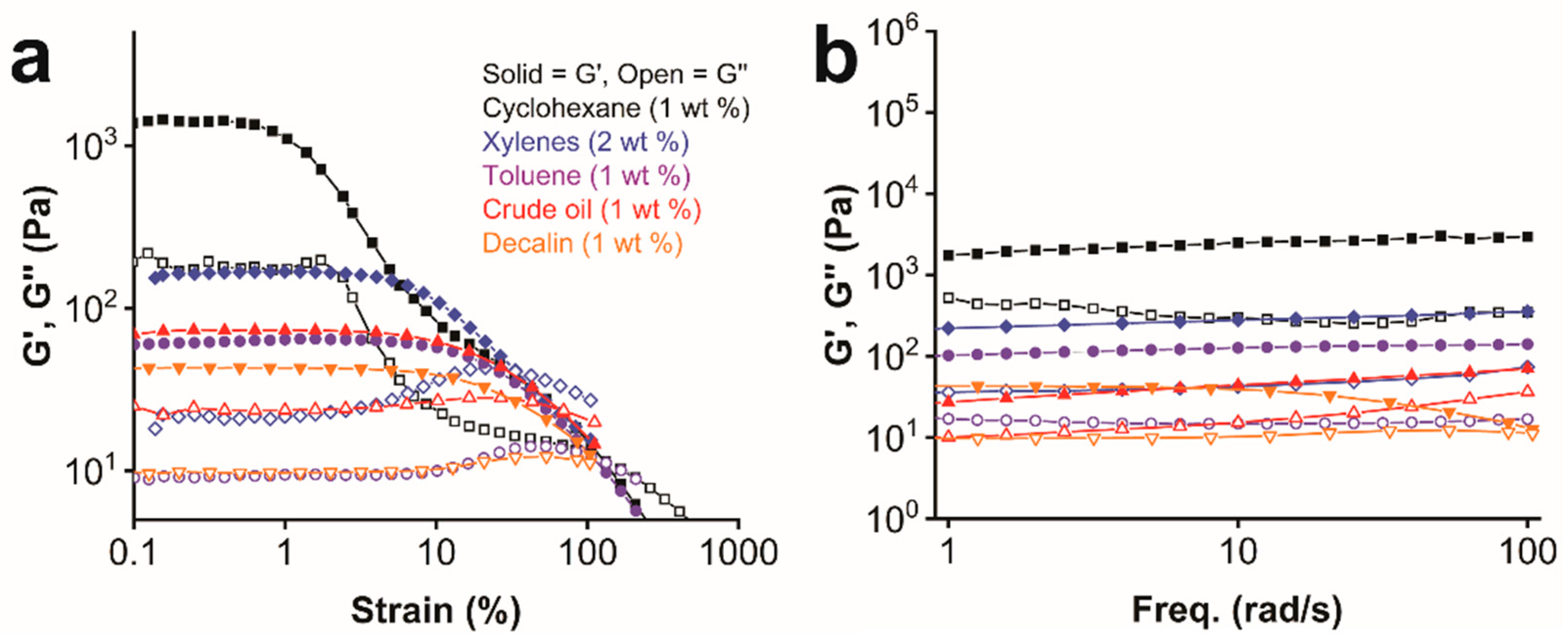
2.5. Rheological Measurements
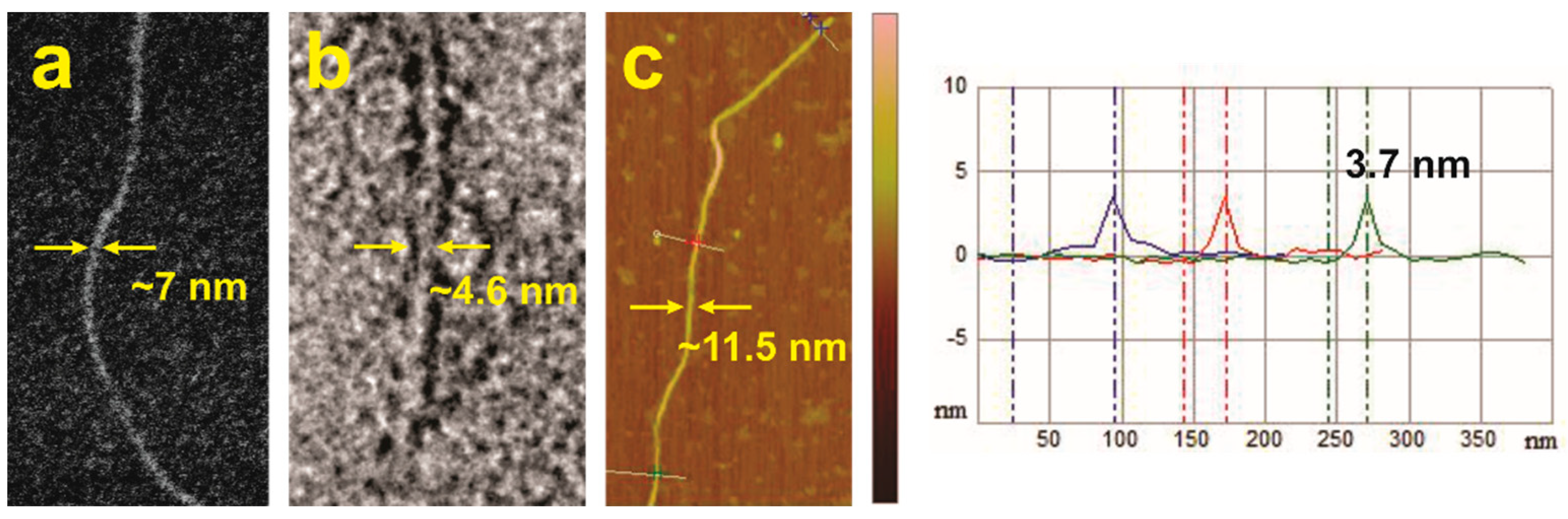
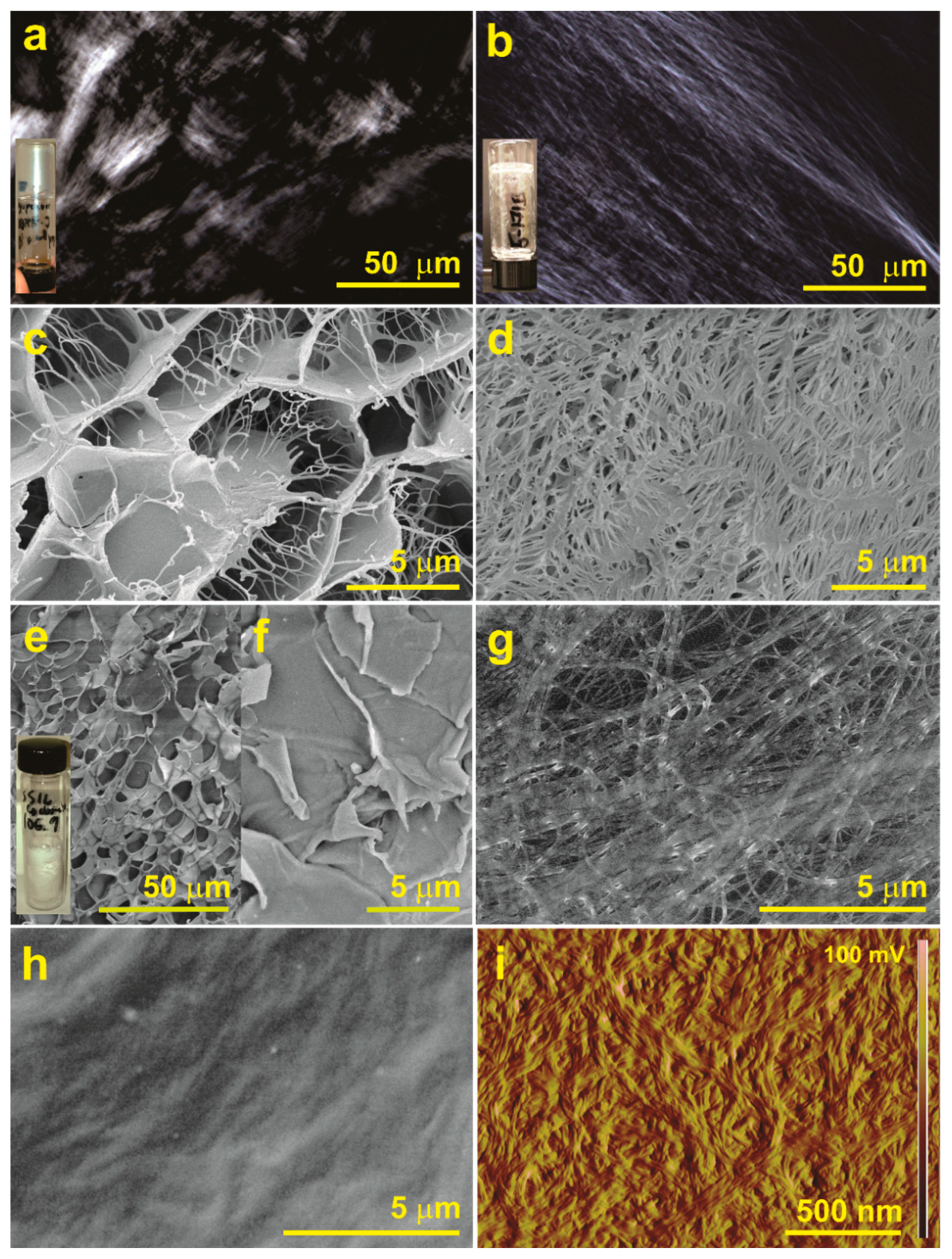
2.6. Microscopy Studies
2.7. Nuclear Magnetic Resonance (NMR) Spectroscopy
2.8. ATR-FTIR Spectroscopy
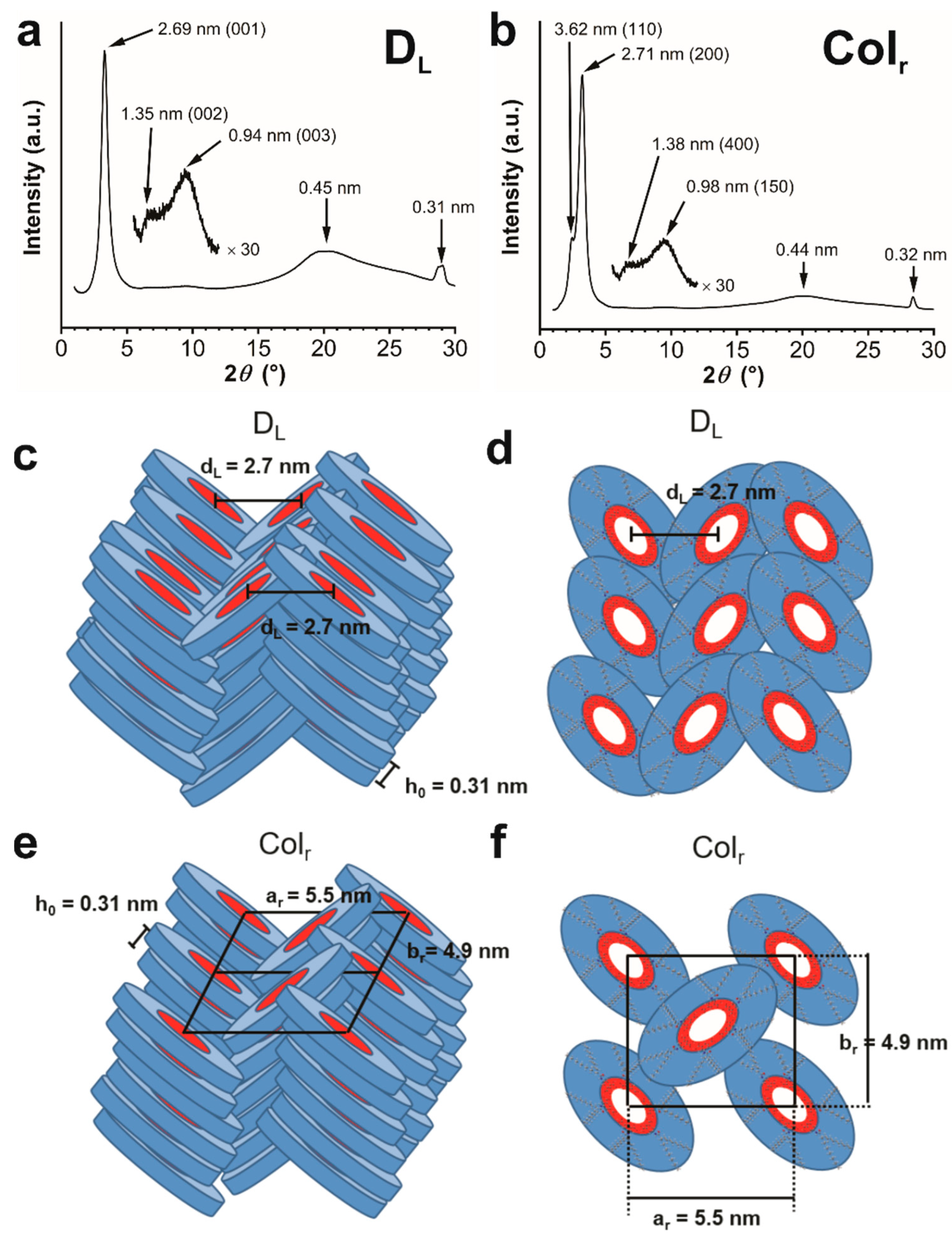
2.9. Powder X-ray Diffraction
2.10. Phase-Selective Gelation
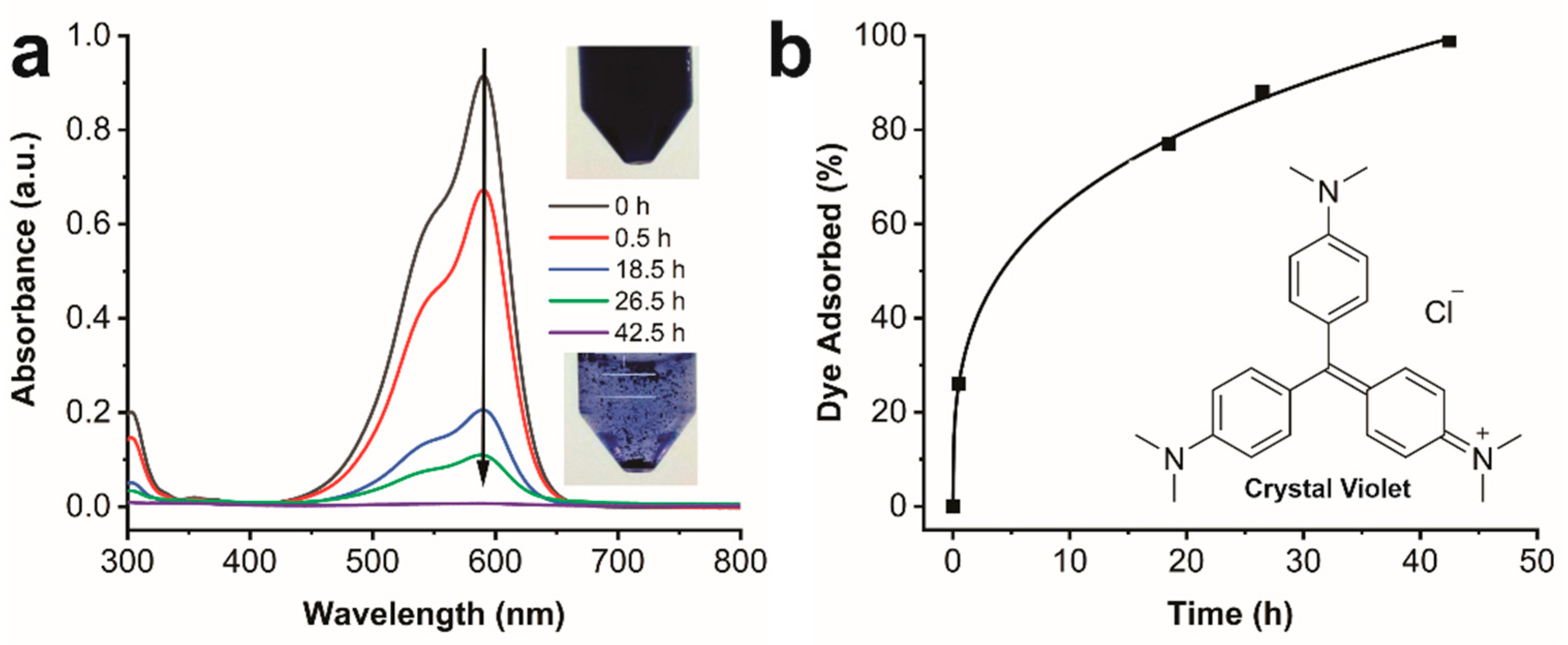
2.11. Dye Removal from Water
3. Conclusions
4. Materials and Methods
4.1. General Information
4.2. Synthesis and Characterization of Gelators ISA4, ISA16L, ISA12, ISA16, and ISA24
4.3. Nuclear Magnetic Resonance (NMR) Spectroscopy
4.4. Mass Spectrometry (MS)
4.5. Gelation Test
4.6. Hansen Solubility Parameters
4.7. Gel-to-Sol Transition Temperature Measurements
4.8. Rheological Measurements
4.9. In Situ Aerogel Preparation (for cryo-SEM)
4.10. Ex Situ Aerogel Preparation
4.11. Thick Xerogel Film Preparation
4.12. Thin Xerogel Film and Self-Assembled Nanostructure Synthesis
4.13. Polarized Optical Microscopy (POM)
4.14. Scanning Electron Microscopy (SEM)
4.15. High-Resolution Transmission Electron Microscopy (HR-TEM)
4.16. Cryo-Scanning Electron Microscopy (cryo-SEM)
4.17. Atomic Force Microscopy (AFM)
4.18. Computer Modeling
4.19. Attenuated Reflectance Fourier Transform Infrared Spectroscopy (ATR-FTIR) Spectroscopy
4.20. Powder X-ray Diffraction (PXRD)
4.21. Crystal Violet (CV) Adsorption
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Terech, P.; Weiss, R.G. Low molecular mass gelators of organic liquids and the properties of their gels. Chem. Rev. 1997, 97, 3133–3160. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.G. The past, present, and future of molecular gels. What is the status of the field, and where is it going? J. Am. Chem. Soc. 2014, 136, 7519–7530. [Google Scholar] [CrossRef] [PubMed]
- Estroff, L.A.; Hamilton, A.D. Water gelation by small organic molecules. Chem. Rev. 2004, 104, 1201–1218. [Google Scholar] [CrossRef] [PubMed]
- Amabilino, D.B.; Smith, D.K.; Steed, J.W. Supramolecular materials. Chem. Soc. Rev. 2017, 46, 2404–2420. [Google Scholar] [CrossRef]
- Liu, M.; Ouyang, G.; Niua, D.; Sang, Y. Supramolecular gelatons: Towards the design of molecular gels. Org. Chem. Front. 2018, 5, 2885–2900. [Google Scholar] [CrossRef]
- Piepenbrock, M.O.M.; Lloyd, G.O.; Clarke, N.; Steed, W. Metal- and anion-binding supramolecular gels. Chem. Rev. 2010, 110, 1960–2004. [Google Scholar] [CrossRef]
