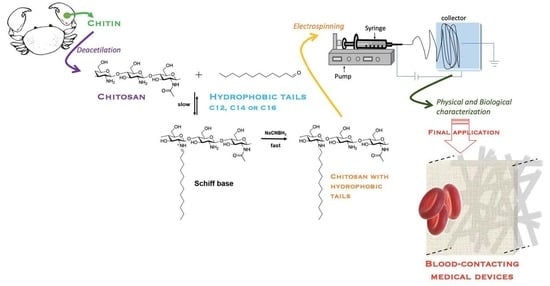
Electrospun Chitosan Functionalized with C12, C14 or C16 Tails for Blood-Contacting Medical Devices
Abstract
:1. Introduction
2. Results and Discussion
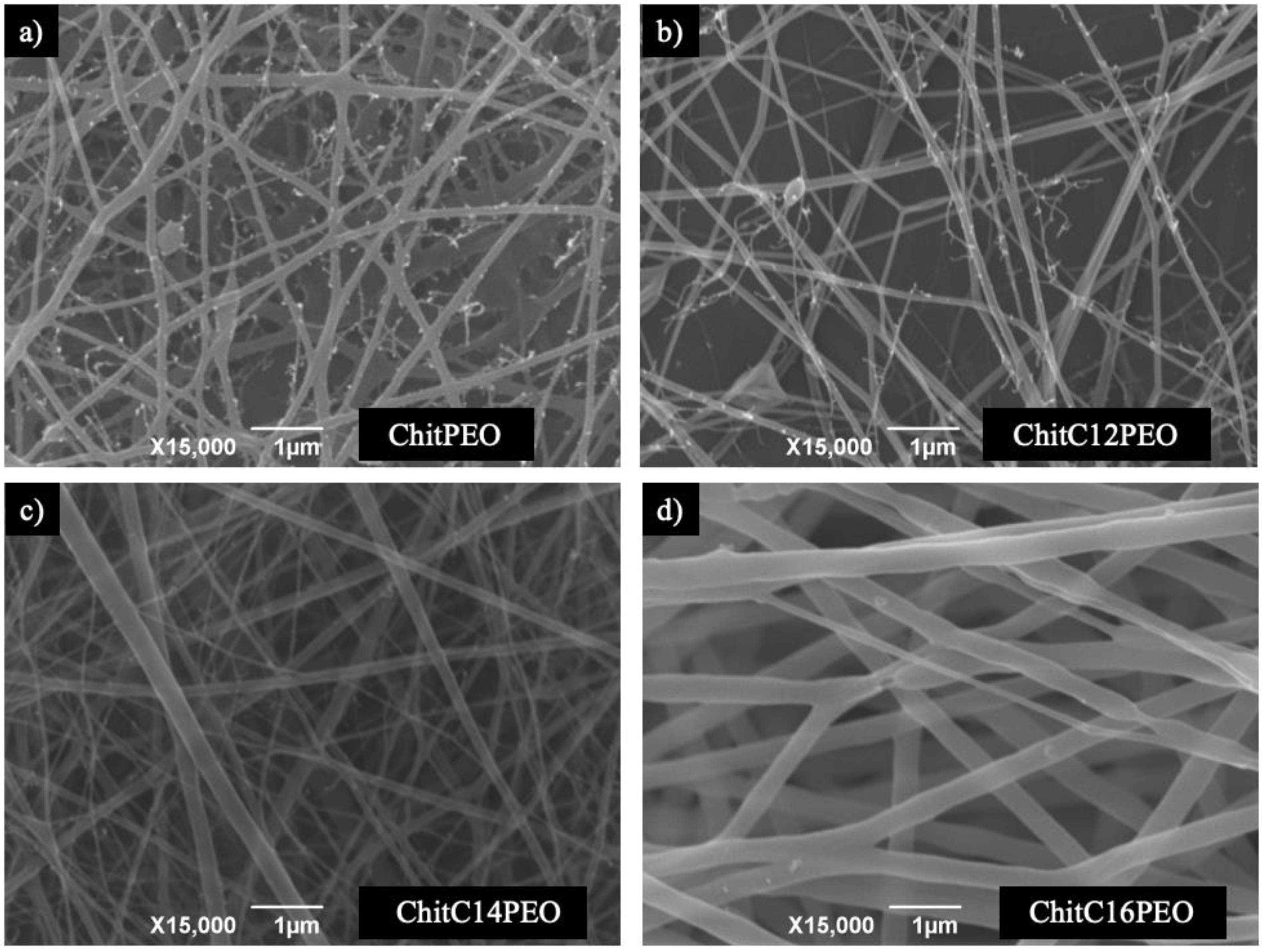
2.1. SEM Analysis
2.2. Young Modulus and Contact Angle Determinations
2.3. XPS Analysis
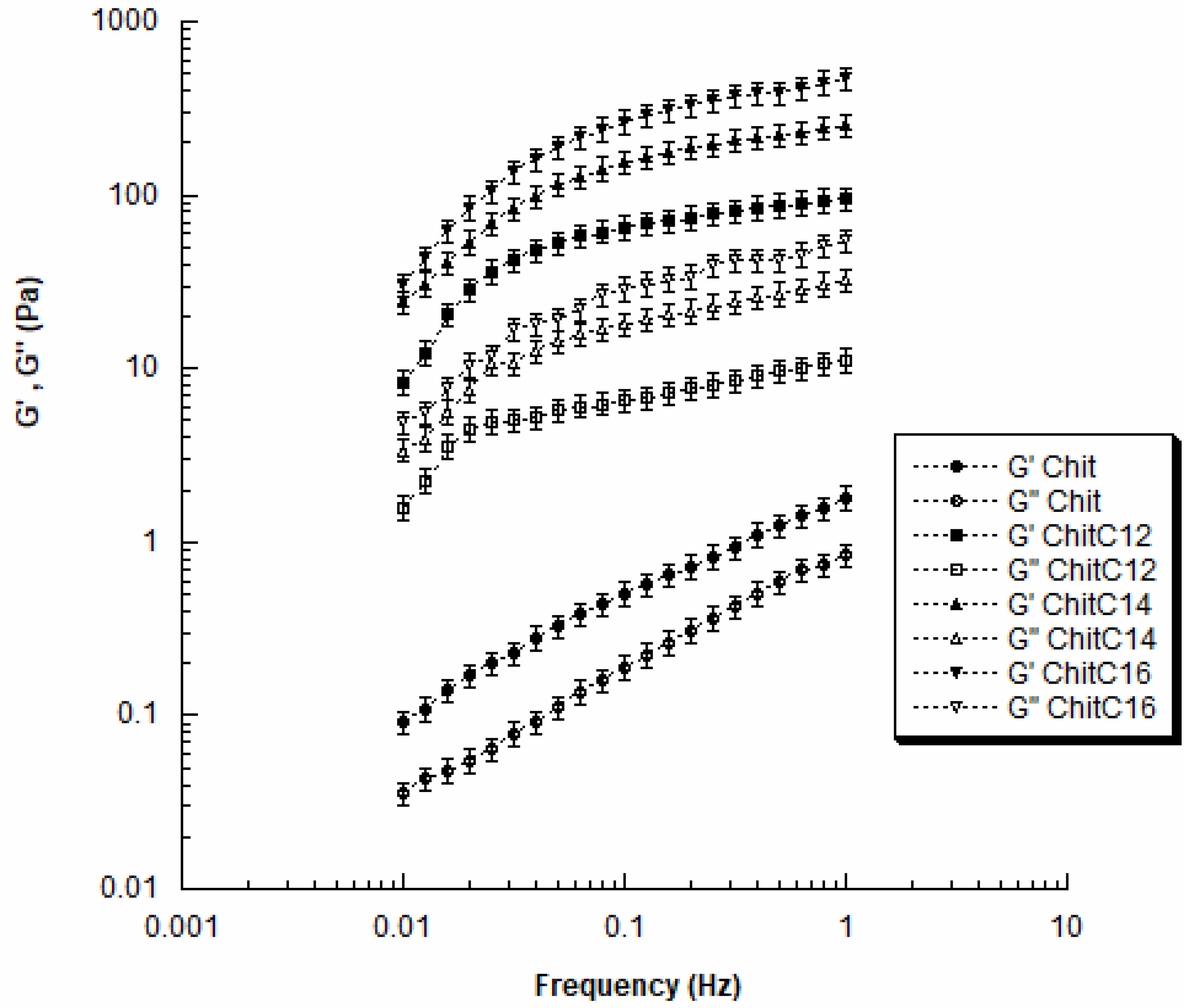
2.4. Dynamic Mechanical Analysis
2.5. Bioassays
2.5.1. Bacteriostatic/Bactericidal Properties of Electrospun Functionalized Chitosan Scaffolds
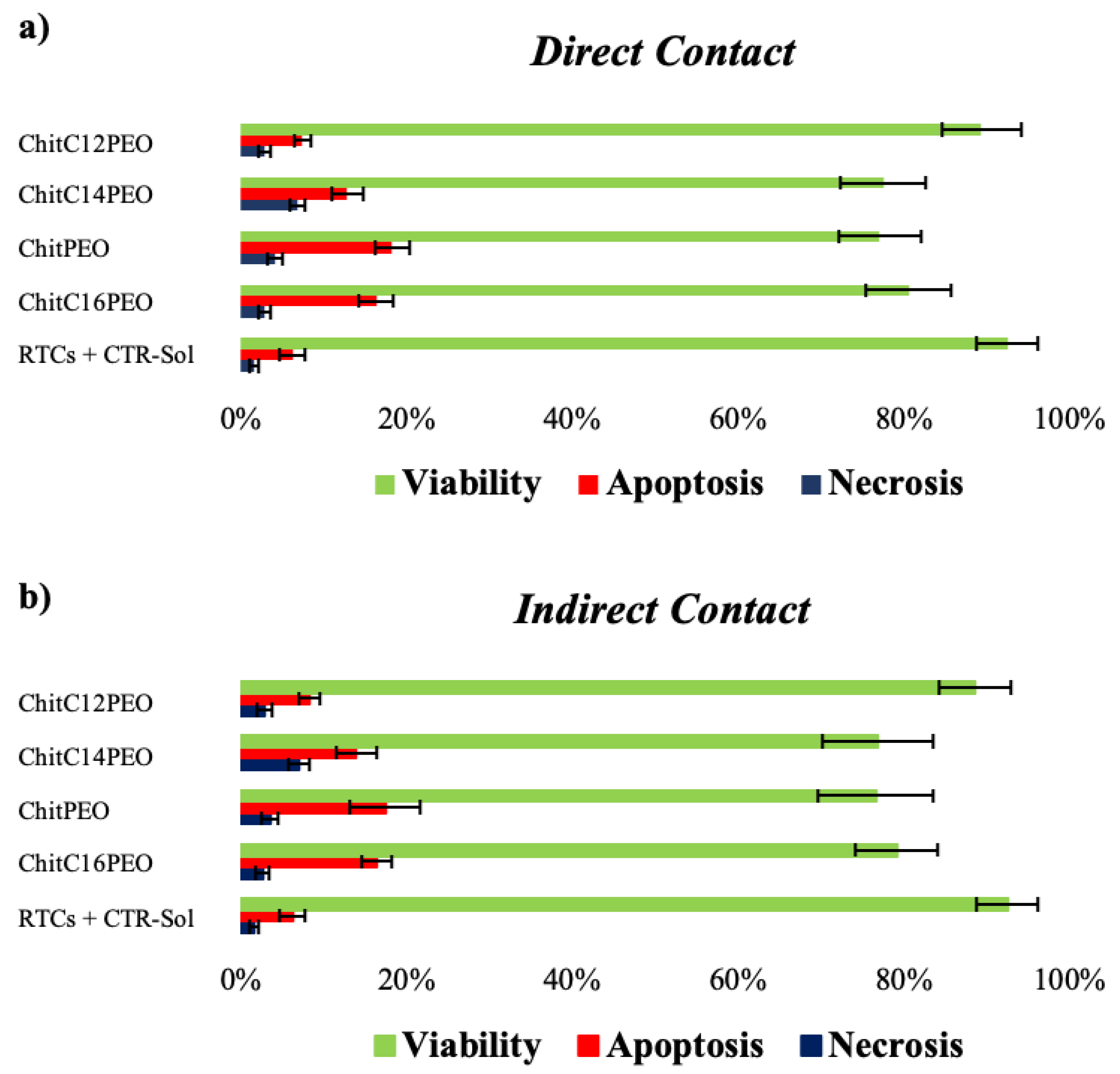
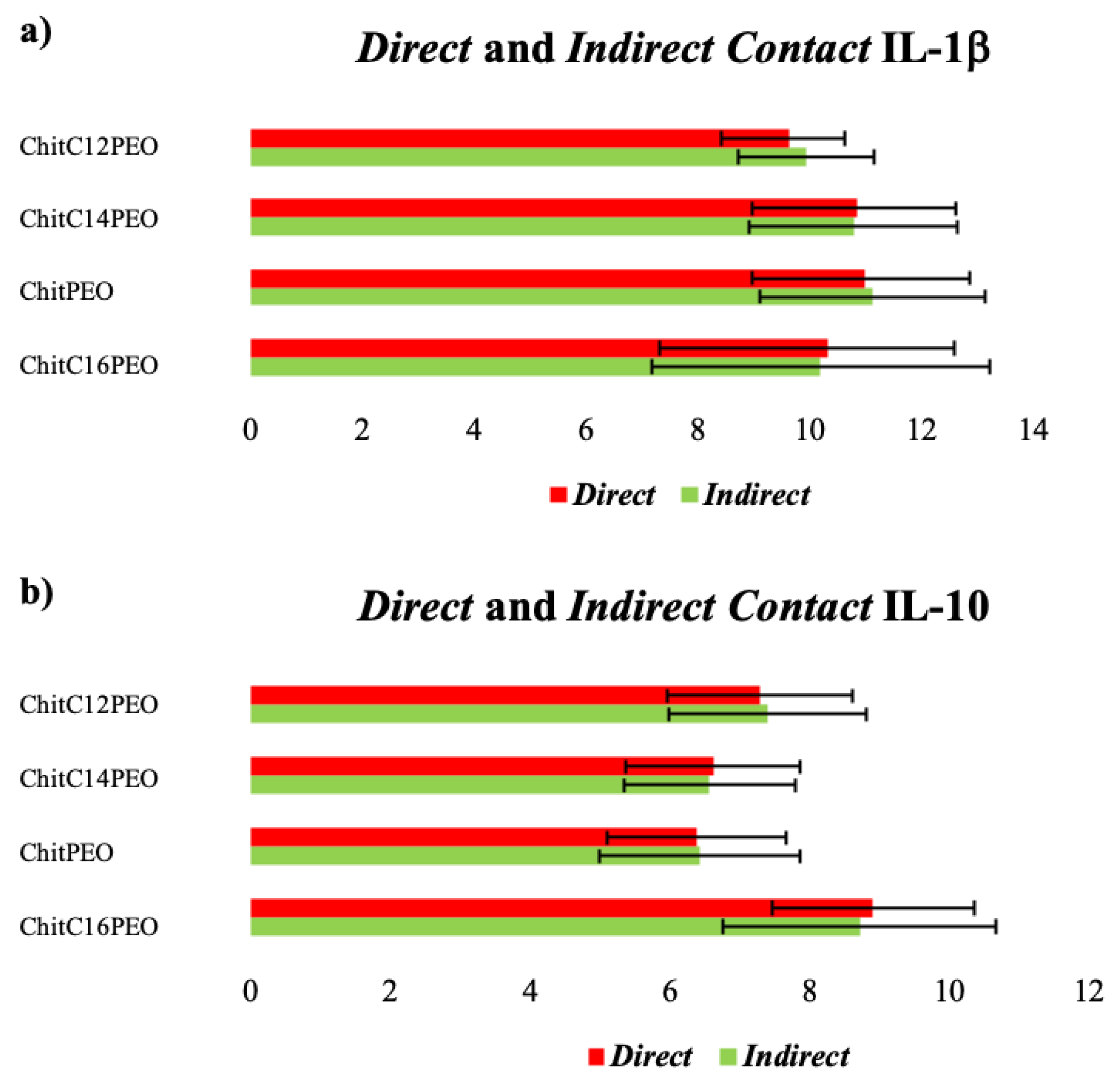
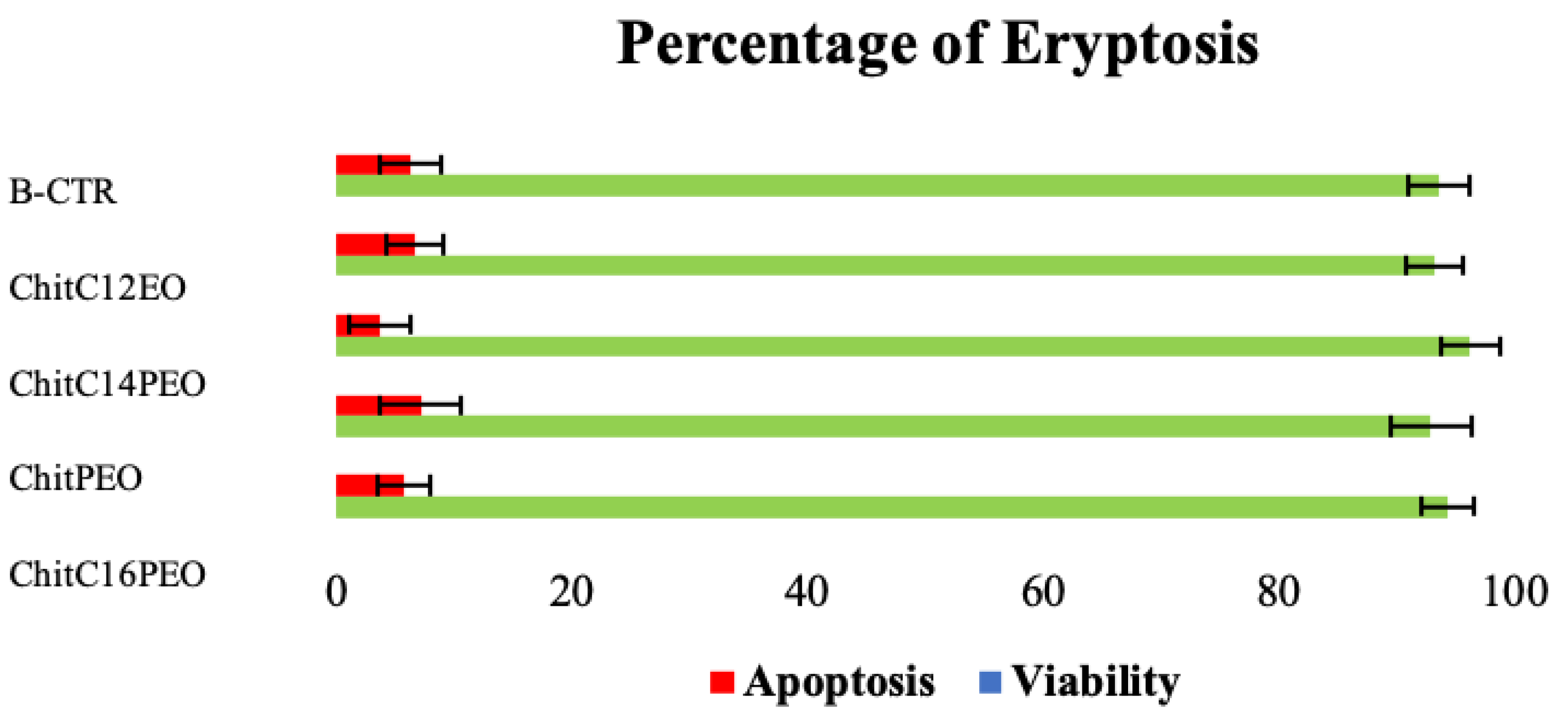
2.5.2. Cellular Properties of Electrospun Functionalized Chitosan Scaffolds
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
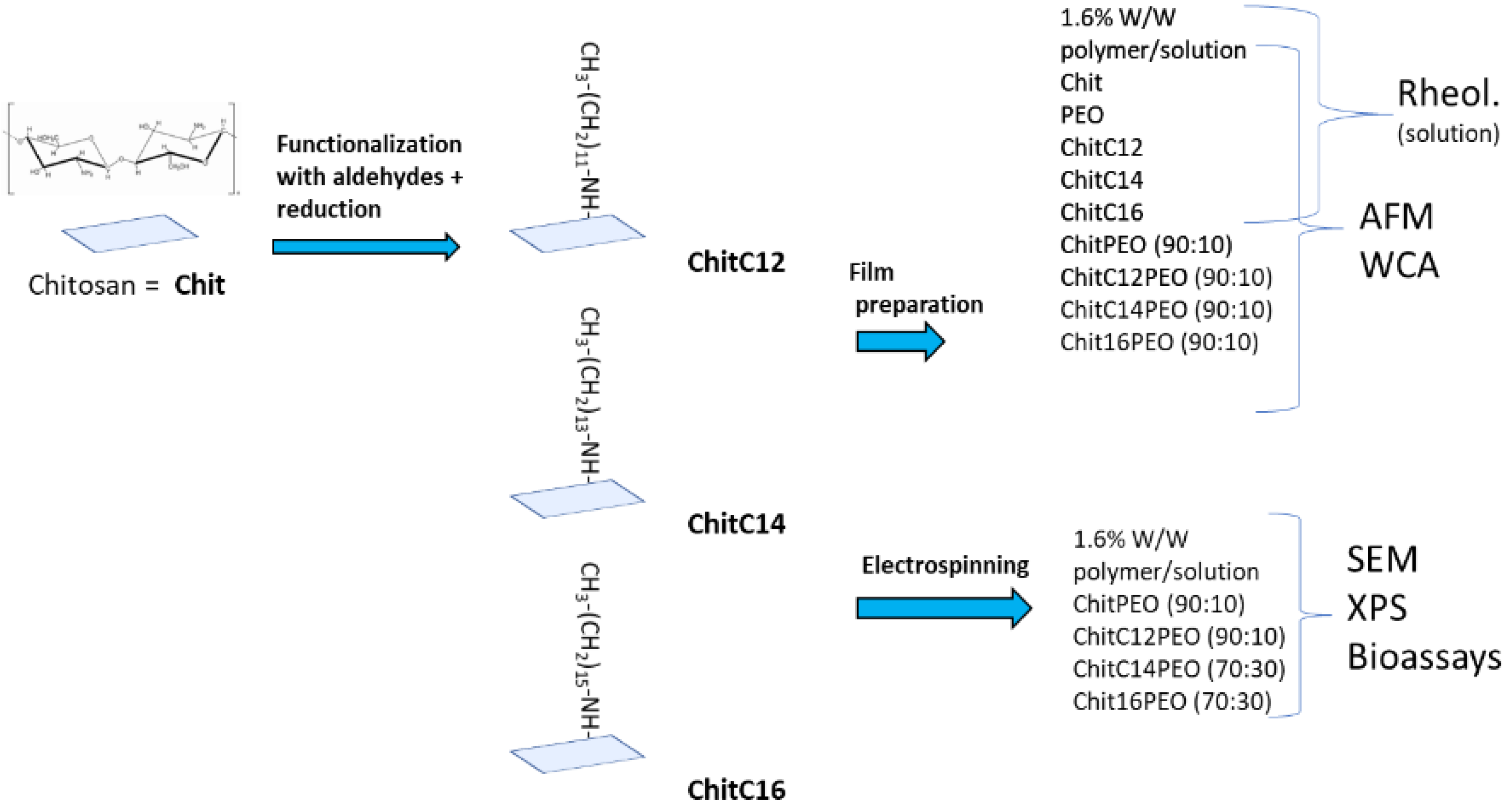
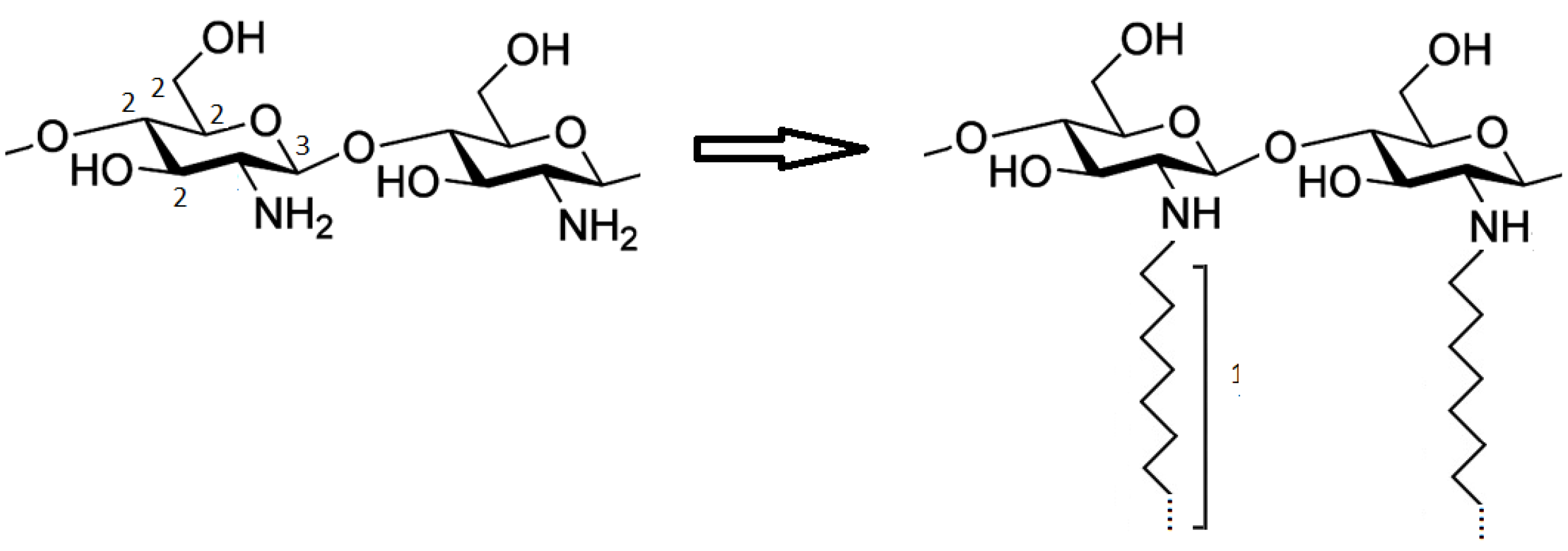
4.2.1. Preparation of ChitC12, ChitC14 and ChitC16
4.2.2. Preparation of Polymer Solutions for Electrospinning
4.2.3. Electrospinning Process
4.2.4. Scanning Electron Microscopy
4.2.5. Preparation of Polymer Solutions for Contact Angle Measurements, Young Moduli Determination and DMA Analysis
4.2.6. Water Contact Angle Measurements
4.2.7. AFM Analysis
4.2.8. XPS Measurements
4.2.9. DMA Analysis
4.2.10. Biological Assays
