The Effect of Composition, Pre-Treatment on the Mechanical and Acoustic Properties of Apple Gels and Freeze-Dried Materials
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characteristics of Apple Gels
2.2. Characteristics of Dried Apple Gels
3. Conclusions
4. Materials and Methods
4.1. Materials and Production of Gels and Dried Materials
4.2. Measurements of Properties of Gels and Dried Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dille, M.; Draget, K.; Hattrem, M. The effect of filler particles on the texture of food gels. In Modifying Food Texture; Chen, J., Rosenthal, A., Eds.; Elsevier/Woodhead Publishing: Amsterdam, The Netherlands, 2015; pp. 183–200. [Google Scholar]
- Periche, A.; Heredia, A.; Escriche, I.; Andrés, A.; Castelló, M.L. Potential use of isomaltulose to produce healthier marshmallows. LWT 2015, 62, 605–612. [Google Scholar] [CrossRef]
- Tiwari, S.; Bhattacharya, S. Fabricated mango pulp-gellan gels: Effect of selected additives on rheological and sensory attributes. J. Food Qual. 2016, 39, 545–558. [Google Scholar] [CrossRef]
- Banerjee, S.; Ravi, R.; Bhattacharya, S. Textural characterisation of gellan and agar based fabricated gels with carrot juice. LWT 2013, 53, 255–261. [Google Scholar] [CrossRef]
- Pająk, P.; Fortuna, T.; Gałkowska, D. Rheological characteristics of sour cherries in gels containing waxy maize and cassava starches. J. Food Qual. 2012, 35, 401–410. [Google Scholar] [CrossRef]
- Genovese, D.B.; Ye, A.; Singh, H. High methoxyl pectin/apple particles composite gels: Effect of particle size and particle concentration on mechanical properties and gel structure. J. Tex. Stud. 2010, 41, 171–189. [Google Scholar] [CrossRef]
- Tiwari, S.; Ravi, R.; Bhattacharya, S. Dehumidifier assisted drying of a model fruit pulp-based gel and sensory attributes. J. Food Sci. 2012, 77, S263–S273. [Google Scholar] [CrossRef]
- Nussinovitch, A.; Jaffe, N.; Gillilov, M. Fractal pore-size distribution on freeze-dried agar-texturized fruit surfaces. Food Hydrocoll. 2004, 18, 825–835. [Google Scholar] [CrossRef]
- Jakubczyk, E.; Kamińska-Dwórznicka, A.; Ostrowska-Ligęza, E.; Górska, A.; Wirkowska-Wojdyła, M.; Mańko-Jurkowska, D.; Górska, A.; Bryś, J. Application of different compositions of apple puree gels and drying methods to fabricate snacks of modified structure, storage stability and hygroscopicity. Appl. Sci. 2021, 11, 10286. [Google Scholar] [CrossRef]
- Ozcelik, M.; Heigl, A.; Kulozik, U.; Ambros, S. Effect of hydrocolloid addition and microwave-assisted freeze drying on the characteristics of foamed raspberry puree. Inn. Food Sci. Emerg. Technol. 2019, 56, 102183. [Google Scholar] [CrossRef]
- Llavata, B.; García-Pérez, J.V.; Simal, S.; Cárcel, J.A. Innovative pre-treatments to enhance food drying: A current review. Curr. Opin. Food Sci. 2020, 35, 20–26. [Google Scholar] [CrossRef]
- Menon, A.; Stojceska, V.; Tassou, S.A. A systematic review on the recent advances of the energy efficiency improvements in non-conventional food drying technologies. Trends Food Sci. Technol. 2020, 100, 67–76. [Google Scholar] [CrossRef]
- Tsami, E.; Krokida, M.K.; Drouzas, A.E. Effect of drying method on the sorption characteristics of model fruit powders. J. Food Eng. 1998, 38, 381–392. [Google Scholar] [CrossRef]
- Tiwari, S.; Bhattacharya, S. Aeration of model gels: Rheological characteristics of gellan and agar gels. J. Food Eng. 2011, 107, 134–139. [Google Scholar] [CrossRef]
- López-Ramírez, A.M.; Duarte-Sierra, A. Avocado jelly: Formulation and optimization of an avocado gel using hydrocolloids. Int. J. Gastron. Food Sci. 2020, 21, 100234. [Google Scholar] [CrossRef]
- Sundaram, J.; Durance, T.D. Water sorption and physical properties of locust bean gum–pectin–starch composite gel dried using different drying methods. Food Hydrocoll. 2008, 22, 1352–1361. [Google Scholar] [CrossRef]
- Jakubczyk, E.; Kamińska-Dwórznicka, A. Effect of addition of chokeberry juice concentrate and foaming agent on the physical properties of agar gel. Gels 2021, 7, 137. [Google Scholar] [CrossRef]
- Nussinovitch, A.; Peleg, M. Mechanical properties of a raspberry product texturized with alginate. J. Food Proc. Preserv. 1990, 14, 267–278. [Google Scholar] [CrossRef]
- Peleg, M. On fundamental issues in texture evaluation and texturization—A view. Food Hydrocoll. 2006, 20, 405–414. [Google Scholar] [CrossRef]
