Stability of Paracetamol Instant Jelly for Reconstitution: Impact of Packaging, Temperature and Humidity
Abstract
:1. Introduction
2. Results and Discussion
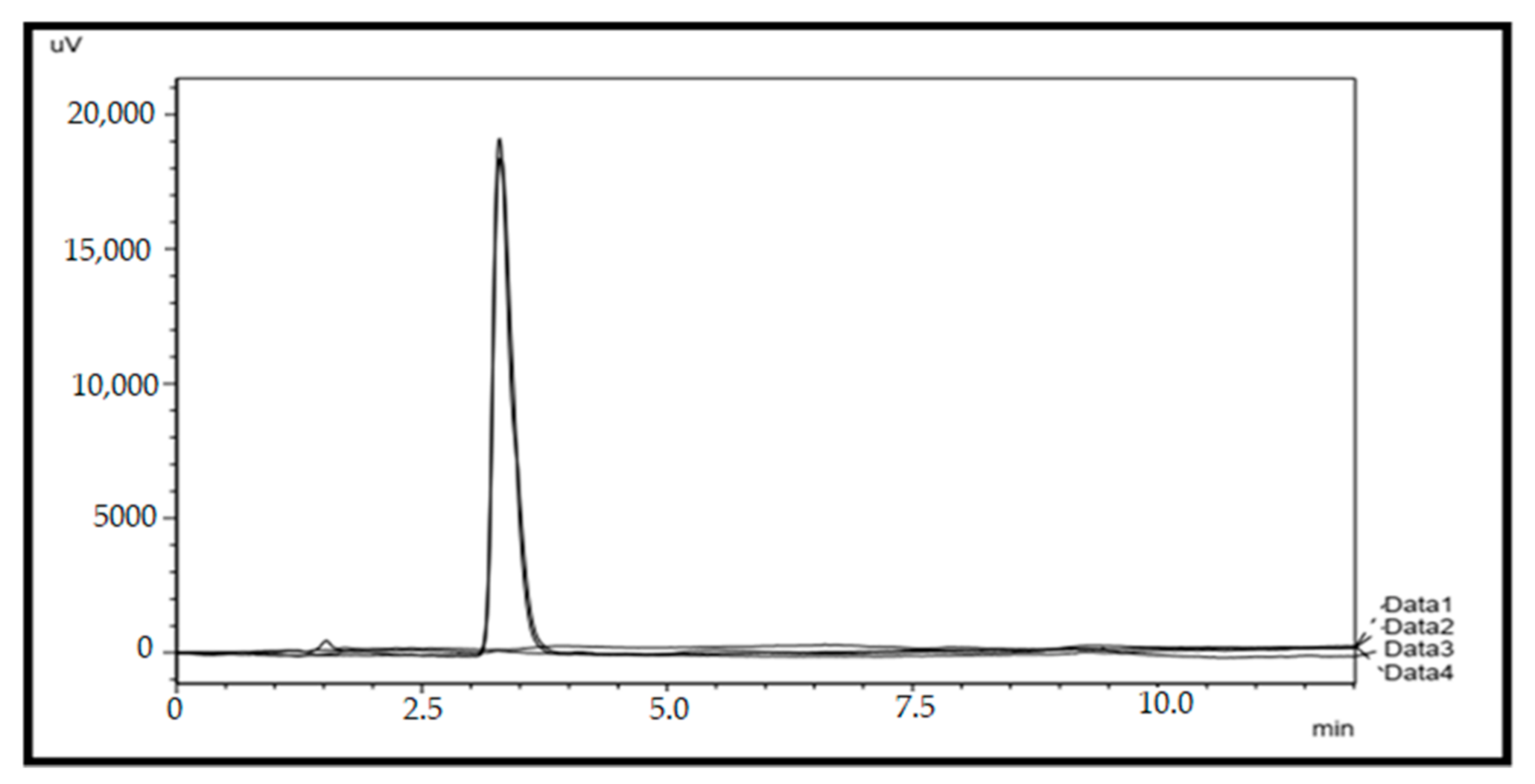
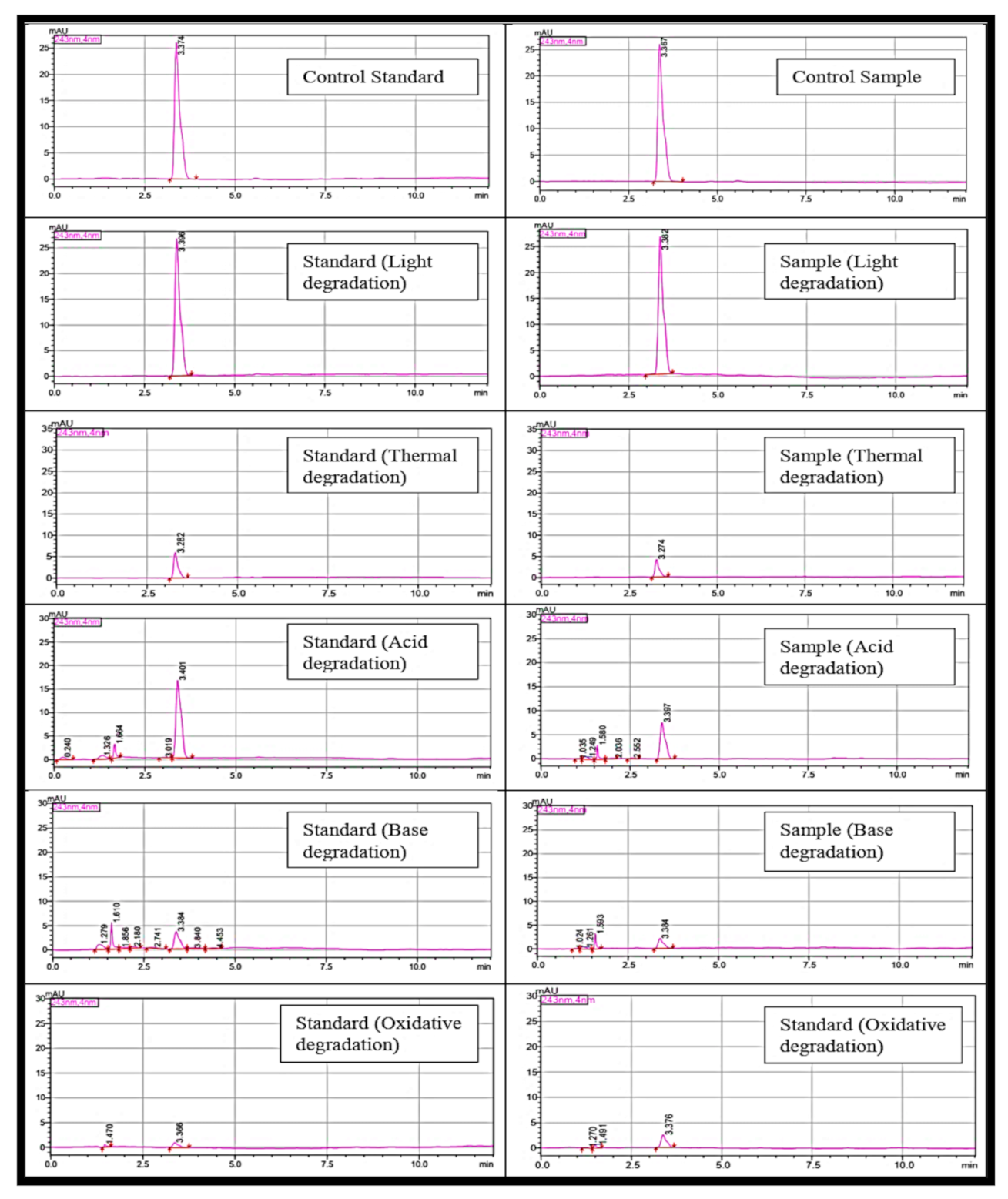
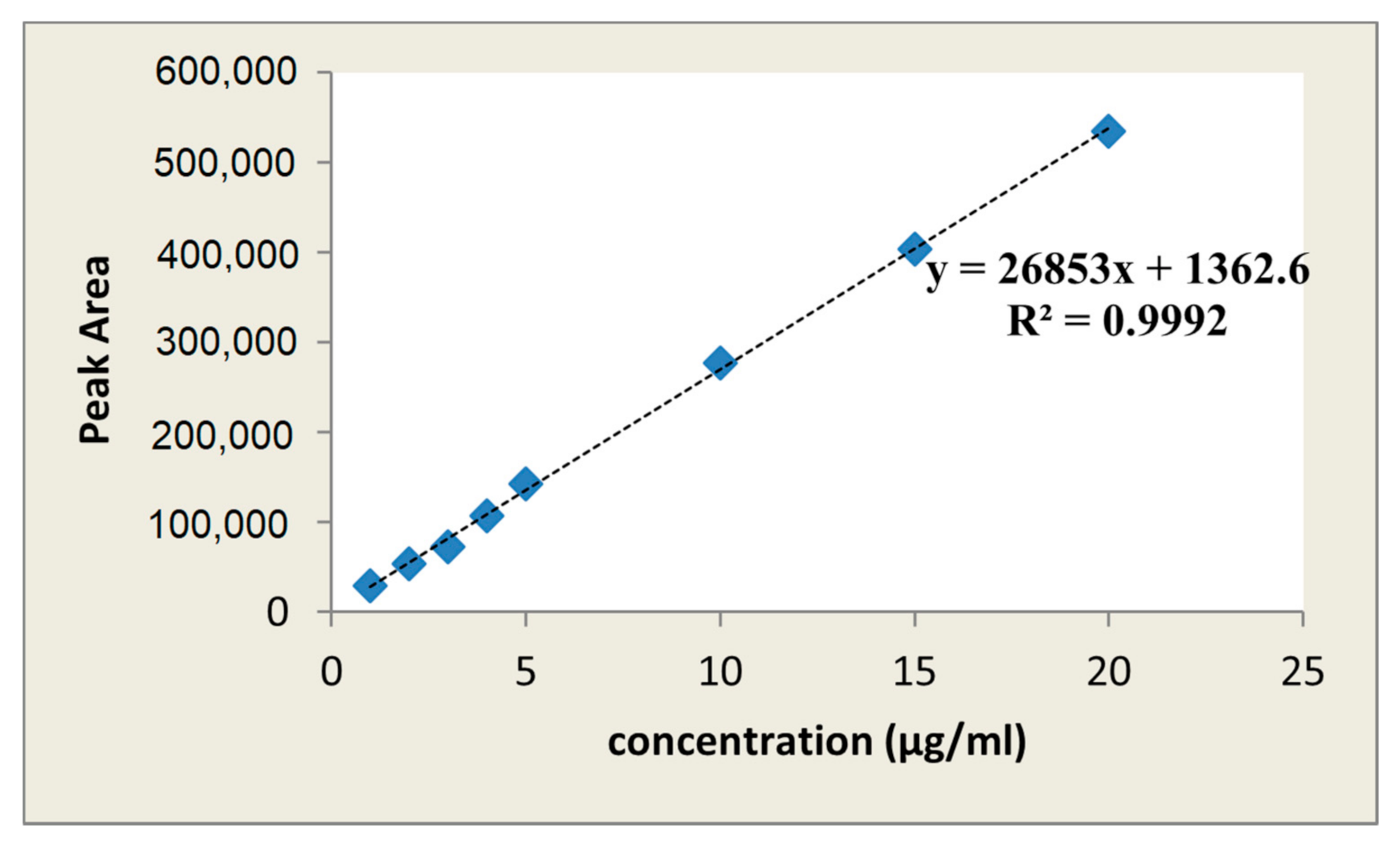
2.1. HPLC Method Validation for Quantification of Paracetamol
2.2. Physical Observation and Moisture Content
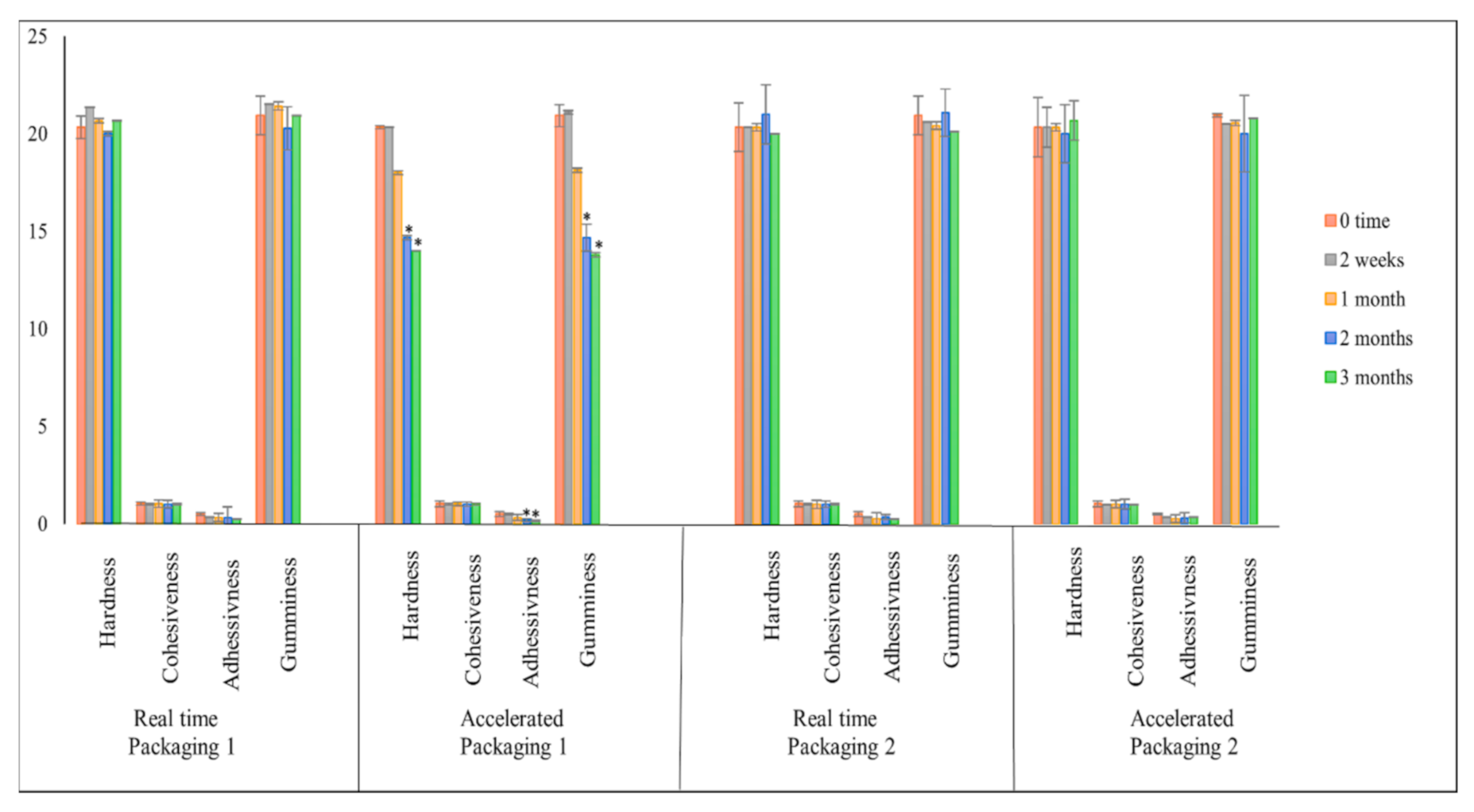
2.3. Texture Profile Analysis (TPA)
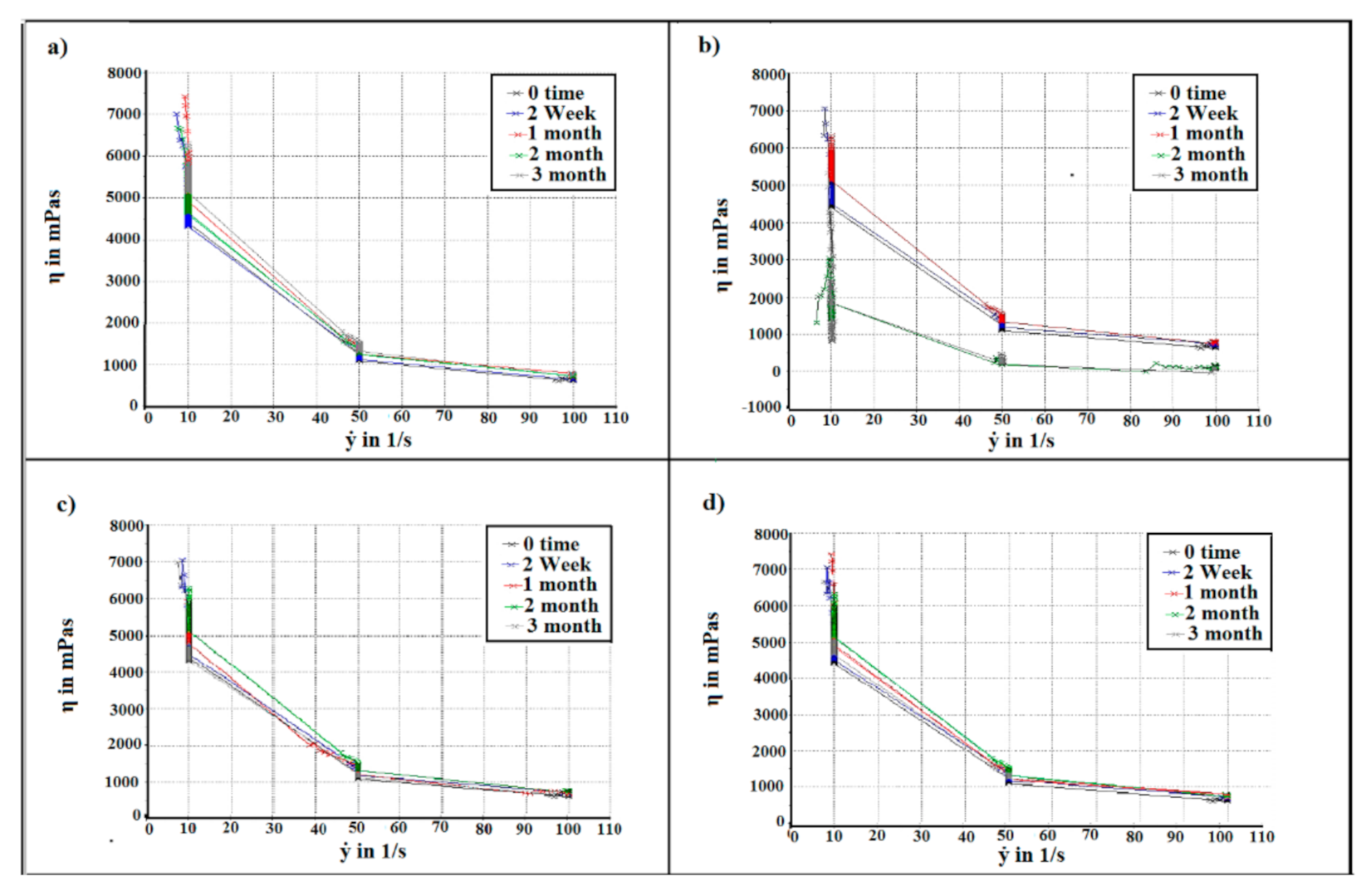
2.4. Flow Behaviour Measurement
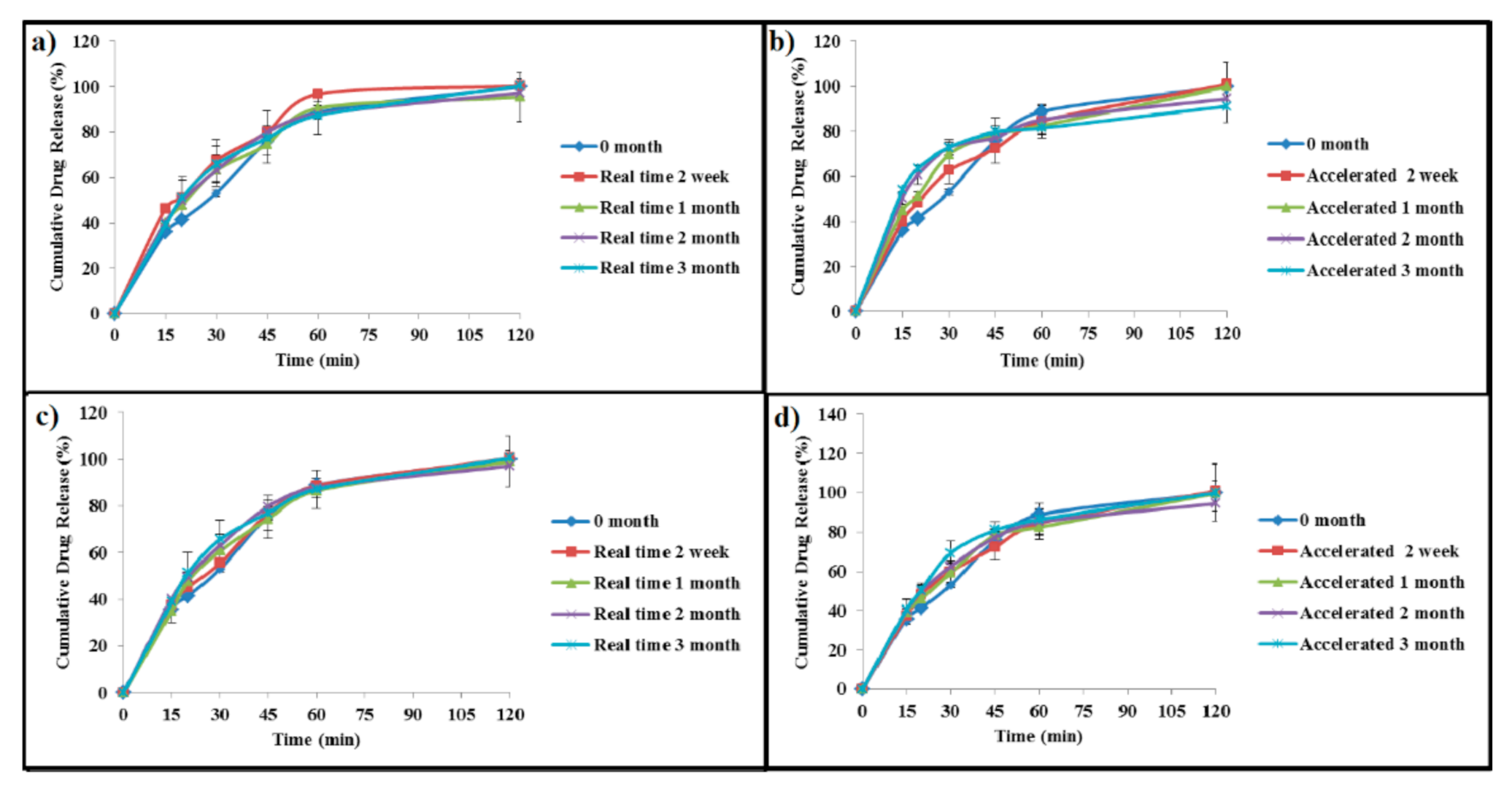
2.5. In Vitro Drug Release
2.6. Paracetamol Content
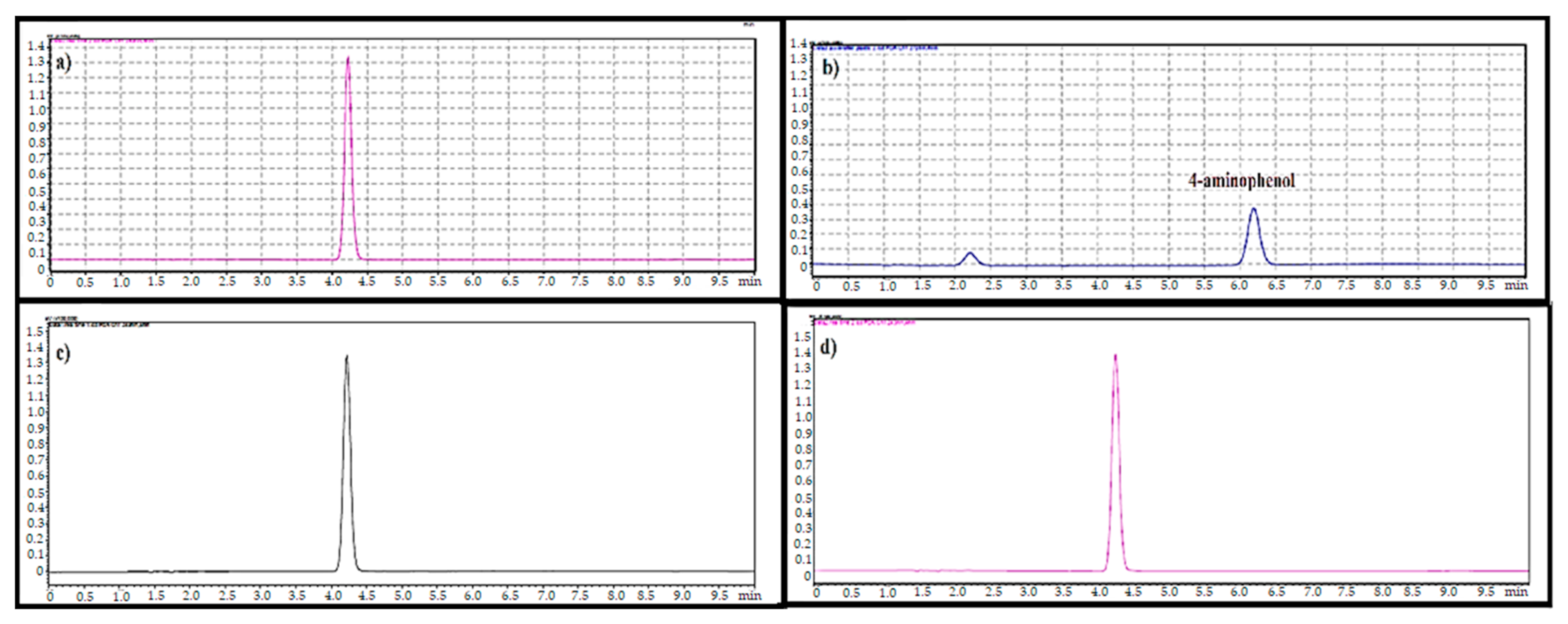
2.7. 4-Aminophenol Level
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Stability Study Protocol
4.2.2. Analytical Method Validation of Paracetamol
4.2.3. Physical Observation
4.2.4. Moisture Content
4.2.5. Texture Profile Analysis (TPA)
4.2.6. Flow Curve Measurement
4.2.7. In Vitro Drug Release
4.2.8. Determination of Drug Content and Shelf Life
4.2.9. 4-Aminophenol Level
4.2.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Nyamweya, N.N.M.; Ngugi, S.G. Development and Evaluation of Pediatric Orally Disintegrating Paracetamol Tablets. East Cent. African J. Pharm. Sci. 2019, 22, 37–44. [Google Scholar]
- Martínez-Terán, M.E.; Hoang-Thi, T.H.; Flament, M.P. Multiparticulate dosage forms for pediatric use. Pediatr. Ther. 2017, 7, 665–2161. [Google Scholar] [CrossRef] [Green Version]
- Almurisi, S.H.; Doolaanea, A.A.; Akkawi, M.E.; Chatterjee, B.; Aljapairai, K.A.S.; Sarker, M.Z.I. Formulation development of paracetamol instant jelly for pediatric use. Drug Dev. Ind. Pharm. 2020, 46, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, S.; Singla, D.; Sakhuja, N. Stability testing of pharmaceutical products. J. App. Pharm. Sci. 2012, 2, 129–138. [Google Scholar]