- Gronwald, O.; Snip, E.; Shinkai, S. Gelators for organic liquids based on self-assembly: A new facet of supramolecular and combinatorial chemistry. Curr. Opin. Colloid Interface Sci. 2002, 7, 148–156. [Google Scholar] [CrossRef]
- Guenet, J.-M. Organogels: Thermodynamics, Structure, Solvent Role, and Properties; Springer: New York, NY, USA, 2016. [Google Scholar]
- Molecular Gels: Structure and Dynamics; Weiss, R.G. (Ed.) The Royal Society of Chemistry: Cambridge, UK, 2018; Volume 25. [Google Scholar]
- Hirst, A.R.; Escuder, B.; Miravet, J.F.; Smith, D.K. High-tech applications of self-assembling supramolecular nanostructured gel-phase materials: From regenerative medicine to electronic devices. Angew. Chem. Int. Ed. 2008, 47, 8002–8018. [Google Scholar] [CrossRef]
- Sangeetha, N.M.; Maitra, U. Supramolecular gels: Functions and uses. Chem. Soc. Rev. 2005, 34, 821–836. [Google Scholar] [CrossRef] [Green Version]
- Escuder, B.; Miravet, J. Functional Molecular Gels, 1st ed.; Royal Society of Chemistry: Cambridge, UK, 2014. [Google Scholar]
- Esposito, C.L.; Kirilov, P.; Roullin, V.G. Organogels, promising drug delivery systems: An update of state-of-the-art and recent applications. J. Control. Release 2018, 271, 1–20. [Google Scholar] [CrossRef]
- Mayr, J.; Saldia, C.; Diaz, D.D. Release of small bioactive molecules from physical gels. Chem. Soc. Rev. 2018, 47, 1484–1515. [Google Scholar] [CrossRef] [PubMed]
- Chivers, P.R.A.; Smith, D.K. Shaping and structuring supramolecular gels. Nat. Rev. Mater 2019, 4, 463–468. [Google Scholar] [CrossRef] [Green Version]
- Panja, S.; Panjab, A.; Ghosh, K. Supramolecular gels in cyanide sensing: A review. Mater. Chem. Front. 2021, 5, 584–602. [Google Scholar] [CrossRef]
- Bellotto, O.; Cringoli, M.C.; Perathoner, S.; Fornasiero, P.; Marchesan, S. Peptide gelators to template inorganic nanoparticle formation. Gels 2021, 7, 14. [Google Scholar] [CrossRef]
- Fang, W.; Zhang, Y.; Wu, J.; Liu, C.; Zhu, H.; Tu, T. Recent advances in supramolecular gels and catalysis. Chem. Asian J. 2018, 13, 712–729. [Google Scholar] [CrossRef]
- Ohsedo, Y. Low-molecular-weight gelators as base materials for ointments. Gels 2016, 2, 13. [Google Scholar] [CrossRef]
- Singh, A.; Aunzanneau, F.I.; Rogers, M.A. Advances in edible oleogel technologies—A decade in review. Food Res. Int. 2017, 97, 307–317. [Google Scholar] [CrossRef]
- Ajayaghosh, A.; Praveen, V.K.; Vijayakumar, C. Organogels as scaffolds for excitation energy transfer and light harvesting. Chem. Soc. Rev. 2008, 37, 109–122. [Google Scholar] [CrossRef]
