Repurposing Lovastatin Cytotoxicity against the Tongue Carcinoma HSC3 Cell Line Using a Eucalyptus Oil-Based Nanoemulgel Carrier
Abstract
:1. Introduction
2. Results and Discussion
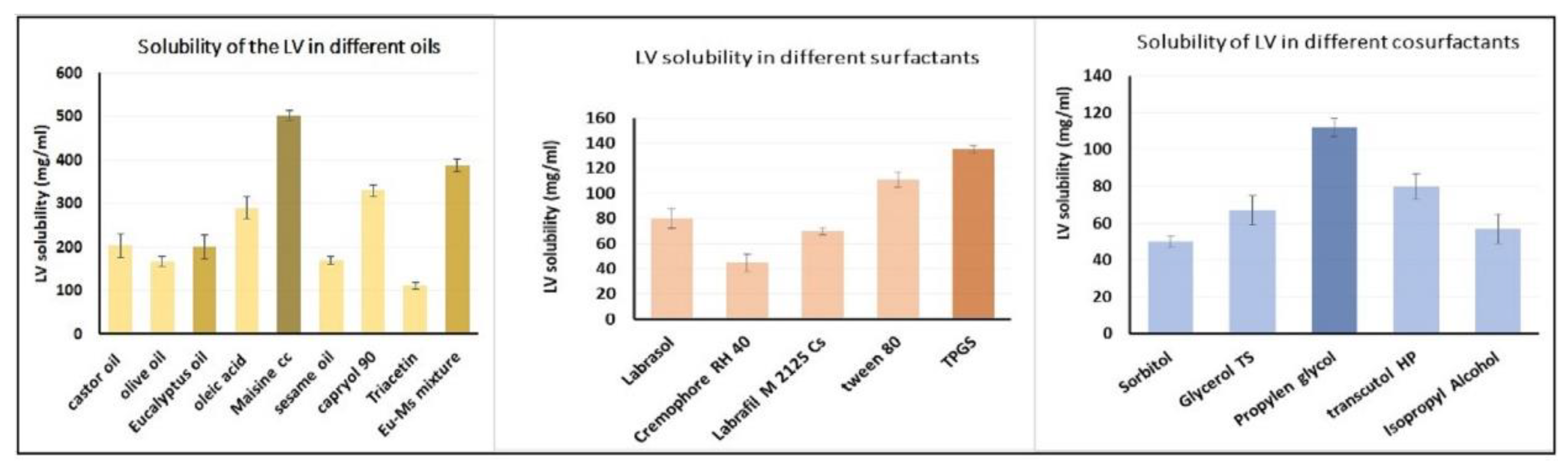
2.1. Estimation of LV Solubility in Various SNEDDS Components
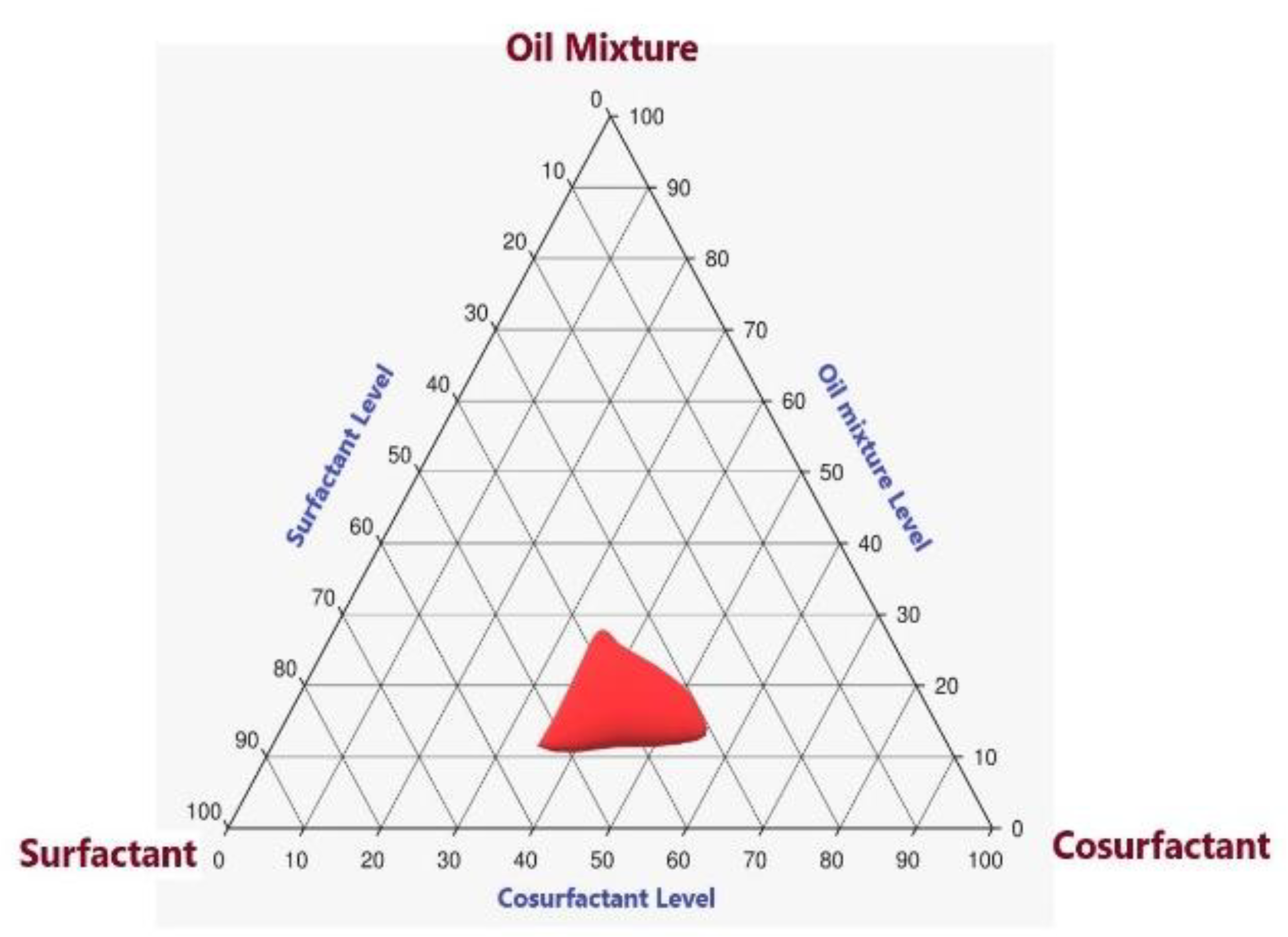
2.2. Pseudoternary-Phase Diagram for LV in Various Solvent Systems
2.3. Assessment of LV-Eu-SNEDDS
2.3.1. Visual Inspection of LV-Eu-SNEDDS
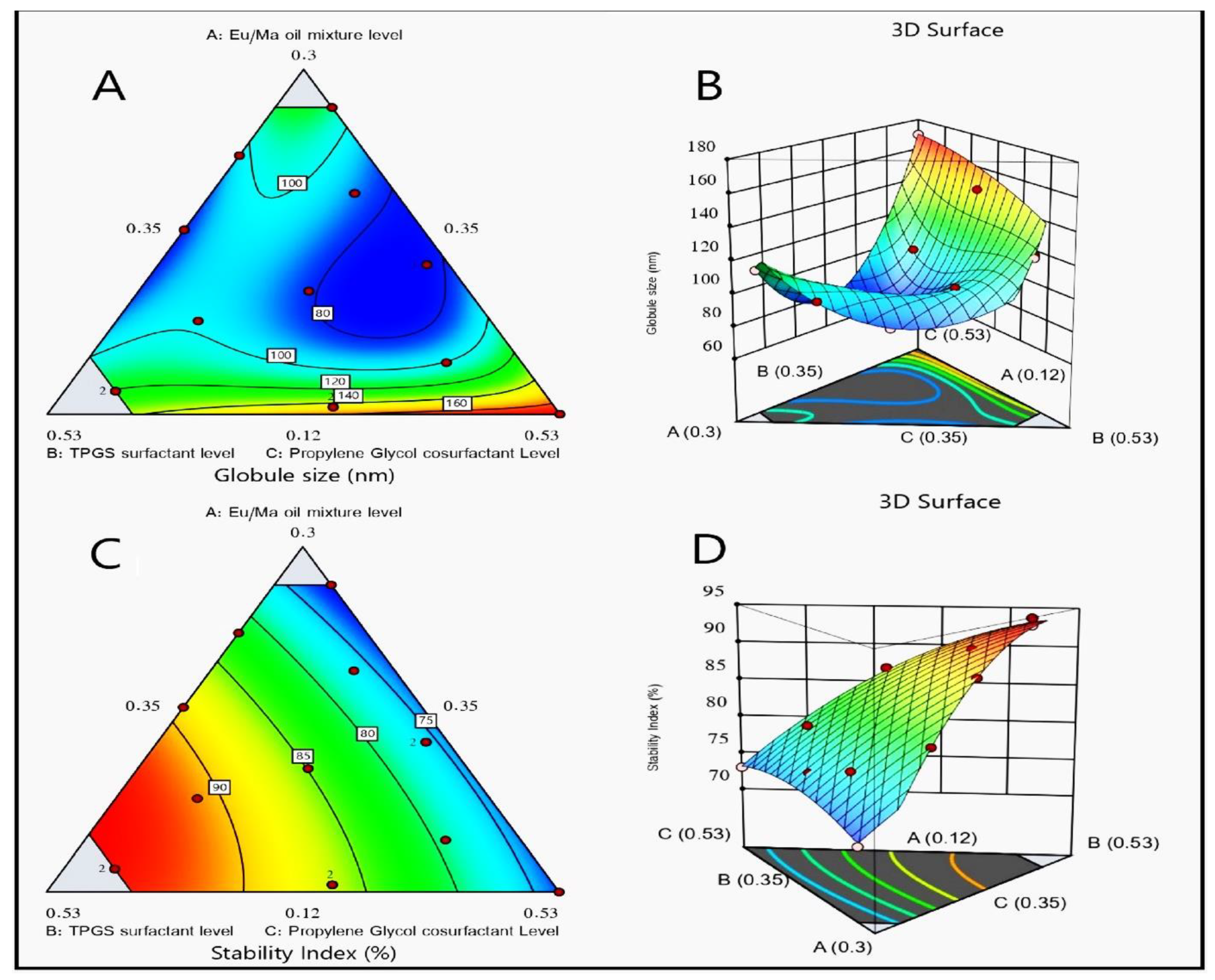
2.3.2. Determination of Droplet Sizes of LV-Eu-SNEDDS
2.3.3. Stability Index Assessment
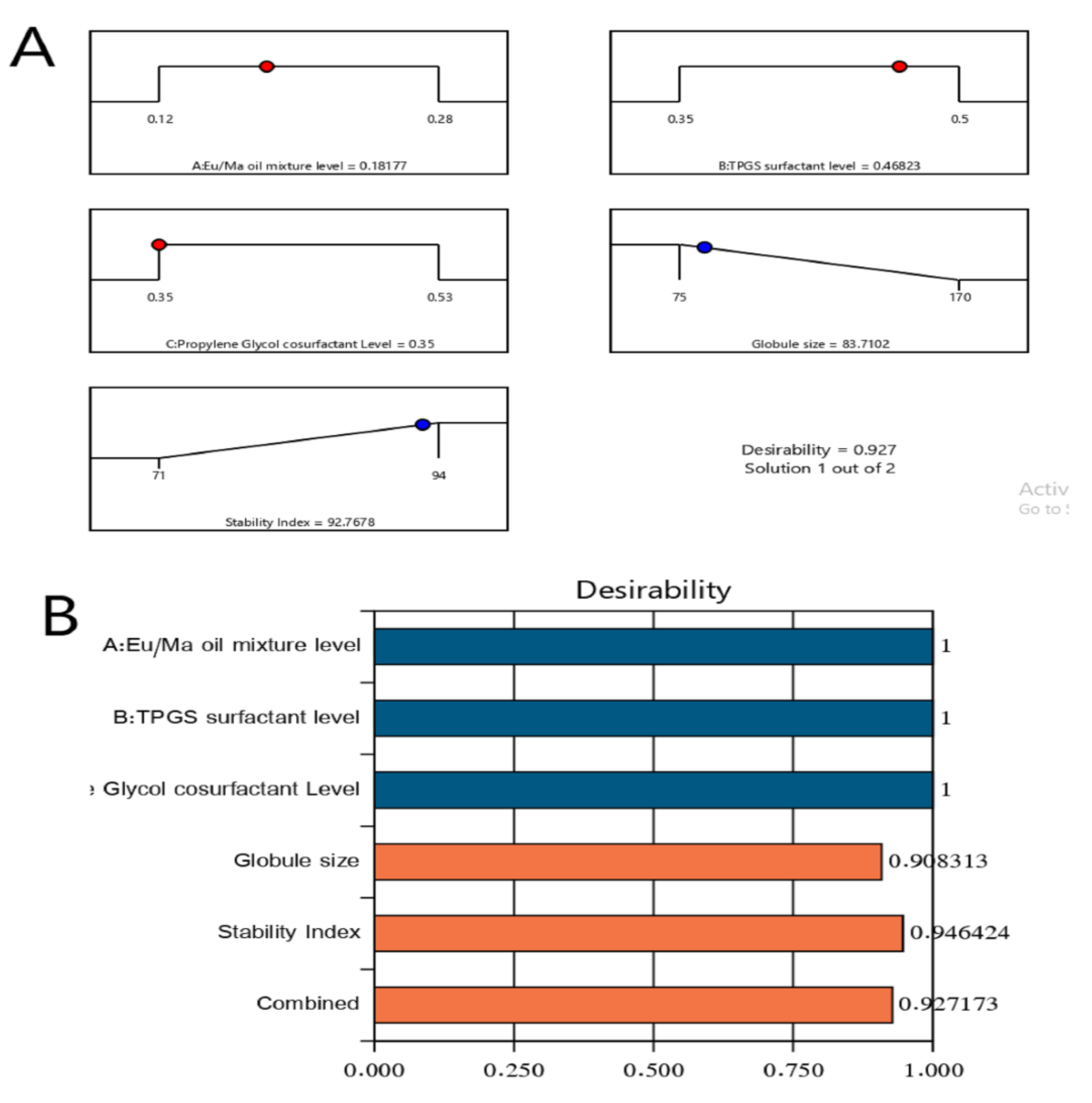
2.4. Optimization of LV-Eu-SNEDDS
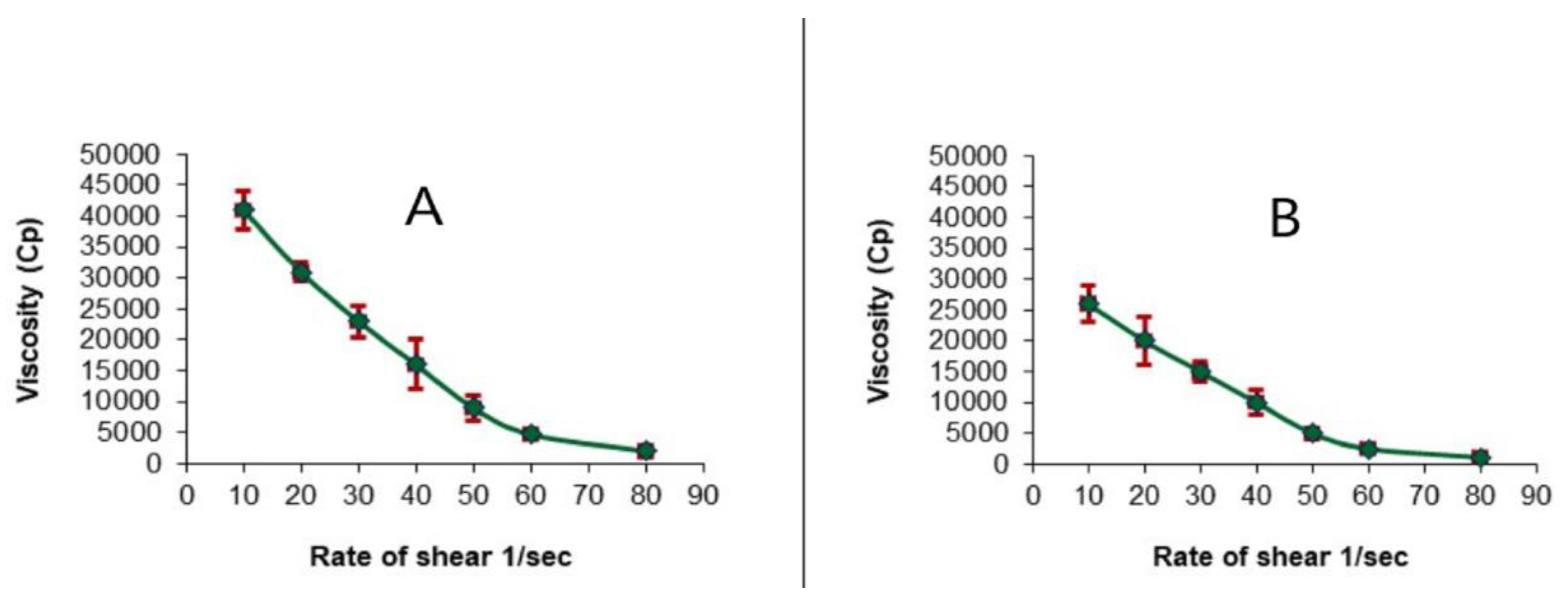
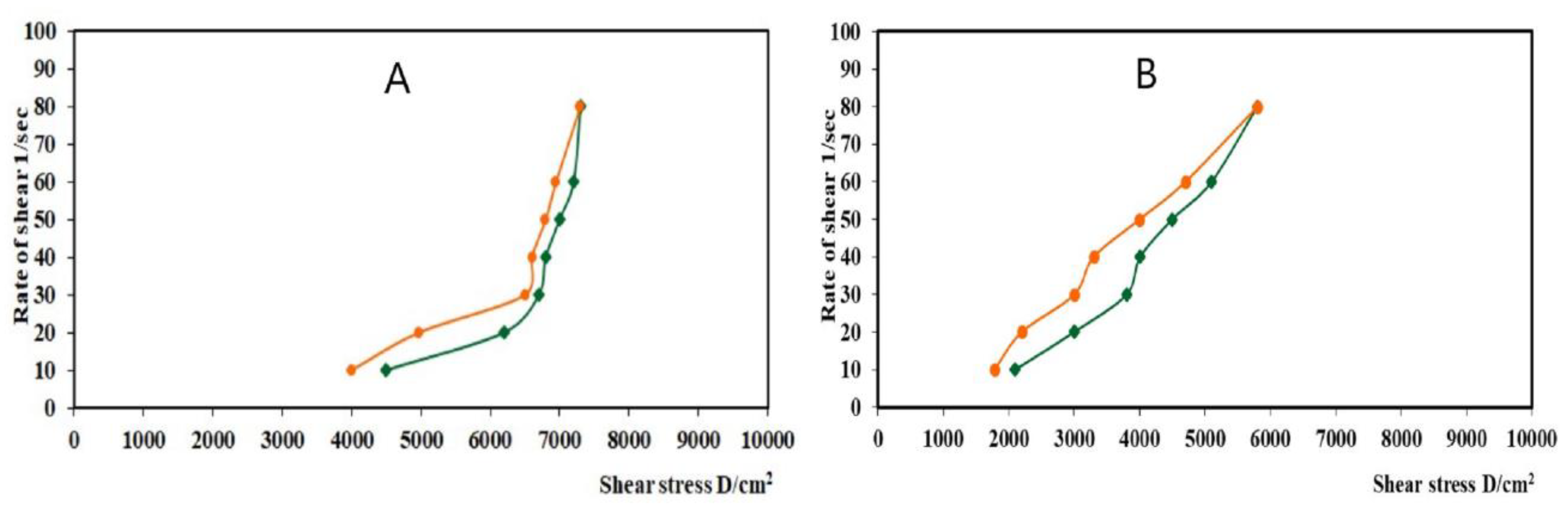
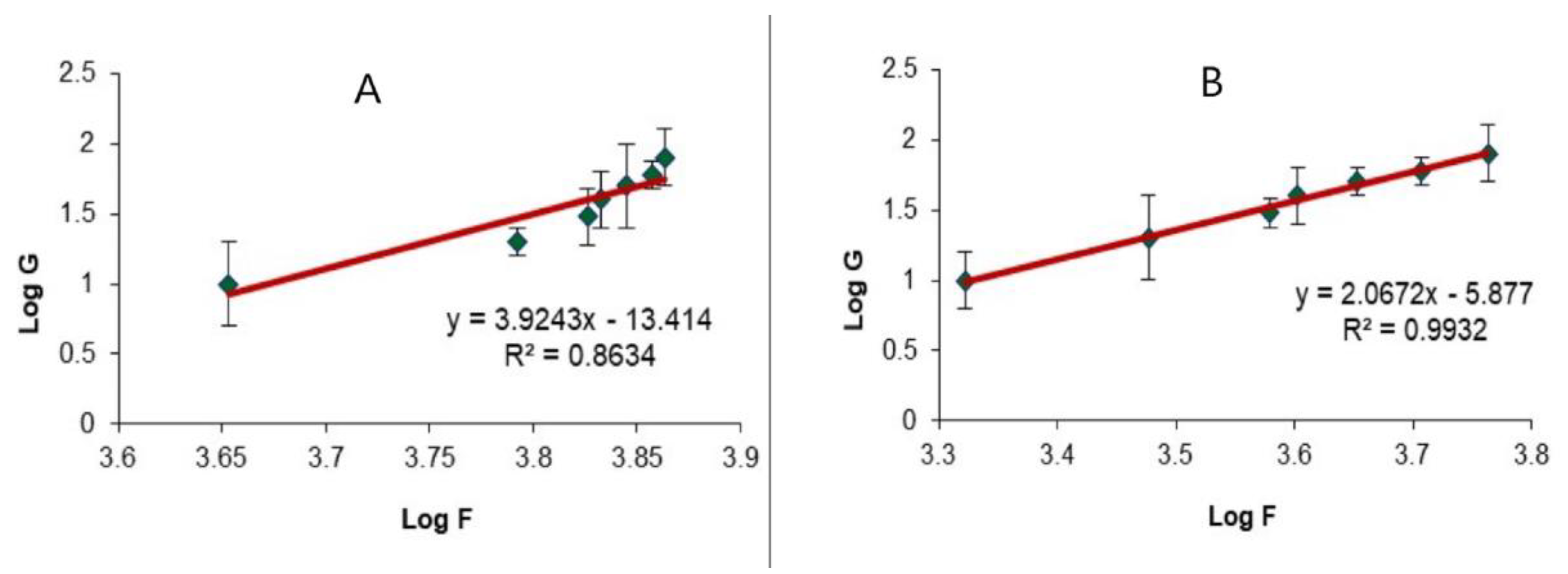
2.5. Evaluation of the Optimized LV-Eu-Nanoemulgel
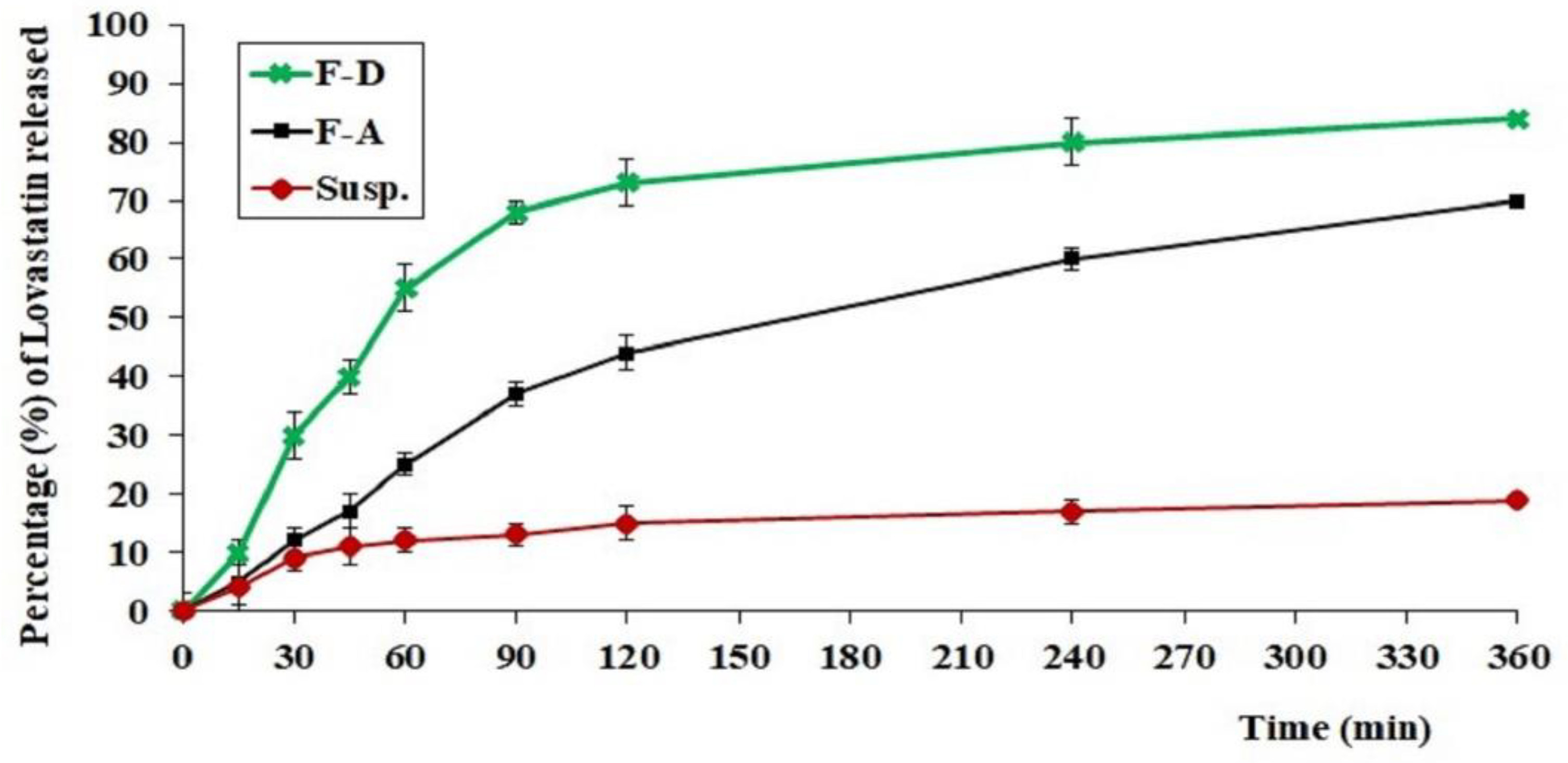
2.6. In Vitro Release of LV from Different Tested Formulations
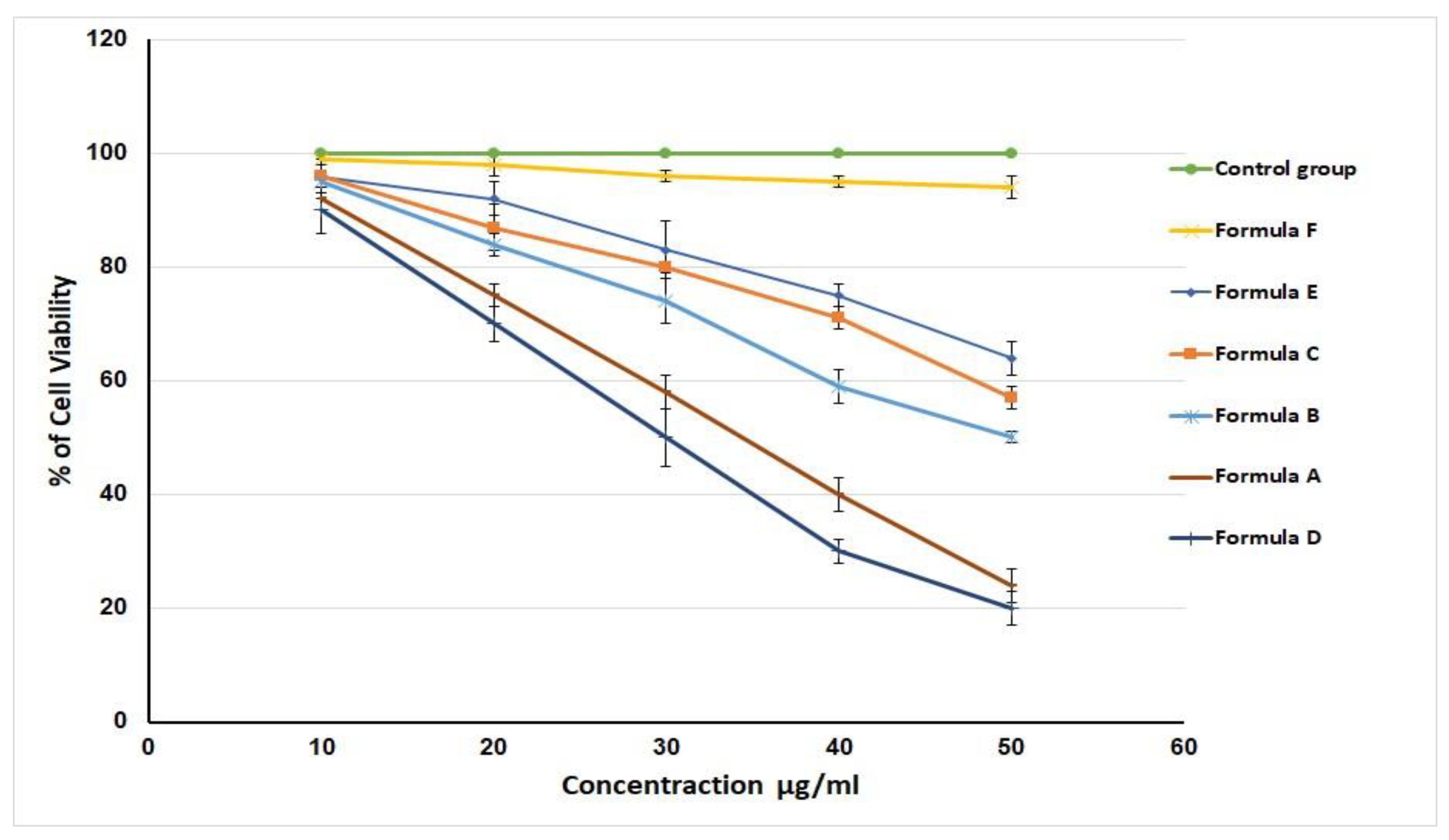
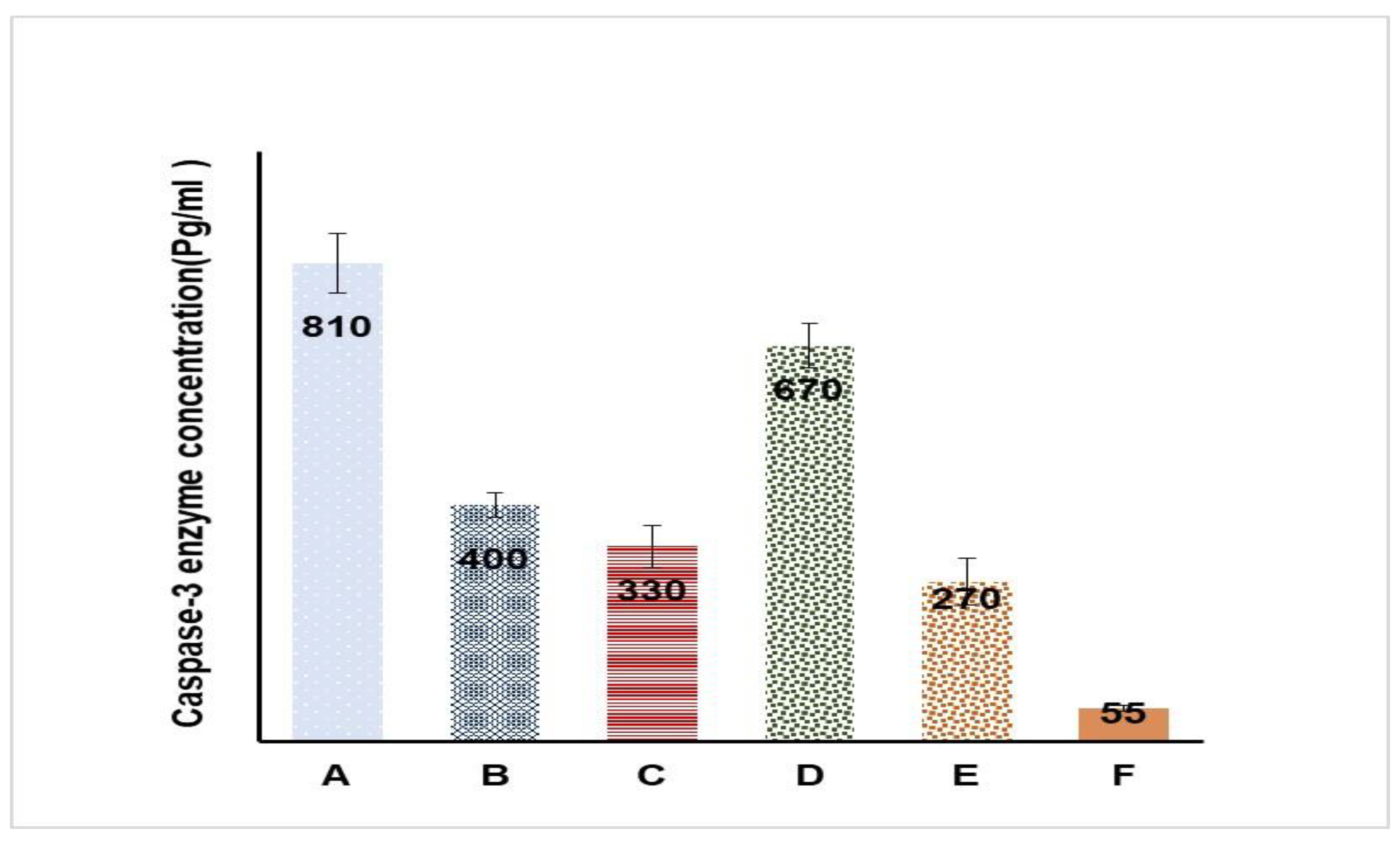
2.7. In Vitro Cell Viability and Caspase-3 Enzyme Assay
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Estimation of LV Solubility in Various SNEDDS Components
4.3. Pseudoternary-Phase Diagram for LV in Various Solvent Systems
4.4. Optimization of LV-Loaded SNEDDS as per Mixture Design
4.5. Preparation of LV-Eu-NEs
4.6. Assessment of the LV-Eu-SNEDDS
4.6.1. Emulsification Ability
4.6.2. Determination of Droplet Size of the Different LV-Eu-SNEDDS
4.6.3. Stability Studies
4.7. Optimization of LV-Eu-SNEDDS
4.8. Preparation and Characterization of the Optimized LV-Eu-Nanoemulgel
4.8.1. Rheological Evaluation of the Optimized LV-Eu-Nanoemulgel
4.8.2. In Vitro Release of the Different Formulations of LV-Eu-SNEDDS-Loaded Gels
4.9. In Vitro Cell Viability Assay
4.10. Caspase-3 Enzyme Assay
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rivera, C. Essentials of oral cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 11884–11894. [Google Scholar]
- Ettinger, K.S.; Ganry, L.; Fernandes, R.P. Oral Cavity Cancer. Oral Maxillofac. Surg. Clin. N. Am. 2019, 31, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Sultana, J.; Bashar, A.; Molla, M.R. New Management Strategies of Oral Tongue Cancer in Bangladesh. J. Maxillofac. Oral Surg. 2014, 13, 394–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scully, C.; Bagan, J. Oral squamous cell carcinoma overview. Oral Oncol. 