A Co-Polymerizable Linker for the Covalent Attachment of Fibronectin Makes pHEMA Hydrogels Cell-Adhesive
Abstract
:1. Introduction
2. Results and Discussion
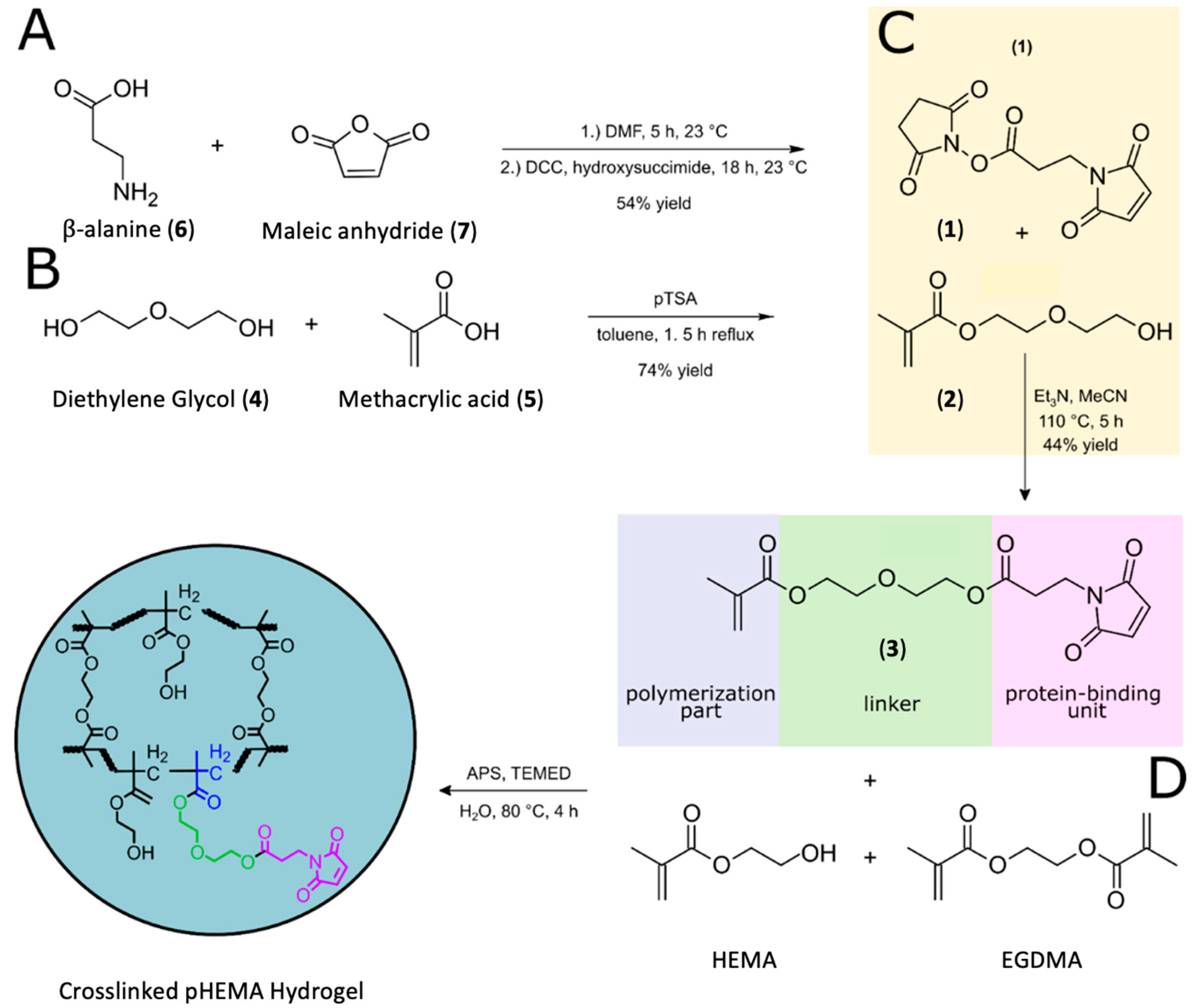
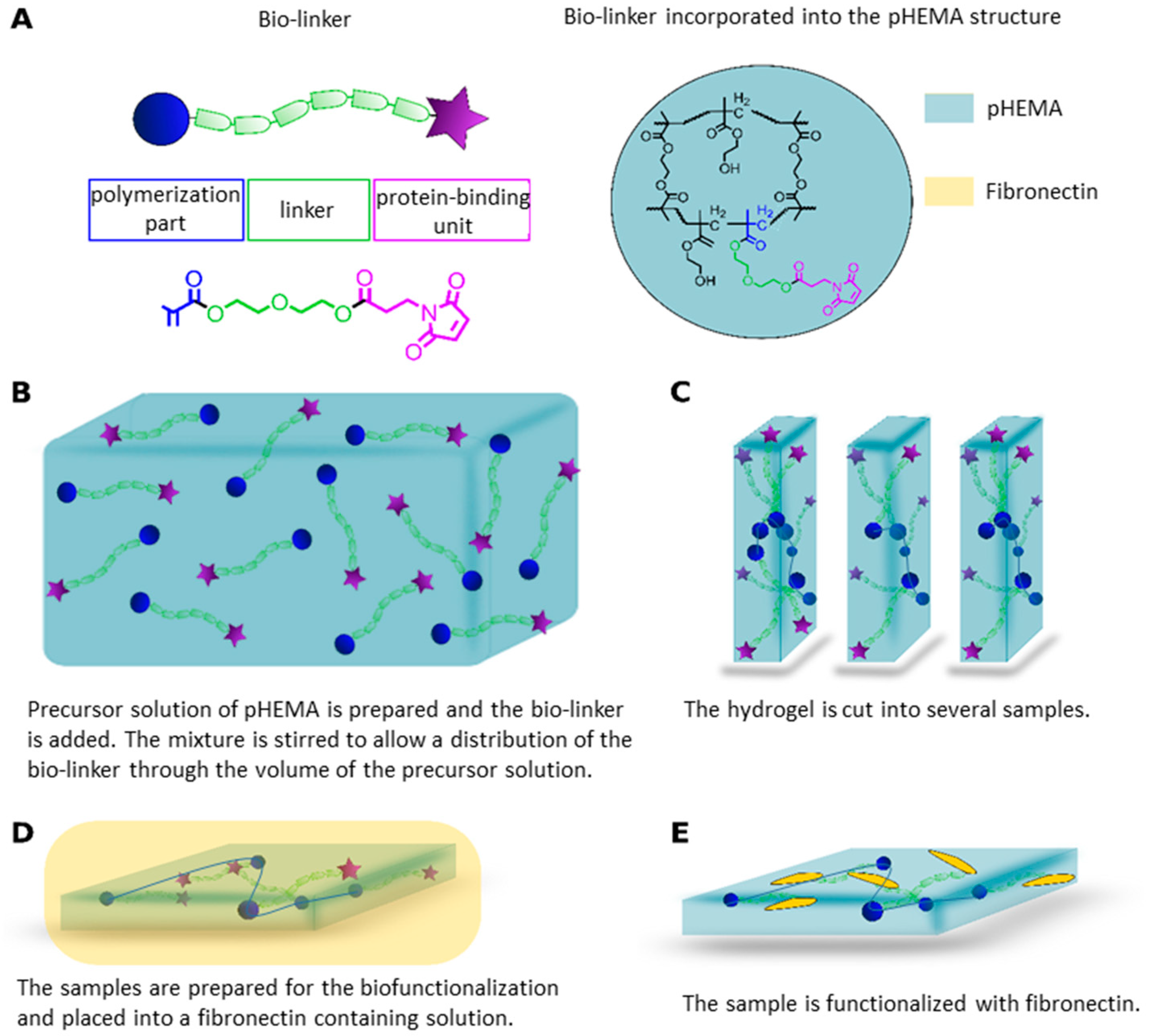
2.1. Bio-Linker Synthesis
2.2. Polymer Synthesis
2.3. Rheology Measurements for Demonstration of Viscoelasticity of Hydrogels
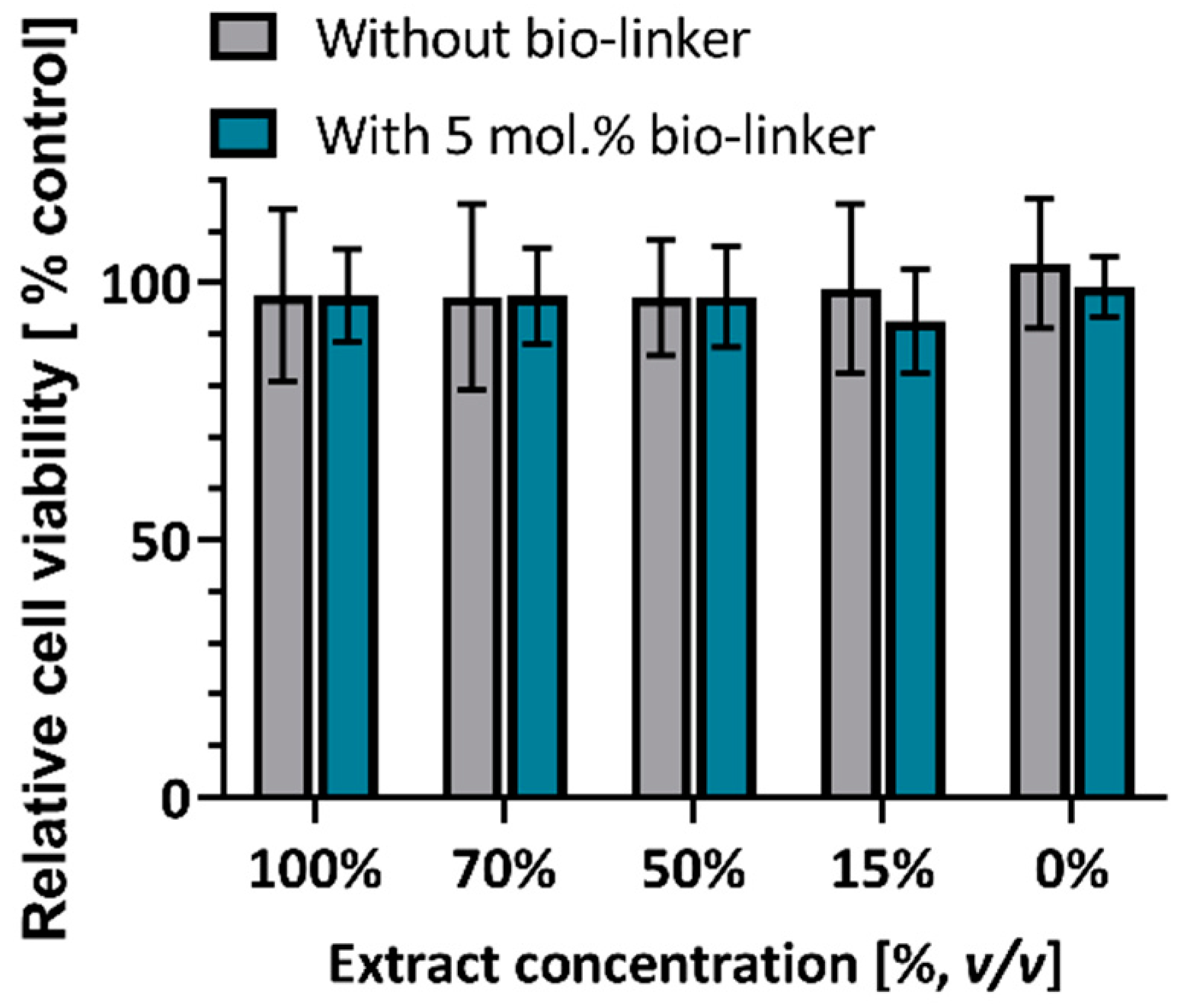
2.4. MTT Assays for Assesment of Biocompatibility of Hydrogels
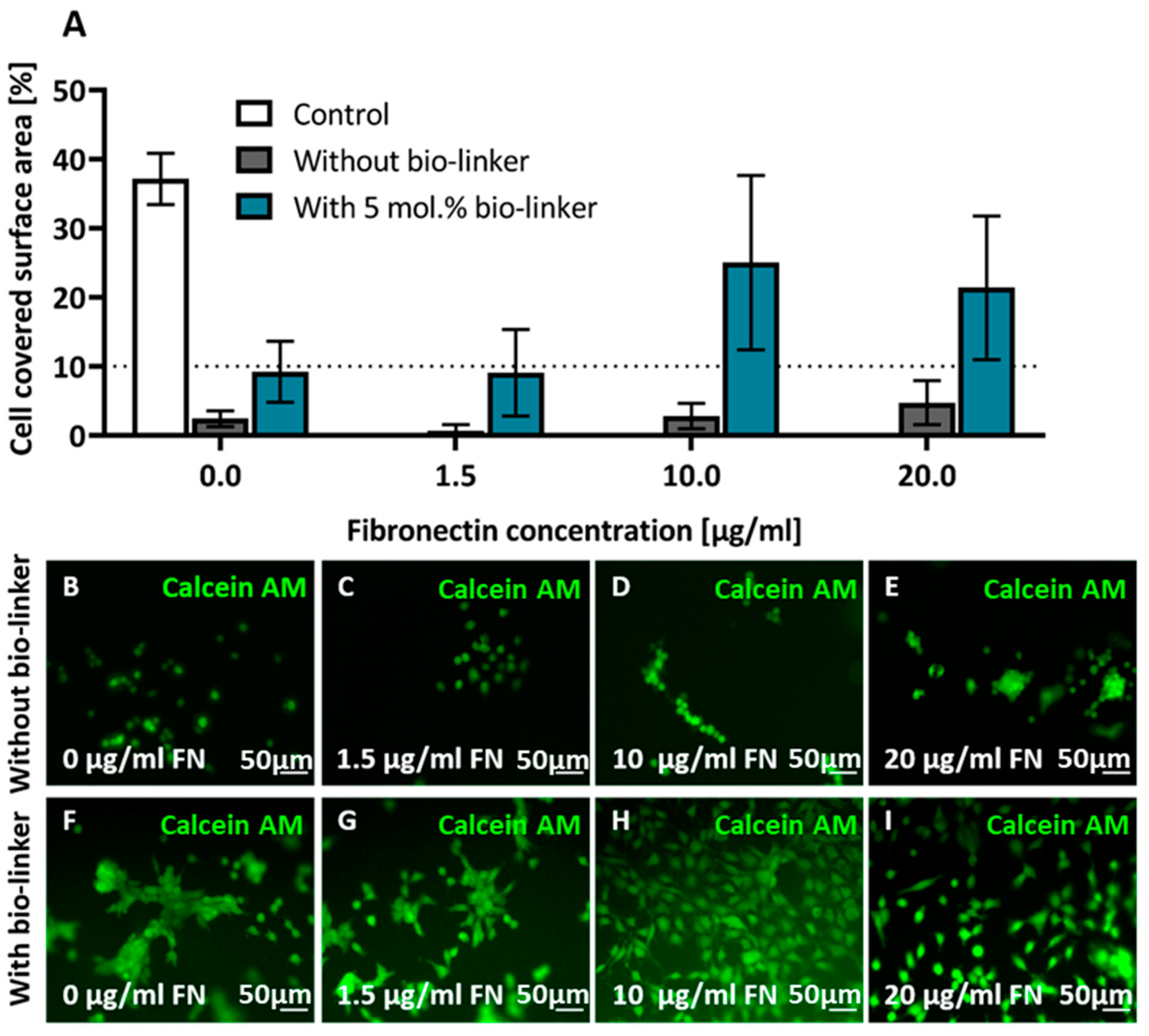
2.5. Cell Adhesion on pHEMA Modified with Bio-Linker (3)
3. Conclusions
4. Materials and Methods
4.1. pHEMA Polymer Synthesis
4.2. Rheology
4.3. Cell Culture
4.4. MTT Assay
4.5. Hydrogel-Functionalisation with Fibronectin
4.6. Cell Adhesion Assay
4.7. Cell Imaging
4.8. Image/Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bershadsky, A.; Chausovsky, A.; Becker, E.; Lyubimova, A.; Geiger, B. Involvement of microtubules in the control of adhesion-dependent signal transduction. Curr. Biol. 1996, 6, 1279–1289. [Google Scholar] [CrossRef] [Green Version]
- Ahearne, M. Introduction to cell—hydrogel mechanosensing. Interface Focus 2014, 4, 20130038. [Google Scholar] [CrossRef] [PubMed]
- Ruprecht, V.; Monzo, P.; Ravasio, A.; Yue, Z.; Makhija, E.; Strale, P.O.; Gauthier, N.; Shivashankar, V.G.; Studer, V.; Albiges-Rizo, C.; et al. How cells respond to environmental cues—Insights from bio-functionalized substrates. J. Cell Sci. 2017, 130, 51–61. [Google Scholar] [CrossRef] [Green Version]
- Ross, A.M.; Jiang, Z.; Bastmeyer, M.; Lahann, J. Physical Aspects of Cell Culture Substrates: Topography, Roughness, and Elasticity. Small 2012, 8, 336–355. [Google Scholar] [CrossRef] [Green Version]
- Huebsch, N.; Lippens, E.; Lee, K.; Mehta, M.; Koshy, S.T.; Darnell, M.C.; Desai, R.M.; Madl, C.M.; Xu, M.; Zhao, X.; et al. Matrix elasticity of void-forming hydrogels controls transplanted-stem-cell-mediated bone formation. Nat. Mater. 2015, 14, 1269–1277. [Google Scholar] [CrossRef] [Green Version]