- Barrangou, L.M.; Daubert, C.R.; Allen Foegeding, E. Textural properties of agarose gels. I. Rheological and fracture properties. Food Hydrocoll. 2006, 20, 184–195. [Google Scholar] [CrossRef]
- Jakubczyk, E.; Gondek, E.; Tryzno, E. Application of novel acoustic measurement techniques for texture analysis of co-extruded snacks. LWT 2017, 75, 582–589. [Google Scholar] [CrossRef]
- Jakubczyk, E.; Linde, M.; Gondek, E.; Kamińska-Dwórznicka, A.; Samborska, K.; Antoniuk, A. The effect of phytosterols addition on the textural properties of extruded crisp bread. J. Food Eng. 2015, 167, 156–161. [Google Scholar] [CrossRef]
- Ciurzyńska, A.; Marzec, A.; Mieszkowska, A.; Lenart, A. Structure influence on mechanical and acoustic properties of freeze-dried gels obtained with the use of hydrocolloids. J. Texture Stud. 2017, 48, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Erkinbaev, C.; Herremans, E.; Nguyen Do Trong, N.; Jakubczyk, E.; Verboven, P.; Nicolaï, B.; Saeys, W. Contactless and non-destructive differentiation of microstructures of sugar foams by hyperspectral scatter imaging. Innov. Food Sci. Emerg. Technol. 2014, 24, 131–137. [Google Scholar] [CrossRef]
- Herremans, E.; Bongaers, E.; Estrade, P.; Gondek, E.; Hertog, M.; Jakubczyk, E.; Nguyen Do Trong, N.; Rizzolo, A.; Saeys, W.; Spinelli, L.; et al. Microstructure–texture relationships of aerated sugar gels: Novel measurement techniques for analysis and control. Innov. Food Sci. Emerg. Technol. 2013, 18, 202–211. [Google Scholar] [CrossRef]
- Lewicki, P.P.; Marzec, A.; Ranachowski, Z. Acoustic properties of foods. In Food Properties Handbook; Rahman, M.S., Ed.; CRC Press: Boca Raton, FL, USA, 2009; pp. 811–841. [Google Scholar]
- Saeleaw, M.; Schleining, G. A review: Crispness in dry foods and quality measurements based on acoustic–mechanical destructive techniques. J. Food Eng. 2011, 105, 387–399. [Google Scholar] [CrossRef]
- Ramya, V.; Jain, N.K. A Review on osmotic dehydration of fruits and vegetables: An integrated approach. J. Food Proc. Eng. 2017, 40, e12440. [Google Scholar] [CrossRef]
- Shukla, B.; Singh, S. Osmo-convective drying of cauliflower, mushroom and greenpea. J. Food Eng. 2007, 80, 741–747. [Google Scholar] [CrossRef]
- Mathlouthi, M. Water content, water activity, water structure and the stability of foodstuffs. Food Contr. 2001, 12, 409–417. [Google Scholar] [CrossRef]
- Nussinovitch, A.; Kopelman, I.; Mizrahi, S. Mechanical properties of composite fruit products based on hydrocolloid gel, fruit pulp and sugar. LWT 1991, 24, 214–217. [Google Scholar]
- Bayarri, S.; Costell, E.; Duran, L. Influence of low sucrose concentrations on the compression resistance of gellan gum gels. Food Hydrocoll. 2002, 16, 593–597. [Google Scholar] [CrossRef]
- Fiszman, S.; Duran, L. Effects of fruit pulp and sucrose on the compression response of different polysaccharides gel systems. Carbohydr. Polym. 1992, 17, 11–17. [Google Scholar] [CrossRef]
- Kanyuck, K.M.; Mills, T.B.; Norton, I.T.; Norton-Welch, A.B. Temperature influences on network formation of low DE maltodextrin gels. Carbohydr. Polym. 2019, 218, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Loret, C.; Meunier, V.; Frith, W.J.; Fryer, P.J. Rheological characterisation of the gelation behaviour of maltodextrin aqueous solutions. Carbohydr. Polym. 2004, 57, 153–163. [Google Scholar] [CrossRef]
- Normand, V.; Plucknett, K.P.; Pomfret, S.J.; Ferdinando, D.; Norton, I.T. Large deformation mechanical behavior of gelatin-maltodextrin composite gels. J. Appl. Polym. Sci. 2001, 82, 124–135. [Google Scholar] [CrossRef]
- Loret, C.L.; Frith, W.J.; Fryer, P.J. Mechanical properties of maltodextrin gels: Small and large deformation. In Gums and Stabilisers for the Food Industry 12; Phillips, G.O., Williams, P.A., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2004; pp. 116–123. [Google Scholar] [CrossRef]
- Luyten, H.; Vliet, T.V. Acoustic emission, fracture behavior and morphology of dry crispy foods: A discussion article. J. Texture Stud. 2006, 37, 221–240. [Google Scholar] [CrossRef]
- Silva-Espinoza, M.A.; del Mar Camacho, M.; Martínez-Navarrete, N. Use of different biopolymers as carriers for purposes of obtaining a freeze-dried orange snack. LWT 2020, 127, 109415. [Google Scholar] [CrossRef]
- Telis, V.R.N.; Martínez-Navarrete, N. Collapse and color changes in grapefruit juice powder as affected by water activity, glass transition, and addition of carbohydrate polymers. Food Biophysic. 2009, 4, 83–93. [Google Scholar] [CrossRef]