2009, 45, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Ha, P.K.; Chang, S.S.; Glazer, C.A.; Califano, J.A.; Sidransky, D. Molecular techniques and genetic alterations in head and neck cancer. Oral Oncol. 2009, 45, 335–339. [Google Scholar] [CrossRef] [Green Version]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef] [Green Version]
- Mei, Z.; Liang, M.; Li, L.; Zhang, Y.; Wang, Q.; Yang, W. Effects of statins on cancer mortality and progression: A systematic review and meta-analysis of 95 cohorts including 1,111,407 individuals. Int. J. Cancer 2017, 140, 1068–1081. [Google Scholar] [CrossRef]
- Roy, M.; Kung, H.J.; Ghosh, P.M. Statins and prostate cancer: Role of cholesterol inhibition vs. prevention of small GTP-binding proteins. Am. J. Cancer Res. 2011, 1, 542–561. [Google Scholar]
- Langballe, R.; Cronin-Fenton, D.; Dehlendorff, C.; Jensen, M.B.; Ejlertsen, B.; Andersson, M.; Friis, S.; Mellemkjær, L. Statin use and risk of contralateral breast cancer: A nationwide cohort study. Br. J. Cancer 2018, 119, 1297–1305. [Google Scholar] [CrossRef]
- Nguyen, T.; Khan, A.; Liu, Y.; El-Serag, H.B.; Thrift, A.P. The Association Between Statin Use After Diagnosis and Mortality Risk in Patients with Esophageal Cancer: A Retrospective Cohort Study of United States Veterans. Am. J. Gastroenterol. 2018, 113, 1310. [Google Scholar] [CrossRef]
- Couttenier, A.; Lacroix, O.; Vaes, E.; Cardwell, C.R.; De Schutter, H.; Robert, A. Statin use is associated with improved survival in ovarian cancer: A retrospective population-based study. PLoS ONE 2017, 12, e0189233. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Liu, J.; Luo, F.; Lin, Q.; Rosol, T.J.; Deng, X. Anticancer properties of Monascus metabolites. Anticancer Drugs 2014, 25, 735–744. [Google Scholar] [CrossRef]
- Gunde-Cimerman, N.; Cimerman, A. Pleurotus fruiting bodies contain the inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A reductase-lovastatin. Exp. Mycol. 1995, 19, 1–6. [Google Scholar] [CrossRef]
- Crescencio, M.E.; Rodríguez, E.; Páez, A.; Masso, F.A.; Montaño, L.F.; López-Marure, R. Statins inhibit the proliferation and induce cell death of human papilloma virus positive and negative cervical cancer cells. Int. J. Biomed. Sci. 2009, 5, 411–420. [Google Scholar] [PubMed]
