Characteristics of Sodium Alginate/Antarctic Krill Protein Composite Fiber Based on Cellulose Nanocrystals Modification: Rheology, Hydrogen Bond, Crystallization, Strength, and Water-Resistance
Abstract
:1. Introduction
2. Results and Discussion
2.1. Rheological Properties of SA/AKP/CNCs Composite Solution
2.2. Infrared Spectrum Analysis of SA/AKP/CNCs Composite Fiber
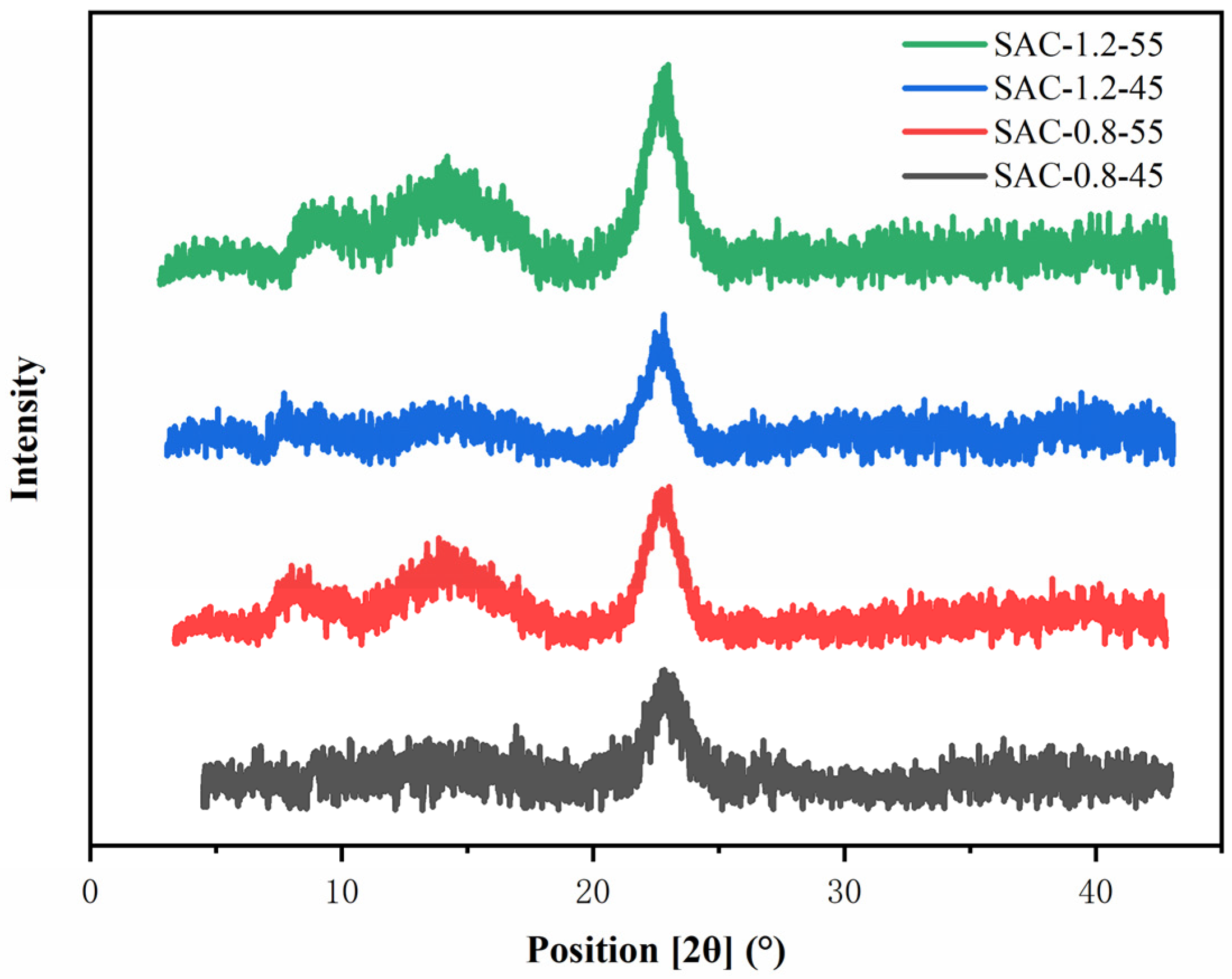
2.3. X-ray Diffraction Analysis of SA/AKP/CNCs Composite Fiber
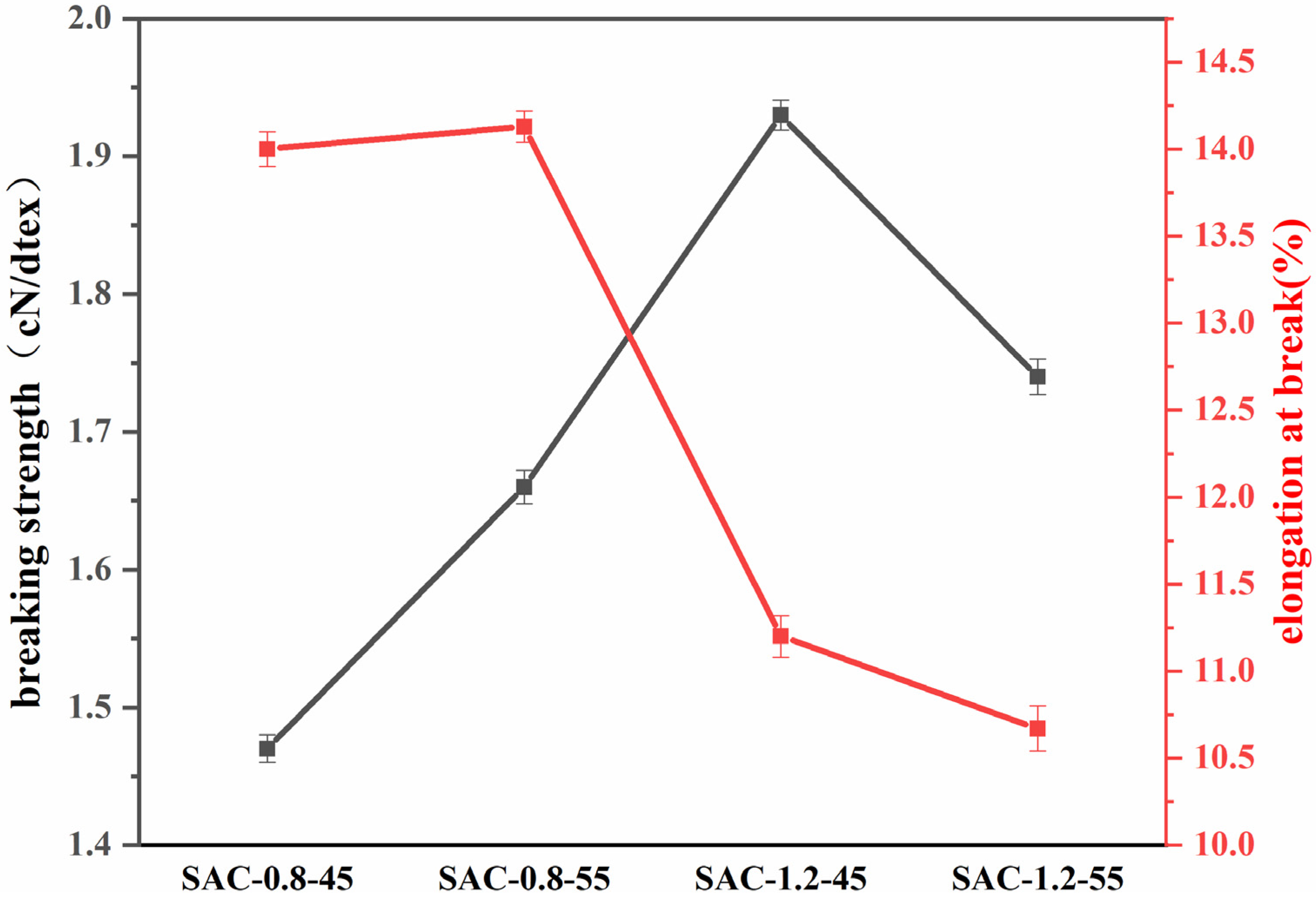
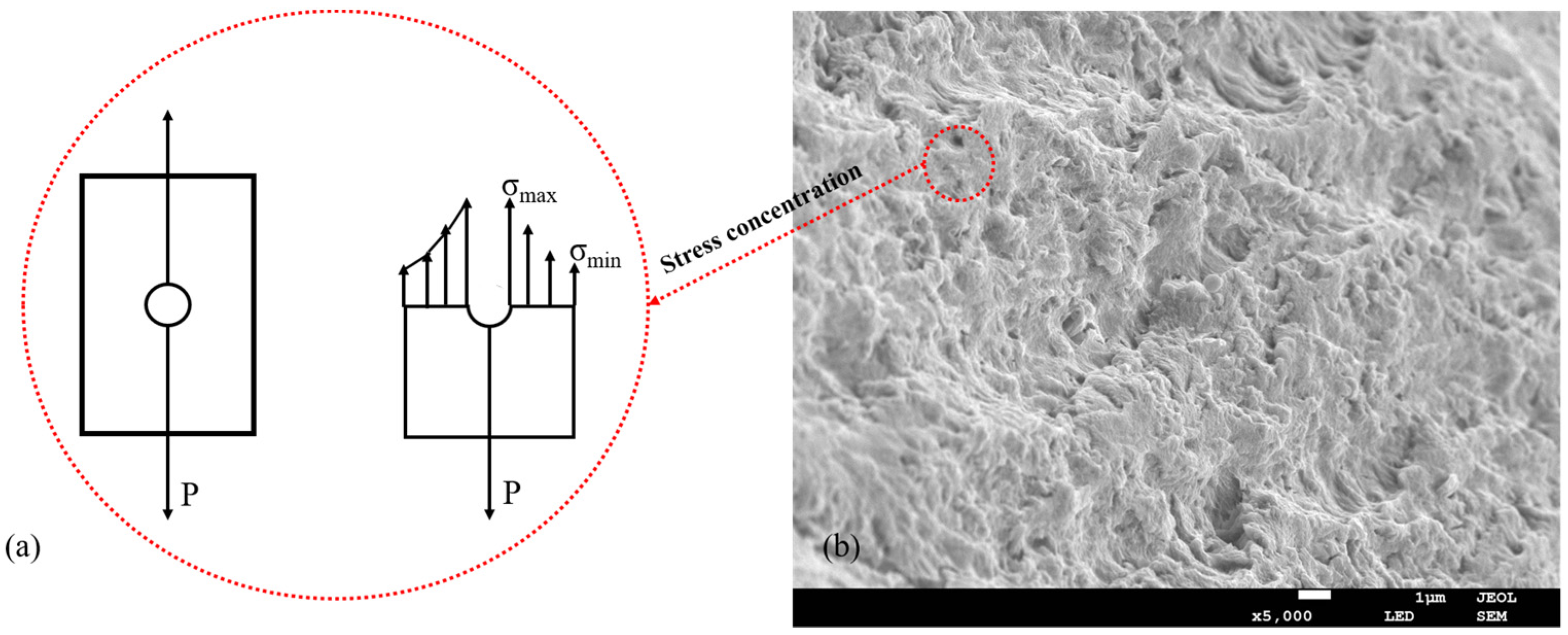
2.4. Mechanical Properties Analysis of SA/AKP/CNCs Composite Fiber
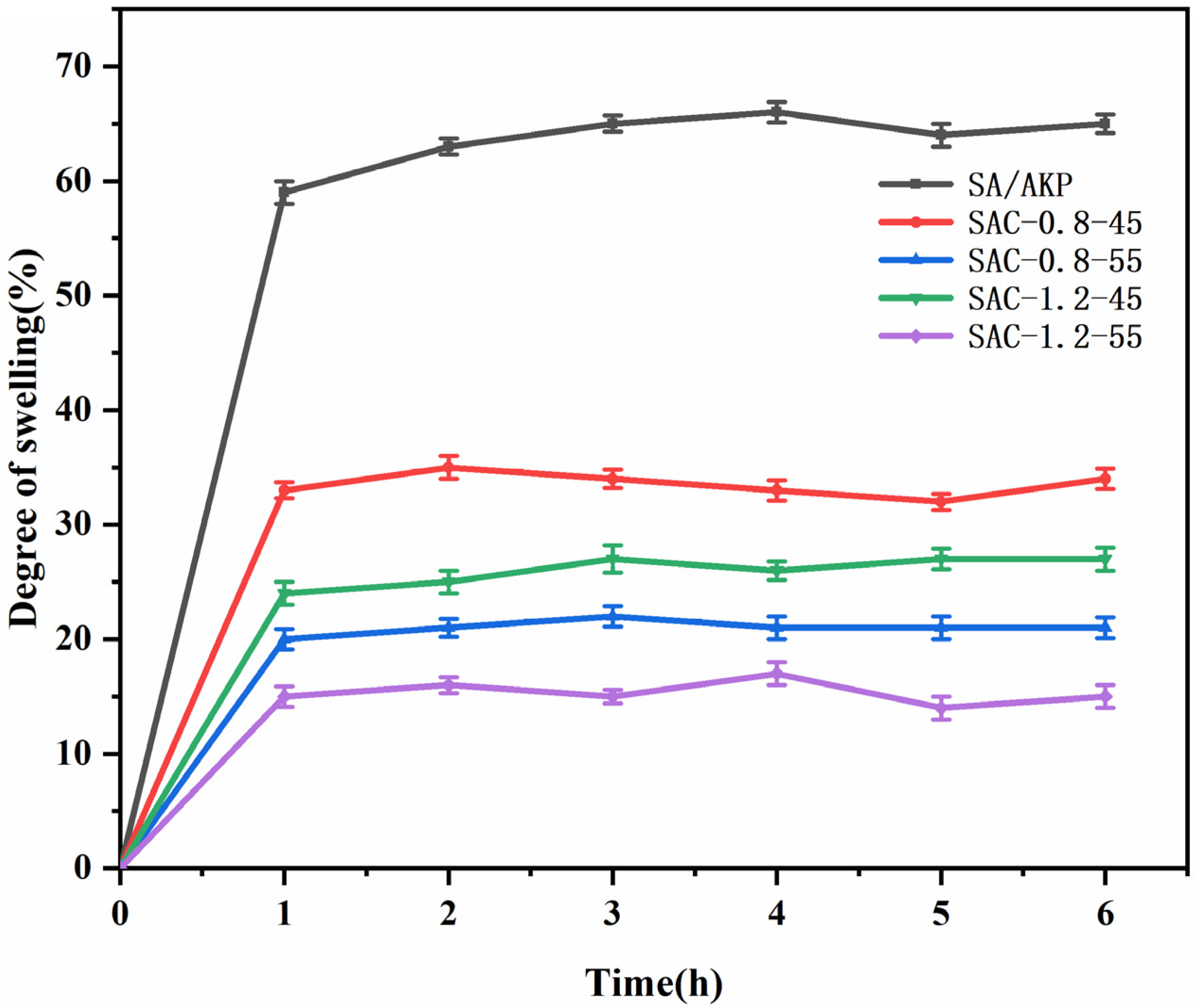
2.5. Water Resistance Analysis of SA/AKP/CNCs Composite Fiber
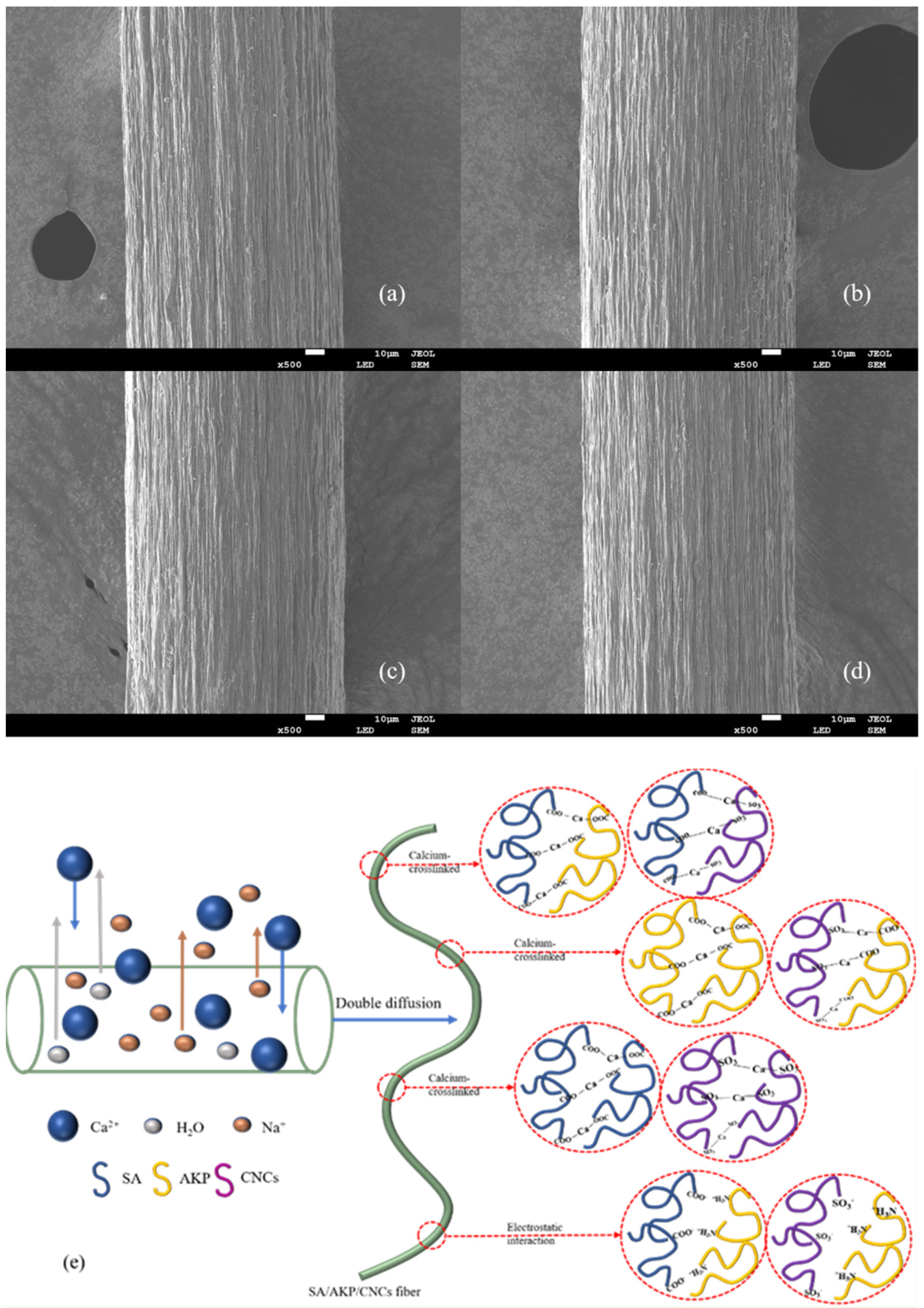
2.6. Scanning Electron Microscopy Analysis of SA/AKP/CNCs Composite Fiber
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of SA/AKP/CNCs Spinning Solution
4.3. Preparation of SA/AKP/CNCs Composite Fiber
4.4. Rheological Property Test
4.5. Fourier Transform Infrared Analysis
4.6. X-ray Diffraction Analysis
4.7. Tensile Test
4.8. Water Absorption Test