- Ohsedo, Y. Low-molecular-weight organogelators as functional materials for oil spill remediation. Polym. Adv. Technol. 2016, 27, 4226–4251. [Google Scholar] [CrossRef]
- Okesola, B.; Smith, D.K. Applying low-molecular weight supramolecular gelators in an environmental setting—Self assembled gels as smart materials for pollutant removal. Chem. Soc. Rev. 2016, 45, 4226–4251. [Google Scholar] [CrossRef] [Green Version]
- Weiss, R.; Terech, P. Molecular Gels Materials with Self-Assembled Fibrillar Networks, 1st ed.; Weiss, R., Terech, P., Eds.; Springer: Dordrect, The Netherlands, 2006; Volume 256. [Google Scholar]
- Menger, F.M.; Lee, S.J. Long organic fibers obtained by noncovalent synthesis. J. Am. Chem. Soc. 1994, 116, 5987–5988. [Google Scholar] [CrossRef]
- Lee, J.S.; Lee, S.; Kim, J.M. Fluorogenic conjugated polymer fibers from amphiphilic diacetylene supramolecules. Macromol. Res. 2008, 16, 73–75. [Google Scholar] [CrossRef]
- Meiners, C.; DeFeyter, S.; Lieser, G.; van Stam, J.; Soltermann, A.; Berghmans, H.; De Schryver, F.C.; Müllen, K. Identification and characterization of aggregates formed by a partly neutralized isophthalic acid derivative in aqueous solution. Langmuir 1999, 15, 3374–3380. [Google Scholar] [CrossRef]
- Valiyaveettil, S.; Enkelrnann, V.; Müllen, K. Supramolecular structures formed from hydrogen-bonded networks of 5-alkoxyisophthalic acid. Chem. Commun. 1994, 2097–2098. [Google Scholar] [CrossRef]
- Togari, Y.; Hirota, S.; Kitagawa, H.; Tsukamoto, Y.; Kobayashi, K. Hydrogen-bonded six-component assembly for capsule formation based on tetra(4-pyridyl) cavitand and isophthalic acid linker and its application to photoresponsive capsule. Org. Biomol. Chem. 2018, 16, 7626–7635. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, S.C.; Zeng, F.; Reichert, D.E.C.; Kolotuchin, S.V. Self-assembling dendrimers. Science 1996, 271, 1095–1098. [Google Scholar] [CrossRef] [PubMed]
- de Feyter, S.; Gesquière, A.; Klapper, M.; Müllen, K.; de Schryver, F.C. Toward two-dimensional supramolecular control of hydrogen-bonded arrays: The case of isophthalic acids. Nano Lett. 2003, 3, 1485–1488. [Google Scholar] [CrossRef]
- Ye, B.-H.; Tong, M.-L.; Chen, X.-M. Metal-organic molecular architectures with 2,2′-bipyridyl-like and carboxylate ligands. Coord. Chem. Rev. 2005, 249, 545–565. [Google Scholar] [CrossRef]
- Mai, H.D.; Kang, P.; Kim, J.K.; Yoo, H. A cobalt supramolecular triple-stranded helicate-based discrete molecular cage. Sci. Rep. 2017, 7, 43448. [Google Scholar] [CrossRef]
