Radiometal-Labeled Chitosan Microspheres as Transarterial Radioembolization Agents against Hepatocellular Carcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
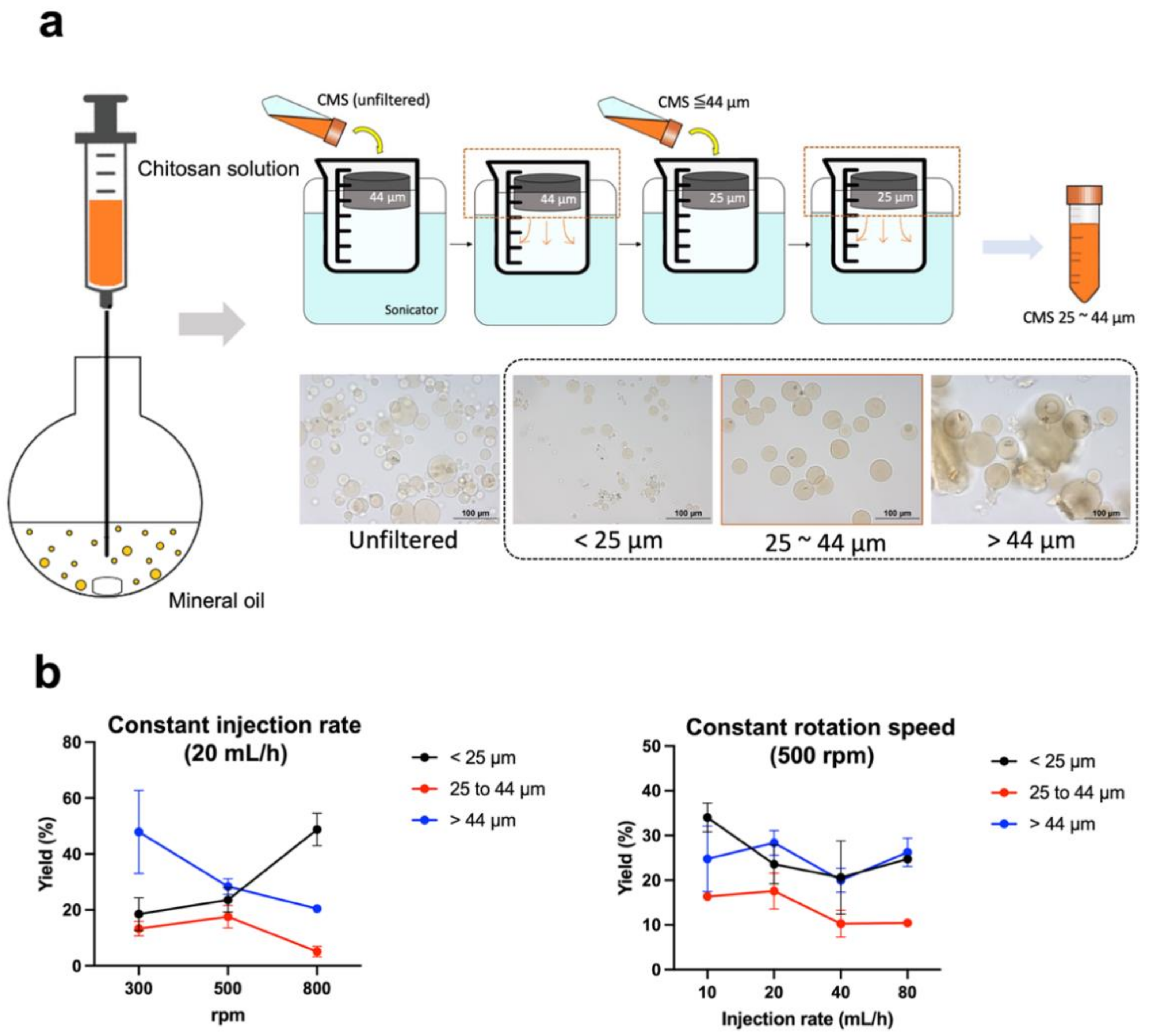
2.2. Preparation of Chitosan Microspheres (CMS)
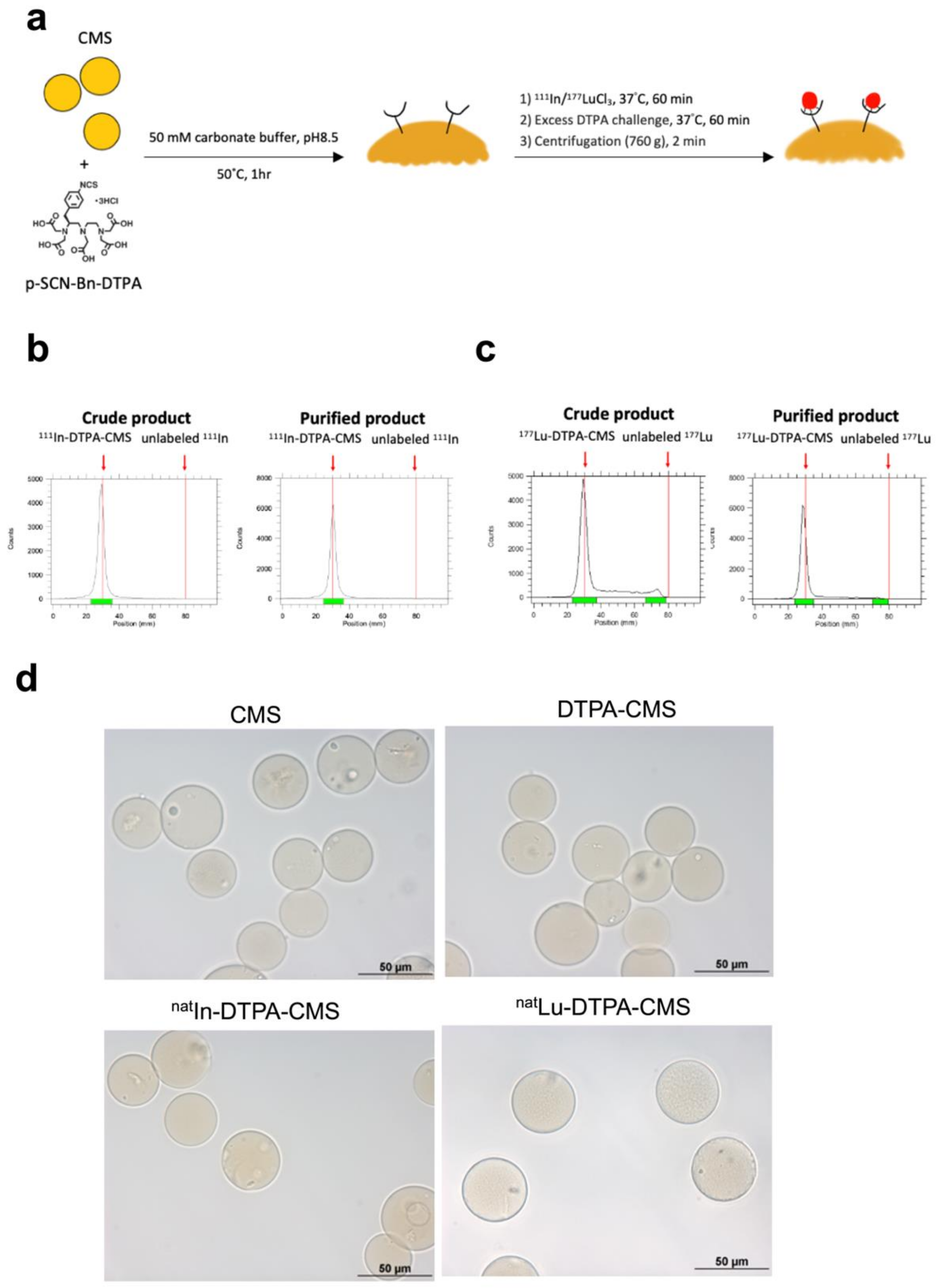
2.3. Preparation of DTPA-Modified CMS
2.4. Radiolabeling of CMS
2.5. In Vitro Stability of 111In- and 177Lu-Labeled DTPA-CMS
2.6. Cell Culture and Xenograft Inoculation
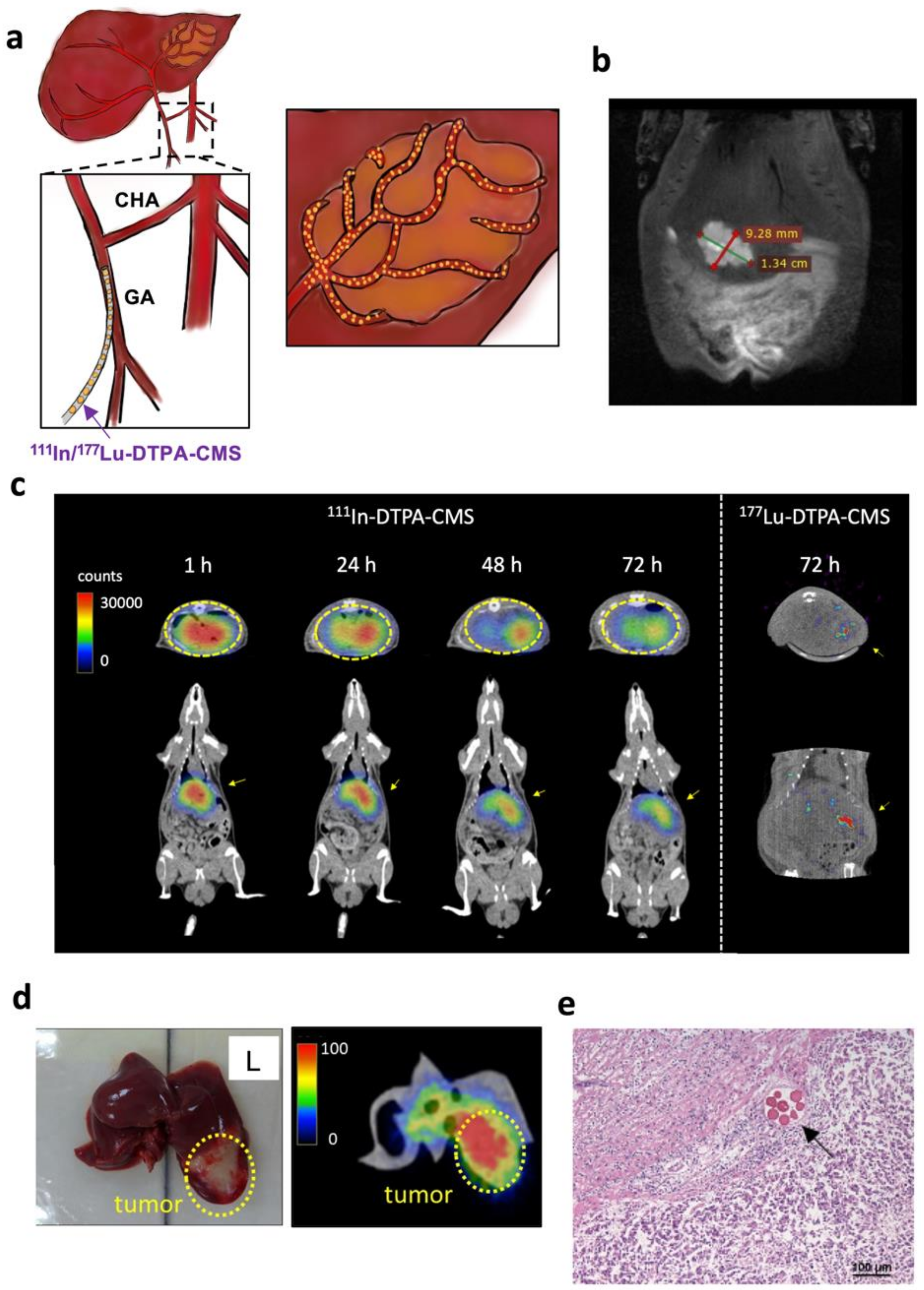
2.7. SPECT/CT Imaging of Radiolabeled CMS
2.8. Biodistribution of Radiolabeled CMS
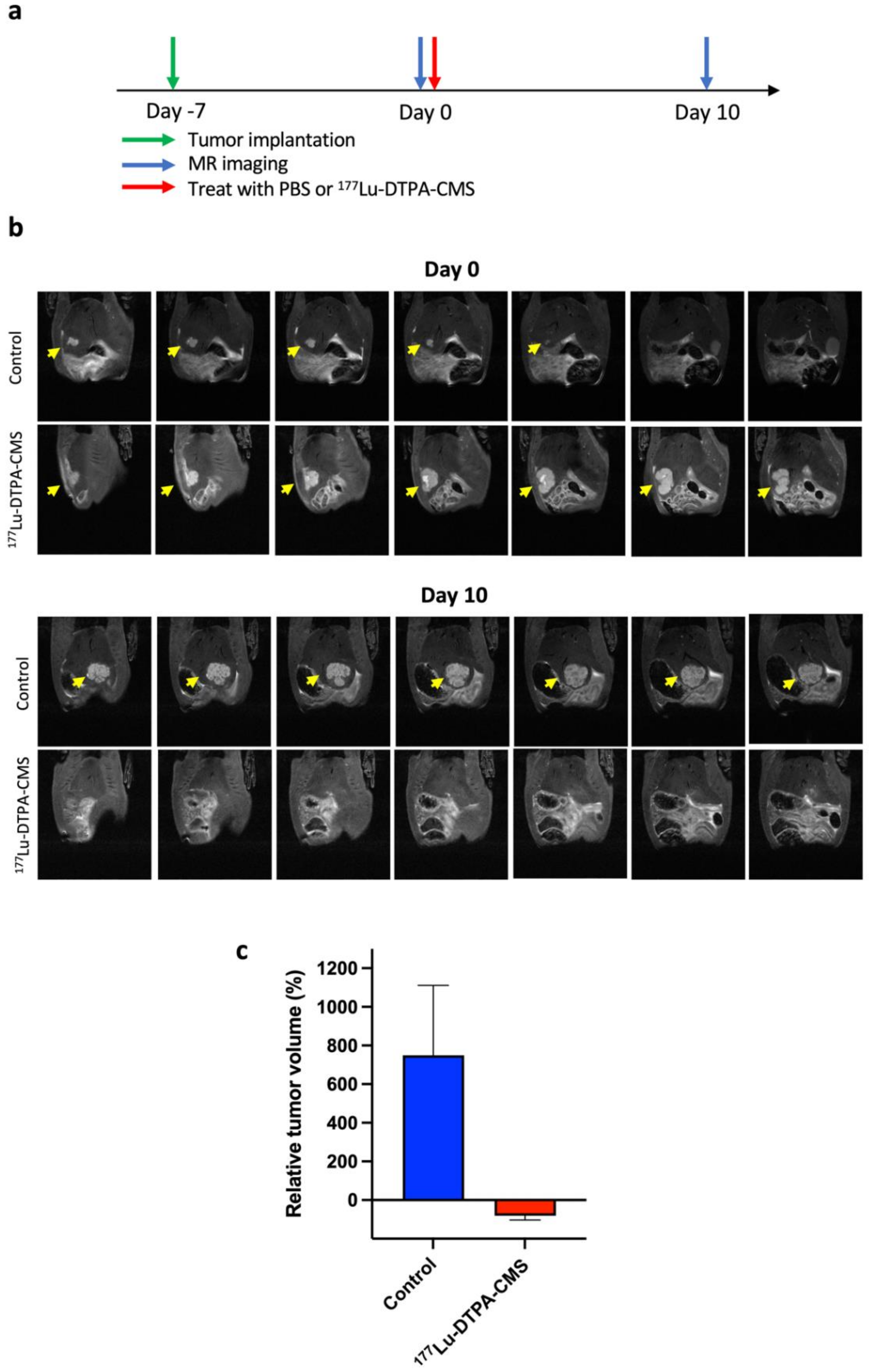
2.9. The Therapeutic Efficacy of 177Lu-DTPA-CMS
2.10. Hematoxylin and Eosin Staining
2.11. Dosimetry of 177Lu-DTPA-CMS
2.12. Statistical Analysis
3. Results
3.1. Production and Radiolabeling of Chitosan Microspheres (CMS)
3.2. Stability of the Radiolabeled Chitosan Microspheres
3.3. SPECT Imaging of Hepatoma-Bearing Rats
3.4. Biodistribution Studies
3.5. The Therapeutic Efficacy of 177Lu-DTPA-CMS
3.6. Estimation of Dosimetry in Human Organs in the Treatment of 177Lu-DTPA-CMS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Serag, H.B. Epidemiology of Viral Hepatitis and Hepatocellular Carcinoma. Gastroenterology 2012, 142, 1264–1273.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashtari, S. Hepatocellular carcinoma in Asia: Prevention strategy and planning. World J. Hepatol. 