- Cooke, D.; Gidley, M.; Hedges, N. Thermal properties of polysaccharides at low moisture: II. Molecular order and control of dissolution temperature in agar. J. Therm. Anal. Calorim. 1996, 47, 1485–1498. [Google Scholar] [CrossRef]
- Cassanelli, M.; Norton, I.; Mills, T. Interaction of mannitol and sucrose with gellan gum in freeze-dried gel systems. Food Biophys. 2018, 13, 304–315. [Google Scholar] [CrossRef] [Green Version]
- Velazquez-Gutierrez, S.K.; Figueira, A.C.; Rodriguez-Huezo, M.E.; Roman-Guerrero, A.; Carrillo-Navas, H.; Perez-Alonso, C. Sorption isotherms, thermodynamic properties and glass transition temperature of mucilage extracted from chia seeds (Salvia hispanica L.). Carbohydr. Polym. 2015, 121, 411–419. [Google Scholar] [CrossRef]
- Kilara, A.; Sengupta, T. Multi-textured foods. In Food Texture Design and Optimization; Dar, Y.L., Light, J.M., Eds.; John Wiley & Sons: Oxford, UK, 2014; pp. 159–221. [Google Scholar]
- Nussinovitch, A.; Corradini, M.; Normand, M.; Peleg, M. Effect of starch, sucrose and their combinations on the mechanical and acoustic properties of freeze-dried alginate gels. Food Res. Inter. 2001, 34, 871–878. [Google Scholar] [CrossRef]
- Roos, Y.H. Glass transition temperature and its relevance in food processing. Annu. Rev. Food Sci. Technol. 2010, 1, 469–496. [Google Scholar] [CrossRef] [PubMed]
- Slade, L.; Levine, H. Beyond water activity: Recent advances based on an alternative approach to the assessment of food quality and safety. Crit. Rev. Food Sci. Nutr. 1991, 30, 115–360. [Google Scholar] [CrossRef] [PubMed]
- Telis, V.; Sobral, P. Glass transitions and state diagram for freeze-dried pineapple. LWT 2001, 34, 199–205. [Google Scholar] [CrossRef]
- Mara Righetto, A.; Maria Netto, F. Effect of encapsulating materials on water sorption, glass transition and stability of juice from immature acerola. Int. J. Food Propert. 2005, 8, 337–346. [Google Scholar] [CrossRef]
- Gabas, A.; Telis, V.; Sobral, P.; Telis-Romero, J. Effect of maltodextrin and arabic gum in water vapor sorption thermodynamic properties of vacuum dried pineapple pulp powder. J. Food Eng. 2007, 82, 246–252. [Google Scholar] [CrossRef]
- Roos, Y.H.; Drusch, S. Phase Transitions in Foods; Academic Press: Oxford, UK, 2015; p. 380. [Google Scholar]
- Farahnaky, A.; Mansoori, N.; Majzoobi, M.; Badii, F. Physicochemical and sorption isotherm properties of date syrup powder: Antiplasticizing effect of maltodextrin. Food Bioprod. Processing 2016, 98, 133–141. [Google Scholar] [CrossRef]
- Pérez-Alonso, C.; Beristain, C.I.; Lobato-Calleros, C.; Rodríguez-Huezo, M.E.; Vernon-Carter, E.J. Thermodynamic analysis of the sorption isotherms of pure and blended carbohydrate polymers. J. Food Eng. 2006, 77, 753–760. [Google Scholar] [CrossRef]
- Djendoubi Mrad, N.; Bonazzi, C.; Boudhrioua, N.; Kechaou, N.; Courtois, F. Influence of sugar composition on water sorption isotherms and on glass transition in apricots. J. Food Eng. 2012, 111, 403–411. [Google Scholar] [CrossRef]
- Roopa, B.; Bhattacharya, S. Texturized alginate gels: Screening experiments to identify the important variables on gel formation and their properties. LWT 2010, 43, 1403–1408. [Google Scholar] [CrossRef]
- Kasapis, S. Glass transition phenomena in dehydrated model systems and foods: A review. Dry. Technol. 2005, 23, 731–757. [Google Scholar] [CrossRef]
- Pittia, P.; Sacchetti, G. Antiplasticization effect of water in amorphous foods. A review. Food Chem. 2008, 106, 1417–1427. [Google Scholar] [CrossRef]
- Mascia, L.; Kouparitsas, Y.; Nocita, D.; Bao, X. Antiplasticization of polymer materials: Structural aspects and effects on mechanical and diffusion-controlled properties. Polymers 2020, 12, 769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seow, C.; Cheah, P.; Chang, Y. Antiplasticization by water in reduced-moisture food systems. J. Food Sci. 1999, 64, 576–581. [Google Scholar] [CrossRef]
- Iglesias, H.; Galmarini, M.V.; Díaz Barrios, L.F.; Chirife, J. Kinetics of Water Sorption and Sugar Crystallization in Freeze-Dried Bananas Previously Immersed in Concentrated Sucrose and Trehalose Solution. An. Asoc. Química Argent. 2017, 104. Available online: https://repositorio.uca.edu.ar/handle/123456789/5499 (accessed on 8 November 2021).