- Björkhem-Bergman, L.; Acimovic, J.; Torndal, U.B.; Parini, P.; Eriksson, L.C. Lovastatin prevents carcinogenesis in a rat model for liver cancer. Effects of ubiquinone supplementation. Anticancer Res. 2010, 30, 1105–1112. [Google Scholar] [PubMed]
- Murakami, R.; Chen, C.; Lyu, S.Y.; Lin, C.E.; Tzeng, P.C.; Wang, T.F.; Chang, J.C.; Shieh, Y.H.; Chen, I.F.; Huang, S.K.; et al. Lovastatin lowers the risk of breast cancer: A population-based study using logistic regression with a random effects model. SpringerPlus 2016, 5, 1932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, B.; Rao, C.V.; Bhendwal, S.; Ramey, W.R.; Shirin, H.; Reddy, B.S.; Holt, P.R. Lovastatin augments sulindac-induced apoptosis in colon cancer cells and potentiates chemopreventive effects of sulindac. Gastroenterology 1999, 117, 838–847. [Google Scholar] [CrossRef]
- Gilman, K.A.A.; Han, S.; Won, Y.W.; Putnam, C.W. Complex interactions of lovastatin with 10 chemotherapeutic drugs: A rigorous evaluation of synergism and antagonism. BMC Cancer 2021, 21, 356. [Google Scholar]
- Zhou, J.; Zhou, D. Improvement of oral bioavailability of lovastatin by using nanostructured lipid carriers. Drug Des. Dev. Ther. 2015, 9, 5269–5275. [Google Scholar] [CrossRef] [Green Version]
- Neelam, A.; Hany, O.; Sherwani, S.K.; Jabeen, S.; Nangyal, H. Phytochemical and Bioactivity of Commercially Available Eucalyptus Oil against Human Pathogens. South Asian J. Life Sci. 2014, 2, 8–11. [Google Scholar] [CrossRef] [Green Version]
- Guimarães, R.; Sousa, M.J.; Ferreira, I.C.F.R. Contribution of essential oils and phenolics to the antioxidant properties of aromatic plants. Ind. Crops Prod. 2010, 32, 152–156. [Google Scholar] [CrossRef]
- Bayala, B.; Bassole, I.H.; Gnoula, C.; Nebie, R.; Yonli, A.; Morel, L.; Figueredo, G.; Nikiema, J.-B.; Lobaccaro, J.-M.A.; Simpore, J. Chemical composition, antioxidant, anti-infammatory and antiproliferative activities of essential oils of plants from Burkina Faso. PLoS ONE 2014, 9, e92122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Döll-Boscardin, P.M.; Sartoratto, A.; Sales Maia, B.H.; de Paula, J.P.; Nakashima, T.; Farago, P.V.; Kanunfre, C.C. In Vitro Cytotoxic Potential of Essential Oils of Eucalyptus benthamii and Its Related Terpenes on Tumor Cell Lines. Evid.