RTCs Culture
Test by Direct and Indirect Contact
Test on Extracts with Blood
Sterilization Test with Ethanol 20%
Sterilization Test with Ethanol 70%
Membrane Sterilization Test
Antibacterial Properties
1st Antibacterial Property Test
Second Antibacterial Property Test
Membrane Negative Control
Membrane Sterilization Test
Liquid Test for Evaluating Membrane Antibacterial Properties
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| C12 | hydrocarburic tails with 12 methylene units |
| C14 | hydrocarburic tails with 14 methylene units |
| C16 | hydrocarburic tails with 16 methylene units |
| DA | acetylation degree |
| SEM | scanning electron microscope |
| XPS | X-ray photoelectron spectroscopy |
| DNA | deoxyribonucleic acid |
| DMA | dynamic mechanical analysis |
| AFM | atomic force microscopy |
| RTCs | renal tubular cells |
| WCA | water contact angle |
| PEO | polyethylene oxide |
| ChitC12 | Chitosan-C12 |
| ChitC14 | Chitosan-C14 |
| ChitC16 | Chitosan-C16 |
| ChitC12PEO | Chitosan-C12 with the addition of PEO |
| ChitC14PEO | Chitosan-C14 with the addition of PEO |
| ChitC16PEO | Chitosan-C16 with the addition of PEO |
| ChitPEO | Chitosan with the addition of PEO |
| Es | values distribution of Young modulus |