4.9. Scanning Electron Microscopic Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stinson-Bagby, K.L.; Roberts, R.; Foster, E.J. Effective cellulose nanocrystal imaging using transmission electron microscopy. Carbohydr. Polym. 2018, 186, 429–438. [Google Scholar] [CrossRef]
- Tang, J.; Sisler, J.; Grishkewich, N.; Tam, K.C. Functionalization of cellulose nanocrystals for advanced applications. J. Colloid Interface Sci. 2017, 494, 397–409. [Google Scholar] [CrossRef]
- Uto, T.; Yamamoto, K.; Kadokawa, J.-I. Cellulose Crystal Dissolution in Imidazolium-Based Ionic Liquids: A Theoretical Study. J. Phys. Chem. B 2018, 122, 258–266. [Google Scholar] [CrossRef]
- Peng, B.L.; Dhar, N.; Liu, H.L.; Tam, K.C. Chemistry and applications of nanocrystalline cellulose and its derivatives: A nanotechnology perspective. Can. J. Chem. Eng. 2011, 89, 1191–1206. [Google Scholar] [CrossRef]
- Ye, H.-M.; Wang, C.-S.; Zhang, Z.-Z.; Yao, S.-F. Effect of cellulose nanocrystals on the crystallization behavior and enzymatic degradation of poly(butylene adipate). Carbohydr. Polym. 2018, 189, 99–106. [Google Scholar] [CrossRef]
- Sukyai, P.; Anongjanya, P.; Bunyahwuthakul, N.; Kongsin, K.; Harnkarnsujarit, N.; Sukatta, U.; Sothornvit, R.; Chollakup, R. Effect of cellulose nanocrystals from sugarcane bagasse on whey protein isolate-based films. Food Res. Int. 2018, 107, 528–535. [Google Scholar] [CrossRef]