2015, 7, 1708–1717. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Memon, K.; Lewandowski, R.J.; Kulik, L.; Riaz, A.; Mulcahy, M.F.; Salem, R. Radioembolization for Primary and Metastatic Liver Cancer. Semin. Radiat. Oncol. 2011, 21, 294–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastiaannet, R.; Kappadath, S.C.; Kunnen, B.; Braat, A.J.A.T.; Lam, M.G.E.H.; De Jong, H.W.A.M. The physics of radioembolization. EJNMMI Phys. 2018, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Kallini, J.R.; Gabr, A.; Thorlund, K.; Balijepalli, C.; Ayres, D.; Kanters, S.; Ebrahim, S.; Mills, E.; Lewandowski, R.J.; Salem, R. Comparison of the Adverse Event Profile of TheraSphere((R)) with SIR-Spheres((R)) for the Treatment of Unresectable Hepatocellular Carcinoma: A Systematic Review. Cardiovasc. Intervent. Radiol. 2017, 40, 1033–1043. [Google Scholar] [CrossRef]
- Bhangoo, M.S.; Karnani, D.R.; Hein, P.N.; Giap, H.; Knowles, H.; Issa, C.; Steuterman, S.; Pockros, P.; Frenette, C. Radioembolization with Yttrium-90 microspheres for patients with unresectable hepatocellular carcinoma. J. Gastrointest. Oncol. 2015, 6, 469–478. [Google Scholar] [CrossRef]
- Chiang, P.-F.; Peng, C.-L.; Shih, Y.-H.; Cho, Y.-H.; Yu, C.-S.; Kuo, Y.-M.; Shieh, M.-J.; Luo, T.-Y. Biodegradable and Multifunctional Microspheres for Treatment of Hepatoma through Transarterial Embolization. ACS Biomater. Sci. Eng. 2018, 4, 3425–3433. [Google Scholar] [CrossRef]