- Tembhare, E.; Gupta, K.R.; Umekar, M.J. An approach to drug stability studies and shelf-life determination. Arch. Curr. Res. Int 2019, 19, 1–20. [Google Scholar] [CrossRef]
- Kopp, S. Stability testing of pharmaceutical products in a global environment. RAJ Pharma 2006, 5, 291–294. [Google Scholar]
- Haywood, A.; Mangan, M.; Glass, B. Stability implications of repackaging paracetamol tablets into dose administration aids. J. Pharm. Pract. Res. 2006, 36, 25–28. [Google Scholar] [CrossRef] [Green Version]
- Florence, A.T.; Attwood, D. Physicochemical principles of pharmacy. In Manufacture, Formulation and Clinical Use; Pharmaceutical Press: London, UK, 2015. [Google Scholar]
- Li, H.; Nadig, D.; Kuzmission, A.; Riley, C.M. Prediction of the changes in drug dissolution from an immediate-release tablet containing two active pharmaceutical ingredients using an accelerated stability assessment program (ASAP prime®). AAPS Open 2016, 2, 7. [Google Scholar] [CrossRef] [Green Version]
- Harun-Or-Rashid, M.; Biswas, S.; Bhuiyan, J.R.; Abdullah-Al-Mamun, M.; Islam, M.F. Evaluation of Photo-degradation of Paracetamol Tablet in Various Packaging Modes in Bangladesh. IJPRS 2014, 3, 1–2. [Google Scholar]
- Yamashita, S.; Iguchi, K.; Noguchi, Y.; Sakai, C.; Yokoyama, S.; Ino, Y.; Hayashi, H.; Teramachi, H.; Sako, M.; Sugiyama, T. Changes in the quality of medicines during storage under LED lighting and consideration of countermeasures. J. Pharm. Health Care Sci. 2018, 4, 1–6. [Google Scholar] [CrossRef]