- Khan, F.; Ahmad, S.R. Polysaccharides and Their Derivatives for Versatile Tissue Engineering Application. Macromol. Biosci. 2013, 13, 395–421. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Işık, B.; Uğraşkan, V. Adsorption of methylene blue on sodium alginate–flax seed ash beads: Isotherm, kinetic and thermodynamic studies. Int. J. Biol. Macromol. 2021, 167, 1156–1167. [Google Scholar] [CrossRef]
- Zakerikhoob, M.; Abbasi, S.; Yousefi, G.; Mokhtari, M.; Noorbakhsh, M.S. Curcumin-incorporated crosslinked sodium alginate-g-poly (N-isopropyl acrylamide) thermo-responsive hydrogel as an in-situ forming injectable dressing for wound healing: In vitro characterization and in vivo evaluation. Carbohydr. Polym. 2021, 271, 118434. [Google Scholar] [CrossRef]
- Sanchez-Ballester, N.M.; Bataille, B.; Soulairol, I. Sodium alginate and alginic acid as pharmaceutical excipients for tablet formulation: Structure-function relationship. Carbohydr. Polym. 2021, 270, 118399. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Zhou, J.; Huang, T.; Zhang, Z.; Xing, Q.; Zhou, X.; Zhang, K.; Yao, M.; Cheng, T.; Wang, X.; et al. Sodium alginate/collagen/stromal cell-derived factor-1 neural scaffold loaded with BMSCs promotes neurological function recovery after traumatic brain injury. Acta Biomater. 