-Based Complement. Alternat. Med. 2012, 2012, 342652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lahmadi, G.; Lahmar, A.; Znati, M.; Elaieb, M.T.; Khouja, M.L.; Ascrizzi, R.; Flamini, G.; Harrath, A.H.; Chekir-Ghedira, L.; Jannet, H.B. Chemical Composition and Cytotoxic Activity of Eucalyptus torquata Luehm. and Eucalyptus salmonophloia F. Muell. Trunk Bark Essential Oils against Human SW620 and MDA-MB-231 Cancer Cell Lines. Chem. Biodivers. 2021, 18, e2100315. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.F.; El-Menshawe, S.F.; Khallaf, R.A.; Rabea, Y.K. A novel transdermal nanoethosomal gel of lercanidipine HCl for treatment of hypertension: Optimization using Box-Benkhen design, in vitro and in vivo characterization. Drug Deliv. Transl. Res. 2020, 10, 227–240. [Google Scholar] [CrossRef]
- Salem, H.F.; Nafady, M.M.; Ewees, M.G.E.; Hassan, H.; Khallaf, R.A. Rosuvastatin calcium-based novel nanocubic vesicles capped with silver nanoparticles-loaded hydrogel for wound healing management: Optimization employing Box-Behnken design: In vitro and in vivo assessment. J. Liposome Res. 2021, 11, 45–61. [Google Scholar] [CrossRef]
- Ali, S.A.; Sindi, A.M.; Mair, Y.H.; Khallaf, R.A. Oral gel loaded by ethotransfersomes of antifungal drug for oral thrush: Preparation, characterization, and assessment of antifungal activity. J. Drug Del. Sci. Technol. 2021, 66, 102841. [Google Scholar] [CrossRef]
- Hosny, K.M.; Alhakamy, N.A.; Sindi, A.M.; Khallaf, R.A. Coconut Oil Nanoemulsion Loaded with a Statin Hypolipidemic Drug for Management of Burns: Formulation and In Vivo Evaluation. Pharmaceutics 2020, 12, 1061. [Google Scholar] [CrossRef]
- Hosny, K.M.; Sindi, A.M.; Alkhalidi, H.M.; Kurakula, M.; Hassan, A.H.; Bakhaidar, R.B.; Abualsunun, W.A.; Almehmady, A.M.; Khames, A.; Rizg, W.Y.; et al. Development of omega-3 loxoprofen-loaded nanoemulsion to limit the side effect associated with NSAIDs in treatment of tooth pain. Drug Deliv. 2021, 28, 741–751. [Google Scholar] [CrossRef]
- Hosny, K.M.; Khallaf, R.A.; Asfour, H.Z.; Rizg, W.Y.; Alhakamy, N.A.; Sindi, A.M.; Alkhalidi, H.M.; Abualsunun, W.A.; Bakhaidar, R.B.; Almehmady, A.M.; et al. Development and Optimization of Cinnamon Oil Nanoemulgel for Enhancement of Solubility and Evaluation of Antibacterial, Antifungal and Analgesic Effects against Oral Microbiota. Pharmaceutics 2021, 13, 1008. [Google Scholar] [CrossRef]
- Hosny, K.M.; Asfour, H.Z.; Rizg, W.Y.; A Alhakamy, N.; Sindi, A.M.; Alkhalidi, H.M.; A Abualsunun, W.; Bakhaidar, R.B.; Almehmady, A.M.; Akeel, S.K.; et al. Formulation, Optimization, and Evaluation of Oregano Oil Nanoemulsions for the Treatment of Infections Due to Oral Microbiota. Int. J. Nanomed. 2021, 16, 5465–5478. [Google Scholar] [CrossRef] [PubMed]