| G′ | storage modulus |
| G″ | loss modulus |
| ANOVA | analysis of variance |
| CTR-Sol | complete RTCs medium |
| MPO | myeloperoxidase |
| B-CTR | blood control |
| DMT | Derjaguin–Müller–Toporov |
| FITC | Phenyl-isothiocyanate |
| RBC | red blood cells |
References
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Kainer, M.A.; Lynfield, R.; Maloney, M.; McAllister-Hollod, L.; Nadle, J.; et al. Multistate Point-Prevalence Survey of Health Care–Associated Infections. N. Engl. J. Med. 2014, 370, 1198–1208. [Google Scholar] [CrossRef] [Green Version]
- Guggenbichler, J.P.; Assadian, O.; Boeswald, M.; Kramer, A. Incidence and clinical implication of nosocomial infections associated with implantable biomaterials–catheters, ventilator-associated pneumonia, urinary tract infections. GMS Krankenh. Interdiszip 2011, 6, Doc18. [Google Scholar] [CrossRef]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [Green Version]
- Jaffer, I.H.; Fredenburgh, J.C.; Hirsh, J.; Weitz, J.I. Medical device-induced thrombosis: What causes it and how can we prevent it? J. Thromb. Haemost. 2015, 13, S72–S81. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Kumar, M.N.V.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Takahashi, T.; Imai, M.; Suzuki, I.; Sawai, J. Growth inhibitory effect on bacteria of chitosan membranes regulated with deacetylation degree. Biochem. Eng. J. 2008, 40, 485–491. [Google Scholar] [CrossRef]