- Harnkarnsujarit, N.; Charoenrein, S. Effect of water activity on sugar crystallization and β-carotene stability of freeze-dried mango powder. J. Food Eng. 2011, 105, 592–598. [Google Scholar] [CrossRef]
- Zogzas, N.; Maroulis, Z.; Marinos-Kouris, D. Densities, shrinkage and porosity of some vegetables during air drying. Dry. Technol. 1994, 12, 1653–1666. [Google Scholar] [CrossRef]
- Jakubczyk, E.; Gondek, E.; Kamińska-Dwórznicka, A.; Samborska, K.; Wiktor, A.; Królikowski, K. A complex approach to assessing properties of aerated agar-fructose gels: Application of acoustic emission technique. Food Hydrocoll. 2019, 91, 66–75. [Google Scholar] [CrossRef]
- Ostrowska-Ligęza, E.; Dolatowska-Żebrowska, K.; Wirkowska-Wojdyła, M.; Bryś, J.; Górska, A. Comparison of thermal characteristics and fatty acids composition in raw and roasted cocoa beans from Peru (Criollo) and Ecuador (Forastero). Appl. Sci. 2021, 11, 2698. [Google Scholar] [CrossRef]
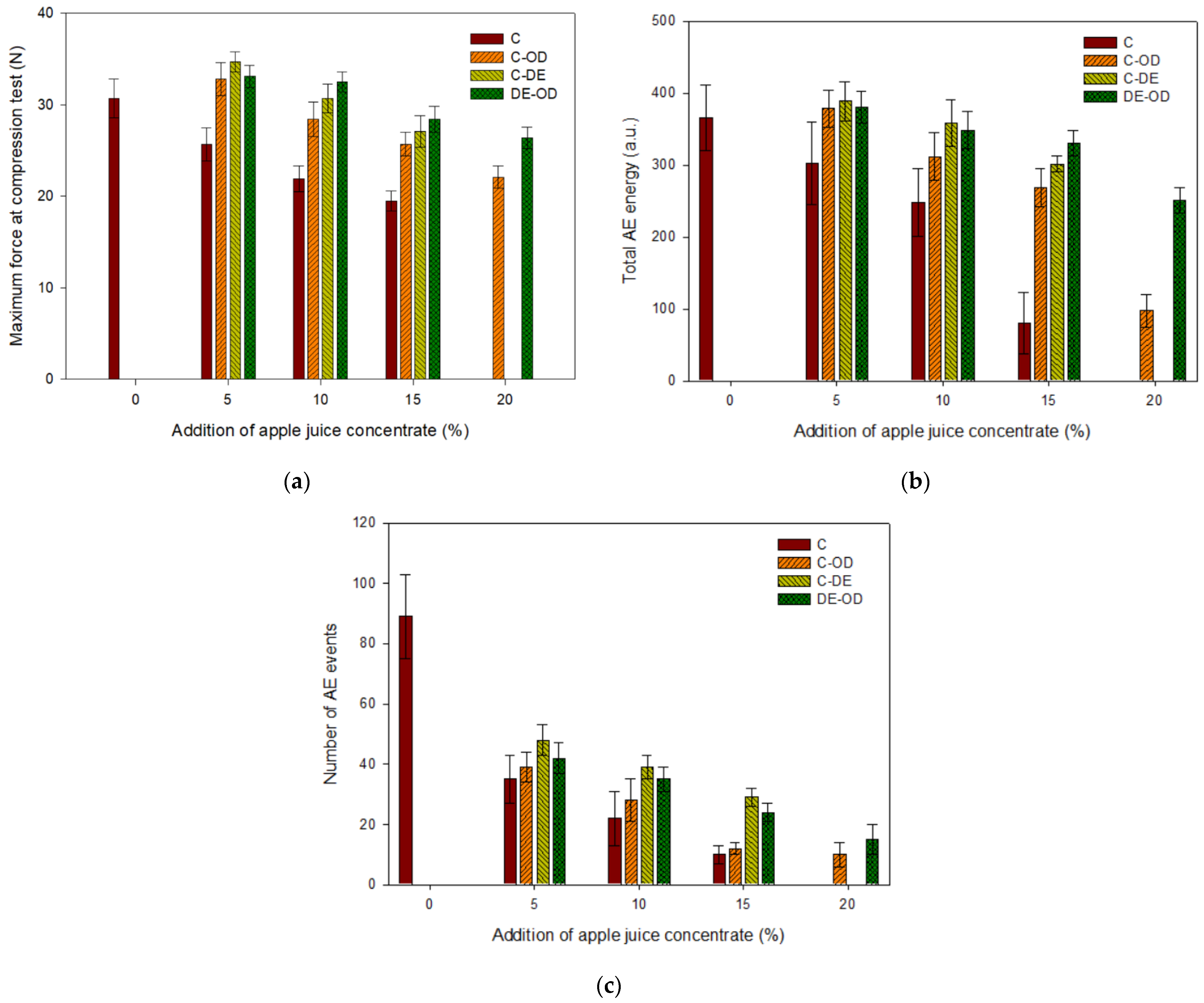
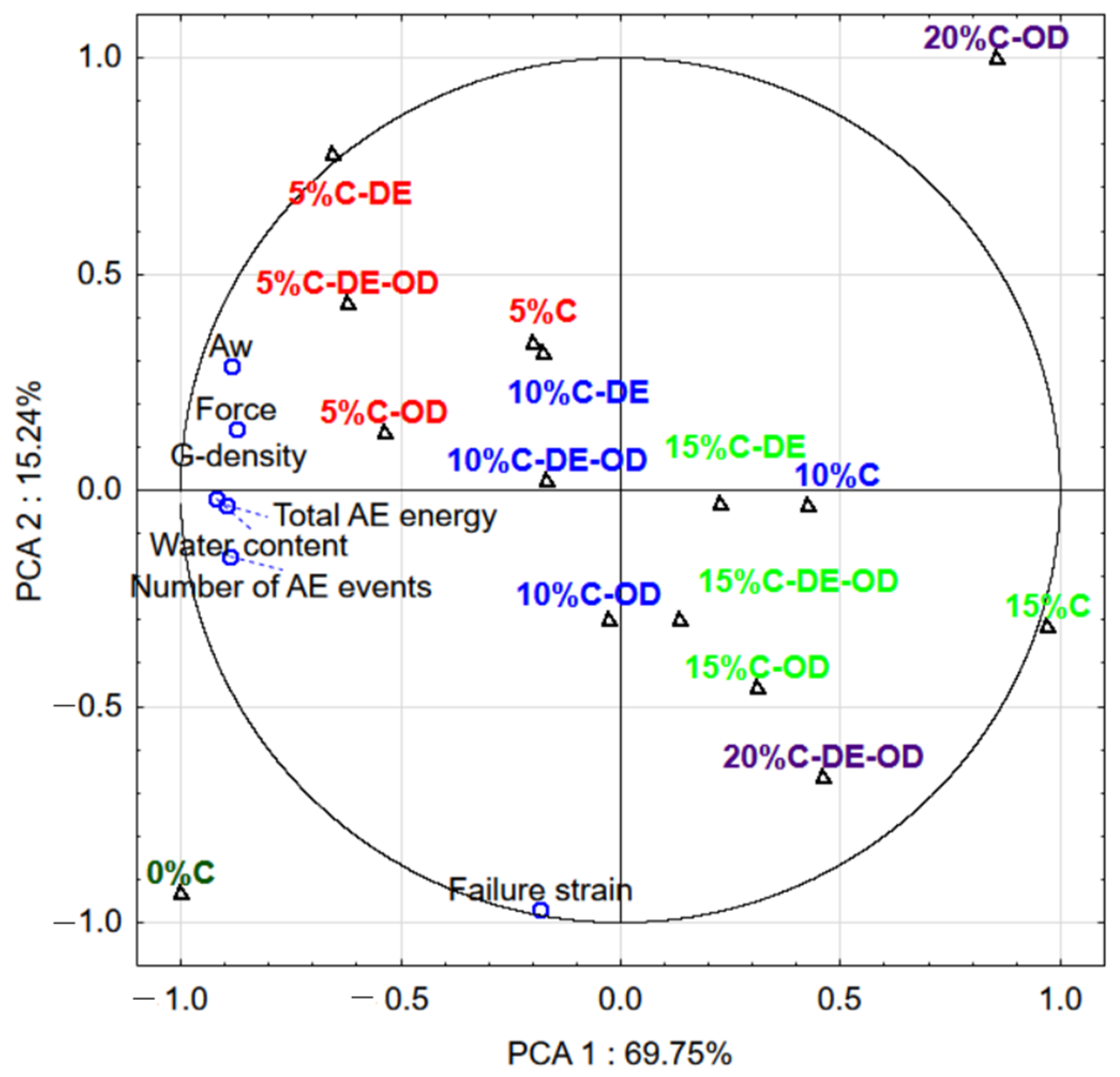

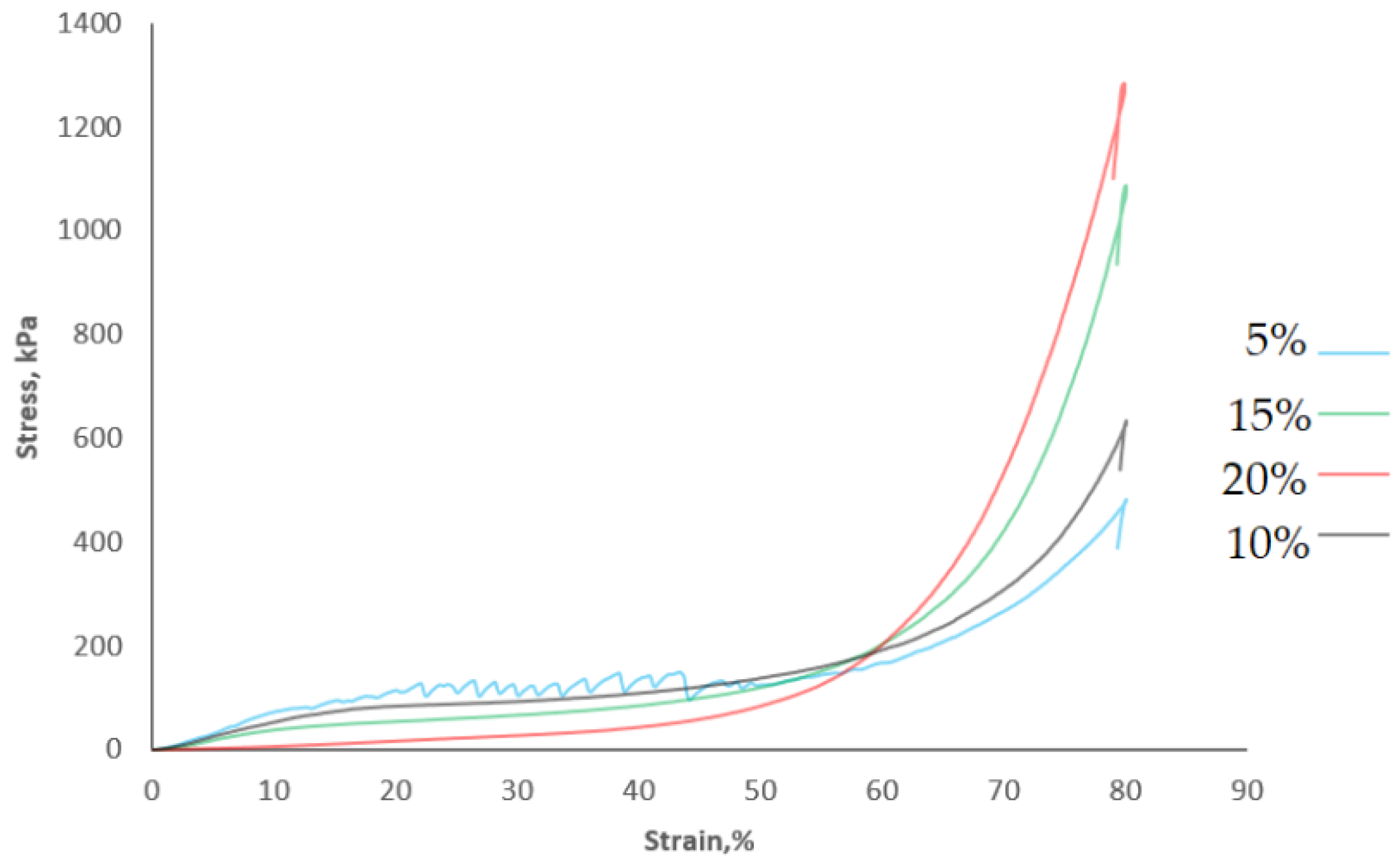


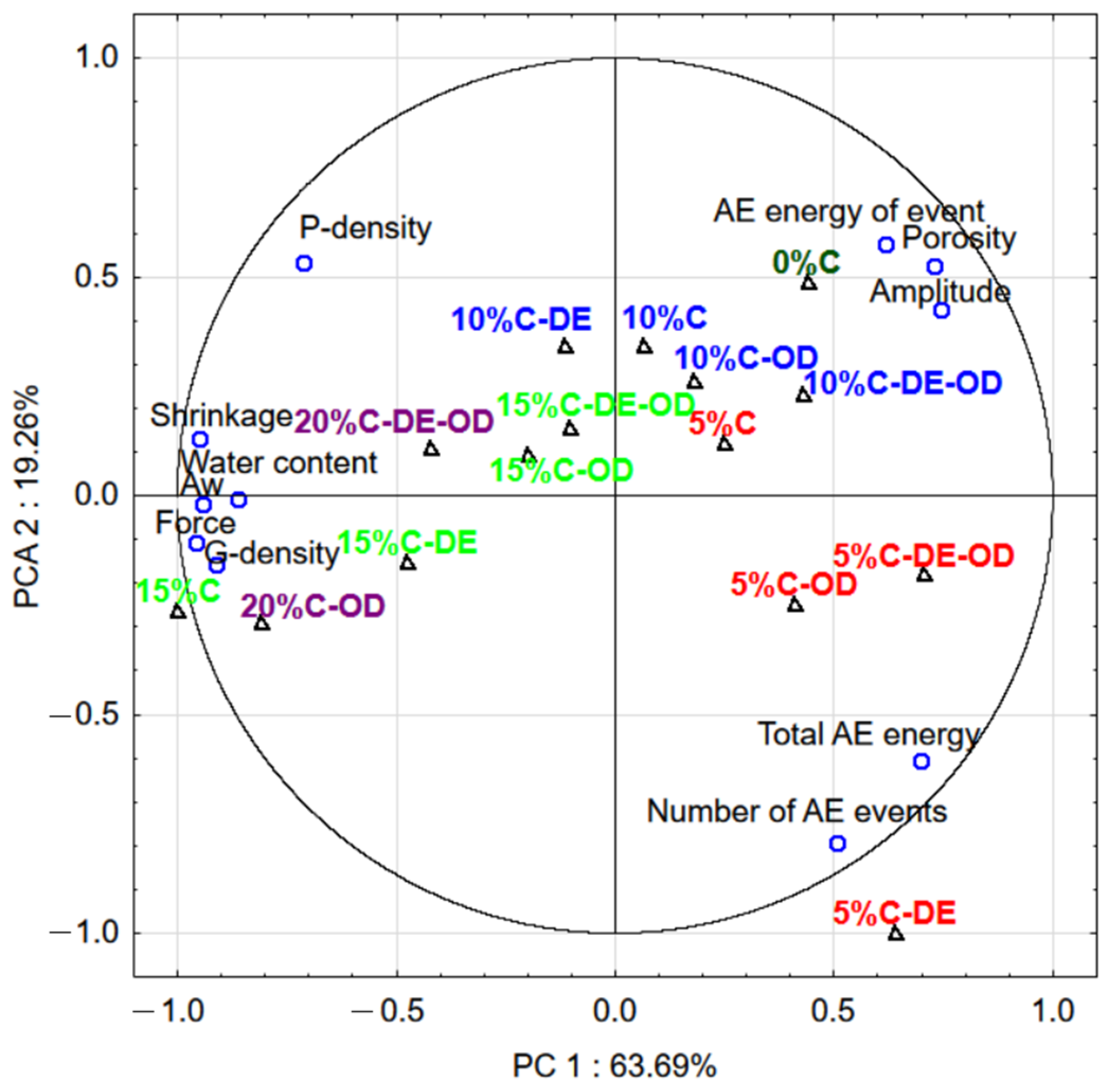
| Samples | Water Content, % | Water Activity | Geometric Density, g∙cm−3 | Failure Strain, % |
|---|---|---|---|---|
| 0%C | 98.1 ± 0.1 a* | 0.991 ± 0.004 ab | 1.12 ± 0.03 g | 40.1 ± 1.2 a |
| 5%C | 93.6 ± 0.2 c | 0.988 ± 0.001 bc | 1.14 ± 0.01 fg | 35.4 ± 0.9 ef |
| 10%C | 87.4 ± 0.1 g | 0.985 ± 0.002 bc | 1.20 ± 0.01 cdef | 36.4 ± 0.8 cdef |