- Aithal, G.C.; Narayan, R.; Nayak, U.Y. Nanoemulgel: A Promising Phase in Drug Delivery. Curr. Pharm. Des. 2020, 26, 279–291. [Google Scholar] [CrossRef]
- Anand, K.; Ray, S.; Rahman, M.; Shaharyar, A.; Bhowmik, R.; Bera, R.; Karmakar, S. Nano-emulgel: Emerging as a Smarter Topical Lipidic Emulsion-based Nanocarrier for Skin Healthcare Applications. Recent Pat. Anti-Infect. Drug Discov. 2019, 14, 16–35. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, H.; Gorain, B.; Pandey, M.; Chatterjee, L.A.; Sengupta, P.; Das, A.; Molugulu, N.; Kesharwani, P. Recent Update on Nanoemulgel as Topical Drug Delivery System. J. Pharm. Sci. 2017, 106, 1736–1751. [Google Scholar] [CrossRef] [PubMed]
- Abou-Taleb, H.A.; Khallaf, R.A.; Abdel-Aleem, J.A. Intranasal niosomes of nefopam with improved bioavailability: Preparation, optimization, and in-vivo evaluation. Drug Des. Dev. Ther. 2018, 12, 3501–3516. [Google Scholar] [CrossRef] [Green Version]
- Hosny, K.M.; Rizg, W.Y.; Khallaf, R.A. Preparation and Optimization of In Situ Gel Loaded with Rosuvastatin-Ellagic Acid Nanotransfersomes to Enhance the Anti-Proliferative Activity. Pharmaceutics 2020, 12, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khallaf, R.A.; Aboud, H.M.; Sayed, O.M. Surface modified niosomes of olanzapine for brain targeting via nasal route; preparation, optimization, and in vivo evaluation. J. Liposome Res. 2020, 30, 163–173. [Google Scholar] [CrossRef]
- Choi, S.J.; McClements, D.J. Nanoemulsions as delivery systems for lipophilic nutraceuticals: Strategies for improving their formulation, stability, functionality and bioavailability. Food Sci. Biotechnol. 2020, 29, 149–168. [Google Scholar] [CrossRef]
- Chuacharoen, T.; Prasongsuk, S.; Sabliov, C.M. Effect of Surfactant Concentrations on Physicochemical Properties and Functionality of Curcumin Nanoemulsions Under Conditions Relevant to Commercial Utilization. Molecules 2019, 24, 2744. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Patel, S.; Sheng, Y.; Pal, D.; Mitra, A.K. Statistical design for formulation optimization of hydrocortisone butyrate-loaded PLGA nanoparticles. AAPS PharmSciTech 2014, 15, 569–587. [Google Scholar] [CrossRef] [Green Version]