2021, 131, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Guo, J.; Yu, Y.; An, Q.; Wang, L.; Li, S.; Huang, X.; Mu, S.; Qi, S. Hydrogen bonds of sodium alginate/Antarctic krill protein composite material. Carbohydr. Polym. 2016, 142, 275–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Guo, J.; Zhao, M.; Zhang, R.; Guan, F. Hydrogen bonding in chitosan/Antarctic krill protein composite system: Study on construction and enhancement mechanism. Int. J. Biol. Macromol. 2020, 142, 513–520. [Google Scholar] [CrossRef]
- Li, F.; Zheng, Y.; Wang, B. Rheological Behaviors of Graphene Oxide/Polyacrylonitrile Spinning Solutions. Mater. Sci. Forum 2017, 898, 2187–2196. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Tang, J.; Lan, A.; Yang, Y.; Gibril, M.; Yu, M. Efficient preparation of high concentration cellulose solution with complex DMSO/ILs solvent. J. Polym. Res. 2016, 23, 32. [Google Scholar] [CrossRef]
- Pearson, F.G.; Marchessault, R.H.; Liang, C.Y. Infrared spectra of crystalline polysaccharides. V. Chitin. J. Polym. Sci. 1960, 43, 101–116. [Google Scholar] [CrossRef]