- Calvino, M.M.; Lisuzzo, L.; Cavallaro, G.; Lazzara, G.; Milioto, S. Non-isothermal thermogravimetry as an accelerated tool for the shelf-life prediction of paracetamol formulations. Thermochim. Acta 2021, 700, 178940. [Google Scholar] [CrossRef]
- Zadbuke, N.; Shahi, S.; Gulecha, B.; Padalkar, A.; Thube, M. Recent trends and future of pharmaceutical packaging technology. J. Pharm. Bioallied Sci. 2013, 5, 98. [Google Scholar] [CrossRef]
- Mogna, G. Material Impermeable to Humidity and Oxygen for Packaging Dietary Products, Cosmetics and Medicinal Specialities. U.S. Patent 10,384,847, 20 August 2019. [Google Scholar]
- Qiu, Y.; Chen, Y.; Zhang, G.G.Z.; Yu, L.; Mantri, R.V. Developing Solid Oral Dosage Forms: Pharmaceutical Theory and Practice; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Capra, P.; Musitelli, G.; Faccioli, M.; Briasco, B.; Perugini, P. Protocol and specimen set up for mechanical evaluation of cosmetic packaging. World J. Pharm. Res. 2016, 5, 217–233. [Google Scholar]
- Carstensen, J.T.; Rhodes, C. Drug Stability, Revised, and Expanded: Principles and Practices; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Dejaegher, B.; Jimidar, M.; de Smet, M.; Cockaerts, P.; Smeyers-Verbeke, J.; Heyden, Y.V. Improving method capability of a drug substance HPLC assay. J. Pharm. Biomed. Anal. 2006, 2, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Waterman, K.C.; MacDonald, B.C. Package selection for moisture protection for solid, oral drug products. J. Pharm. Sci. 2010, 99, 4437–4452. [Google Scholar] [CrossRef]
- Ola, M.; Bhaskar, R.; Patil, P. Dry syrup: An overview. Indian J. Pharm. Biol. Res. 2018, 6, 30–38. [Google Scholar] [CrossRef]