| 15%C | 83.4 ± 0.2 l | 0.983 ± 0.001 c | 1.23 ± 0.02 abcd | 37.4 ± 0.5 bcde |
| 5%C-OD | 95.4 ± 0.4 b | 0.988 ± 0.003 bc | 1.13 ± 0.01 g | 36.4 ± 1.4 cdef |
| 10%C-OD | 90.6 ± 0.1 e | 0.986 ± 0.001 bc | 1.18 ± 0.03 defg | 37.8 ± 0.5 bcd |
| 15%C-OD | 88.1 ± 0.1 g | 0.985 ± 0.003 bc | 1.17 ± 0.02 defg | 38.4 ± 0.4 abc |
| 20%C-OD | 85.3 ± 0.2 h | 0.983 ± 0.002 c | 1.20 ± 0.01 cdef | 32.4± 0.1 g |
| 5%C-DE | 92.1 ± 0.2 d | 0.994 ± 0.003 a | 1.16 ± 0.02 efg | 35.0 ± 0.8 f |
| 10%C-DE | 89.0 ± 0.5 f | 0.990 ± 0.003 abc | 1.23 ± 0.03 abcd | 35.9 ± 0.6 def |
| 15%C-DE | 85.9 ± 0.4 h | 0.987 ± 0.002 bc | 1.27 ± 0.01 ab | 36.9 ± 0.4 cdef |
| 5%C-DE-OD | 93.8 ± 0.2 c | 0.993 ± 0.002 ab | 1.15 ± 0.02 efg | 36.0 ± 0.3 def |
| 10%C-DE-OD | 89.1 ± 0.1 f | 0.988 ± 0.005 abc | 1.21 ± 0.03 bcde | 37.0 ± 0.5 bcdef |
| 15%C-DE-OD | 87.4 ± 0.2 g | 0.986± 0.002 bc | 1.25 ± 0.01 abc | 37.9 ± 0.3 bcd |
| 20%C-DE-OD | 85.3 ± 0.4 h | 0.984 ± 0.002 c | 1.29 ± 0.02 a | 39.1 ± 0.7 ab |
| Samples | Water Content, % | Water Activity | Geometric Density, g∙cm−3 | Pycnometric Density, g∙cm−3 | Porosity, % | Shrinkage |
|---|---|---|---|---|---|---|
| 0%C | 8.12 ± 0.22 d* | 0.239 ± 0.003 g | 0.16 ± 0.04 i | 0.65 ± 0.02 h | 77.4 ± 3.6 a | 0.258 ± 0.032 ef |
| 5%C | 5.69 ± 0.03 g | 0.245 ± 0.002 fg | 0.44 ± 0.01 fgh | 1.12 ± 0.04 d | 61.2 ± 0.5 cd | 0.152 ± 0.061 gh |
| 10%C | 6.24 ± 0.15 g | 0.249 ± 0.009 fg | 0.47 ± 0.01 fg | 1.27 ± 0.05 c | 63.3 ± 0.5 c | 0.432 ± 0.021 cd |