- Sautter, A.; Thalacker, C.; Heise, B.; Würthner, F. Hydrogen bond-directed aggregation of diazadibenzoperylene dyes in lowpolarity solvents and the solid state. Proc. Nat. Acad. Sci. USA 2002, 99, 4993–4996. [Google Scholar] [CrossRef] [Green Version]
- Wei, M.-H.; Li, B.; David, R.L.A.; Jones, S.C.; Sarohia, V.; Schmitigal, J.A.; Kornfield, J.A. Megasupramolecules for safer, cleaner fuel by end association of long telechelic polymers. Science 2015, 350, 72–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Espinosa, L.M.; Balog, S.; Weder, C. Isophthalic acid−pyridine h-bonding: Simplicity in the design of mechanically robust phase-segregated supramolecular polymers. ACS Macro Lett. 2014, 31, 540–543. [Google Scholar] [CrossRef]
- Alcala, R.; Martinez-Carrera, S. The crystal structure of isophthalic acid. Acta Cryst. B 1972, 28, 1671–1677. [Google Scholar] [CrossRef]
- Enkelmann, V.; Valiyaveettil, S.; Möessner, G.; Müllen, K. Self-assembly of 5-alkoxyisophthalic acids: Alkyl chain length dependence for the formation of channel-type and sheet-type structures. Supramol. Sci. 1995, 2, 3–7. [Google Scholar] [CrossRef]
- Yang, J.; Marendaz, J.L.; Geib, S.J.; Hamilton, A.D. Hydrogen bonding control of self-assembly: Simple isophthalic acid derivatives form cyclic hexameric aggregates. Tet. Lett. 1994, 35, 3665–3668. [Google Scholar] [CrossRef]
- Potluri, V.K.; Hamilton, A.D. Isophthalic acid-derived organogelators. J. Supramol. Chem. 2002, 2, 321–326. [Google Scholar] [CrossRef]
- Lv, C.; Takeda, T.; Hoshino, N.; Akutagawa, T. Tubular and lamellar hydrogen-bonding molecular assemblies of isophthalic acid derivatives bearing a –CONHCnH2n+1 chain. RSC Adv. 2018, 8, 22250–22258. [Google Scholar] [CrossRef] [Green Version]
- Zafar, A.; Yang, J.; Geib, S.J.; Hamilton, A.D. Linked bis-isophthalic acid derivatives as building blocks in the design of self-assembling structures. Tet. Lett. 1996, 37, 2327–2330. [Google Scholar] [CrossRef]
- Sahoo, P.; Adarsh, N.N.; Chacko, G.E.; Raghavan, S.R.; Puranik, V.G.; Dastidar, P. Combinatorial library of primaryalkylammonium dicarboxylate gelators: A supramolecular synthon approach. Langmuir 2009, 25, 8742–8750. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, S.; Xiang, S.; Huang, J.; Chen, L.; Su, C.-Y. Heterometallic coordination polymer gels based on a rigid, bifunctional ligand. Chem. Eur. J. 2011, 17, 2369–2372. [Google Scholar] [CrossRef]