- Sun, R.; Liu, X.; Yu, Y.; Miao, J.; Leng, K.; Gao, H. Preparation process optimization, structural characterization and in vitro digestion stability analysis of Antarctic krill (Euphausia superba) peptides-zinc chelate. Food Chem. 2021, 340, 128056. [Google Scholar] [CrossRef]
- Shah, R.; Huang, S.; Pingali, S.V.; Sawada, D.; Pu, Y.; Rodriguez, M.; Ragauskas, A.J.; Kim, S.H.; Evans, B.R.; Davison, B.H.; et al. Hemicellulose–Cellulose Composites Reveal Differences in Cellulose Organization after Dilute Acid Pretreatment. Biomacromolecules 2019, 20, 893–903. [Google Scholar] [CrossRef]
- Zheng, Q.; Du, M.; Yang, B.; Wu, G. Relationship between dynamic rheological behavior and phase separation of poly(methyl methacrylate)/poly(styrene-co-acrylonitrile) blends. Polymer 2001, 42, 5743–5747. [Google Scholar] [CrossRef]
- Chen, X.; Sakaguchi, M. Transition behavior from Mode I cracking to crystallographic cracking in a Ni-base single crystal superalloy. Int. J. Fatigue 2020, 132, 105400. [Google Scholar] [CrossRef]
- Lee, J.-E.; Kim, Y.E.; Lee, G.-H.; Kim, M.J.; Eom, Y.; Chae, H.G. The effect of cellulose nanocrystals (CNCs) on the microstructure of amorphous polyetherimide (PEI)-based nanocomposite fibers and its correlation with the mechanical properties. Compos. Sci. Technol. 2020, 200, 108452. [Google Scholar] [CrossRef]