- Slaughter, V.B.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in regenerative medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef] [Green Version]
- Patel, J.M.; Loebel, C.; Saleh, K.S.; Wise, B.C.; Bonnevie, E.D.; Miller, L.M.; Carey, J.L.; Burdick, J.A.; Mauck, R.L. Tissue Engineering: Stabilization of Damaged Articular Cartilage with Hydrogel-mediated Reinforcement and Sealing. Adv. Healthc. Mater. 2021, 10, 2100315. [Google Scholar] [CrossRef]
- Lee-Thedieck, C.; Rauch, N.; Fiammengo, R.; Klein, G.; Spatz, J.P. Impact of substrate elasticity on human hematopoietic stem and progenitor cell adhesion and motility. J. Cell Sci. 2012, 125, 3765–3775. [Google Scholar] [CrossRef] [Green Version]
- Hadden, W.J.; Young, J.L.; Holle, A.W.; McFetridge, M.L.; Kim, D.Y.; Wijesinghe, P.; Taylor-Weiner, H.; Wen, J.H.; Lee, A.R.; Bieback, K.; et al. Stem cell migration and mechanotransduction on linear stiffness gradient hydrogels. Proc. Natl. Acad. Sci. USA 2017, 114, 5647–5652. [Google Scholar] [CrossRef] [Green Version]
- Jungst, T.; Smolan, W.; Schacht, K.; Scheibel, T.; Groll, J. Strategies and Molecular Design Criteria for 3D Printable Hydrogels. Chem. Rev. 2016, 116, 1496–1539. [Google Scholar] [CrossRef] [PubMed]
- Gutekunst, S.B.; Siemsen, K.; Huth, S.; Möhring, A.; Hesseler, B.; Timmermann, M.; Paulowicz, I.; Mishra, Y.K.; Siebert, L.; Adelung, R.; et al. 3D Hydrogels Containing Interconnected Microchannels of Subcellular Size for Capturing Human Pathogenic Acanthamoeba Castellanii. ACS Biomater. Sci. Eng. 2019, 5, 1784–1792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendes, B.B.; Daly, A.C.; Reis, R.L.; Domingues, R.M.A.; Gomes, M.E.; Burdick, J.A. Injectable Hyaluronic Acid and Platelet Lysate-derived Granular Hydrogels for Biomedical Applications. Acta Biomater. 2021, 119, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Passos, M.F.; Dias, D.R.C.; Bastos, G.N.T.; Jardini, A.L.; Benatti, A.C.B.; Dias, C.G.B.T.; Maciel Filho, R. pHEMA hydrogels: Synthesis, kinetics and in vitro tests. J. Therm. Anal. Calorim. 2016, 125, 361–368. [Google Scholar] [CrossRef]