- Wood, D.M.; Greenland, B.W.; Acton, A.L.; Rodríguez-Llansola, F.; Murray, C.A.; Cardin, C.J.; Miravet, J.F.; Escuder, B.; Hamley, I.W.; Hayes, W. Ph-Tunable Hydrogelators for Water Purification: Structural Optimisation And Evaluation. Chem. Eur. J. 2012, 18, 2692–2699. [Google Scholar] [CrossRef] [PubMed]
- Mallick, A.; Schön, E.-M.; Panda, T.; Sreenivas, K.; Díaz, D.D.; Banerjee, R. Fine-tuning the balance between crystallization and gelation and enhancement of CO2 uptake on functionalized calcium based MOFs and metallogels. J. Mater. Chem. 2012, 22, 14951–14963. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wang, T.; Liu, M. Supramolecular polymer hydrogels from bolaamphiphilic l-histidine and benzene dicarboxylic acids: Thixotropy and significant enhancement of Eu III fluorescence. Chem. Eur. J. 2012, 18, 14650–14659. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.; Li, L.; Zhang, J.; Tan, X.; Cui, H.; Shi, J.; Hu, J.; Chen, L.; Su, C.Y.; James, S.L. Porous organic–inorganic hybrid aerogels based on Cr3+/Fe3+ and rigid bridging carboxylates. J. Mater. Chem. 2012, 22, 1862–1867. [Google Scholar] [CrossRef]
- Yan, X.; Li, S.; Cook, T.R.; Ji, X.; Yao, Y.; Pollock, J.B.; Shi, Y.; Yu, G.; Li, J.; Huang, F.; et al. Hierarchical self-assembly: Well-defined supramolecular nanostructures and metallohydrogels via amphiphilic discrete organoplatinum(ii) metallacycles. J. Am. Chem. Soc. 2013, 135, 14036–14039. [Google Scholar] [CrossRef]
- Bag, K.; Sukul, P.K.; Santra, D.C.; Roy, A.; Malik, S. Proton induced aggregation of water soluble isophthalic acid appended arylene diimides: Justification with perylene derivative. RSC Adv. 2016, 6, 34027–34037. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, X.; Zhao, M.; Lu, F.; Zheng, L. Formation of supermolecular chiral gels from L-aspartic acid-based perylenebisimides and benzene dicarboxylic acids. New J. Chem. 2017, 41, 7643–7649. [Google Scholar] [CrossRef]
- Häring, M.; Rodríguez-López, J.; Grijalvo, S.; Tautz, M.; Eritja, R.; Martín, V.S.; Díaz, D.D. Isosteric substitution of 4H-1,2,4-triazole by 1H-1,2,3-triazole in isophthalic derivative enabled hydrogel formation for controlled drug delivery. Mol. Pharmaceutics 2018, 15, 2963–2972. [Google Scholar]
- Zacharias, S.C.; Ramon, G.; Bourne, S.A. Supramolecular metallogels constructed from carboxylate gelators. Soft Matter 2018, 14, 4505–4519. [Google Scholar] [CrossRef]
- Sarkar, K.; Dastidar, P. Rational approach towards designing metallogels from aurea-functionalized pyridyl dicarboxylate: Anti-inflammatory, anticancer, and drug delivery. Chem. Asian J. 2019, 14, 194–204. [Google Scholar] [CrossRef]