- Bao, D.; Liu, L.; Sun, T.; Han, Y.; Meng, F.; Zhao, M.; Yu, Y.; Guo, J.; Zhang, S. Solid solid phase change (SSPC) chitosan-g-mPEG fiber with improved mechanical performance via in-situ wet spinning process. Carbohydr. Polym. 2020, 240, 116313. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Guo, J.; Zhao, M.; Gong, Y.; Qiao, B. Effect of Coagulation Bath Temperature on Mechanical, Morphological, and Thermal Properties of Cellulose/Antarctic Krill Protein Composite Fibers. Langmuir 2020, 36, 5647–5653. [Google Scholar] [CrossRef]
- Song, J.; Guo, J.; Zhang, S.; Gong, Y. Properties of cellulose/Antarctic krill protein composite fibers prepared in different coagulation baths. Int. J. Biol. Macromol. 2018, 114, 334–340. [Google Scholar] [CrossRef]
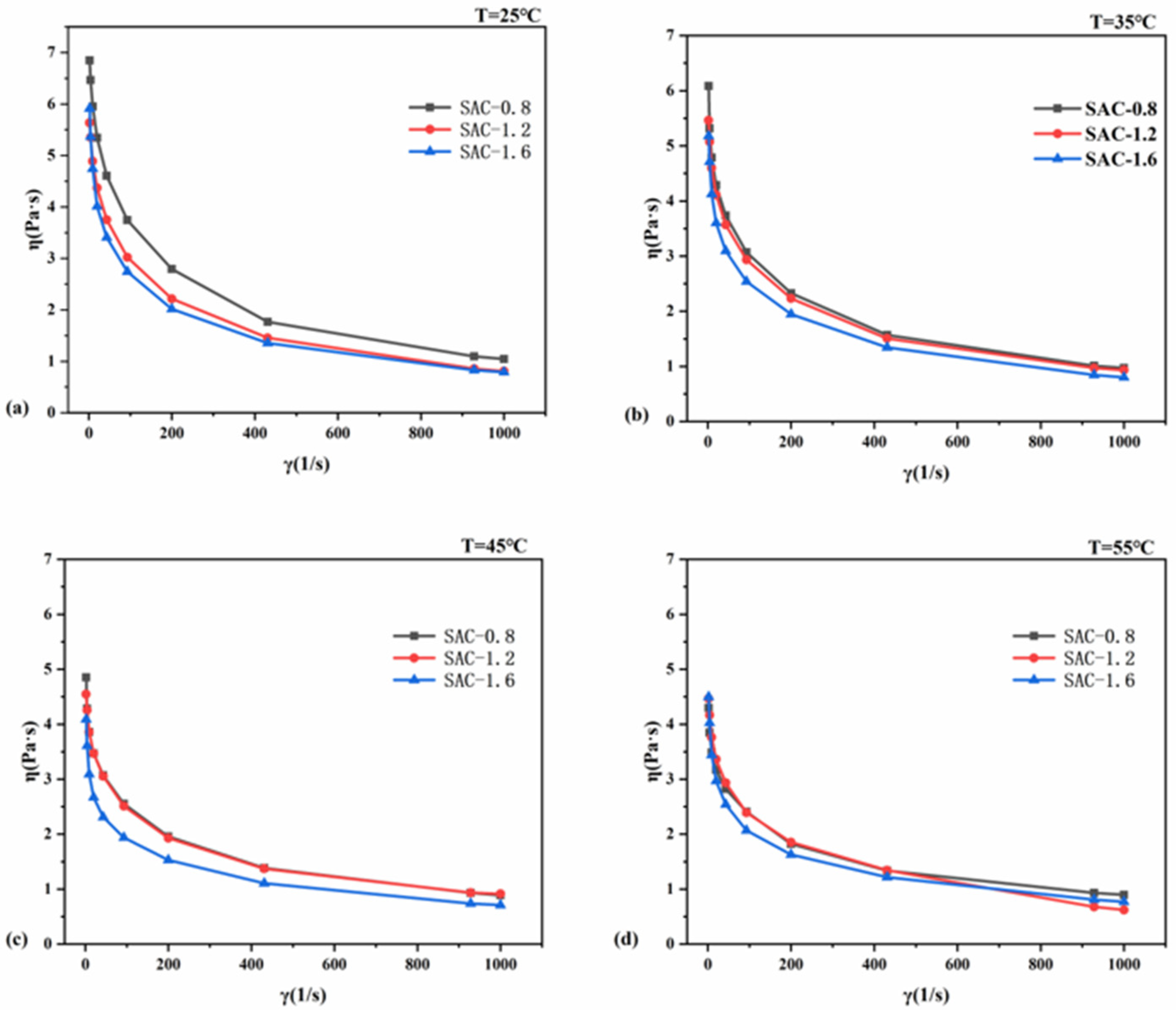
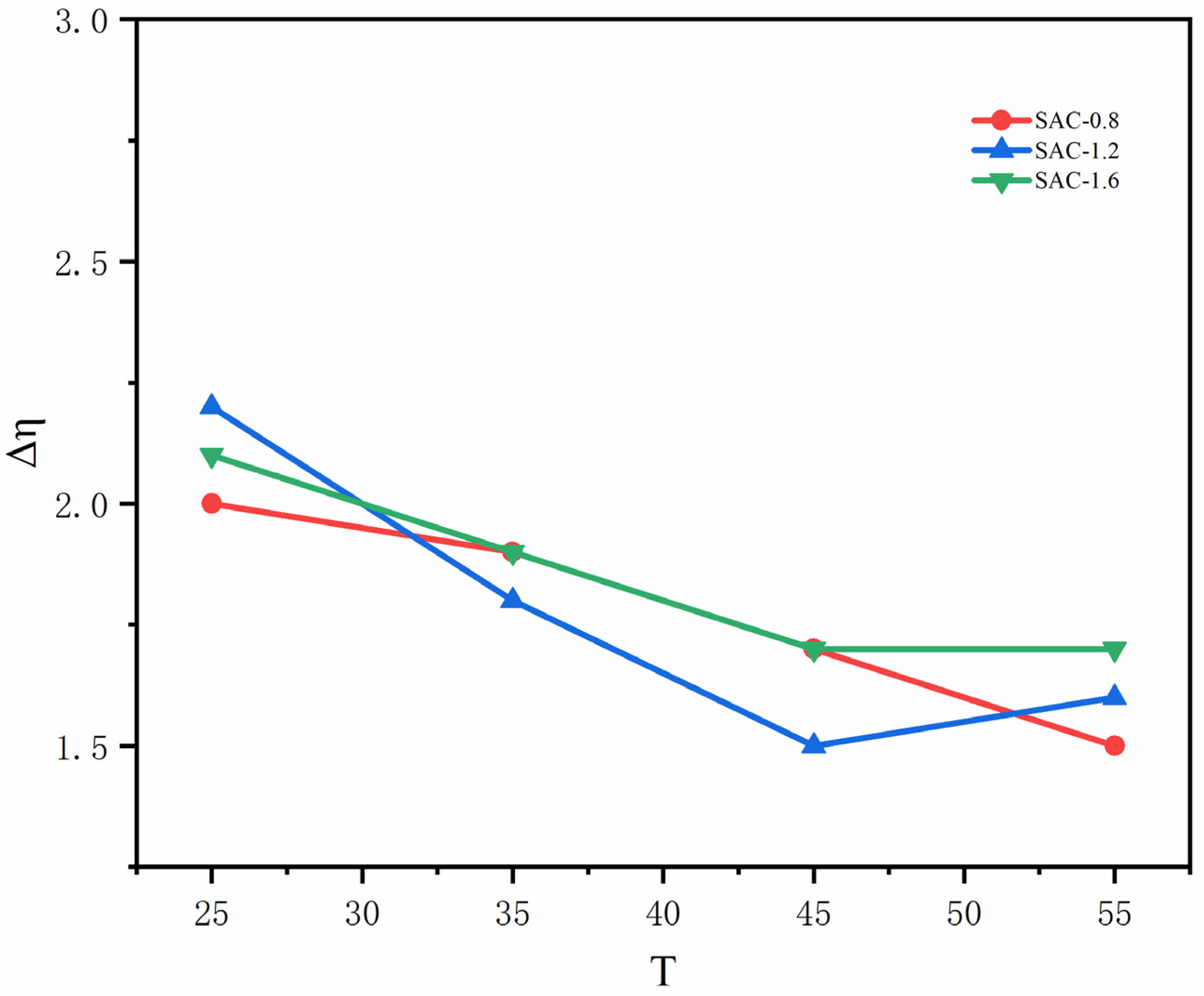

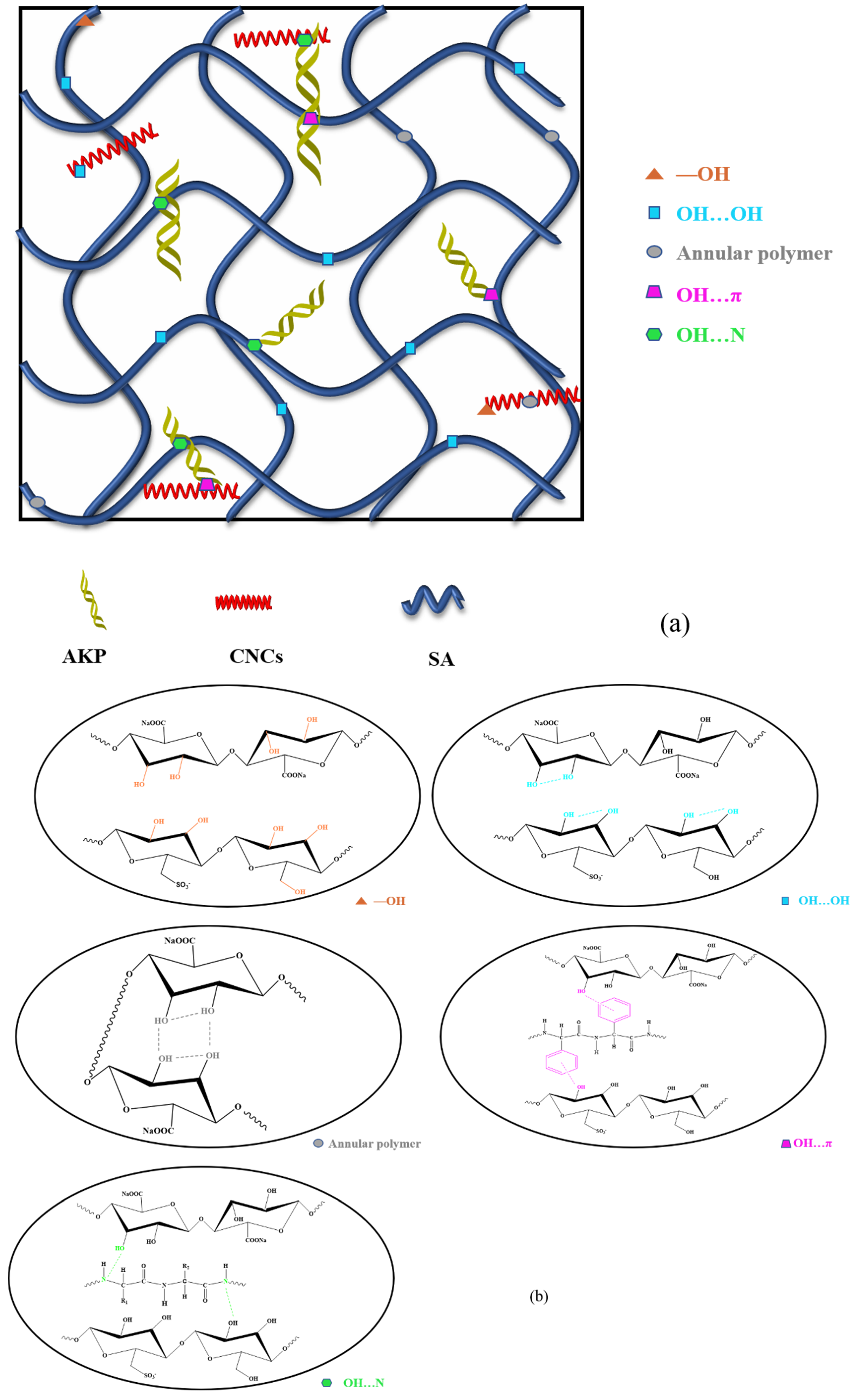
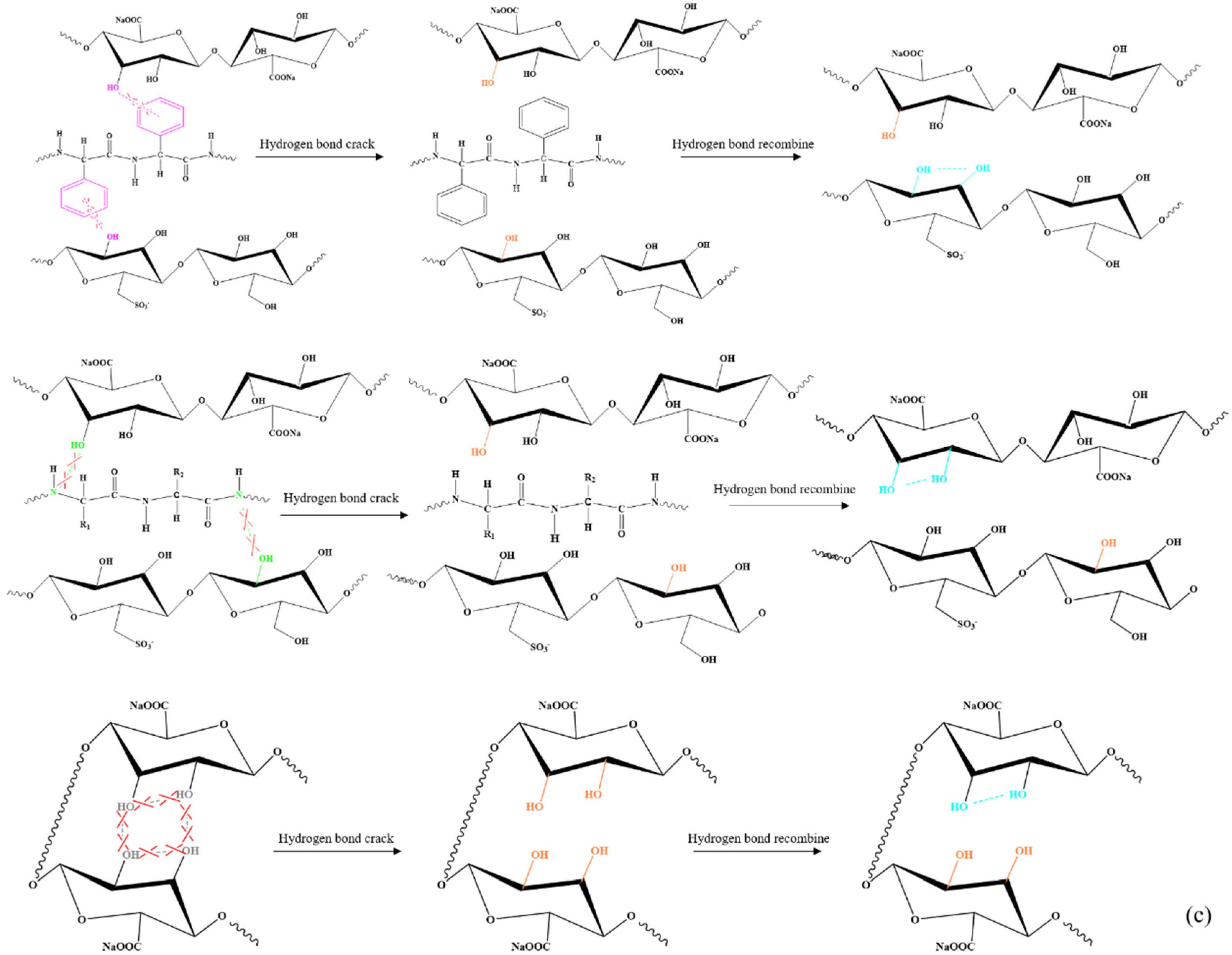
| Sample | 25 °C | 35 °C | 45 °C | 55 °C |
|---|---|---|---|---|
| SAC-0.8 | 0.69 | 0.71 | 0.73 | 0.75 |
| SAC-1.2 | 0.68 | 0.71 | 0.74 | 0.73 |
| SAC-1.6 | 0.67 | 0.70 | 0.72 | 0.72 |
| Sample | Hydrogen Bond Type | Abbreviations | Wave Number/cm−1 | Relative Strength/% | ||