- Atzet, S.; Curtin, S.; Trinh, P.; Bryant, S.; Ratner, B. Degradable poly(2-hydroxyethyl methacrylate)-co-polycaprolactone hydrogels for tissue engineering scaffolds. Biomacromolecules 2008, 9, 3370–3377. [Google Scholar] [CrossRef] [Green Version]
- Michel, S.S.E.; Dutertre, F.; Denbow, M.; Galan, M.C.; Briscoe, W. Facile Synthesis of Chitosan-Based Hydrogels and Microgels through Thiol-Ene Photoclick Crosslinking. ACS Appl. Bio Mater. 2019, 2, 3257–3268. [Google Scholar] [CrossRef]
- Klebe, R.J.; Bentley, K.L.; Schoen, R.C. Adhesive substrates for fibronectin. J. Cell. Physiol. 1981, 109, 481–488. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [Green Version]
- Hersel, U.; Dahmen, C.; Kessler, H. RGD modified polymers: Biomaterials for stimulated cell adhesion and beyond. Biomaterials 2003, 24, 4385–4415. [Google Scholar] [CrossRef]
- Ribeiro, A.J.S.; Denisin, A.K.; Wilson, R.E.; Pruitt, B.L. For whom the cells pull: Hydrogel and micropost devices for measuring traction forces. Methods 2016, 94, 51–64. [Google Scholar] [CrossRef] [Green Version]
- Naahidi, S.; Jafari, M.; Logan, M.; Wang, Y.; Yuan, Y.; Bae, H.; Dixon, B.; Chen, P. Biocompatibility of hydrogel-based scaffolds for tissue engineering applications. Biotechnol. Adv. 2017, 35, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Michel, S.S.E.; Rogers, S.E.; Briscoe, W.; Galan, M.C. Tuneable Thiol-Ene Photo Crosslinked Chitosan-Based Hydrogels for Biomedical Applications. ACS Appl. Bio Mater. 2020, 3, 8075–8083. [Google Scholar] [CrossRef] [PubMed]
- Qazi, T.H.; Burdick, J.A. Granular Hydrogels for Endogenous Tissue Repair. Biomater. Biosyst. 2021, 1, 100008. [Google Scholar] [CrossRef]
- Duarte, C.D.F.; Lindsay, C.D.; Roth, J.G.; LeSavage, B.L.; Seymour, A.J.; Krajina, B.A.; Ribeiro, R.; Costa, P.F.; Blaeser, A.; Heilshorn, S.C. Bioprinting Cell- and Spheroid-Laden Protein-Engineered Hydrogels as Tissue-on-Chip Platforms. Front. Bioeng. Biotechnol. 2020, 8, 374–386. [Google Scholar] [CrossRef] [PubMed]