- Bellich, B.; D’Agostino, I.; Semeraro, S.; Gamini, A.; Cesàro, A. “The Good, the Bad and the Ugly” of Chitosans. Mar. Drugs 2016, 14, 99. [Google Scholar] [CrossRef] [Green Version]
- Phil, L.; Naveed, M.; Mohammad, I.S.; Bo, L.; Bin, D. Chitooligosaccharide: An evaluation of physicochemical and biological properties with the proposition for determination of thermal degradation products. Biomed. Pharmacother. 2018, 102, 438–451. [Google Scholar] [CrossRef]
- Hirano, S.; Tsuchida, H.; Nagao, N. N-acetylation in chitosan and the rate of its enzymic hydrolysis. Biomaterials 1989, 10, 574–576. [Google Scholar] [CrossRef]
- Secchi, V.; Franchi, S.; Ciccarelli, D.; Dettin, M.; Zamuner, A.; Serio, A.; Iucci, G.; Battocchio, C. Biofunctionalization of TiO2 Surfaces with Self-Assembling Layers of Oligopeptides Covalently Grafted to Chitosan. ACS Biomater. Sci. Eng. 2019, 5, 2190–2199. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P. Silver nanoparticles embedded in zeolite membranes: Release of silver ions and mechanism of antibacterial action. Int. J. Nanomed. 2011, 6, 1833. [Google Scholar] [CrossRef] [Green Version]