- Hasan, N.; Chaiharn, M.; Toor, U.A.; Mirani, Z.A.; Sajjad, G.; Shetr, N.; Alziz, M.; Siddiqui, F.A. Development, validation and application of RP-HPLC method: Simultaneous determination of antihistamine and preservatives with paracetamol in liquid formulations and human serum. Open Med. Chem. J. 2016, 10, 33. [Google Scholar] [CrossRef] [Green Version]
- Blessy, M.; Patel, R.D.; Prajapati, P.N.; Agrawal, Y.K. Development of forced degradation and stability indicating studies of drugs—A review. J. Pharm. Anal. 2014, 4, 159–165. [Google Scholar] [CrossRef] [Green Version]
- Yamazaki, N.; Taya, K.; Shimokawa, K.; Ishii, F. The most appropriate storage method in unit-dose package and correlation between color change and decomposition rate of aspirin tablets. Int. J. Pharm. 2010, 396, 105–110. [Google Scholar] [CrossRef]
- Mochizuki, K.; Takayama, K. Prediction of color changes in acetaminophen solution using the time–temperature superposition principle. Drug Dev. Ind. Pharm. 2016, 42, 1050–1057. [Google Scholar] [CrossRef]
- Garrido, J.I.; Lozano, J.E.; Genovese, D.B. Effect of formulation variables on rheology, texture, colour, and acceptability of apple jelly: Modelling and optimization. LWT-Food Sci. Technol. 2015, 62, 325–332. [Google Scholar] [CrossRef]
- Yusof, N.; Jaswir, I.; Jamal, P.; Jami, M.S. Texture Profile Analysis (TPA) of the jelly dessert prepared from halal gelatin extracted using High Pressure Processing (HPP). Malays. J. Fundam. Appl. Sci 2019, 15, 604–608. [Google Scholar] [CrossRef]
- Mutlu, C.; Tontul, S.A.; Erbaş, M. Production of a minimally processed jelly candy for children using honey instead of sugar. LWT 2018, 93, 499–505. [Google Scholar] [CrossRef]