- Vibhute, A.M.; Sureshan, K.M. How far are we in combating marine oil spills by using phase-selective organogelators? ChemSusChem 2020, 13, 5343–5360. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Krishnan-Ghosh, Y. First report of phase selective gelation of oil from oil/water mixtures. Possible implications toward containing oil spills. Chem. Commun. 2001, 2, 185–186. [Google Scholar] [CrossRef]
- Jadhav, S.R.; Vemula, P.K.; Kumar, R.; Raghavan, S.R.; John, G. Sugar-derived phase-selective molecular gelators as model solidifiers for oil spills. Angew. Chem. Int. Ed. 2010, 49, 1–5. [Google Scholar]
- Ray, S.; Das, A.K.; Banerjee, A. Ph-responsive, bolaamphiphile-based smart metallo-hydrogels as potential dye-adsorbing agents, water purifier, and vitamin b12 carrier. Chem. Mater. 2007, 19, 1633–1639. [Google Scholar] [CrossRef]
- Adhikari, B.; Palui, G.; Banerjee, A. Self-assembling tripeptide based hydrogels and their use in removal of dyes from waste-water. Soft Matter 2009, 5, 3452–3460. [Google Scholar] [CrossRef]
- Marullo, S.; Meli, A.; Dintcheva, N.T.; D’Anna, F. Environmentally friendly eutectogels comprising L-amino acids and deep eutectic solvents: Efficient materials for wastewater treatment. ChemPlusChem 2020, 85, 301–311. [Google Scholar] [CrossRef]
- ter Halle, A.; Claparols, C.; Garrigues, J.C.; Franceschi-Messant, S.; Perez, E. Development of an extraction method based of new porous organogel materials coupled with liquid chromatography-mass spectrometry for the rapid quantification of bisphenol A in urine. J. Chromatogr. A. 2015, 1414, 1–9. [Google Scholar] [CrossRef]
- Carlini, R.; Makeiff, D.A. Nanosized Particles of Benzimidazolone Pigments. U.S. Patent US7905954B2, 15 March 2011. Available online: https://www.uspto.gov (accessed on 3 May 2022).
- Makeiff, D.A.; Carlini, R. Sterically Bulky Stabilizers. U.S. Patent US9067878B2, 23 April 2013. Available online: https://www.uspto.gov (accessed on 3 May 2022).
- Makeiff, D.A.; Carlini, R. Self-Assembled Nanostructures. U.S. Patent US8703988B2, 22 April 2014. Available online: https://www.uspto.gov (accessed on 3 May 2022).
- Makeiff, D.A.; Carlini, R. Organogel Compositions Comprising Alkylated Aromatic Acids. U.S. Patent US9067878B2, 30 June 2015. Available online: https://www.uspto.gov (accessed on 3 May 2022).
- Makeiff, D.A.; Carlini, R. Phase Selective Gelation with Alkylated Aromatic Acid Compounds. U.S. Patent US9623435B2, 18 April 2017. Available online: https://www.uspto.gov (accessed on 3 May 2022).