- Abdelbary, A.; Salem, H.F.; Khallaf, R.A.; Ali, A.M. Mucoadhesive niosomal in situ gel for ocular tissue targeting: In vitro and in vivo evaluation of lomefloxacin hydrochloride. Pharm. Dev. Technol. 2017, 22, 409–417. [Google Scholar] [CrossRef]
- Ghica, M.V.; Hîrjău, M.; Lupuleasa, D.; Dinu-Pîrvu, C.E. Flow and Thixotropic Parameters for Rheological Characterization of Hydrogels. Molecules 2016, 21, 786. [Google Scholar] [CrossRef] [PubMed]
- Bogman, K.; Peyer, A.K.; Török, M.; Küsters, E.; Drewe, J. HMG-CoA reductase inhibitors and P-glycoprotein modulation. Br. J. Pharmacol. 2001, 132, 1183–1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, L.; Zhu, G.; Shang, J.; Chen, X.; Zhang, C.; Ji, X.; Zhang, Q.; Wei, Y. An overview on the biological activity and anti-cancer mechanism of lovastatin. Cell Signal. 2021, 87, 110122. [Google Scholar] [CrossRef] [PubMed]
- El-Leithy, E.S.; Shaker, D.S.; Ghorab, M.K.; Abdel-Rashid, R.S. Evaluation of mucoadhesive hydrogels loaded with diclofenac sodium-chitosan microspheres for rectal administration. AAPS PharmSciTech 2010, 11, 1695–1702. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.T.; Delfino, S.F.; Feinberg, C.E.; Johnson, M.F.; Nappi, V.L.; Olinger, J.T.; Schwab, A.P.; Swanson, H.I. Current and Emerging Uses of Statins in Clinical Therapeutics: A Review. Lipid Insights 2016, 9, 13–29. [Google Scholar] [CrossRef] [Green Version]
- Fitria, A.; Hanifah, S.; Chabib, L.; Uno, A.M.; Munawwarah, H.; Atsil, N.; Pohara, H.A.; Weuanggi, D.A.; Syukri, Y. Design and characterization of propolis extract loaded self-nano emulsifying drug delivery system as immunostimulant. Saudi Pharm. J. 2021, 29, 625–634. [Google Scholar] [CrossRef]
- Dantas, M.G.; Reis, S.A.; Damasceno, C.M.; Rolim, L.; Rolim-Neto, P.J.; Carvalho, F.O.; Quintans-Junior, L.; da Silva Almeida, J.R.G. Development and Evaluation of Stability of a Gel Formulation Containing the Monoterpene Borneol. Sci. World J. 2016, 2016, 7394685. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Formulation Code | Independent Variables (Mixture Components) | Ependent Variables | ||||
|---|---|---|---|---|---|---|
| A | B | C | Y1 (nm) | Y2 (%) | PDI | |
| SNEDD-1 | 0.123 | 0.427 | 0.448 | 147 ± 1.2 | 86 ± 1.0 | 0.05 ± 0.002 |
| SNEDD-2 | 0.120 | 0.350 | 0.530 | 170 ± 0.5 | 73 ± 0.5 | 0.30 ± 0.010 |
| SNEDD-3 | 0.123 | 0.427 | 0.448 | 145 ± 1.1 | 87 ± 0.2 | 0.10 ± 0.004 |