- Siemsen, K.; Rajput, S.; Rasch, F.; Taheri, F.; Adelung, R.; Lammerding, J.; Selhuber-Unkel, C. Tunable 3D Hydrogel Microchannel Networks to Study Confined Mammalian Cell Migration. Adv. Healthc. Mater. 2021, 10, 2100625. [Google Scholar] [CrossRef] [PubMed]
- Caliari, S.R.; Burdick, J.A. A practical guide to hydrogels for cell culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayhan, H.; Ayhan, F. Water based PHEMA hydrogels for controlled drug delivery. Turk Biyokim. Derg. 2018, 43, 228–239. [Google Scholar] [CrossRef]
- Daly, A.C.; Riley, L.A.; Segura, T.; Burdick, J.A. Hydrogel Microparticles for Biomedical Applications. Nat. Rev. Mater. 2020, 5, 20–43. [Google Scholar] [CrossRef]
- Chatterjee, S.; Upadhyay, P.; Mishra, M.; Srividya, M.; Akshara, M.R.; Kamali, N.; Zaidi, Z.S.; Iqbal, S.F.; Misra, S.K. Advances in chemistry and composition of soft materials for drug releasing contact lenses. RSC Adv. 2020, 10, 36751–36777. [Google Scholar] [CrossRef]
- Guiseppi-Elie, A.; Dong, C.; Dinu, C.Z. Crosslink density of a biomimetic poly(HEMA)-based hydrogel influences growth and proliferation of attachment dependent RMS 13 cells. J. Mater. Chem. 2012, 22, 19529–19539. [Google Scholar] [CrossRef]
- Varaprasad, K.; Raghavendra, G.M.; Jayaramudu, T.; Yallapu, M.M.; Sadiku, R. A mini review on hydrogels classification and recent developments in miscellaneous applications. J. Mater. Sci. Eng. C 2017, 79, 958–971. [Google Scholar] [CrossRef] [PubMed]
- Hanak, B.W.; Hsieh, C.-y.; Donaldson, W.; Browd, S.R.; Lau, K.K.S.; Shain, W. Reduced cell attachment to poly(2-hydroxyethyl methacrylate)-coated ventricular catheters in vitro. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 1268–1279. [Google Scholar] [CrossRef] [PubMed]
- Zainuddin, Z.; Barnard, Z.; Keen, I.; Hill, D.J.T.; Chirila, V.T.; Harkin, D.G. PHEMA hydrogels modified through the grafting of phosphate groups by ATRP support the attachment and growth of human corneal epithelial cells. J. Biomater. Appl. 2008, 23, 147–168. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Wu, T.; Xu, C.; Langenbach, K.J.; Elliott, J.T.; Vogt, B.D.; Beers, K.L.; Amis, E.J.; Washburn, N.R. Tuning cell adhesion on gradient poly(2-hydroxyethyl methacrylate)-grafted surfaces. Langmuir 2005, 21, 12309–12314. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Ren, T.; Zhu, J.; Mao, Z.; Gao, C. Adsorption of plasma proteins and fibronectin on poly(hydroxylethyl methacrylate) brushes of different thickness and their relationship with adhesion and migration of vascular smooth muscle cells. Regen. Biomater. 2014, 1, 17–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, T.; Mao, Z.; Guo, J.; Gao, C. Directional migration of vascular smooth muscle cells guided by a molecule weight gradient of poly(2-hydroxyethyl methacrylate) brushes. Langmuir 2013, 29, 6386–6395. [Google Scholar] [CrossRef]
- Minett, T.W.; Tighe, B.J.; Lydon, M.J.; Rees, D.A. Requirements for cell spreading on polyHEMA coated culture substrates. Cell Biol. Int. Rep. 1984, 8, 151–159. [Google Scholar] [CrossRef]
- Ravi, M.; Paramesh, V.; Kaviya, S.R.; Anuradha, E.; Solomon, F.D.P. 3D Cell Culture Systems: Advantages and Applications. J. Cell. Physiol. 2015, 230, 16–26. [Google Scholar] [CrossRef]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef] [Green Version]
- Aubin, H.; Nichol, J.W.; Hutson, C.B.; Bae, H.; Sieminski, A.L.; Cropek, D.M.; Akhyari, P.; Khademhosseini, A. Directed 3D cell alignment and elongation in microengineered hydrogels. Biomaterials 2010, 31, 6941–6951. [Google Scholar] [CrossRef] [Green Version]
- Paterson, S.M.; Shadforth, A.M.A.; Shaw, J.A.; Brown, D.H.; Chirila, V.T.; Baker, V.M. Improving the cellular invasion into PHEMA sponges by incorporation of the RGD peptide ligand: The use of copolymerization as a means to functionalize PHEMA sponges. J. Mater. Sci. Eng. C 2013, 33, 4917–4922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charrier, E.E.; Pogoda, K.; Wells, R.G.; Janmey, P.A. Control of cell morphology and differentiation by substrates with independently tunable elasticity and viscous dissipation. Nat. Commun. 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thermo Fisher, S. Crosslinkers Technical Handbook; Thermo Fisher: Waltham, MA, USA, 2012. [Google Scholar]