- De La Vega, J.C.; Esquinas, P.L.; Rodríguez-Rodríguez, C.; Bokharaei, M.; Moskalev, I.; Liu, D.; Saatchi, K.; Häfeli, U.O. Radioembolization of Hepatocellular Carcinoma with Built-In Dosimetry: First in vivo Results with Uniformly-Sized, Biodegradable Microspheres Labeled with (188)Re. Theranostics 2019, 9, 868–883. [Google Scholar] [CrossRef]
- Rodrigues, S.; Dionísio, M.; López, C.R.; Grenha, A. Biocompatibility of Chitosan Carriers with Application in Drug Delivery. J. Funct. Biomater. 2012, 3, 615–641. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Li, Y.; Gong, N.; Zhao, X. Properties and Biocompatibility of Chitosan Films Modified by Blending with Peg. Biomaterials 2002, 23, 2641–2648. [Google Scholar] [CrossRef]
- Sahoo, D.; Sahoo, S.; Mohanty, P.; Sasmal, S.; Nayak, P.L. Chitosan: A New Versatile Bio-polymer for Various Applications. Des. Monomers Polym. 2009, 12, 377–404. [Google Scholar] [CrossRef]
- Lee, J.Y.; Chu, S.H.; Jeon, J.Y.; Lee, M.K.; Park, J.H.; Lee, D.C.; Lee, J.-W.; Kim, N.-K. Effects of 12 weeks of probiotic supplementation on quality of life in colorectal cancer survivors: A double-blind, randomized, placebo-controlled trial. Dig. Liver Dis. 2014, 46, 1126–1132. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.; Kim, K.I.; Kwon, J.; Kim, B.S.; Jeong, H.-J.; Jang, S.J.; Oh, P.-S.; Park, H.S.; Lim, S.T.; Sohn, M.-H. (131)I-labeled chitosan hydrogels for radioembolization: A preclinical study in small animals. Nucl. Med. Biol. 2017, 52, 16–23. [Google Scholar] [CrossRef]