| 15%C | 17.42 ± 0.62 a | 0.601 ± 0.002 a | 0.95 ± 0.01 a | 1.28 ± 0.03 c | 26.0 ± 0.5 h | 0.753 ± 0.042 a |
| 5%C-OD | 4.46 ± 0.09 h | 0.158 ± 0.006 i | 0.51 ± 0.05 ef | 0.99 ± 0.01 e | 50.2 ± 2.9 ef | 0.065 ± 0.015 hi |
| 10%C-OD | 6.96 ± 0.12 f | 0.277 ± 0.001 e | 0.41 ± 0.03 gh | 1.17 ± 0.01 d | 65.8 ± 1.5 bc | 0.199 ± 0.034 fg |
| 15%C-OD | 7.76 ± 0.46 e | 0.306 ± 0.003 d | 0.57 ± 0.01 de | 1.34 ± 0.01 c | 57.7 ± 0.4 cd | 0.414 ± 0.042 cd |
| 20%C-OD | 12.17 ± 0.03 b | 0.451 ± 0.002 b | 0.99 ± 0.01 a | 1.42 ± 0.01 ab | 30.5 ± 0.4 h | 0.601 ± 0.022 b |
| 5%C-DE | 4.32 ± 0.13 h | 0.184 ± 0.013 h | 0.42 ± 0.03 gh | 0.75 ± 0.02 g | 45.3 ± 2.3 fg | 0.012 ± 0.017 i |
| 10%C-DE | 8.36 ± 0.16 de | 0.400 ± 0.008 c | 0.64 ± 0.01 cd | 1.31 ± 0.01 c | 51.4 ±0.4 e | 0.595 ± 0.021 b |
| 15%C-DE | 8.13 ± 0.14 e | 0.401 ± 0.003 c | 0.74 ± 0.01 b | 1.30 ± 0.03 c | 43.3 ± 0.4 g | 0.516 ± 0.043 bc |
| 5%C-DE-OD | 4.30 ± 0.21 h | 0.137 ± 0.002 j | 0.36 ± 0.04 h | 0.89 ± 0.01 f | 61.0 ± 2.6 cd | 0.072 ± 0.053 hi |
| 10%C-DE-OD | 3.96 ± 0.14 h | 0.149 ± 0.005 ij | 0.44 ± 0.04 fgh | 1.40 ± 0.04 ab | 69.5 ±1.6 b | 0.099 ± 0.032 ghi |
| 15%C-DE-OD | 5.99 ± 0.05 g | 0.258 ± 0.009 f | 0.71 ± 0.03 bc | 1.41 ± 0.04 ab | 50.4 ±1.2 ef | 0.357 ± 0.019 de |
| 20%C-DE-OD | 9.78 ± 0.07 c | 0.403 ± 0.007 c | 0.79 ± 0.01 b | 1.43 ± 0.02 a | 45.0 ± 0.4 g | 0.507 ± 0.026 bc |
| Samples | Tg onset | Tg midpoint | Tg endpoint |
|---|---|---|---|