- Singh, V.; Guizani, N.; Al-Alawi, A.; Claereboudt, M.; Rahman, M.S. Instrumental texture profile analysis (TPA) of date fruits as a function of its physico-chemical properties. Ind. Crops Prod. 2013, 50, 866–873. [Google Scholar] [CrossRef]
- Rahman, M.S.; Al-Shamsi, Q.; Abdullah, A.; Claereboudt, M. Classification of commercial Omani halwa by physico-chemical properties and instrumental texture profile analysis (TPA). Ital. J. Food Sci. 2012, 24, 292–304. [Google Scholar]
- Zema, M.; Mapelli, L.G.; Marconi, M.G. Pharmaceutical Composition for Rapid Suspension in Water. U.S. Patent 5,306,506, 26 April 1994. [Google Scholar]
- Kluk, A.; Sznitowska, M. Application properties of oral gels as media for administration of minitablets and pellets to paediatric patients. Int. J. Pharm. 2014, 460, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Doolaanea, A.A.; Bahari, A. Advantages of jelly over liquid formulations for pediatrics. J. Formul. Sci. Bioavailab. 2017, 1, 1000102. [Google Scholar]
- Zahn, M. Handbook of Stability Testing in Pharmaceutical Development; Springer: New York, NY, USA, 2008; pp. 43–91. [Google Scholar]
- Beaman, J.; Whitlock, M.; Wallace, R.; Edwards, A. The scientific basis for the duration of stability data required at the time of submission. J. Pharm. Sci. 2010, 99, 2538–2543. [Google Scholar] [CrossRef] [PubMed]
- de Bleye, C.; Dumont, E.; Rozet, E.; Sacré, P.-Y.; Chavez, P.-F.; Netchacovitch, L.; Piel, G.; Hubert, P.; Ziemons, E. Determination of 4-aminophenol in a pharmaceutical formulation using surface enhanced Raman scattering: From development to method validation. Talanta 2013, 116, 899–905. [Google Scholar] [CrossRef]