- Kim, K.; Kim, S.-J. Lu-177-Based Peptide Receptor Radionuclide Therapy for Advanced Neuroendocrine Tumors. Nucl. Med. Mol. Imaging 2018, 52, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Viana, R.D.S.; Costa, L.A.D.M.; Harmon, A.C.; Gomes Filho, M.A.; Falcão, E.H.; Vicente, M.G.; Junior, S.A.; Mathis, J.M. 177Lu-Labeled Eu-Doped Mesoporous SiO2 Nanoparticles as a Theranostic Radiopharmaceutical for Colorectal Cancer. ACS Appl. Nano Mater. 2020, 3, 8691–8701. [Google Scholar] [CrossRef]
- Sobol, N.; Sutherlin, L.; Cedrowska, E.; Schorp, J.; Rodriguez-Rodriguez, C.; Sossi, V.; Lattimer, J.; Miller, D.C.; Pevsner, P.; Robertson, J.D. Synthesis and targeting of gold-coated (177)Lu-containing lanthanide phosphate nanoparticles—A potential theranostic agent for pulmonary metastatic disease. APL Bioeng. 2018, 2, 016101. [Google Scholar] [CrossRef] [Green Version]
- Ryden, T.; Lagerlöf, J.H.; Hemmingsson, J.; Marín, I.; Svensson, J.; Båth, M.; Gjertsson, P.; Bernhardt, P. Fast GPU-based Monte Carlo code for SPECT/CT reconstructions generates improved (177)Lu images. EJNMMI Phys. 2018, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zhang, L.; Li, W.; Lei, C.; Cao, Y.; Wang, Y.; Wang, Z.; Pang, H. Preparation and SPECT/CT Imaging of (177)Lu-Labeled Peptide Nucleic Acid (PNA) Targeting CITED1: Therapeutic Evaluation in Tumor-Bearing Nude Mice. OncoTargets Ther. 2020, 13, 487–496. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Sun, X.; Yin, H.; Wang, T.; Li, Y.; Zhou, C.; Zhou, H.; He, S.; Cong, H. Chitosan Microsphere Used as an Effective System to Deliver a Linked Antigenic Peptides Vaccine Protect Mice Against Acute and Chronic Toxoplasmosis. Front. Cell. Infect. Microbiol. 2018, 8, 163. [Google Scholar] [CrossRef]
- Luo, C.; Yang, Q.; Lin, X.; Qi, C.; Li, G. Preparation and drug release property of tanshinone IIA loaded chitosan-montmorillonite microspheres. Int. J. Biol. Macromol. 2019, 125, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Min, Q.; Liu, J.; Li, J.; Wan, Y.; Wu, J. Chitosan-Polylactide/Hyaluronic Acid Complex Microspheres as Carriers for Controlled Release of Bioactive Transforming Growth Factor-beta1. Pharmaceutics 2018, 10, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villicaña-Molina, E.; Pacheco-Contreras, E.; Aguilar-Reyes, E.A.; Patiño, C.A.L. Pectin and chitosan microsphere preparation via a water/oil emulsion and solvent evaporation method for drug delivery. Int. J. Polym. Mater. Polym. Biomater. 2019, 69, 467–475. [Google Scholar] [CrossRef]
- Price, E.W.; Orvig, C. Matching chelators to radiometals for radiopharmaceuticals. Chem. Soc. Rev. 2014, 43, 260–290. [Google Scholar] [CrossRef]