| 0%C | 88.74 | 94.03 | 98.12 |
| 5%C | 27.90 | 33.11 | 39.41 |
| 15%C | −35.24 | −24.36 | −29.75 |
| 5%C-OD | 46.13 | 52.75 | 59.00 |
| 15%C-OD | 9.99 | 16.91 | 23.82 |
| 5%C-DE | 40.43 | 44.68 | 48.93 |
| 15%C-DE | −2.93 | 0.54 | 8.01 |
| 5%C-DE-OD | 56.15 | 61.63 | 67.11 |
| 15%C-DE-OD | 7.00 | 12.56 | 18.13 |
| 20%C-DE-OD | −24.44 | −16.32 | −20.81 |
| Samples | AE Energy of Event, pJ | Amplitude, mV |
|---|---|---|
| 0%C | 3890.3 ± 1779.0 a* | 545.7 ± 162.9 ab |
| 5%C | 2358.5 ± 832.6 ab | 457.0 ± 160.3 ab |
| 10%C | 4186.3 ± 1655.6 a | 718.7 ± 181.9 a |
| 15%C | 1232.2 ± 745.2 ab | 125.3 ± 32.1 cde |
| 5%C-OD | 1767.4 ± 641.9 ab | 425.1 ± 85.6 abc |
| 10%C-OD | 3407.3 ± 1608.7 ab | 497.50 ± 52.8 ab |
| 15%C-OD | 1699.5 ± 922.1 ab | 353.5 ± 51.5 bcde |
| 20%C-OD | 452.1 ± 99.4 b | 85.4 ± 11.1 e |
| 5%C-DE | 2013.2 ± 935.4 ab | 458.0 ± 118.0 ab |
| 10%C-DE | 3688.2 ± 999.9 a | 681.7 ± 147.6 a |
| 15%C-DE | 1201.1 ± 101.1 ab | 98.8 ± 14.7 ed |
| 5%C-DE-OD | 3887.7 ± 840.4 a | 675.6 ± 88.9 ab |
| 10%C-DE-OD | 2872.9 ± 804.8 ab | 603.7 ± 159.3 ab |
| 15%C-DE-OD | 2349.8 ± 936.5 ab | 403.9 ± 54.3 abcde |
| 20%C-DE-OD | 2321.1 ± 935.6 ab | 416.0 ± 64.8 abcd |
| Type of Gel | Apple Juice Concentrate, % | Agar Powder, % | Maltodextrin, % | Water, % |
|---|---|---|---|---|
| 0%C | 0 | 2.0 | 0.0 | 98.0 |
| 5%C | 5 | 2.0 | 0.0 | 93.0 |
| 10%C | 10 | 2.0 | 0.0 | 88.0 |
| 15%C | 15 | 2.0 | 0.0 | 83.0 |
| 5%C-DE | 5.0 | 2.0 | 1.8 | 91.2 |
| 10%C-DE | 10.0 | 2.0 | 3.5 | 84.5 |