- Greiner, A.; Wendorff, J.H. Electrospinning: A Fascinating Method for the Preparation of Ultrathin Fibers. Angew. Chem. Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Mattanavee, W.; Suwantong, O.; Puthong, S.; Bunaprasert, T.; Hoven, V.P.; Supaphol, P. Immobilization of Biomolecules on the Surface of Electrospun Polycaprolactone Fibrous Scaffolds for Tissue Engineering. ACS Appl. Mater. Interfaces 2009, 1, 1076–1085. [Google Scholar] [CrossRef]
- Humes, H.; Fissell, W.; Tiranathanagul, K. The future of hemodialysis membranes. Kidney Int. 2006, 69, 1115–1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engler, A.; Richert, L.; Wong, J.Y.; Picart, C.; Discher, D.E. Surface probe measurements of the elasticity of sectioned tissue, thin gels and polyelectrolyte multilayer films: Correlations between substrate stiffness and cell adhesion. Surf. Sci. 2004, 570, 142–154. [Google Scholar] [CrossRef] [Green Version]
- Powell, C. X-ray Photoelectron Spectroscopy Database XPS, Version 4.1; NIST Standard Reference Database: Gaithersburg, MD, USA, 1989; Volume 20. [CrossRef]
- Briggs, D. Handbook of X-ray Photoelectron Spectroscopy; Wanger, C.D., Riggs, W.M., Davis, L.E., Moulder, J.F., Muilenberg, G.E., Eds.; Perkin-Elmer Corp., Physical Electronics Division: Eden Prairie, MN, USA, 1979; Volume 190, p. $195. [Google Scholar]
- Watts, J.F. High Resolution XPS of Organic Polymers: The Scienta ESCA 300 Database; Beamson, G., Briggs, D., Eds.; John Wiley & Sons: Chichester, UK, 1992; Volume 280, p. £195. [Google Scholar]
- Brocca, A.; Virzì, G.M.; Pasqualin, C.; Pastori, S.; Marcante, S.; De Cal, M.; Ronco, C. Cardiorenal Syndrome Type 5: In Vitro Cytotoxicity Effects on Renal Tubular Cells and Inflammatory Profile. Anal. Cell. Pathol. 2015, 2015, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Chandy, T.; Sharma, C.P. Prostaglandin E1-immobilized poly (vinyl alcohol)-blended chitosan membranes: Blood compatibility and permeability properties. J. Appl. Polym. Sci. 1992, 44, 2145–2156. [Google Scholar] [CrossRef]
- Amiji, M.M. Permeability and blood compatibility properties of chitosan-poly(ethylene oxide) blend membranes for haemodialysis. Biomaterials 1995, 16, 593–599. [Google Scholar] [CrossRef]
- Pakravan, M.; Heuzey, M.-C.; Ajji, A. Core–Shell Structured PEO-Chitosan Nanofibers by Coaxial Electrospinning. Biomacromolecules 2012, 13, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Sader, J.E.; Chon, J.; Mulvaney, P. Calibration of rectangular atomic force microscope cantilevers. Rev. Sci. Instrum. 1999, 70, 3967–3969. [Google Scholar] [CrossRef] [Green Version]