- Garin, E.; Rolland, Y.; Laffont, S.; Edeline, J. Clinical impact of (99m)Tc-MAA SPECT/CT-based dosimetry in the radioembolization of liver malignancies with (90)Y-loaded microspheres. Eur. J. Pediatr. 2015, 43, 559–575. [Google Scholar] [CrossRef] [Green Version]
- Haste, P.; Tann, M.; Persohn, S.; LaRoche, T.; Aaron, V.; Mauxion, T.; Chauhan, N.; Dreher, M.R.; Johnson, M.S. Correlation of Technetium-99m Macroaggregated Albumin and Yttrium-90 Glass Microsphere Biodistribution in Hepatocellular Carcinoma: A Retrospective Review of Pretreatment Single Photon Emission CT and Posttreatment Positron Emission Tomography/CT. J. Vasc. Interv. Radiol. 2017, 28, 722–730.e1. [Google Scholar] [CrossRef]
- Dash, A.; Pillai, M.R.A.; Knapp, F.F., Jr. Production of (177)Lu for Targeted Radionuclide Therapy: Available Options. Nucl. Med. Mol. Imaging 2015, 49, 85–107. [Google Scholar] [CrossRef] [Green Version]

| 111In-DTPA-CMS a | 177Lu-DTPA-CMS | ||||
|---|---|---|---|---|---|
| Organ b | 1 h | 24 h | 48 h | 72 h | 72 h |
| Blood | 0.01 ± 0.01 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | N.D. c |
| Heart | 0.02 ± 0.02 | 0.01 ± 0.01 | N.D. | N.D. | N.D. |
| Lung | 0.34 ± 0.15 | 0.33 ± 0.31 | 0.11 ± 0.01 | 0.15 ± 0.20 | 0.04 ± 0.04 |
| Liver | 5.31 ± 2.07 | 3.10 ± 0.52 | 3.15 ± 0.75 | 3.57 ± 0.51 | 4.48 ± 0.94 |
| Stomach | 0.66 ± 0.82 | 0.02 ± 0.03 | 0.17 ± 0.23 | 0.24 ± 0.28 | 0.25 ± 0.50 |