- Makeiff, D.A.; Cho, J.Y.; Godbert, N.; Smith, B.; Azyat, K.; Wagner, A.; Kulka, M.; Carlini, R. Supramolecular gels from alkylated benzimidazolone derivatives. J. Mol. Liq. 2021, 339, 116723–116737. [Google Scholar] [CrossRef]
- Murata, K.; Aoki, M.; Suzuki, T.; Harada, T.; Kawabata, H.; Komori, T.; Ohseto, F.; Ueda, K.; Shinkai, S. Thermal and light control of the sol-gel phase transition in cholesterol-based organic gels. Novel helical aggregation modes as detected by circular dichroism and electron microscopic observation. J. Am. Chem. Soc. 1994, 116, 6664–6676. [Google Scholar] [CrossRef]
- Luboradzki, R.; Gronwald, O.; Ikeda, A.; Shinkai, S. Sugar-integrated “supergelators” which can form organogels with 0.03–0.05% [g mL−1]. Chem. Lett. 2000, 29, 1148–1149. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters A User’s Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Lan, Y.; Corradini, M.G.; Weiss, R.G.; Raghavanc, S.R.; Rogers, M.A. To gel or not to gel: Correlating molecular gelation with solvent parameters. Chem. Soc. Rev. 2015, 44, 6035–6058. [Google Scholar] [CrossRef] [PubMed]
- Nasr, P.; Corradini, M.G.; Hill, J.; Read, S.T.; Rosendahl, S.M.; Weiss, R.G.; Auzanneau, F.-I.; Rogers, M.A. Hansen solubility parameters clarify the role of the primary and secondary hydroxyl groups on the remarkable self-assembly of 1:3,2:4-dibenzylidene sorbitol. J. Phys. Chem. C 2020, 124, 2645–26466. [Google Scholar] [CrossRef]
- Nunes, D.R.; Reche-Tamayo, M.; Ressouche, E.; Raynal, M.; Isare, B.; Foury-Leylekian, P.; Albouy, P.-A.; Brocorens, P.; Lazzaroni, R.; Bouteiller, L. Organogel formation rationalized by Hansen solubility parameters: Shift of the gelation sphere with the gelator structure. Langmuir 2019, 35, 7970–7977. [Google Scholar] [CrossRef] [PubMed]
- Raynal, M.; Bouteiller, L. Organogel formation rationalized by Hansen solubility parameters. Chem. Commun. 2011, 47, 8271–8273. [Google Scholar] [CrossRef] [PubMed]
- Abbott, S.; Hansen, C.M.; Yamamoto, H. Hansen Solubility Parameters in Practice, 5th ed.; Hansen-Solubility.com: Boca Raton, FL, USA, 2015. [Google Scholar]
- Takahashi, A.; Sakai, M.; Kato, T. Melting temperature of thermally reversible gel. VI. Effect of branching on the sol-gel transition of polyethylene gels. Polym. J. 1980, 12, 335–341. [Google Scholar] [CrossRef]
- Makarević, J.; Jokić, M.; Frkanec, L.; Katalenić, D.; Žinić, M. Gels with exceptional thermal stability formed by bis(amino acid) oxalamide gelators and solvents of low polarity. Chem. Commun. 2002, 19, 2238–2239. [Google Scholar] [CrossRef] [PubMed]
- Dawn, A.; Kumari, H. Low molecular weight supramolecular gels under shear: Rheology as the tool for elucidating structure–function correlation. Chem. Eur. J. 2018, 24, 762–776. [Google Scholar] [CrossRef]
- Yu, G.; Wan, X.; Han, C.; Huang, F. Characterization of supramolecular gels. Chem. Soc. Rev. 2013, 42, 6697–6722. [Google Scholar] [CrossRef]
- Ikeda, S.; Nishinari, K. “Weak gel”-type rheological properties of aqueous dispersions of nonaggregated κ-carrageenan helices. J. Agric. Food Chem. 2001, 49, 4436–4441. [Google Scholar] [CrossRef]
- Collin, D.; Covis, R.; Allix, F.; Jamart-Grégoire, B.; Martinoty, P. Jamming transition in solutions containing organogelator molecules of amino-acid type: Rheological and calorimetry experiments. Soft Matter 2013, 9, 2947–2958. [Google Scholar] [CrossRef]
- Samorí, P.; Francke, V.; Mangel, T.; Müllen, K.; Rabe, J.P. Poly-para-phenylene-ethynylene assemblies for a potential molecular nanowire: An SFM study. Opt. Mater. 1998, 9, 390–393. [Google Scholar] [CrossRef]
- Banno, M.; Yamaguchi, T.; Nagai, K.; Kaiser, C.; Hecht, S.; Yashima, E. Optically active, amphiphilic poly(meta-phenylene ethynylene)s: Synthesis, hydrogen-bonding enforced helix stability, and direct AFM observation of their helical structures. J. Am. Chem. Soc. 2012, 134, 8718–8728. [Google Scholar] [CrossRef] [PubMed]
- Tasaki-Handa, T.; Abe, Y.; Ooi, K. Fabrication of a monolithic cryogel from the cyclohexane organogel of a coordination polymer based on a phosphoester. RSC Adv. 2016, 6, 71404–71408. [Google Scholar] [CrossRef]
- Tripathi, A.; Parsons, G.N.; Khan, S.A.; Rojas, O.J. Synthesis of organic aerogels with tailorable morphology and strength by controlled solvent swelling following Hansen solubility. Sci. Rep. 2018, 8, 2106. [Google Scholar] [CrossRef] [PubMed]
- Mears, L.L.E.; Draper, E.R.; Castilla, A.M.; Zhuola, H.S.; Dietrich, B.; Nolan, M.C.; Smith, G.N.; Doutch, J.; Rogers, S.; Akhtar, R.; et al. Drying affects the fiber network in low molecular weight hydrogels. Biomacromolecules 2017, 18, 3531–3540. [Google Scholar] [CrossRef]