| SNEDD-4 | 0.132 | 0.500 | 0.368 | 122 ± 2.1 | 93 ± 1.3 | 0.32 ± 0.005 |
| SNEDD-5 | 0.198 | 0.357 | 0.444 | 75 ± 0.2 | 75 ± 0.3 | 0.09 ± 0.001 |
| SNEDD-6 | 0.198 | 0.357 | 0.444 | 76 ± 0.5 | 76 ± 0.5 | 0.25 ± 0.011 |
| SNEDD-7 | 0.216 | 0.433 | 0.350 | 80 ± 1.2 | 89 ± 1.0 | 0.29 ± 0.004 |
| SNEDD-8 | 0.184 | 0.406 | 0.409 | 80 ± 1.4 | 84 ± 1.3 | 0.17 ± 0.002 |
| SNEDD-9 | 0.168 | 0.452 | 0.378 | 99 ± 3.1 | 91 ± 2.2 | 0.18 ± 0.006 |
| SNEDD-10 | 0.146 | 0.376 | 0.476 | 102 ± 2.7 | 80 ± 2.0 | 0.23 ± 0.003 |
| SNEDD-11 | 0.132 | 0.500 | 0.368 | 120 ± 1.01 | 94 ± 3.1 | 0.28 ± 0.005 |
| SNEDD-12 | 0.280 | 0.350 | 0.370 | 110 ± 0.9 | 71 ± 0.9 | 0.33 ± 0.012 |
| SNEDD-13 | 0.254 | 0.395 | 0.350 | 96 ± 0.5 | 82 ± 0.8 | 0.19 ± 0.008 |
| SNEDD-14 | 0.235 | 0.364 | 0.400 | 89 ± 1.0 | 78 ± 1.2 | 0.22 ± 0.003 |
| SNEDD-15 | 0.123 | 0.427 | 0.448 | 147 ± 2.1 | 86 ± 1.5 | 0.20 ± 0.006 |
| SNEDD-16 | 0.120 | 0.350 | 0.530 | 170 ± 2.3 | 73 ± 0.4 | 0.09 ± 0.008 |
| Dependent Variables | R2 | Adjusted R2 | Predicted R2 | F-Value | p-Value | Adequate Precision |
|---|---|---|---|---|---|---|
| Y1 | 0.9987 | 0.9967 | 0.8595 | 494.12 | 0.0001 | 69.33 |
| Y2 | 0.9926 | 0.9880 | 0.9761 | 215.13 | 0.0001 | 40.72 |
| Solution | Eu/Ma Oil Mixture % | TPGS % | Propylene Glycol % | Droplet Size (nm) | Stability Index (%) | Desirability |
|---|---|---|---|---|---|---|
| Predicated value | 0.182 | 0.468 | 0.350 | 83.7 | 92.7 | 0.927 |
| Experimental value | 0.182 | 0.468 | 0.350 | 85 | 93 | 0.927 |
| Formulation | Sample Size | Mean ± SD | D-Value | p-Value |
|---|---|---|---|---|
| A | 6 | 810 ± 103 | 0.3603 | 0.3327 |
| B | 6 | 400 ± 26 | 0.2834 | 0.6265 |
| C | 6 | 330 ± 33 | 0.2222 | 0.8714 |
| D | 6 | 670± 65 | 0.2410 | 0.8049 |
| E | 6 | 270 ± 50 | 0.2311 | 0.8612 |
| F | 6 | 55 ± 6.4 | 0.2144 | 0.8949 |
| Treatments Pair | Tukey HSD Q Statistic | Tukey HSD p-Value | Tukey HSD Inference |
|---|---|---|---|
| A vs. B | 17.1126 | 0.0010053 | p < 0.01 |
| A vs. C | 20.0505 | 0.0010053 | p < 0.01 |
| A vs. D | 5.5557 | 0.0056425 | p < 0.01 |
| A vs. E | 22.2993 | 0.0010053 | p < 0.01 |
| A vs. F | 31.5448 | 0.0010053 | p < 0.01 |
| B vs. C | 2.9380 | 0.3254137 | insignificant |
| B vs. D | 11.5569 | 0.0010053 | p < 0.01 |
| B vs. E | 5.1867 | 0.0110464 | p < 0.05 |
| B vs. F | 14.4322 | 0.0010053 | p < 0.01 |
| C vs. D | 14.4949 | 0.0010053 | p < 0.01 |
| C vs. E | 2.2487 | 0.5977232 | insignificant |