| S.I. | 1.08 ± 1.67 | 0.01 ± 0.01 | 0.03 ± 0.06 | 0.01 ± 0.00 | 0.04 ± 0.07 |
| L.I. | 0.41 ± 0.79 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.00 | 0.00 ± 0.01 |
| Spleen | 1.13 ± 2.25 | 0.01 ± 0.00 | 0.05 ± 0.02 | 0.03 ± 0.03 | 0.09 ± 0.16 |
| Pancreas | 1.40 ± 2.69 | 0.01 ± 0.01 | 0.14 ± 0.15 | 0.09 ± 0.16 | 0.26 ± 0.52 |
| Kidney | 0.29 ± 0.51 | 0.05 ± 0.00 | 0.06 ± 0.02 | 0.09 ± 0.03 | 0.05 ± 0.09 |
| Bladder | 0.11 ± 0.20 | 0.05 ± 0.08 | 0.00 ± 0.01 | 0.01 ± 0.00 | N.D. |
| Muscle | 0.02 ± 0.04 | N.D. | N.D. | N.D. | N.D. |
| Bone | 0.07 ± 0.14 | 0.01 ± 0.01 | 0.00 ± 0.00 | 0.01 ± 0.00 | 0.05 ± 0.09 |
| Tumor | 13.75 ± 6.31 | 12.71 ± 6.37 | 12.87 ± 3.80 | 12.65 ± 3.13 | 13.11 ± 6.53 |
| T/L ratio | 2.58 ± 0.83 | 4.09 ± 2.07 | 4.06 ± 0.77 | 3.71 ± 1.53 | 2.91 ± 1.32 |
| LSF(%) d | 0.83 ± 0.82 | 0.75 ± 0.26 | 0.79 ± 0.24 | 1.01 ± 1.08 | 0.05 ± 0.05 |
| Organs b | Dose (mSv/MBq) |
|---|---|
| Adrenals | 0.04 |
| Brain | 0.01 |
| Breasts | 0.02 |
| Gallbladder wall | 0.06 |
| LLI wall | 0.01 |
| Small intestine | 0.02 |
| Stomach wall | 0.02 |
| ULI wall | 0.02 |
| Heart wall | 0.03 |
| Kidneys | 0.03 |
| Liver | 2.34 |
| Lungs | 0.09 |
| Muscle | 0.01 |
| Ovaries | 0.01 |
| Pancreas | 0.11 |
| Red marrow | 0.01 |
| Osteogenic cells | 0.04 |
| Skin | 0.01 |
| Spleen | 0.01 |
| Testes | 0.01 |
| Thymus | 0.02 |
| Thyroid | 0.01 |
| Urinary bladder wall | 0.01 |