|---|---|---|---|---|---|---|
| SAC-0.8-45 | I | Free hydroxyl | -OH | 3651 | 1.5 | 1.5 |
| III | Intramolecular hydrogen bond | OH…OH | 3438 | 72 | 85.5 | |
| IV | Annular polymer | 3227 | 13.5 | |||
| II | Intermolecular hydrogen bond | OH…π | 3585 | 10 | 13 | |
| V | OH…N | 3110 | 3 | |||
| SAC-0.8-55 | I | Free hydroxyl | -OH | 3652 | 2 | 2 |
| III | Intramolecular hydrogen bond | OH…OH | 3439 | 69 | 86 | |
| IV | Annular polymer | 3232 | 17 | |||
| II | Intermolecular hydrogen bond | OH…π | 3581 | 11 | 12 | |
| V | OH…N | 3104 | 1 | |||
| SAC-1.2-45 | I | Free hydroxyl | -OH | 3652 | 2 | 2 |
| III | Intramolecular hydrogen bond | OH…OH | 3440 | 67 | 84 | |
| IV | Annular polymer | 3232 | 17 | |||
| II | Intermolecular hydrogen bond | OH…π | 3581 | 12 | 14 | |
| V | OH…N | 3104 | 2 | |||
| SAC-1.2-55 | I | Free hydroxyl | -OH | 3642 | 2 | 2 |
| III | Intramolecular hydrogen bond | OH…OH | 3446 | 61 | 85 | |
| IV | Annular polymer | 3240 | 24 | |||
| II | Intermolecular hydrogen bond | OH…π | 3579 | 11 | 13 | |
| V | OH…N | 3104 | 2 | |||
| Sample | Crystallinity (%) |
|---|---|
| SAC-0.8-45 | 14 |
| SAC-0.8-55 | 19 |
| SAC-1.2-45 | 17 |