- Derjaguin, B.V.; Muller, V.M.; Toporov, Y.P. Effect of contact deformations on the adhesion of particles. J. Colloid Interface Sci. 1975, 53, 314–326. [Google Scholar] [CrossRef]
- van der Hauwaert, C.; Savary, G.; Gnemmi, V.; Glowacki, F.; Pottier, N.; Bouillez, A.; Maboudou, P.; Zini, L.; Leroy, X.; Cauffiez, C.; et al. Isolation and Characterization of a Primary Proximal Tubular Epithelial Cell Model from Human Kidney by CD10/CD13 Double Labeling. PLoS ONE 2013, 8, e66750. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Sample | C/O | C/N | C1/C2 | C1/C3 | N2/N1 |
|---|---|---|---|---|---|
| ChitPEO (90:10) | 1.78 | 14.2 | 0.7 | 2.1 | 0.10 |
| ChitC12PEO (90:10) | 2.44 | 26 | 0.76 | 2.6 | - |
| ChitC14PEO (70:30) | 3.45 | 117 | 1.8 | 6.8 | - |
| ChitC16PEO (70:30) | 4.03 | 172 | 3.0 | 7.8 | - |
| Chit | 2.49 | 15.8 | 1.63 | 4.54 | 0.15 |
| ChitC12 | 2.78 | 19.4 | 2.25 | 5.52 | - |
| ChitC14 | 2.99 | 20.4 | 3.48 | 6.79 | - |