| Uterus | 0.01 |
| Tumor (4.0 g) c | 1.01 |
| Total body | 0.08 |
| Effective dose | 0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, H.-W.; Lo, Y.-H.; Chang, D.-Y.; Li, J.-J.; Chang, W.-Y.; Chen, C.-H.; Chang, C.-H.; Chen, C.-L.; Wang, H.-E.; Liu, R.-S.; et al. Radiometal-Labeled Chitosan Microspheres as Transarterial Radioembolization Agents against Hepatocellular Carcinoma. Gels 2022, 8, 180. https://doi.org/10.3390/gels8030180
Chan H-W, Lo Y-H, Chang D-Y, Li J-J, Chang W-Y, Chen C-H, Chang C-H, Chen C-L, Wang H-E, Liu R-S, et al. Radiometal-Labeled Chitosan Microspheres as Transarterial Radioembolization Agents against Hepatocellular Carcinoma. Gels. 2022; 8(3):180. https://doi.org/10.3390/gels8030180
Chicago/Turabian StyleChan, Hui-Wen, Yi-Hsuan Lo, Deng-Yuan Chang, Jia-Je Li, Wen-Yi Chang, Chih-Hao Chen, Chih-Hsien Chang, Chuan-Lin Chen, Hsin-Ell Wang, Ren-Shyan Liu, and et al. 2022. "Radiometal-Labeled Chitosan Microspheres as Transarterial Radioembolization Agents against Hepatocellular Carcinoma" Gels 8, no. 3: 180. https://doi.org/10.3390/gels8030180
APA StyleChan, H.-W., Lo, Y.-H., Chang, D.-Y., Li, J.-J., Chang, W.-Y., Chen, C.-H., Chang, C.-H., Chen, C.-L., Wang, H.-E., Liu, R.-S., & Wu, C.-Y. (2022). Radiometal-Labeled Chitosan Microspheres as Transarterial Radioembolization Agents against Hepatocellular Carcinoma. Gels, 8(3), 180. https://doi.org/10.3390/gels8030180