| 15%C-DE | 15.0 | 2.0 | 5.0 | 78.0 |
| Type of Gel | Agar Powder, % | Maltodextrin, % | Water, % |
|---|---|---|---|
| 5%C-OD | 2.0 | 0.0 | 98.0 |
| 10%C-OD | 2.0 | 0.0 | 98.0 |
| 15%C-OD | 2.0 | 0.0 | 98.0 |
| 20%C-OD | 2.0 | 0.0 | 98.0 |
| 5%C-DE-OD | 2.0 | 1.8 | 96.2 |
| 10%C-DE-OD | 2.0 | 3.5 | 94.5 |
| 15%C-DE-OD | 2.0 | 5.0 | 93.0 |
| 20%C-DE-OD | 2.0 | 7.0 | 91.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakubczyk, E.; Kamińska-Dwórznicka, A.; Ostrowska-Ligęza, E. The Effect of Composition, Pre-Treatment on the Mechanical and Acoustic Properties of Apple Gels and Freeze-Dried Materials. Gels 2022, 8, 110. https://doi.org/10.3390/gels8020110
Jakubczyk E, Kamińska-Dwórznicka A, Ostrowska-Ligęza E. The Effect of Composition, Pre-Treatment on the Mechanical and Acoustic Properties of Apple Gels and Freeze-Dried Materials. Gels. 2022; 8(2):110. https://doi.org/10.3390/gels8020110
Chicago/Turabian StyleJakubczyk, Ewa, Anna Kamińska-Dwórznicka, and Ewa Ostrowska-Ligęza. 2022. "The Effect of Composition, Pre-Treatment on the Mechanical and Acoustic Properties of Apple Gels and Freeze-Dried Materials" Gels 8, no. 2: 110. https://doi.org/10.3390/gels8020110
APA StyleJakubczyk, E., Kamińska-Dwórznicka, A., & Ostrowska-Ligęza, E. (2022). The Effect of Composition, Pre-Treatment on the Mechanical and Acoustic Properties of Apple Gels and Freeze-Dried Materials. Gels, 8(2), 110. https://doi.org/10.3390/gels8020110