- Kouwer, P.H.J.; Koepf, M.; Le Sage, V.A.A.; Jaspers, M.; van Buul, A.M.; Eksteen-Akeroyd, Z.H.; Woltinge, T.; Schwartz, E.; Kitto, H.J.; Hoogenboom, R.; et al. Responsive biomimetic networks from polyisocyanopeptide hydrogels. Nature 2013, 493, 651–655. [Google Scholar] [CrossRef] [Green Version]
- Escuder, B.; Lusar, M.L.; Miravet, J.F. Insight on the NMR study of supramolecular gels and its application to monitor molecular recognition on self-assembled fibers. J. Org. Chem. 2006, 71, 7747–7752. [Google Scholar] [CrossRef]
- Shapiro, Y.E. Structure and dynamics of hydrogels and organogels: An NMR spectroscopy approach. Prog. Polym. Sci. 2011, 36, 1184. [Google Scholar] [CrossRef]
- Duncan, D.C.; Whitten, D.G. 1H NMR Investigation of the composition, structure, and dynamics of cholesterol-stilbene tethered dyad organogels. Langmuir 2000, 16, 6445–6452. [Google Scholar] [CrossRef]
- Wallace, M.; Iggo, J.; Adams, D.J. Using solution state NMR spectroscopy to probe NMR invisible gelators. Soft Matter 2015, 11, 7739–7747. [Google Scholar] [CrossRef] [Green Version]
- Zeng, F.; Zimmerman, S.C.; Kolotuchin, S.V.; Reichert, D.E.C.; Ma, Y. Supramolecular polymer chemistry: Design, synthesis, characterization, and kinetics, thermodynamics, and fidelity of formation of self-assembled dendrimers. Tetrahedron 2002, 58, 825–843. [Google Scholar] [CrossRef]
- Balakrishnan, C.; Manonmani, M.; Ahamed, S.R.; Vinitha, G.; Meenakshisundarama, S.P.; Sockalingam, R.M. Supramolecular cocrystals of O-H---O hydrogen bonded 18-crown-6 with isophthalic acid derivatives: Hirshfeld surface analysis and thirdorder nonlinear optical properties. Acta Cryst. 2020, B76, 241–251. [Google Scholar] [CrossRef]
- Kato, T.; Fréchet, J.M.J.; Wilson, P.; Saito, T.; Uryu, T.; Fujishima, A.; Jin, C.; Kaneuchi, F. Hydrogen-bonded liquid crystals. Novel mesogens incorporating nonmesogenic bipyridyl compounds through complexation between H-bond donor and acceptor moieties. Bull. Chem. Soc. Jpn. 1993, 66, 3581–3584. [Google Scholar] [CrossRef]
- Godbert, N.; Crispini, A.; Ghedhini, M.; Carini, M.; Chiaravalloti, F.; Ferrise, A. LCDiXRay: A user-friendly program for powder diffraction indexing of columnar liquid crystals. J. Appl. Cryst. 2014, 47, 668–669. [Google Scholar] [CrossRef] [Green Version]
- Prasad, S.K.; Rao, S.S.; Chrandrasekhar, S.; Kumar, S. X-ray studies on the columnar structures of discotic liquid crystals. Mol. Cryst. Liq. Cryst. 2003, 396, 121–139. [Google Scholar] [CrossRef]
- Gearba, R.I.; Lehmann, M.; Levin, J.; Ivanov, D.A.; Koch, M.H.J.; Barberá, J.; Debije, M.G.; Piris, J.; Geerts, Y.H. Tailoring discotic mesophases: Columnar order enforced with hydrogen bonds. Adv. Mater. 2003, 15, 1614–1618. [Google Scholar] [CrossRef]
- Scarpelli, F.; Ionescu, A.; Aiello, I.; La Deda, M.; Crispini, A.; Ghedini, M.; Brunelli, E.; Sesti, S.; Godbert, N. High order in a self-assembled iridium(III) complex gelator towards nanostructured IrO2 thin films. Chem. Asian J. 2017, 12, 2703–2710. [Google Scholar] [CrossRef]
- Rodríguez-Llansola, F.; Escuder, B.; Miravet, J.F.; Hermida-Merino, D.; Hamley, I.W.; Cardin, C.J.; Hayes, W. Selective and highly efficient dye scavenging by a pH-responsive molecular hydrogelator. Chem. Commun. 2010, 46, 7960–7962. [Google Scholar] [CrossRef] [Green Version]
- Terech, P.; Rossat, C.; Volino, F. On the measurement of phase transition temperatures in physical molecular organogels. J. Colloid Interface Sci. 2000, 227, 363–370. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makeiff, D.A.; Cho, J.-Y.; Smith, B.; Carlini, R.; Godbert, N. Self-Assembly of Alkylamido Isophthalic Acids toward the Design of a Supergelator: Phase-Selective Gelation and Dye Adsorption. Gels 2022, 8, 285. https://doi.org/10.3390/gels8050285
Makeiff DA, Cho J-Y, Smith B, Carlini R, Godbert N. Self-Assembly of Alkylamido Isophthalic Acids toward the Design of a Supergelator: Phase-Selective Gelation and Dye Adsorption. Gels. 2022; 8(5):285. https://doi.org/10.3390/gels8050285
Chicago/Turabian StyleMakeiff, Darren A., Jae-Young Cho, Bradley Smith, Rina Carlini, and Nicolas Godbert. 2022. "Self-Assembly of Alkylamido Isophthalic Acids toward the Design of a Supergelator: Phase-Selective Gelation and Dye Adsorption" Gels 8, no. 5: 285. https://doi.org/10.3390/gels8050285
APA StyleMakeiff, D. A., Cho, J.-Y., Smith, B., Carlini, R., & Godbert, N. (2022). Self-Assembly of Alkylamido Isophthalic Acids toward the Design of a Supergelator: Phase-Selective Gelation and Dye Adsorption. Gels, 8(5), 285. https://doi.org/10.3390/gels8050285