- Adegoke, O.A.; Thomas, O.E.; Amao, S.A.; Agboola, S.O.; Omotosho, A.E. A new method for the microdetermination of Para-aminophenol in generic brands of paracetamol tablets. Arab J. Basic Appl. Sci. 2019, 26, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, I.; Shaikh, R.H. Stability of paracetamol in packaged tablet formulations. Pak. J. Pharm. Sci. 1993, 6, 37–45. [Google Scholar] [PubMed]
- Al-Japairai, K.A.S.; Chatterjee, B.; Mahmood, S.; Almurisi, S.H. A bioanalytical method for quantification of telmisartan in rat plasma; development, validation and application to pharmacokinetic study. Res. J. Pharm. Technol. 2021, 14, 2139–2144. [Google Scholar] [CrossRef]
- Kishino, T.; Inoue, Y.; Inoue, K.; Saito, K.; Kondo, M.; Otsukal, K.; Terukina, T.; Hattori, Y.; Otsuka, M. redictive Evaluation of Pharmaceutical Properties of Ulinastatin-Containing Vaginal Suppositories as a Hospital Preparation by Near-Infrared Spectroscopy. Chem. Pharm. Bull. 2018, 66, 589–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krokida, M.K.; Maroulis, Z.B.; Saravacos, G.D. Rheological properties of fluid fruit and vegetable puree products: Compilation of literature data. Int. J. food Prop. 2001, 4, 179–200. [Google Scholar] [CrossRef]
- Mohamad, M.; Roheena, J. Stability studies for the determination of shelf life of aceclofenac formulation. Der Pharm. Lett. 2012, 4, 483–486. [Google Scholar]
- AlAani, H.; Alnukkary, Y.; Alashkar, I. Stability and kinetic studies for the estimation of shelf life of chloramphenicol, dexamethasone sodium phosphate, and tetrahydrozoline hydrochloride ophthalmic solution. Int. J. Pharm. Sci. Rev. Res. 2015, 30, 327–330. [Google Scholar]