- Argraves, W.S.; Suzuki, S.; Arai, H.; Thompson, K.; Pierschbacher, M.D.; Ruoslahti, E. Amino acid sequence of the human fibronectin receptor. J. Cell Biol. 1987, 105, 1183–1190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koteliansky, V.E.; Glukhova, M.A.; Benjamin, V.M.; Smirnov, V.N.; Filimonov, V.V.; Zalite, O.M.; Venyaminov, S.Y. A Study of the Structure of Fibronectin. Eur. J. Biochem. 1981, 119, 619–624. [Google Scholar] [CrossRef]
- Ravasco, J.M.; Faustino, H.; Trindade, A.; Gois, P.M.P. Bioconjugation with Maleimides: A Useful Tool for Chemical Biology. Chem. Eur. J. 2019, 25, 43–59. [Google Scholar] [CrossRef]
- Dolan, C.; Drouet, F.; Ware, D.C.; Brothers, P.J.; Jin, J.; Brimble, M.A.; Williams, D.E. A new high-capacity metal ion-complexing gel containing cyclen ligands. RSC Adv. 2016, 6, 23645–23652. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, S.; Zhong, W.; Zhou, X.; Li, S. Development and Properties of Valine-Alanine based Antibody-Drug Conjugates with Monomethyl Auristatin E as the Potent Payload. Int. J. Mol. Sci. 2017, 18, 1860. [Google Scholar] [CrossRef]
- Achilias, D.S.; Siafaka, P.I. Polymerization Kinetics of Poly(2-Hydroxyethyl Methacrylate) Hydrogels and Nanocomposite Materials. Processes 2017, 5, 21. [Google Scholar] [CrossRef] [Green Version]
- Menter, P. Acrylamide Polymerization—A Practical Approach; tech note 1156; Bio-Rad Laboratories, Alfred Nobel Drive: Hercules, CA, USA, 2000. [Google Scholar]
- Chaudhuri, O.; Gu, L.; Klumpers, D.; Darnell, M.; Bencherif, S.A.; Weaver, J.C.; Huebsch, N.; Lee, H.-p.; Lippens, E.; Duda, G.N.; et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 2016, 15, 326. [Google Scholar] [CrossRef] [Green Version]
- Gutekunst, S.B.; Grabosch, C.; Kovalev, A.; Gorb, S.N.; Selhuber-Unkel, C. Influence of the PDMS substrate stiffness on the adhesion of Acanthamoeba castellanii. Beilstein J. Nanotechnol. 2014, 5, 1393–1398. [Google Scholar] [CrossRef] [Green Version]