| ChitC16 | 3.37 | 29.5 | 5.17 | 7.53 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dettin, M.; Roso, M.; Messina, G.M.L.; Iucci, G.; Peluso, V.; Russo, T.; Zamuner, A.; Santi, M.; Milan Manani, S.; Zanella, M.; et al. Electrospun Chitosan Functionalized with C12, C14 or C16 Tails for Blood-Contacting Medical Devices. Gels 2022, 8, 113. https://doi.org/10.3390/gels8020113
Dettin M, Roso M, Messina GML, Iucci G, Peluso V, Russo T, Zamuner A, Santi M, Milan Manani S, Zanella M, et al. Electrospun Chitosan Functionalized with C12, C14 or C16 Tails for Blood-Contacting Medical Devices. Gels. 2022; 8(2):113. https://doi.org/10.3390/gels8020113
Chicago/Turabian StyleDettin, Monica, Martina Roso, Grazia M. L. Messina, Giovanna Iucci, Valentina Peluso, Teresa Russo, Annj Zamuner, Marta Santi, Sabrina Milan Manani, Monica Zanella, and et al. 2022. "Electrospun Chitosan Functionalized with C12, C14 or C16 Tails for Blood-Contacting Medical Devices" Gels 8, no. 2: 113. https://doi.org/10.3390/gels8020113
APA StyleDettin, M., Roso, M., Messina, G. M. L., Iucci, G., Peluso, V., Russo, T., Zamuner, A., Santi, M., Milan Manani, S., Zanella, M., Battocchio, C., Marletta, G., Modesti, M., Rassu, M., De Cal, M., & Ronco, C. (2022). Electrospun Chitosan Functionalized with C12, C14 or C16 Tails for Blood-Contacting Medical Devices. Gels, 8(2), 113. https://doi.org/10.3390/gels8020113