- Akhtar, N.; Talegaonkar, S.; Khar, R.K.; Jaggi, M. A validated stability-indicating LC method for estimation of etoposide in bulk and optimized self-nano emulsifying formulation: Kinetics and stability effects. Saudi Pharm. J. 2013, 21, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Hassan, I.H.; Mohamed, F.; Haris, M.S. Analytical Method Validation (AMV) of Paracetamol Honey Suspension. IIUM Med. J. Malays. 2016, 15, 78. [Google Scholar] [CrossRef]



| Type | Conditions |
|---|---|
| Products in primary containers permeable to water vapour | 30 ± 2 °C/75 ± 5% RH |
| Products in primary containers impermeable to water vapour | 30 ± 2 °C/RH not specified |
| Accelerated studies | 40 ± 2 °C/75 ± 5% RH |
| Intra-Day | Inter-Day | |||||
|---|---|---|---|---|---|---|
| Level | Peak Area | % Recovery | % RSD | Peak Area | % Recovery | %RSD |
| 100% spiked sample | 278,093 | 99.01 ± 1.38 | 1.4 | 269,518 | 98.5 ± 1.18 | 1.19 |
| 279,699 | 279,012 | |||||
| 271,627 | 273,302 | |||||
| 271,175 | 271,424 | |||||
| 271,656 | 271,871 | |||||
| 271,630 | 271,740 | |||||
| Packaging | Storage Conditions | 0 Time | 2 Week | 1 Month | 2 Month | 3 Months |
|---|---|---|---|---|---|---|
| Packaging 1 | Real-time | 2.57 ± 0.29 | 3.57 ± 0.29 * | 6.71 ± 0.20 * | 7.38 ± 0.1 * | 7.48 ± 0.18 * |
| Accelerated | 8.71 ± 0.19 * | 10.35 ± 0.16 * | 13.4 ± 0.18 * | 15.16 ± 0.29 * | ||
| Packaging 2 | Real-time | 2.6 ± 0.3 | 2.82 ± 0.13 | 2.89 ± 0.18 | 3.05 ± 0.12 | |
| Accelerated | 2.68 ± 0.47 | 2.91 ± 0.57 | 2.92 ± 0.23 | 2.89 ± 0.99 |
| Packaging Material | Sample | 0 Time | 2 Week | 1 Month | 2 Month | 3 Months |
|---|---|---|---|---|---|---|
| Packaging 1 | Real-time | 103.27 ± 0.08 | 102.48 ± 0.60 | 102.37 ± 0.33 | 101.87 ± 0.11 | 101.65 ± 0.75 |