| C vs. F | 11.4942 | 0.0010053 | p < 0.01 |
| D vs. E | 16.7436 | 0.0010053 | p < 0.01 |
| D vs. F | 25.9891 | 0.0010053 | p < 0.01 |
| E vs. F | 9.2455 | 0.0010053 | p < 0.01 |
| Component | Level | Response | Goal | |
|---|---|---|---|---|
| Low | High | |||
| Eu/Ma oil mixture level; (A) | 0.12 | 0.28 | Mean globule size (Y1) Stability Index (Y2) | Minimize Maximize |
| TPGS surfactant level; (B) | 0.35 | 0.5 | ||
| Propylene Glycol cosurfactant Level; (C) | 0.35 | 0.53 | ||
| Formulation | Composition |
|---|---|
| A | Hydrogel loaded with optimized LV-Eu –SNEDDs |
| B | Hydrogel loaded with NE formulated with castor oil instead of Eu/Ma oil |
| C | Hydrogel loaded with NE formulated without LV |
| D | Aqueous dispersion LV-Eu –SNEDDs without using of carbopol 934 gelling agent |
| E | Physical mixture of LV and Eu oil |
| F | Carbopol 934 hydrogel (plain) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizg, W.Y.; Hosny, K.M.; Mahmoud, S.S.; Kammoun, A.K.; Alamoudi, A.J.; Tayeb, H.H.; Bukhary, H.A.; Badr, M.Y.; Murshid, S.S.A.; Alfayez, E.; et al. Repurposing Lovastatin Cytotoxicity against the Tongue Carcinoma HSC3 Cell Line Using a Eucalyptus Oil-Based Nanoemulgel Carrier. Gels 2022, 8, 176. https://doi.org/10.3390/gels8030176
Rizg WY, Hosny KM, Mahmoud SS, Kammoun AK, Alamoudi AJ, Tayeb HH, Bukhary HA, Badr MY, Murshid SSA, Alfayez E, et al. Repurposing Lovastatin Cytotoxicity against the Tongue Carcinoma HSC3 Cell Line Using a Eucalyptus Oil-Based Nanoemulgel Carrier. Gels. 2022; 8(3):176. https://doi.org/10.3390/gels8030176
Chicago/Turabian StyleRizg, Waleed Y., Khaled M. Hosny, Samar S. Mahmoud, Ahmed K. Kammoun, Abdulmohsin J. Alamoudi, Hossam H. Tayeb, Haitham A. Bukhary, Moutaz Y. Badr, Samar S. A. Murshid, Eman Alfayez, and et al. 2022. "Repurposing Lovastatin Cytotoxicity against the Tongue Carcinoma HSC3 Cell Line Using a Eucalyptus Oil-Based Nanoemulgel Carrier" Gels 8, no. 3: 176. https://doi.org/10.3390/gels8030176
APA StyleRizg, W. Y., Hosny, K. M., Mahmoud, S. S., Kammoun, A. K., Alamoudi, A. J., Tayeb, H. H., Bukhary, H. A., Badr, M. Y., Murshid, S. S. A., Alfayez, E., Ali, S. A., Mushtaq, R. Y., & Abualsunun, W. A. (2022). Repurposing Lovastatin Cytotoxicity against the Tongue Carcinoma HSC3 Cell Line Using a Eucalyptus Oil-Based Nanoemulgel Carrier. Gels, 8(3), 176. https://doi.org/10.3390/gels8030176