| SAC-1.2-55 | 27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shan, J.; Guo, J.; Guan, F.; Li, F.; Di, C. Characteristics of Sodium Alginate/Antarctic Krill Protein Composite Fiber Based on Cellulose Nanocrystals Modification: Rheology, Hydrogen Bond, Crystallization, Strength, and Water-Resistance. Gels 2022, 8, 139. https://doi.org/10.3390/gels8030139
Shan J, Guo J, Guan F, Li F, Di C. Characteristics of Sodium Alginate/Antarctic Krill Protein Composite Fiber Based on Cellulose Nanocrystals Modification: Rheology, Hydrogen Bond, Crystallization, Strength, and Water-Resistance. Gels. 2022; 8(3):139. https://doi.org/10.3390/gels8030139
Chicago/Turabian StyleShan, Jicheng, Jing Guo, Fucheng Guan, Feng Li, and Chunqiu Di. 2022. "Characteristics of Sodium Alginate/Antarctic Krill Protein Composite Fiber Based on Cellulose Nanocrystals Modification: Rheology, Hydrogen Bond, Crystallization, Strength, and Water-Resistance" Gels 8, no. 3: 139. https://doi.org/10.3390/gels8030139
APA StyleShan, J., Guo, J., Guan, F., Li, F., & Di, C. (2022). Characteristics of Sodium Alginate/Antarctic Krill Protein Composite Fiber Based on Cellulose Nanocrystals Modification: Rheology, Hydrogen Bond, Crystallization, Strength, and Water-Resistance. Gels, 8(3), 139. https://doi.org/10.3390/gels8030139