| Accelerated | 102.50 ± 0.30 | 99.8 ± 1.02 | 93.7 ± 0.55 | 89.44 ± 2.22 | ||
| Packaging 2 | Real-time | 102.37 ± 1.6 | 102.22 ± 1.03 | 101.87 ± 0.11 | 101.98 ± 0.04 | |
| Accelerated | 102.78 ± 2.17 | 102.35 ± 2.04 | 101.87 ± 0.12 | 102.08 ± 0.09 |
| Packaging Material | Temperature °C | Slop | Kdeg (Days −1) | t90% | |
|---|---|---|---|---|---|
| Days | Years | ||||
| Packaging 1 | 30 | 0.00007 | 0.00016 | 651 | 1.78 |
| 40 | 0.0007 | 0.0016 | 65 | 0.18 | |
| Packaging 2 | 30 | 0.00005 | 0.000115 | 913 | 2.5 |
| 40 | 0.00006 | 0.000138 | 761 | 2.08 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamed Almurisi, S.; AL-Japairai, K.; Alshammari, F.; Alheibshy, F.; Sammour, R.M.F.; Doolaanea, A.A. Stability of Paracetamol Instant Jelly for Reconstitution: Impact of Packaging, Temperature and Humidity. Gels 2022, 8, 144. https://doi.org/10.3390/gels8030144
Hamed Almurisi S, AL-Japairai K, Alshammari F, Alheibshy F, Sammour RMF, Doolaanea AA. Stability of Paracetamol Instant Jelly for Reconstitution: Impact of Packaging, Temperature and Humidity. Gels. 2022; 8(3):144. https://doi.org/10.3390/gels8030144
Chicago/Turabian StyleHamed Almurisi, Samah, Khater AL-Japairai, Farhan Alshammari, Fawaz Alheibshy, Rana M.F. Sammour, and Abd Almonem Doolaanea. 2022. "Stability of Paracetamol Instant Jelly for Reconstitution: Impact of Packaging, Temperature and Humidity" Gels 8, no. 3: 144. https://doi.org/10.3390/gels8030144
APA StyleHamed Almurisi, S., AL-Japairai, K., Alshammari, F., Alheibshy, F., Sammour, R. M. F., & Doolaanea, A. A. (2022). Stability of Paracetamol Instant Jelly for Reconstitution: Impact of Packaging, Temperature and Humidity. Gels, 8(3), 144. https://doi.org/10.3390/gels8030144