- Meakin, J.R.; Hukins, D.W.L.; Aspden, R.M.; Imrie, C.T. Rheological properties of poly(2-hydroxyethyl methacrylate) (pHEMA) as a function of water content and deformation frequency. J. Mater. Sci. Mater. Med. 2003, 14, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, J.M.; Oyen, M.L. Hydrogel Composite Materials for Tissue Engineering Scaffolds. JOM 2013, 65, 505–516. [Google Scholar] [CrossRef]
- Peppas, N.A.; Huang, Y.; Torres-Lugo, M.; Ward, J.H.; Zhang, J. Physicochemical Foundations and Structural Design of Hydrogels in Medicine and Biology. Annu. Rev. Biomed. Eng. 2000, 2, 9–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Discher, D.E.; Janmey, P.; Wang, Y.-L. Tissue Cells Feel and Respond to the Stiffness of Their Substrate. Science 2005, 310, 1139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, E.; Kumar, S.; Lee, J.; Jang, J.; Park, J.H.; Chang, M.C.; Kwon, I.; Lee, J.S.; Huh, I.Y. Modified hydrogels based on poly(2-hydroxyethyl methacrylate) (pHEMA) with higher surface wettability and mechanical properties. Macromol. Res. 2017, 25, 704–711. [Google Scholar] [CrossRef]
- Eschbach, F.O.; Huang, S.J. Hydrophilic-Hydrophobic Binary Systems of Poly(2-hydroxyethyl methacrylate) and Polycaprolactone. Part I: Synthesis and Characterization. J. Bioact. Compat. Polym. 1994, 9, 29–54. [Google Scholar] [CrossRef]
- Ulu, A.; Balcioglu, S.; Birhanli, E.; Sarimeseli, A.; Keskin, R.; Koytepe, S.; Ates, B. Poly(2-hydroxyethyl methacrylate)/boric acid composite hydrogel as soft contact lens material: Thermal, optical, rheological, and enhanced antibacterial properties. J. Appl. Polym. Sci. 2018, 135, 46575. [Google Scholar] [CrossRef]
- Technical Committee ISO/TC 194 (Biological Evaluation of Medical Devices). Biological Evaluation of Medical Devices Part 5: Tests for Cytotoxicity (ISO Standard No. 10993-5), 2009 International Organization for Standardization Web Site. Available online: https://www.iso.org/standard/36406.html (accessed on 21 February 2022).
- Technical Committee ISO/TC 194 (Biological Evaluation of Medical Devices). Biological Evaluation of Medical Devices Part 1: Evaluation and Testing within a Risk Management Process (ISO Standard No. 10993-1), 2018 International Organization for Standardization Web Site. Available online: https://www.iso.org/standard/68936.html (accessed on 21 February 2022).
- Omidian, H.; Park, K.; Kandalam, U.; Rocca, J.G. Swelling and Mechanical Properties of Modified HEMA-based Superporous Hydrogels. J. Bioact. Compat. Polym. 2010, 25, 483–497. [Google Scholar] [CrossRef]
- Jhaveri, S.J.; Hynd, M.R.; Dowell-Mesfin, N.; Turner, J.N.; Shain, W.; Ober, C.K. Release of Nerve Growth Factor from HEMA Hydrogel-Coated Substrates and Its Effect on the Differentiation of Neural Cells. Biomacromolecules 2009, 10, 174–183. [Google Scholar] [CrossRef]
- Badea, A.; McCracken, J.M.; Tillmaand, E.G.; Kandel, M.E.; Oraham, A.W.; Mevis, M.B.; Rubakhin, S.S.; Popescu, G.; Sweedler, V.J.; Nuzzo, R.G. 3D-Printed pHEMA Materials for Topographical and Biochemical Modulation of Dorsal Root Ganglion Cell Response. ACS Appl. Mater. Interfaces 2017, 9, 30318–30328. [Google Scholar] [CrossRef]
- Cox, E.A.; Sastry, S.K.; Huttenlocher, A. Integrin-mediated Adhesion Regulates Cell Polarity. Mol. Biol. Cell 2001, 12, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schumacher, L.; Siemsen, K.; Appiah, C.; Rajput, S.; Heitmann, A.; Selhuber-Unkel, C.; Staubitz, A. A Co-Polymerizable Linker for the Covalent Attachment of Fibronectin Makes pHEMA Hydrogels Cell-Adhesive. Gels 2022, 8, 258. https://doi.org/10.3390/gels8050258
Schumacher L, Siemsen K, Appiah C, Rajput S, Heitmann A, Selhuber-Unkel C, Staubitz A. A Co-Polymerizable Linker for the Covalent Attachment of Fibronectin Makes pHEMA Hydrogels Cell-Adhesive. Gels. 2022; 8(5):258. https://doi.org/10.3390/gels8050258
Chicago/Turabian StyleSchumacher, Laura, Katharina Siemsen, Clement Appiah, Sunil Rajput, Anne Heitmann, Christine Selhuber-Unkel, and Anne Staubitz. 2022. "A Co-Polymerizable Linker for the Covalent Attachment of Fibronectin Makes pHEMA Hydrogels Cell-Adhesive" Gels 8, no. 5: 258. https://doi.org/10.3390/gels8050258
APA StyleSchumacher, L., Siemsen, K., Appiah, C., Rajput, S., Heitmann, A., Selhuber-Unkel, C., & Staubitz, A. (2022). A Co-Polymerizable Linker for the Covalent Attachment of Fibronectin Makes pHEMA Hydrogels Cell-Adhesive. Gels, 8(5), 258. https://doi.org/10.3390/gels8050258






